-
PDF
- Split View
-
Views
-
Cite
Cite
Joseph A Picoraro, Dale Lee, Caren A Heller, Alandra Weaver, Jeffrey S Hyams, Laurie S Conklin, Anthony Otley, David Ziring, Subra Kugathasan, Joel R Rosh, Andrew Mulberg, Lee A Denson, Michael D Kappelman, Andrew B Grossman, Athos Bousvaros, K T Park, Pediatric Inflammatory Bowel Disease Clinical Innovations Meeting of the Crohn’s & Colitis Foundation: Charting the Future of Pediatric IBD, Inflammatory Bowel Diseases, Volume 25, Issue 1, January 2019, Pages 27–32, https://doi.org/10.1093/ibd/izy205
Close - Share Icon Share
Abstract
The Crohn’s & Colitis Foundation has facilitated transformational research in pediatric inflammatory bowel disease (IBD), through the RISK and PROTECT studies, that has laid the groundwork for a comprehensive understanding of molecular mechanisms of disease and predictors of therapeutic response in children. Despite these advances, children have lacked timely and informed access to the latest therapeutic advancements in IBD. The Crohn's & Colitis Foundation convened a Pediatric Resource Organization for Kids with Inflammatory Intestinal Diseases (PRO-KIIDS) Clinical Innovations Meeting at the inaugural Crohn's and Colitis Congress in January 2018 to devise how to advance the care of children with IBD. The working group selected 2 priorities: (1) accelerating therapies to children with IBD and (2) stimulating investigator-initiated research while fostering sustainable collaboration; and proposed 2 actions: (a) the convening of a task force to specifically address how to accelerate pharmacotherapies to children with IBD and (b) the funding of a multicenter clinical and translational research study that incorporates the building of critical research infrastructure.
INTRODUCTION
Children and adolescents with inflammatory bowel disease (IBD) have unique needs due to the importance of supporting growth and development. With the highest peak incidence of new diagnosis of IBD occurring before 20 years of age and the expectation of lifelong therapy, the development of innovative therapies for children and adolescents is a top priority and a current gap in patient care.1 Children and adolescents furthermore provide unique insights into the pathophysiology and natural history of IBD due to the strong genetic association and decreased impact of environmental factors such as smoking and medications.
The Crohn’s & Colitis Foundation has actively supported pediatric research, initially by funding pediatric investigator-initiated studies. In 2003, the Foundation sponsored a clinical trials meeting that led to the development of criteria for differentiating between Crohn’s disease (CD) and ulcerative colitis (UC)2 and to the development of a composite clinical scoring system for pediatric ulcerative colitis.3, 4 In 2005, the first Challenges in Pediatric IBD meeting was held, which identified a number of research priorities, including genetics, bone health, growth, behavioral health, and quality improvement.5 Subsequent initiatives arising from this meeting included a funded initiative on pediatric skeletal health (led by Francisco Sylvester)6 and the development of the multicenter Pediatric RISK Stratification study (led by Subra Kugathasan and Lee Denson). Success from the Foundation-funded RISK study in turn led to an National Institutes of Health–funded study evaluating predictors of complicated pediatric UC (the PROTECT study, led by Jeffrey Hyams). As these large cohort studies near completion, the Foundation convened a Pediatric IBD Clinical Innovations Meeting at the inaugural Crohn’s and Colitis Congress in January 2018 to devise how to accelerate the delivery of new therapies to children and adolescents with IBD.
The Pediatric Resource Organization for Kids with Inflammatory Intestinal Diseases (PRO-KIIDS) Clinical Innovations meeting involved representation from a wide number of stakeholders, including academia, industry, government, the Foundation, and parents of children with IBD (see the “Acknowledgments” for a full list of participants). At the conclusion of this meeting, 2 primary priorities were finalized: (1) expediting delivery of new therapies to children with IBD and (2) developing another large multicenter clinical and translational study for the PRO-KIIDS network.
STATEMENT OF PROBLEM
Children and adolescents with IBD lack data-driven and timely access to the newest pharmacotherapies available to adult patients. Research advances, including large-scale pediatric trials necessary to bring new therapies to pediatric patients, require investment of significant resources to stimulate and sustain collaboration across various stakeholders.
CURRENT PROGRESS
Collaborative research from the Foundation’s PRO-KIIDS network has transformed our understanding of the multifactorial etiology of IBD, including genetics, the intestinal microbiome, environmental exposures, and immunological factors.7 The RISK study, which enrolled 1096 children with newly diagnosed CD with follow-up over approximately 3 years, characterized the natural history of pediatric CD and demonstrated that early anti–tumor necrosis factor–alpha therapy was associated with a reduction in penetrating complications (abscess and internal fistulae), though not a reduction in fibrostenosing disease.8 A predictive model furthermore identified specific demographic, serologic, and transcriptomic elements associated with disease complications.9 The PROTECT study, a prospective cohort of 428 children with newly diagnosed UC, evaluated standardized therapy for UC in children and identified key predictors of treatment response.10 Both RISK and PROTECT are landmark PRO-KIIDS network studies that have accelerated the understanding of critically important aspects of CD and UC. They have fostered the development of risk stratification to individualize treatment strategies aimed to optimize long-term health, beginning with childhood IBD diagnosis.
BUILDING ON SUCCESS
Although the RISK and PROTECT multicenter studies laid foundations for the development of therapeutic strategies for pediatric IBD, therapeutic options for children with IBD remain limited. The most transformative therapy for IBD, infliximab, did not achieve a Food and Drug Administration (FDA) label for pediatric patients until 8 years after the label was approved for adults with IBD. During this period, infliximab was utilized off-label for children with CD and UC refractory to thiopurines; corticosteroids, with their extensive adverse effects, were employed broadly. The challenges of off-label use of medications for pediatric IBD and other pediatric diseases persist. Approximately 50% of pediatric medications in chronically ill children are used off-label to benefit individual patients. According to the American Academy of Pediatrics position statement on off-label use, “Off label use remains a persistent public health issue, especially, for infants, young children, and children with rare illnesses…. Much work remains to be done to ensure the best possible practice for therapeutic decision-making in pediatrics.”11 Currently, adalimumab in UC, certolizumab in CD, ustekinumab in CD, and vedolizumab in CD and UC all have adult but not pediatric indications.
At present, off-label use of medications for pediatric IBD is widespread despite the absence of rigorous pediatric pharmacokinetic (pK) and pharmacodynamic (pD) data. This has resulted in the extrapolation of adult pK/pD data to children despite known differences in metabolic profile and physiology.12 Though many new medications are available to adults, the lack of pediatric studies incorporated into drug development has severely limited the informed use of potentially disease course–altering therapies in children. Numerous obstacles have made the execution of pediatric trials difficult, including a lack of uniform assessments of disease activity and clinical trial end points, suboptimal partnership with industry during drug development, lack of pediatric-specific multicenter research infrastructure, lesser financial incentive when compared with drug development for adult patients, and heightened logistical barriers and ethical issues in pediatric clinical trials.
In light of these issues specific to pediatric research, we propose (1) the development of a task force to specifically address the need to accelerate the delivery of new therapeutics to children with IBD and (2) the continued growth of rigorous, well-coordinated research in the pediatric population through the ongoing investment in novel investigator-initiated research studies, and with an explicit focus on creating sustainable infrastructure to support future pediatric trials.
ACCELERATE THERAPIES TO CHILDREN WITH IBD
Pediatric gastroenterologists act as strong advocates for their patients, prescribing potentially life-altering medications in the absence of pK/pD and safety data, and often encounter third-party payer hurdles. The Pediatric Research Equity Act, enacted in the United States in 2003,13 gives the FDA authority to mandate pharmaceutical companies to provide safety and effectiveness data for pediatric patients. However, the time frame is open, and market incentives to expedite the process are lacking. The 21st Century Cures Act, enacted in 2016,14 may accelerate drug approvals in general, but the implications for children remain unclear. Pediatric gastroenterologists need adequate dosing and safety data to effectively prescribe medications to children; a system that fosters the availability of safer and more effective therapies to adults without inclusion of children further disadvantages this “vulnerable” population. Reform is necessary and can be achieved, through both mandates and incentives, and by partnering with regulatory bodies and pharmaceutical companies, to ensure this critical information is available at the time of initial FDA approval.
Several strategies may provide the requisite data to appropriately guide the dosing of new medications in pediatric patients and could concomitantly lead to pediatric indication in a timely manner—inclusion of adolescent (12–17 years) subjects in adult trials, extrapolation of adult pK/pD data, and creating a system to gather pK/pD phase II pediatric studies during late phase II/phase III clinical trials in adults. Adolescent inclusion could lead to approved indication for 70% of the pediatric IBD population, but study of drugs in a population of research subjects with less experience with placebo-controlled trials may disincentivize pharmaceutical companies to pursue this approach. Extrapolation may be more feasible and, if paired with early pediatric pK/pD data, could sufficiently inform pediatric dosing.
Most IBD is treated with immune-suppressing agents, which run rare but serious risks of opportunistic infections and lymphoma. Such complications are particularly concerning to parents of children, who desire accurate data regarding the long-term risks of medication. Obtaining accurate safety data on immune-modifying therapies in IBD necessitates robust, multicenter, longitudinal, and coordinated safety monitoring. Industry-sponsored registries, such as DEVELOP and CAPE, provide crucial data but are proprietary. A unified public drug safety registry, such as the CARRAnet registry developed by the pediatric rheumatology community, would provide more valuable information about the natural history of pediatric IBD than individual company registries that only assess the safety of a single drug.
In addition to dosing and safety, global harmonization of clinical end points in pediatric IBD is essential. Currently, there is a lack of uniform data types and standard measures. The Clinical Data Interchange Standards Consortium (CDISC) facilitates the harmonization of terminology to be used across parties invested in clinical research, including the pharmaceutical industry, government agencies, academia, health care providers, subjects, patients, and technology providers, among others.15 CDISC standards have been applied to specific disease states, resulting in unified data elements pertinent to the disease, known as therapeutic area user guides (TAUGs). TAUGs have accelerated the discovery of biomarkers in polycystic kidney disease and malaria. The development of a TAUG for pediatric IBD, or IBD generally, would advance global harmonization of data and may accelerate drug development through reduction of inefficiency and fostering of collaboration.
A recent major change in the design of pediatric and adult IBD clinical trials has been the desire of regulatory bodies to de-emphasize composite end points of disease activity (eg, the Pediatric Crohn Disease Activity Index [PCDAI] and the Pediatric Ulcerative Colitis Activity Index [PUCAI]). Instead, the FDA and European Medicines Agency (EMA) have supported the development of patient-reported outcomes (PROs) and the need for validated indices of endoscopic disease activity.16, 17 To address the need for reliable and valid PROs, 2 separate projects are underway to develop and validate PROs for Crohn’s disease (TUMMY-CD) and ulcerative colitis (TUMMY-UC) in pediatrics. These measures are being developed with the guidance of the FDA and EMA to ensure that they comply with regulatory standards that will apply to future drug development. Open and responsive dialogue between the FDA, EMA, and investigators is crucial to the timely development and validation of TUMMY-CD and TUMMY-UC. A coordinated effort to implement these measures in clinical trial design through partnership with industry should follow. Additional work needs to be done to further validate measurements of endoscopic healing, such as the Simple Endoscopic Score for Crohn’s disease (SES-CD), and determine the need for central reading, interobserver variability, and reference timelines for expected endoscopic healing. The correlation between endoscopic disease activity and less invasive assessments (including disease activity measures, PROs, noninvasive biomarkers, and diagnostic imaging) should also be a focus of future research.
The pediatric population provides a unique opportunity to elucidate molecular mechanisms of disease and determine targeted therapeutic approaches that incorporate biomarker guidance. Translational studies of the RISK and PROTECT cohorts hold the potential to provide a paradigm shift in delivering on the promise of applying the right therapy to the right patient at the right time. Incorporation of prospective multi-omic data into clinical trials is crucial, and academic–industry partnerships hold promise.
PROPOSED ACTION 1: CONVENE AND CHARGE TASK FORCE
We propose the convening of a task force with the charge to develop a strategic plan that spans academia, clinicians, industry, regulatory agencies, and patients/families to accelerate new therapies to children with IBD. This task force should be composed of a pediatric gastroenterologist, IBD clinical trial researcher, drug development expert from industry, patient and caregiver, clinical research coordinator, biostatistician/bioinformaticist, regulatory agency representative, formulary expert from a third-party payer, and a pediatric rheumatologist or oncologist with experience in well-developed research infrastructure (Table 1).
| Clinical | Pediatric gastroenterologist |
| Patient and caregiver | |
| Pediatric rheumatologist or oncologist | |
| Research | IBD clinical trial researcher |
| Biostatistician and bioinformaticist | |
| Clinical research coordinator | |
| Industry | Specialist on drug development |
| Regulatory | Agency representative |
| Payer | Formulary expert from third-party payer |
| Clinical | Pediatric gastroenterologist |
| Patient and caregiver | |
| Pediatric rheumatologist or oncologist | |
| Research | IBD clinical trial researcher |
| Biostatistician and bioinformaticist | |
| Clinical research coordinator | |
| Industry | Specialist on drug development |
| Regulatory | Agency representative |
| Payer | Formulary expert from third-party payer |
| Clinical | Pediatric gastroenterologist |
| Patient and caregiver | |
| Pediatric rheumatologist or oncologist | |
| Research | IBD clinical trial researcher |
| Biostatistician and bioinformaticist | |
| Clinical research coordinator | |
| Industry | Specialist on drug development |
| Regulatory | Agency representative |
| Payer | Formulary expert from third-party payer |
| Clinical | Pediatric gastroenterologist |
| Patient and caregiver | |
| Pediatric rheumatologist or oncologist | |
| Research | IBD clinical trial researcher |
| Biostatistician and bioinformaticist | |
| Clinical research coordinator | |
| Industry | Specialist on drug development |
| Regulatory | Agency representative |
| Payer | Formulary expert from third-party payer |
The task force would determine the optimal strategy to ensure that dosing for pediatric IBD is available for newly developed therapies at the time of FDA approval (Fig. 1). This may include clinician/industry partnership during drug development and advocacy for federal legislation. The task force would outline the optimal design of a patient safety registry in pediatric IBD. This may align with current efforts underway. The task force would determine the optimal method to federate clinical trial data for pediatric IBD. This may include the creation of a TAUG with CDISC standards, a uniform structure of pediatric study plans submitted to the FDA by industry, the use of “basket trial” methodology, and an overarching infrastructure for clinical trials, such as a master protocol or a shared control group. The task force may also clarify the role of biomarker development and investigation during drug development.
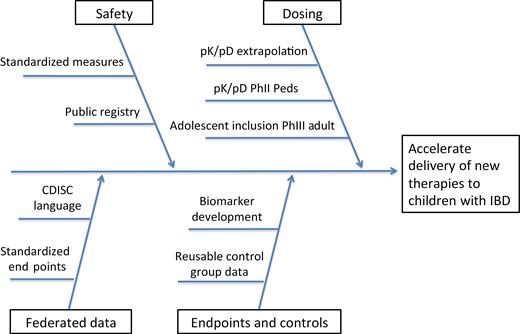
Opportunities to advance pharmacotherapies in pediatric IBD. Standardized measures include acvity indices, patient-reported outcomes, endoscopic score. Abbreviations: pD, pharmacodynamic; PhII, phase 2; PhIII, phase 3; pK, pharmacokine c.
PROPOSED ACTION 2: COPRODUCTION OF NEW RESEARCH INITIATIVES WITH INFRASTRUCTURE
To build upon the collaborative research framework from RISK and PROTECT, we propose that future studies within the PRO-KIIDS network foster innovative investigator-initiated pilot studies while developing sustainable infrastructure for future trials. Support for pilot studies will help facilitate the development of young investigators and also help fund the study of high-risk, high-reward hypotheses with the potential to advance pediatric IBD research. Executing the completion of these projects in tandem with infrastructure building will allow continuity of research questions and expedite novel questions from hypothesis-generating to validation and clinical application.
The infrastructure to help PRO-KIIDS sites be “trial-ready” may include creation of database resources geared toward supporting clinical trials, developing centralized resources for sample collection and reading, and providing resources for research coordinator support and training (Fig. 2). Building of infrastructure that is transferrable among projects will benefit future investigators, eliminate waste, and expedite study design and implementation. Infrastructure development should incorporate nonexclusive resources that will facilitate interested sites to join any given PRO-KIIDS research consortium study.
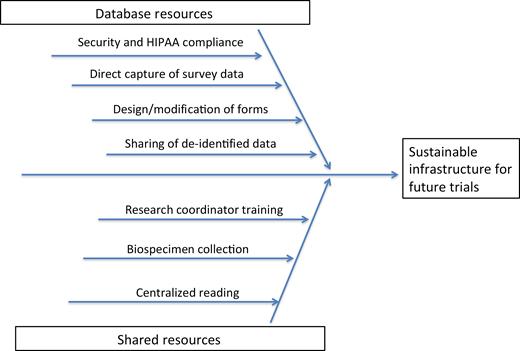
Innovative pediatric studies on the role of genetics and environmental triggers may provide unique insight into understanding IBD pathogenesis. This knowledge may facilitate the development of novel technologies and therapeutic approaches that impact both children and adults. Projects assessing the validity of adult measures (ie, Short Endoscopic Index of CD Severity [SES-CD]) or endoscopic Mayo score in children may help facilitate the incorporation of children into adult trials, and similar projects to define end points in pediatric trials will also accelerate the delivery of therapies to children.
CONCLUSION
The future of IBD holds great promise for children. The Crohn’s & Colitis Foundation has facilitated transformational research in RISK and PROTECT that has laid the groundwork for a comprehensive understanding of molecular mechanisms of disease and predictors of therapeutic response in children. Despite these advances, children have lacked timely and informed access to the latest therapeutic advancements in IBD. The working group of the Pediatric IBD Clinical Innovations meeting at the inaugural Crohn’s & Colitis Foundation Congress selected 2 priorities to advance the care of children with IBD: (1) accelerating therapies to children with IBD and (2) stimulating investigator-initiated research while fostering sustainable collaboration. We recommend the convening of a task force to specifically address how to accelerate pharmacotherapies to children with IBD and the funding of a multicenter clinical and translational research study that incorporates the building of critical research infrastructure. The PRO-KIIDS network has helped establish a foundation for pediatric IBD research, and notable opportunities exist for further breakthroughs utilizing a strong collaborative approach.
Conflicts of interest: Caren Heller is an employee of the Crohn’s & Colitis Foundation. Alandra Weaver is an employee of the Crohn’s & Colitis Foundation. Jeffrey Hyams has served on the advisory boards of AbbVie and Janssen. He has consulted for Allergan, Boehringer-Ingelheim, Eli Lilly, Mallinkrodt, Pfizer, Receptos, and Roche. Laurie Conklin is a part-time employee of ReveraGen Biopharma, LLC. She has served on the advisory board of Janssen. Anthony Otley has received research support from AbbVie, Janssen, Astellas, Shire, and Takeda. He has served on the advisory boards of AbbVie and Janssen. David Ziring has served on the speakers’ bureau for Janssen. Subra Kugathasan has served on the advisory boards and consulted for AbbVie and Janssen. He has served on the data and safety monitoring board for Takeda. Joel Rosh has served on the advisory boards and consulted for AbbVie, Celgene, Janssen, Luitpold, and Pfizer. He has received research funding from Abbvie and Janssen. Andrew Mulberg is an employee and shareholder of Amicus Therapeutics, Inc. Lee Denson has received research support from Janssen. Michael Kappelman has received research support from AbbVie and Johnson & Johnson. He has consulted for AbbVie, Eli Lilly, Johnson & Johnson, and Pfizer. He is a shareholder in Johnson & Johnson. Athos Bousvaros has received research support from AbbVie and Prometheus Laboratories. He has performed research for Janssen. He has served on the data and safety monitoring board for Shire. He has received royalties from Up to Date. K. T. Park has received research support from AbbVie Inc., Buhlmann Laboratories, Takeda, and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. He has consulted for Inova Diagnostics and Prometheus Laboratories.
Supported by:
Members of the Pediatric IBD Clinical Innovations Workgroup: Clinical Trial Design Issues Affecting Regulatory Approval: Richard Colletti, Marla Dubinsky, Stacy Kahn, Jessica Lee, Rick Strauss, Harland Winter, David Ziring, Eric Zuckerman; Optimal Use of Diagnostic Modalities and Currently Available Therapies: Ajay Gulati, Anne Griffiths, Shehzad Saeed, Mel Heyman, Francisco Sylvester, Jeremy Adler, Robert Baldassano, Jess Kaplan; Changing the Treatment Paradigm for Children: Michael Rosen, Sandra Kim, David Suskind, Richard Kellermeyer, Paul Rufo, Jonathan Moses.
Participants: Jeremy Adler, MD, Robert Baldassano, MD, Athos Bousvaros, MD, MPH, Richard B. Colletti, MD, Laurie Conklin, MD, Lee A. Denson, MD, Marla Dubinsky, MD, Anne Griffiths, MD, Andrew Grossman, MD, Ajay Gulati, MD, Caren A. Heller, MD, Mel Heyman, MD, Jeffrey S. Hyams, MD, Laura Lee Johnson, PhD, Stacy Kahn, MD, Jess Kaplan, MD, Michael Kappelman, MD, MPH, Richard Kellermayer, MD, PhD, Sandra Kim, MD, Subra Kugathasan, MD, Dale Lee, MD, Jessica Lee, MD, Jonathan D. Moses, MD, Andrew Mulberg, MD, Anthony Otley, MD, K. T. Park, MD, MS, Joseph A. Picoraro, MD, Brent Polk, MD, Shervin Rabizadeh, MD, Michael Rosen, MD, MSCI, Joel Rosh, MD, Paul Rufo, MD, Shehzad Saeed, MD, Scott Snapper, MD, PhD, Rick Strauss, MD, David Suskind, MD, Alandra Weaver, MPH, Harland Winter, MD, Eric Zuckerman, DO.
ACKNOWLEDGMENTS
We would like to thank all meeting participants for their insights and input. Financial support for this workshop was provided, in part, by sponsorships from AbbVie and Eli Lilly and Company.
REFERENCES
Author notes
These authors are co-first authors.



