-
PDF
- Split View
-
Views
-
Cite
Cite
Weston Bettner, Anthony Rizzo, Steven Brant, Sharon Dudley-Brown, Jonathan Efron, Sandy Fang, Susan Gearhart, Michael Marohn, Alyssa Parian, Maryam Kherad Pezhouh, Joanna Melia, Bashar Safar, Brindusa Truta, Elizabeth Wick, Mark Lazarev, Low Incidence of Dysplasia and Colorectal Cancer Observed among Inflammatory Bowel Disease Patients with Prolonged Colonic Diversion, Inflammatory Bowel Diseases, Volume 24, Issue 5, May 2018, Pages 1092–1098, https://doi.org/10.1093/ibd/izx102
Close - Share Icon Share
Abstract
In inflammatory bowel disease (IBD), many scenarios call for fecal diversion, leaving behind defunctionalized bowel. The theoretical risk of colorectal cancer (CRC) in this segment is frequently cited as a reason for resection. To date, no studies have characterized the incidence of neoplasia in the diverted colorectal segments of IBD patients.
A retrospective cohort analysis was conducted for IBD patients identified through a tertiary care center pathology database. Patients that had undergone colorectal diversion and were diverted for ≥ 1 year were included. Incidence of diverted dysplasia/CRC was calculated for Crohn’s disease (CD) and ulcerative colitis (UC) with respect to diverted patient-years (dpy) and patient-years of disease (pyd).
In total, 154 patients comprising 754 dpy and 1984 pyd were analyzed. Only 2 cases of diverted colorectal dysplasia (CD 1, UC 1) and 1 case of diverted CRC (UC) were observed. In the UC cohort (n = 75), the rate of diversion-associated CRC was 4.5 cases/1000 dpy (95% CI 0.11–25/1000) or 1.5 cases/1000 pyd (95% CI 0.04–8.2/1000). In the CD cohort (n = 79), no patients developed CRC, although a dysplasia rate of 1.9 cases/1000 dpy (95% CI 0.05–11/1000) or 0.77 cases/1000 pyd (95% CI 0.02–4.3/1000) was observed. All patients developing neoplasia had disease duration > 10 years and microscopic inflammation.
Diverted dysplasia occurred infrequently with rates overlapping those reported in registries for IBD-based rectal cancers. Neoplasia was undetected in patients with < 10 pyd, regardless of diversion duration, suggesting low yield for endoscopic surveillance before this time.
INTRODUCTION
An estimated 0.4% of North Americans have an inflammatory bowel disease (IBD) diagnosis with approximately 13% of ulcerative colitis (UC) patients and 29% of Crohn’s disease (CD) patients requiring intestinal resection within 7 years of diagnosis.1–3 UC patients commonly undergo 2 or 3 stage restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA). CD patients operated on for refractory disease or severe perianal disease frequently undergo a staged approach with a diverting ostomy.4 Re-anastomosis may be delayed, sometimes indefinitely, on account of refractory disease, concern for poor wound healing, lack of clinical follow-up, or patient preference.5
While the risk of colorectal cancer (CRC) in IBD populations has been well-described,6–8 a relative paucity of data exist regarding the risk of CRC in diverted IBD bowel segments. Case reports of cancer in diverted large bowel and rectal stumps have been published.4, 9–17 The current risk remains unclear, particularly in light of recent evidence suggesting a decreasing risk of CRC amongst IBD populations over time,8, 17 a trend that has been attributed to better pharmacological control of inflammation, improved colonoscopic surveillance, increased implementation of colectomy, and an effect of aging IBD cohorts, with sick patients previously censored.8, 18 Currently, there are no consensus guidelines to inform surveillance endoscopy for diverted segments.
The aim of the present study was to analyze a cohort of IBD patients with prolonged diversion, defined as ≥12 months, to characterize the incidence of dysplasia or cancer observed in chronically diverted colorectal segments.
MATERIALS AND METHODS
Patients
A retrospective chart review was conducted on IBD patients undergoing colonic resection at the Johns Hopkins Hospital from 1990–2015. The hospital’s pathology database was queried using the following search parameters: “Crohn’s” AND “diversion, takedown, or resection” and “Ulcerative Colitis” AND “diversion, takedown, or resection.” For each identified patient, the electronic medical record (EMR) was manually reviewed to confirm the diagnosis of IBD, and patients were included for the study if they had: (1) a confirmed diagnosis of UC or CD, (2) a diverted colorectal segment for ≥12 months, and (3) a resection and/or endoscopy of the diverted segment after ≥12 months.
Calculations and Statistical Analyses
Duration of colonic diversion, duration of disease, rate of dysplasia/cancer, and diverted bowel length were calculated using data from the EMR (See Supplemental Tables 4, 5). The duration of colonic diversion was calculated using the length of time between the initial diversion and the last recorded endoscopy, resection, or reanastomosis of the diverted segment. For patients undergoing multiple diversions and reanastomoses, only the longest period of continuous diversion was included for analysis. Disease duration was calculated using length of time between the age at diagnosis and the last recorded procedure that provided pathology (endoscopy, resection, or reanastomosis of the diverted segment).
Diverted bowel dysplasia and cancer rates were calculated in the context of both diverted patient-years (dpy) and patient-years of disease (pyd). Confidence intervals were calculated using the function poisson.test in RStudio (Version 0.99.903) assuming a Poisson distribution for incidence (see Supplemental Methods).19
Diverted bowel length was calculated using the largest dimension recorded during surgical resection or the farthest depth probed on endoscopy, whichever was larger. Diverted bowel length figures were computed for the subset of patients within each cohort for which we were able to extract bowel length measurements from surgical pathology or endoscopy reports.
ETHICAL CONSIDERATIONS
The research protocol was approved by the Johns Hopkins Medicine Institutional Review Board.
RESULTS
The pathology database query initially identified 1179 CD and 753 UC patients as potential study candidates. Of these 1932 prospective IBD patients, 79 CD and 75 UC patients met inclusion criteria (Fig. 1).
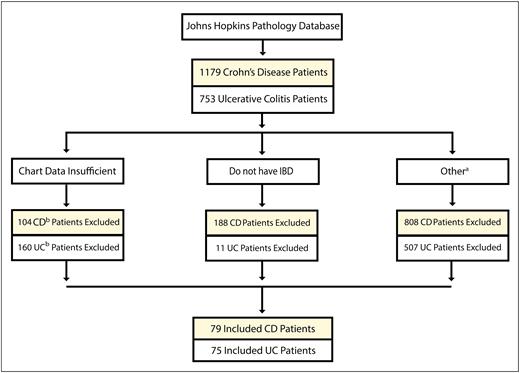
Patient selection flow chart for CD and UC. IBD–Inflammatory Bowel Disease. aDiverted for less than a year, never diverted, diverted but lost to follow-up, diverted but no histopathological data, dysplasia in the resection margins at the time of diversion (see Supplemental Methods). bCD and UC.
CD patients were more likely to be female than UC patients (61% vs 44%, respectively) (Table 1). CD patients most commonly had a penetrating phenotype (44%), ileocolonic involvement (60%), and concomitant perianal disease (68%). UC patients predominantly had pancolitis (64%). Age at diagnosis was earlier for CD versus UC patients (22 years vs 29 years) and disease duration was longer for CD versus UC patients (16 years vs 6.4 years).
| Disease . | CD (n = 79) Number (Percent)a . | UC (n = 75) Number (Percent) . |
|---|---|---|
| Demographics | ||
| Sex | ||
| Male/Female | 31 (39%) / 48 (61%) | 42 (56%) / 33 (44%) |
| Race | ||
| White | 64 (81%) | 62 (82%) |
| African American | 13 (17%) | 7 (9.2%) |
| Disease Characteristics | ||
| Age at Diagnosis (years): median (range) | 22 (4–82) | 29 (4–79) |
| Disease Duration (years): median (range) | 16 (1.7–53) | 6.4 (1–31) |
| Family History of CRC | 8 (10%) | 4 (5.3%) |
| Personal History of PSC | 0 (0%) | 4 (5.3%) |
| Montreal Criteria for CDb | ||
| B1 (Nonstricturing and nonpenetrating) | 23 (32%) | ----- |
| B2 (Stricturing) | 17 (24%) | ----- |
| B3 (Penetrating) | 32 (44%) | ----- |
| p (Perianal Modifier) | 51 (68%) | ----- |
| Location | ||
| L1 (Ileal) | 1 (1.3%) | ----- |
| L2 (Colonic) | 30 (39%) | ----- |
| L3 (Ileocolonic) | 46 (60%) | ----- |
| Concomitant L4 (Upper GI) | 14 (18%) | ----- |
| Age at Diagnosis (years) | ||
| A1 (≤ 16) | 22 (29%) | ----- |
| A2 (17–40) | 41 (54%) | ----- |
| A3 (≥ 40) | 13 (17%) | ----- |
| Montreal Criteria for UC | ||
| UC Disease Location | ||
| E1 (Ulcerative Proctitis) | ----- | 0 (0%) |
| E2 (Left Sided or Distal) | ----- | 24 (32%) |
| E3 (Extensive or Pancolitis) | ----- | 49 (64%) |
| Unknown | ----- | 2 (2.7%) |
| Disease . | CD (n = 79) Number (Percent)a . | UC (n = 75) Number (Percent) . |
|---|---|---|
| Demographics | ||
| Sex | ||
| Male/Female | 31 (39%) / 48 (61%) | 42 (56%) / 33 (44%) |
| Race | ||
| White | 64 (81%) | 62 (82%) |
| African American | 13 (17%) | 7 (9.2%) |
| Disease Characteristics | ||
| Age at Diagnosis (years): median (range) | 22 (4–82) | 29 (4–79) |
| Disease Duration (years): median (range) | 16 (1.7–53) | 6.4 (1–31) |
| Family History of CRC | 8 (10%) | 4 (5.3%) |
| Personal History of PSC | 0 (0%) | 4 (5.3%) |
| Montreal Criteria for CDb | ||
| B1 (Nonstricturing and nonpenetrating) | 23 (32%) | ----- |
| B2 (Stricturing) | 17 (24%) | ----- |
| B3 (Penetrating) | 32 (44%) | ----- |
| p (Perianal Modifier) | 51 (68%) | ----- |
| Location | ||
| L1 (Ileal) | 1 (1.3%) | ----- |
| L2 (Colonic) | 30 (39%) | ----- |
| L3 (Ileocolonic) | 46 (60%) | ----- |
| Concomitant L4 (Upper GI) | 14 (18%) | ----- |
| Age at Diagnosis (years) | ||
| A1 (≤ 16) | 22 (29%) | ----- |
| A2 (17–40) | 41 (54%) | ----- |
| A3 (≥ 40) | 13 (17%) | ----- |
| Montreal Criteria for UC | ||
| UC Disease Location | ||
| E1 (Ulcerative Proctitis) | ----- | 0 (0%) |
| E2 (Left Sided or Distal) | ----- | 24 (32%) |
| E3 (Extensive or Pancolitis) | ----- | 49 (64%) |
| Unknown | ----- | 2 (2.7%) |
Percentages were calculated out of the total number of patients in each cohort–CD (79), UC (75). In several cases, the denominator was changed to reflect the number of scorable individuals within each cohort. All numbers calculated to 2 significant figures where appropriate unless otherwise specified.
aThe listed values are the number and (percent), respectively, unless otherwise stated.
bSee Supplemental Methods Section for more information on the Montreal Criteria.32
| Disease . | CD (n = 79) Number (Percent)a . | UC (n = 75) Number (Percent) . |
|---|---|---|
| Demographics | ||
| Sex | ||
| Male/Female | 31 (39%) / 48 (61%) | 42 (56%) / 33 (44%) |
| Race | ||
| White | 64 (81%) | 62 (82%) |
| African American | 13 (17%) | 7 (9.2%) |
| Disease Characteristics | ||
| Age at Diagnosis (years): median (range) | 22 (4–82) | 29 (4–79) |
| Disease Duration (years): median (range) | 16 (1.7–53) | 6.4 (1–31) |
| Family History of CRC | 8 (10%) | 4 (5.3%) |
| Personal History of PSC | 0 (0%) | 4 (5.3%) |
| Montreal Criteria for CDb | ||
| B1 (Nonstricturing and nonpenetrating) | 23 (32%) | ----- |
| B2 (Stricturing) | 17 (24%) | ----- |
| B3 (Penetrating) | 32 (44%) | ----- |
| p (Perianal Modifier) | 51 (68%) | ----- |
| Location | ||
| L1 (Ileal) | 1 (1.3%) | ----- |
| L2 (Colonic) | 30 (39%) | ----- |
| L3 (Ileocolonic) | 46 (60%) | ----- |
| Concomitant L4 (Upper GI) | 14 (18%) | ----- |
| Age at Diagnosis (years) | ||
| A1 (≤ 16) | 22 (29%) | ----- |
| A2 (17–40) | 41 (54%) | ----- |
| A3 (≥ 40) | 13 (17%) | ----- |
| Montreal Criteria for UC | ||
| UC Disease Location | ||
| E1 (Ulcerative Proctitis) | ----- | 0 (0%) |
| E2 (Left Sided or Distal) | ----- | 24 (32%) |
| E3 (Extensive or Pancolitis) | ----- | 49 (64%) |
| Unknown | ----- | 2 (2.7%) |
| Disease . | CD (n = 79) Number (Percent)a . | UC (n = 75) Number (Percent) . |
|---|---|---|
| Demographics | ||
| Sex | ||
| Male/Female | 31 (39%) / 48 (61%) | 42 (56%) / 33 (44%) |
| Race | ||
| White | 64 (81%) | 62 (82%) |
| African American | 13 (17%) | 7 (9.2%) |
| Disease Characteristics | ||
| Age at Diagnosis (years): median (range) | 22 (4–82) | 29 (4–79) |
| Disease Duration (years): median (range) | 16 (1.7–53) | 6.4 (1–31) |
| Family History of CRC | 8 (10%) | 4 (5.3%) |
| Personal History of PSC | 0 (0%) | 4 (5.3%) |
| Montreal Criteria for CDb | ||
| B1 (Nonstricturing and nonpenetrating) | 23 (32%) | ----- |
| B2 (Stricturing) | 17 (24%) | ----- |
| B3 (Penetrating) | 32 (44%) | ----- |
| p (Perianal Modifier) | 51 (68%) | ----- |
| Location | ||
| L1 (Ileal) | 1 (1.3%) | ----- |
| L2 (Colonic) | 30 (39%) | ----- |
| L3 (Ileocolonic) | 46 (60%) | ----- |
| Concomitant L4 (Upper GI) | 14 (18%) | ----- |
| Age at Diagnosis (years) | ||
| A1 (≤ 16) | 22 (29%) | ----- |
| A2 (17–40) | 41 (54%) | ----- |
| A3 (≥ 40) | 13 (17%) | ----- |
| Montreal Criteria for UC | ||
| UC Disease Location | ||
| E1 (Ulcerative Proctitis) | ----- | 0 (0%) |
| E2 (Left Sided or Distal) | ----- | 24 (32%) |
| E3 (Extensive or Pancolitis) | ----- | 49 (64%) |
| Unknown | ----- | 2 (2.7%) |
Percentages were calculated out of the total number of patients in each cohort–CD (79), UC (75). In several cases, the denominator was changed to reflect the number of scorable individuals within each cohort. All numbers calculated to 2 significant figures where appropriate unless otherwise specified.
aThe listed values are the number and (percent), respectively, unless otherwise stated.
bSee Supplemental Methods Section for more information on the Montreal Criteria.32
The most common reason for diversion was refractory perianal disease in CD patients (49%) and treatment refractory disease in UC (91%) (Table 2). Compared to UC, the CD cohort had a longer duration of diversion (median 4.2 years vs 1.7 years) but fewer endoscopies per diverted years (one every 3.4 years vs every 2.1 years). Microscopically active inflammation was present in the majority of CD and UC patients (82% and 95%, respectively). Stricturing in CD (27%) and pseudopolyp formation in UC (33%) were the most common macroscopic findings at the time of endoscopy or surgical resection.
| Disease . | CD (n = 79) Number (Percent)a . | UCs (n = 75) Number (Percent) . |
|---|---|---|
| Top Reasons for Diversion | ||
| Perianal Disease | 39 (49%) | ----- |
| Perforation | 18 (23%) | ----- |
| Unknown | 10 (13%) | ----- |
| Treatment Refractory Disease | ----- | 68 (91%) |
| Dysplasia | ----- | 4 (5.3%) |
| Toxic Megacolon | ----- | 2 (2.7%) |
| Otherb | 15 (19%) | 1 (1.3%) |
| Diversion Characteristics | ||
| Age at Diversion (years): median (range) | 36 (5–82) | 32 (5.7–82) |
| Diversion Duration (years): median (range) | 4.2 (1–29) | 1.7 (1–19) |
| Ostomy Characteristics | ||
| Diverted Segment Type: | ||
| Hartmann’s Pouch | 60 (76%) | 75 (100%) |
| Other | 19 (24%) | ----- |
| Length (cm): median (range) | 18 (2–100) | 16 (0.5–68) |
| Endoscopy Detailsc | ||
| Patients Undergoing Endoscopy | 69 (76%) | 75 (100%) |
| Number of Endoscopies: median (range) | 2 (0–12) | 1 (0–6) |
| Total Number of Endoscopies | 155 | 106 |
| Average Time Between Endoscopies | 3.4 years | 2.1 years |
| Findings on Endoscopy or Resection | ||
| Microscopically Active Inflammation | 63 (82%) | 71 (95%) |
| Stricturing/Narrowing | 21 (27%) | 5 (6.7%) |
| Fistula | 15 (19%) | 3 (4.0%) |
| Inflammatory Pseudopolyps/ Nodules | 11 (14%) | 25 (33%) |
| Nondysplastic Polyps | 0 (0%) | 6 (8.0%) |
| Disease . | CD (n = 79) Number (Percent)a . | UCs (n = 75) Number (Percent) . |
|---|---|---|
| Top Reasons for Diversion | ||
| Perianal Disease | 39 (49%) | ----- |
| Perforation | 18 (23%) | ----- |
| Unknown | 10 (13%) | ----- |
| Treatment Refractory Disease | ----- | 68 (91%) |
| Dysplasia | ----- | 4 (5.3%) |
| Toxic Megacolon | ----- | 2 (2.7%) |
| Otherb | 15 (19%) | 1 (1.3%) |
| Diversion Characteristics | ||
| Age at Diversion (years): median (range) | 36 (5–82) | 32 (5.7–82) |
| Diversion Duration (years): median (range) | 4.2 (1–29) | 1.7 (1–19) |
| Ostomy Characteristics | ||
| Diverted Segment Type: | ||
| Hartmann’s Pouch | 60 (76%) | 75 (100%) |
| Other | 19 (24%) | ----- |
| Length (cm): median (range) | 18 (2–100) | 16 (0.5–68) |
| Endoscopy Detailsc | ||
| Patients Undergoing Endoscopy | 69 (76%) | 75 (100%) |
| Number of Endoscopies: median (range) | 2 (0–12) | 1 (0–6) |
| Total Number of Endoscopies | 155 | 106 |
| Average Time Between Endoscopies | 3.4 years | 2.1 years |
| Findings on Endoscopy or Resection | ||
| Microscopically Active Inflammation | 63 (82%) | 71 (95%) |
| Stricturing/Narrowing | 21 (27%) | 5 (6.7%) |
| Fistula | 15 (19%) | 3 (4.0%) |
| Inflammatory Pseudopolyps/ Nodules | 11 (14%) | 25 (33%) |
| Nondysplastic Polyps | 0 (0%) | 6 (8.0%) |
Percentages were calculated out of the total number of patients in each cohort–CD (79), UC (75). In several cases, the denominator was changed to reflect the number of scorable individuals within each cohort. All numbers calculated to 2 significant figures where appropriate unless otherwise specified.
aThe listed values are the number and percent, respectively, unless otherwise stated.
bOther causes for diversion in CD were: bleeding/diverticulitis/diarrhea–6 (7.6%), medically refractory disease–3 (3.8%), structuring–3 (3.8%), colonic mass–2 (3.0%), and severe C. difficile–1 (1.0%). Other causes for diversion in UC were fungemia–1 (1.3%). One case of CD had CRC before diversion but this was not the reason for diversion.
cRefers to endoscopies during diversion.
| Disease . | CD (n = 79) Number (Percent)a . | UCs (n = 75) Number (Percent) . |
|---|---|---|
| Top Reasons for Diversion | ||
| Perianal Disease | 39 (49%) | ----- |
| Perforation | 18 (23%) | ----- |
| Unknown | 10 (13%) | ----- |
| Treatment Refractory Disease | ----- | 68 (91%) |
| Dysplasia | ----- | 4 (5.3%) |
| Toxic Megacolon | ----- | 2 (2.7%) |
| Otherb | 15 (19%) | 1 (1.3%) |
| Diversion Characteristics | ||
| Age at Diversion (years): median (range) | 36 (5–82) | 32 (5.7–82) |
| Diversion Duration (years): median (range) | 4.2 (1–29) | 1.7 (1–19) |
| Ostomy Characteristics | ||
| Diverted Segment Type: | ||
| Hartmann’s Pouch | 60 (76%) | 75 (100%) |
| Other | 19 (24%) | ----- |
| Length (cm): median (range) | 18 (2–100) | 16 (0.5–68) |
| Endoscopy Detailsc | ||
| Patients Undergoing Endoscopy | 69 (76%) | 75 (100%) |
| Number of Endoscopies: median (range) | 2 (0–12) | 1 (0–6) |
| Total Number of Endoscopies | 155 | 106 |
| Average Time Between Endoscopies | 3.4 years | 2.1 years |
| Findings on Endoscopy or Resection | ||
| Microscopically Active Inflammation | 63 (82%) | 71 (95%) |
| Stricturing/Narrowing | 21 (27%) | 5 (6.7%) |
| Fistula | 15 (19%) | 3 (4.0%) |
| Inflammatory Pseudopolyps/ Nodules | 11 (14%) | 25 (33%) |
| Nondysplastic Polyps | 0 (0%) | 6 (8.0%) |
| Disease . | CD (n = 79) Number (Percent)a . | UCs (n = 75) Number (Percent) . |
|---|---|---|
| Top Reasons for Diversion | ||
| Perianal Disease | 39 (49%) | ----- |
| Perforation | 18 (23%) | ----- |
| Unknown | 10 (13%) | ----- |
| Treatment Refractory Disease | ----- | 68 (91%) |
| Dysplasia | ----- | 4 (5.3%) |
| Toxic Megacolon | ----- | 2 (2.7%) |
| Otherb | 15 (19%) | 1 (1.3%) |
| Diversion Characteristics | ||
| Age at Diversion (years): median (range) | 36 (5–82) | 32 (5.7–82) |
| Diversion Duration (years): median (range) | 4.2 (1–29) | 1.7 (1–19) |
| Ostomy Characteristics | ||
| Diverted Segment Type: | ||
| Hartmann’s Pouch | 60 (76%) | 75 (100%) |
| Other | 19 (24%) | ----- |
| Length (cm): median (range) | 18 (2–100) | 16 (0.5–68) |
| Endoscopy Detailsc | ||
| Patients Undergoing Endoscopy | 69 (76%) | 75 (100%) |
| Number of Endoscopies: median (range) | 2 (0–12) | 1 (0–6) |
| Total Number of Endoscopies | 155 | 106 |
| Average Time Between Endoscopies | 3.4 years | 2.1 years |
| Findings on Endoscopy or Resection | ||
| Microscopically Active Inflammation | 63 (82%) | 71 (95%) |
| Stricturing/Narrowing | 21 (27%) | 5 (6.7%) |
| Fistula | 15 (19%) | 3 (4.0%) |
| Inflammatory Pseudopolyps/ Nodules | 11 (14%) | 25 (33%) |
| Nondysplastic Polyps | 0 (0%) | 6 (8.0%) |
Percentages were calculated out of the total number of patients in each cohort–CD (79), UC (75). In several cases, the denominator was changed to reflect the number of scorable individuals within each cohort. All numbers calculated to 2 significant figures where appropriate unless otherwise specified.
aThe listed values are the number and percent, respectively, unless otherwise stated.
bOther causes for diversion in CD were: bleeding/diverticulitis/diarrhea–6 (7.6%), medically refractory disease–3 (3.8%), structuring–3 (3.8%), colonic mass–2 (3.0%), and severe C. difficile–1 (1.0%). Other causes for diversion in UC were fungemia–1 (1.3%). One case of CD had CRC before diversion but this was not the reason for diversion.
cRefers to endoscopies during diversion.
The UC cohort was more likely to have dysplasia before diversion with 1 case of colorectal cancer and 4 cases of dysplasia (3 high-grade and 1 low-grade) reported before diversion. By contrast, the CD cohort had 1 case of CRC and 0 cases of dysplasia reported before diversion.
More cases of diverted CRC (UC 1, CD 0) and high-grade dysplasia (HGD) (UC 1, CD 0) were noted in the UC cohort despite fewer total diverted years (UC 221 years, CD 533 years) (Table 3). In the CD cohort, where a single case of low-grade dysplasia (LGD) was reported, the diverted bowel dysplasia rate was 1.9 cases per 1000 dpy (95% CI 0.2–9.1/1000) or 0.77 cases per 1000 pyd (95% CI 0.1–3.6/1000). Stratified for those with > 8 years disease (n = 57), the dysplasia rate was 2.8 cases per 1000 dpy (95% CI 0.05–11/1000) or 0.81 cases per 1000 pyd (95% CI 0.02–4.3/1000). No CD patients developed CRC or HGD.
Incidence of Dysplasia and Cancer in the Diverted Segments of IBD Patients with Prolonged Diversion
| Disease . | UC Cases per dpy Cases per 1000 dpy Cases per pyd‡ Cases per 1000 pyd . | 95% CI (Cases per 1000 dpy) 95% CI (Cases per 1000 pyd) . | CD Cases per dpy Cases per 1000 dpy Cases per pyd Cases per 1000 pyd . | 95% CI (Cases per 1000 dpy) 95% CI (Cases per 1000 pyd) . |
|---|---|---|---|---|
| CRC | 1 case / 221 dpy | 95% CI: (0.11–25) | 0 cases / 533 dpy | 95% CI: (0–6.9) |
| 4.5 cases / 1000 dpy | 0 cases / 1000 dpy | |||
| 1 case / 678 pyd | 95% CI: (0.04–8.2) | 0 cases / 1306 pyd | 95% CI: (0–2.8) | |
| 1.5 cases / 1000 pyd | 0 cases / 1000 pyd | |||
| Dysplasia | 1 case / 221 dpy | 95% CI: (0.11–25) | 1 case / 533 dpy | 95% CI: (0.05–11) |
| 4.5 cases / 1000 dpy | 1.9 cases / 1000 dpy | |||
| 1 case / 678 pyd | 95% CI: (0.04–8.2) | 1 case / 1306 pyd | 95% CI: (0.02–4.3) | |
| 1.5 cases / 1000 pyd | 0.77 cases / 1000 pyd |
| Disease . | UC Cases per dpy Cases per 1000 dpy Cases per pyd‡ Cases per 1000 pyd . | 95% CI (Cases per 1000 dpy) 95% CI (Cases per 1000 pyd) . | CD Cases per dpy Cases per 1000 dpy Cases per pyd Cases per 1000 pyd . | 95% CI (Cases per 1000 dpy) 95% CI (Cases per 1000 pyd) . |
|---|---|---|---|---|
| CRC | 1 case / 221 dpy | 95% CI: (0.11–25) | 0 cases / 533 dpy | 95% CI: (0–6.9) |
| 4.5 cases / 1000 dpy | 0 cases / 1000 dpy | |||
| 1 case / 678 pyd | 95% CI: (0.04–8.2) | 0 cases / 1306 pyd | 95% CI: (0–2.8) | |
| 1.5 cases / 1000 pyd | 0 cases / 1000 pyd | |||
| Dysplasia | 1 case / 221 dpy | 95% CI: (0.11–25) | 1 case / 533 dpy | 95% CI: (0.05–11) |
| 4.5 cases / 1000 dpy | 1.9 cases / 1000 dpy | |||
| 1 case / 678 pyd | 95% CI: (0.04–8.2) | 1 case / 1306 pyd | 95% CI: (0.02–4.3) | |
| 1.5 cases / 1000 pyd | 0.77 cases / 1000 pyd |
The 95% confidence intervals (CI) calculated using RStudio (Version 0.99.903) assuming a Poisson distribution for incidence.
Incidence of Dysplasia and Cancer in the Diverted Segments of IBD Patients with Prolonged Diversion
| Disease . | UC Cases per dpy Cases per 1000 dpy Cases per pyd‡ Cases per 1000 pyd . | 95% CI (Cases per 1000 dpy) 95% CI (Cases per 1000 pyd) . | CD Cases per dpy Cases per 1000 dpy Cases per pyd Cases per 1000 pyd . | 95% CI (Cases per 1000 dpy) 95% CI (Cases per 1000 pyd) . |
|---|---|---|---|---|
| CRC | 1 case / 221 dpy | 95% CI: (0.11–25) | 0 cases / 533 dpy | 95% CI: (0–6.9) |
| 4.5 cases / 1000 dpy | 0 cases / 1000 dpy | |||
| 1 case / 678 pyd | 95% CI: (0.04–8.2) | 0 cases / 1306 pyd | 95% CI: (0–2.8) | |
| 1.5 cases / 1000 pyd | 0 cases / 1000 pyd | |||
| Dysplasia | 1 case / 221 dpy | 95% CI: (0.11–25) | 1 case / 533 dpy | 95% CI: (0.05–11) |
| 4.5 cases / 1000 dpy | 1.9 cases / 1000 dpy | |||
| 1 case / 678 pyd | 95% CI: (0.04–8.2) | 1 case / 1306 pyd | 95% CI: (0.02–4.3) | |
| 1.5 cases / 1000 pyd | 0.77 cases / 1000 pyd |
| Disease . | UC Cases per dpy Cases per 1000 dpy Cases per pyd‡ Cases per 1000 pyd . | 95% CI (Cases per 1000 dpy) 95% CI (Cases per 1000 pyd) . | CD Cases per dpy Cases per 1000 dpy Cases per pyd Cases per 1000 pyd . | 95% CI (Cases per 1000 dpy) 95% CI (Cases per 1000 pyd) . |
|---|---|---|---|---|
| CRC | 1 case / 221 dpy | 95% CI: (0.11–25) | 0 cases / 533 dpy | 95% CI: (0–6.9) |
| 4.5 cases / 1000 dpy | 0 cases / 1000 dpy | |||
| 1 case / 678 pyd | 95% CI: (0.04–8.2) | 0 cases / 1306 pyd | 95% CI: (0–2.8) | |
| 1.5 cases / 1000 pyd | 0 cases / 1000 pyd | |||
| Dysplasia | 1 case / 221 dpy | 95% CI: (0.11–25) | 1 case / 533 dpy | 95% CI: (0.05–11) |
| 4.5 cases / 1000 dpy | 1.9 cases / 1000 dpy | |||
| 1 case / 678 pyd | 95% CI: (0.04–8.2) | 1 case / 1306 pyd | 95% CI: (0.02–4.3) | |
| 1.5 cases / 1000 pyd | 0.77 cases / 1000 pyd |
The 95% confidence intervals (CI) calculated using RStudio (Version 0.99.903) assuming a Poisson distribution for incidence.
In the UC cohort, where a single case of HGD was reported, the dysplasia rate was 4.5 cases per 1000 dpy (95% CI 0.11–25/1000) or 1.5 cases per 1000 pyd (95% CI 0.04–8.2/1000). Stratifying the UC cohort for those who experienced > 8 years of disease (n = 30) gave a dysplasia rate of 7.5 cases per 1000 dpy (95% CI 1.9–42/1000) or 2.0 cases per 1000 pyd (95% CI 0.05–11/1000).
A single case of rectal adenocarcinoma developed in the UC cohort, yielding a cancer rate of 4.5 cases per 1000 dpy (95% CI 0.11–25/1000) or 1.5 cases per 1000 pyd (95% CI 0.04–8.2/1000); stratified for > 8 years of disease, the cancer rate was 7.5 cases per 1000 dpy (95% CI 0.19–42/1000) or 2.0 cases per 1000 pyd (95% CI 0.05–11/1000). Diverted bowel dysplasia was not observed in patients with fewer than 10 pyd (Fig. 2). The earliest case of diverted neoplasia was observed 4.6 years after diversion (UC: CRC) (Fig. 3).
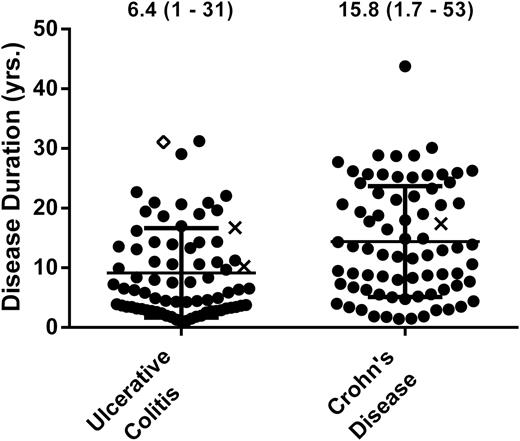
Patient cases plotted by disease duration for UC (n = 76) and CD (n = 75). Four CD cases were censured (see Supplemental Methods). Circles signify patients that did not develop cancer or dysplasia during diversion, x’s signify patients that developed dysplasia during diversion, and diamond’s signify patients that developed cancer during diversion.
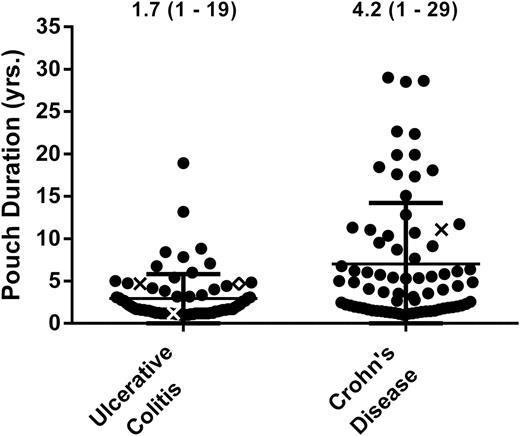
Patient cases plotted by pouch duration for UC (n = 76) and CD (n = 76). Three CD cases were censured (see Supplemental Methods). Circles signify patients that did not develop cancer or dysplasia during diversion, x’s signify patients that developed dysplasia during diversion, and diamond’s signify patients that developed cancer during diversion.
The Cases
CD
The single case of LGD occurred in a 41-year-old white male with a 20.8-year history of ileocolonic and perianal CD who had been diverted for colonic perforation via an ileostomy and transverse colon mucous fistula. Rectal stricturing was noted within 3 months of diversion, preventing endoscopic surveillance. Recommendations for surgical intervention were declined initially. A proctectomy was attempted 7.1 years after the initial diversion but was incomplete distally secondary to dense adhesions. On account of rectal pain and incomplete surveillance, a completion proctectomy was performed after 11.1 years of diversion, revealing LGD in the excised 7.5 cm rectal segment.
UC
Case 1: HGD.
A 46-year-old white man with a 31.5-year history of UC. His medical history was notable for primary sclerosing cholangitis (PSC) complicated by cholangiocarcinoma for which he underwent an orthotopic liver transplant. Multifocal HGD and LGD were discovered during routine colonoscopy, and he underwent a total colectomy with placement of a Brooke ileostomy and a 10 cm Hartmann’s pouch. Histology was notable for indefinite dysplasia at the distal resection margin. He had 3 pouchoscopies during the 4.7 years he remained diverted. A biopsy on the final pouchoscopy revealed high-grade epithelial dysplasia for which he had completion proctectomy.
Case 2: CRC.
The single case of rectal cancer occurred in a 59-year-old white man with a 10.2-year history of UC. He experienced treatment-refractory pancolitis for which he underwent abdominal colectomy with end ileostomy and a 7 cm Hartmann’s pouch. He was subsequently asymptomatic and lost to follow-up, with no endoscopies during the 4.6 years he remained diverted. When he presented for consideration of a J-pouch, sigmoidoscopy revealed a closed-off stricture 4 cm from the anal verge. Proctectomy was preformed revealing a 6.5 cm well-to-moderately differentiated adenocarcinoma, stage pT3.
DISCUSSION
In this retrospective analysis of 154 IBD patients, spanning 754 diversion-years and 1984 disease-years, only a single case of diverted CRC was observed. The computed diverted segment cancer rate [0.50 cases / 1000 pyd (95% CI 0.01– 2.8/1000)] was therefore low and similar to the nondiverted rectal cancer rate reported by Bernstein et al in a population-based study of Canadian IBD patients (0.439 cases / 1000 pyd).20 The similarity in rates may correspond to the at risk total colonic surface area in the majority of our diversions.
The established CRC risk factors in the general IBD population were largely applicable to our diverted cohort. All 3 patients who developed diverted dysplasia or cancer had the following CRC risk factors:1 microscopically active inflammation of the diverted colon2, 18 and long disease duration (all > 10 years).8, 17, 18, 21, 22 Stricturing, a known risk factor for rectal cancer,13, 18, 22 was present in the 1 case of CD dysplasia and the 1 case of UC CRC. In both cases, stricturing prevented endoscopic surveillance of the entire diverted segment for > 4 years. The 1 case of HGD occurred in a UC patient with PSC and liver transplant, factors that increase the likelihood of CRC.13, 18, 23
Chronic colonic inflammation has been postulated as an inciting step for the propagation of IBD-associated neoplasia.5, 13, 24 In diverted bowel, inflammation is exceedingly common with luminal deprivation of short chain fatty acids and changes in bacterial flora implicated as potential driving forces.18, 25 It remains unclear, however, whether diversion-type inflammation is associated with an increased risk of CRC.25 Winther et al5 recently failed to demonstrate any histologic evidence of dysplasia or molecular markers (TP53) of dysplasia in diverted IBD bowel segments despite a preponderance of moderate to severe histological mucosal inflammation. Compared to our study, the sample size was small (n = 42 patients) and did not include patients with perianal CD, a potential risk factor for rectal stump cancer.14 In our study, all cases of dysplasia/cancer occurred in rectal stumps with active inflammation, although further studies are required to determine the role of diversion colitis versus IBD-based inflammation in the progression of diverted segment CRC.13
Comparing the incidence of CRC/dysplasia in IBD patients is challenging as rates differ between geographic region,26 meta-analyses,6, 8, 26 and in referral centers versus population-based studies.8, 23 In our study, a discussion of rectal cancer risk may be more prudent, given the significantly reduced length of diverted colonic segments (median length: 18 cm vs 16 cm for CD and UC, respectively).
To our knowledge, incidence rates of diverted rectal cancer have not been evaluated in recent large IBD studies, though Lutgens et al13 previously reported risk factors for IBD-related rectal stump cancer. In our study, we encountered no cases of rectal cancer in the CD cohort despite 533 dpy and 1306 pyd, a finding that may reflect the low rate of rectal cancer in CD as a whole. Von Roon et al26 in a meta-analysis of all CD patients including those not diverted reported a rectal cancer rate of 0.169 per 1000 patient years of disease. In a study similar to ours, Yamamoto et al4 reviewed the long-term outcome of 69 CD patients who had undergone ileostomy with rectal stump formation between 1962 and 1997, the majority of whom had rectal or perianal involvement. One case of rectal cancer was reported, occurring in a rectovaginal fistula, though neither pyd nor dpy were reported. Our CD cohort likewise had extensive perianal involvement (62%), although 0 cases of CRC were observed. Importantly, and contrary to the study by Yamamoto et al, the majority of our cohort underwent their last endoscopy or resection after the year 2000 (CD 80%, UC 69%), a factor that has bearing since a recent meta-analysis by Jess et al17 reported a decreasing risk in IBD associated CRC from 1979 to 2008 and a meta-analysis by Lutgens et al8 purports a declining trend in CRC rates in IBD patients since the year 2000.
Our UC cohort experienced 1 case of cancer in 678 pyd, a rate of 1.5 cases per 1000 pyd (95% CI 0.04–8.2/1000). This incidence is similar to rectal cancer rates observed in nondiverted UC patients. Karlén et al27 conducted a prospective study of 1547 UC patients in Sweden to determine site-specific rates of cancer. Over 25,464 pyd , they observed 13 rectal cancers, a rate of 0.51 cases per 1000 pyd. This is concordant with what we observed, falling within the 95% CI we calculated for rectal cancer incidence in our UC cohort.
Guidelines for endoscopic surveillance in IBD vary by governing body with the American Gastrointestinal Association recommending annual or biannual surveillance depending on coexisting PSC and the British Society of Gastroenterology recommending surveillance every 1, 3, or 5 years for those with high, medium, or low risk, respectively.22, 28 However, such consensus guidelines do not exist for diverted bowel segments. Based on retrospective case-control data, Lutgens et al13 recommended that surveillance proctoscopies be conducted every 1–2 years in IBD patients with a closed rectal stump, PSC, and a disease duration of greater than 8 years. In the absence of a personal history of CRC or dysplasia, our data suggest that risk stratifying patients based on disease duration, regardless of diversion duration, would be an equitable surveillance strategy as no cases of dysplasia/cancer were discovered before a disease duration of 10 years.
For diverted CD patients, an argument could be made for an interval surveillance period of every 3 years (after an initial 8 years disease duration), as we encountered no cases of HGD/CRC in our CD cohort who underwent an average of 1 endoscopy for every 3.4 diversion years. In contrast, for diverted UC patients, an annual or biannual endoscopy (after an initial 8 years of disease duration) may be prudent as HGD and CRC were discovered in our UC cohort who underwent endoscopy every 2.1 diversion years on average. Cairns et al29 recommended annual sigmoidoscopy for postcolectomy IBD patients with pouch/rectal mucosa and any of the following: previous CRC cancer, dysplasia, or PSC. In our study, all UC patients developing diverted bowel dysplasia or cancer were positive for at least 1 of these variables.
In patients for whom anal or rectal narrowing impedes examination of a diverted bowel segment, the threshold for surgical intervention should be low30 as stricturing can be associated with CRC.13, 18, 22 Of the 3 patients that developed dysplasia or cancer in their diverted bowel remnant, 2 could no longer be assessed by digital rectal exam or endoscopy secondary to severe stricturing.
Our study has several limitations. Our utilization of a tertiary care center may limit the generalizability of our data as IBD patients at referral centers are more likely to have severe disease and, thus, higher cancer risks.8, 23 Moreover, our study included high-risk IBD patients, including those with prior colonic dysplasia/cancer and coexisting PSC.29 However, this being the case, our study would be expected to overestimate dysplasia and cancer in diverted colorectal remnants. Futhermore, the retrospective nature of this study is dependent on the accuracy of previous chart documentation, and whereas this study included chart data from 1990‒2015, a higher proportion of patient charts dated from the 1990s were excluded due to insufficient documentation. As a result, a time-based bias may have been introduced, which has bearing since pharmacotherapies have advanced with time. The median disease duration in our UC cohort (6.4 years) also was shorter than in previous studies,16, 31 and given the increased risk in CRC with disease duration,18 our reported rate of dysplasia/cancer could be an underestimate, particularly in the UC cohort.
CONCLUSIONS
To our knowledge this is the largest study to investigate the rate of dysplasia and colorectal cancer in diverted segments among IBD patients. All IBD patients with diverted bowel dysplasia had multiple CRC risk factors including active inflammation and a disease duration greater than 10 years. Further prospective studies are needed to better define the risk of dysplasia, especially between CD and UC and to guide appropriate endoscopic surveillance intervals.
ACKNOWLEDGMENTS
We would like to acknowledge Ximin Li, ScM, and the Institute for Clinical and Translational Research at the Johns Hopkins School of Public Health for advising the statistical analysis.
Conflicts of Interest: No conflicts of interest are declared.
Supported by: This study was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) that is funded in part by Grant Number UL1 TR001079 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS, or NIH.



