-
PDF
- Split View
-
Views
-
Cite
Cite
Kelly C Cushing, Adeeti Chiplunker, Allie Li, Yun Ju Sung, Taylor Geisman, Li-Shiun Chen, Sharon Cresci, Alexandra M Gutierrez, Smoking Interacts With CHRNA5, a Nicotinic Acetylcholine Receptor Subunit Gene, to Influence the Risk of IBD-Related Surgery, Inflammatory Bowel Diseases, Volume 24, Issue 5, May 2018, Pages 1057–1064, https://doi.org/10.1093/ibd/izx094
Close - Share Icon Share
Abstract
Inflammatory bowel disease (IBD) is a chronic luminal disease with genetic and environmental factors affecting phenotype. This study evaluated the relationship between CHRNA5, a nicotinic receptor subunit gene, and smoking in predicting IBD-related surgery as well as the relationship between CHRNA5 and nicotine dependence.
Participants completed a smoking questionnaire and were genotyped for CHRNA5 rs16969968. Demographic and clinical data were obtained from medical records. Wilcoxon, ANOVA, Chi square, and Fisher’s exact tests were used for comparisons. Logistic regression was used to evaluate the effect of clinical and genetic predictors on surgery, stratified by disease subtype given paradoxical effects of smoking. Kaplan-Meier curves were used to examine the effect of smoking and genotype on time to surgery. (Significance: P < 0.05 for main effects; P < 0.2 for interaction terms)
400 (65.8%) patients had Crohn’s disease (CD) and 208 (34.2%) had ulcerative colitis (UC). 298 (49%) underwent an IBD-related surgery. There was a trend towards significance between rs16969968 and smoking behavior (smoking status [P = 0.05], nicotine dependence [AA > AG > GG; P = 0.08]). Smoking and genotype were not independently associated with surgery in UC or CD. However, interaction between rs16969968 and smoking in predicting surgery was observed for both UC (OR = 2.72; P = 0.05) and CD (OR = 2.88; P = 0.1). CHRNA5 genotype, but not smoking, predicted time to surgery in patients with UC (P = 0.007) but not in patients with CD. The interaction between smoking and genotype was not significantly associated with time to surgery in UC or CD.
The CHRNA5 rs16969968 A variant interacts with smoking to influence IBD-related surgery.
INTRODUCTION
Genetic predisposition likely plays an important role in the development of inflammatory bowel disease (IBD). The largest genome wide association study thus far revealed 163 loci associated with IBD, however, these loci are estimated to account for only 20–25% of the overall risk1 suggesting that there are additional pathways for disease development. Gene-environment interactions represent potential modifiers of disease development and are important areas of investigation.
One modifiable environmental risk factor for patients with IBD is smoking. Smoking is known to influence the natural history and severity of IBD. In Crohn’s disease (CD), smoking is associated with increased flares, increased need for steroids, and increased need for surgery.2, 3 In ulcerative colitis (UC), smoking has been shown to decrease the likelihood of disease development2 but the effect on disease trajectory, including flares and need for surgery, is less clear.2, 4, 5 The paradoxical association between smoking and IBD has yet to be fully explained but its role as an important modulator of disease activity is well-defined.
To date, gene-environment studies have mostly focused on the interaction between smoking and commonly described IBD risk loci, such as IBD4, ATG16L1, IL23R and NOD2.6–10 However, the interaction between smoking and alternative pathways of genetic susceptibility has not been evaluated as thoroughly. Of the non-IBD risk loci studied, CYP2A6 and CHRNA3 have not been reported to have an effect on IBD disease activity11 while CALM3, HLA-DQA1, TRIB1, and IL2/IL21 have been reported to be modified by smoking to influence risk of UC.12 Immunochip data has also been studied and 64 SNPs have been reported to interact with smoking and alter the risk of IBD.13
Nicotinic acetylcholine receptors (nAChRs) are a family of pentameric ligand gated ion channels, which can bind nicotine and acetylcholine, and have been associated with both inflammation and nicotine behavior. CHRNA5 encodes for the alpha 5 subunit of the nAchR (nAChRα5). The CHRNA5 rs16969968 variant is a G to A nucleotide change that results in an amino acid change from aspartic acid (D) to asparagine (N) at position 39814 and has been associated with reduced response to nicotine in the absence of changes in receptor expression or subunit isoform.15 This variant has been associated with increased nicotine dependence and responsiveness to smoking cessation pharmacotherapy in the general population, poor smoking abstinence following myocardial infarction, and increased risk of lung cancer.16–19
Cholinergic receptors have also been implicated in the anti-inflammatory response via diminished tumor necrosis factor (TNF) release from macrophages, activation of JAK2/STAT3 signaling, and induction of regulatory T cells.20, 21 The “cholinergic anti-inflammatory pathway” has been proposed to be important in IBD with most work thus far focusing on the role of the alpha 7 subunit of the nAChR, nAChRα7.22 While nAChRα7 has been shown to mediate vagus nerve inhibition of TNF release from macrophages,20 there have been conflicting results regarding the effect of α7 agonists on colitis.22 The alpha 5 subunit of nAChR, however, has not yet been studied in this clinical setting. nAChRα5 may be important in mediating the luminal immune response as demonstrated by the development of worsened dextran sulphate sodium (DSS) induced colitis in α5 knockout mice, when compared to controls.23 Therefore, we sought to further investigate the role of CHRNA5 in IBD.
There were two aims of this study: 1) to investigate whether the association between CHRNA5 rs16969968 and nicotine dependence observed in other populations (i.e. non-IBD populations) was found in our IBD population and 2) to test for a CHRNA5-smoking interaction in predicting IBD-related surgery, a surrogate of poorly controlled disease.
MATERIALS AND METHODS
Recruitment and data collection
Patients over 18 years of age with an established diagnosis of IBD were prospectively recruited for study participation at the Washington University in Saint Louis gastroenterology and colorectal surgery clinics. Enrollment occurred from July 2014 to June 2016. The study obtained informed consent from participants and approval from the university institutional review board.
Smoking information including history, behaviors, knowledge, and dependence was assessed through a comprehensive questionnaire. Patients self-identified as current, former or never smokers and provided information on years smoked and packs per day (PPD). PPD were subsequently converted to cigarettes per day (CPD) by the research team for purposes of statistical analysis. CPD were categorized as: ≤10, 11–20, 21–30, and ≥31. Nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND).24 Associated demographic, clinical, and surgical data were obtained by independent chart review. Demographic factors included age, sex, and race. Clinical factors included IBD subtype, disease behavior, disease location, disease duration, and medication failure. The diagnosis of IBD was based on endoscopic, pathologic and/or radiologic findings. Study patients who were found not to have IBD were removed from the study prior to analysis. Surgical data obtained included need for IBD-related surgery. IBD-related surgery was used as a surrogate marker for poorly controlled disease and was defined as any surgery performed for an indication related to IBD, including dysplasia. IBD-related surgery represents a significant clinical outcome in IBD and is often reflective of a poorly controlled disease course. Most often, surgery reflects medically refractory disease and/or complications from aggressive or uncontrolled disease activity such as penetrating disease. If an IBD-related surgery was performed, the type of surgery and time from diagnosis to surgery were recorded. Surgeries included abdominal (small bowel resection or strictureplasty, colonic resection, and diversion ostomies) and perianal surgeries in those with CD. (Supplementary Figure 1) Surgeries in the UC cohort included total colectomy with ileorectal anastomosis, segmental resection, and proctocolectomy with end ileostomy or ileal pouch anal anastomosis. For those with more than one surgery, the first surgery was used for all analyses.
DNA extraction
A 3mL EDTA tube of peripheral blood was drawn from each patient and stored at -20ºC. 1 to 3 mL thawed whole blood was mixed with approximately 35 mL Autopure LS Red Blood Cell (RBC) Lysis Solution (Qiagen, Hilden, Germany). The sample was then thoroughly mixed, followed by a 5-minute centrifugation at 3000g. The RBC supernatant was decanted and the resulting cell pellets were mixed with an equal volume of Autopure LS Cell Lysis solution (Qiagen, Hilden, Germany). This was followed by an overnight incubation at room temperature. DNA extraction was then completed with the Autopure LS (Qiagen, Hilden, Germany). Upon completion, samples were placed in a 65C water bath for 1–2 hours and rocked overnight at room temperature. Quality control of all samples was performed using the NanoDrop 8000 (Thermo Scientific, Wilmington, USA). Extracted DNA was then stored at -20°C until genotyping was performed.
Genotyping
Genotyping of CHRNA5 rs16969968 was performed using a TaqMan® assay (TaqMan® assay ID C__26000428_20) according to manufacturer’s instructions (Applied Biosystems, Carlsbad, California). 5 to 10 ng of DNA was amplified by PCR using TaqMan® genotyping master mix and the ABI 7500 real time PCR platform. Allelic discrimination was performed using sequence detection software (Applied Biosystems, Carlsbad, California) and the ABI 7500 real time PCR platform. Genotype call rate was 99.1%. Hardy Weinberg equilibrium was met for rs16969968, with P = 0.84.
Statistical analysis
The sample size was calculated based on the ability to detect a difference in nicotine dependence (FTND < 4 and ≥ 4) by CHRNA5 rs16969968 genotype. Assuming the frequency of the CHRNA5 rs16969968 A allele was 0.33 with 15% having a FTND ≥ 4 and with an odds ratio of 1.4, the estimated sample size was 1181 patients to obtain power of 0.8 and type 1 error of 0.05.
Continuous variables were reported as median +/- interquartile range. Comparison of continuous variables was performed with the Wilcoxon test or ANOVA as appropriate. Categorical variables were reported as absolute number and percentage. Comparisons between categorical variables were performed with the Chi square test or Fisher’s exact test as appropriate. Logistic regression was used to evaluate the effect of clinical and genetic predictors on a surrogate for poorly controlled disease (IBD-related surgery). Logistic regression was performed within each IBD subtype (UC and CD) given the expected paradoxical effects of smoking on disease activity and a different set of covariates of importance for each subtype.
The inclusion of the interaction term was planned a priori based on the hypothesis that there is a known relationship between CHRNA5 and nicotine dependence16, 17 as well as animal data23 demonstrating a relationship between CHRNA5 and intestinal inflammation. In regression analysis for the UC cohort, smoking status was grouped as ever (former/current) smokers because current and former smokers both had the same negative coefficients. In regression for the CD cohort, small bowel (with or without upper GI disease) and large bowel with small bowel and/or upper GI disease both had the same positive coefficients, and therefore, these groups were combined for the final analysis. Time to surgery from diagnosis was examined using Kaplan-Meier curves. A P value of <0.05 was reported as statistically significant. For interaction terms, a P value of <0.20 (lower threshold) was reported as statistically significant to increase the power of identifying interaction effect.25, 26 Analyses were performed using R-studio version 0.99.902.
RESULTS
719 patients were prospectively enrolled in the study. We were unable to reach our goal sample size due to declining recruitment and loss of study personnel. 13 patients had a change in diagnosis and were removed from the study before genotyping occurred. Patients were further excluded for lack of peripheral blood samples for genotyping (n = 29) and non-European ancestry (n = 61) since genotype frequencies revealed substantial variation across race, similar to what has been previously reported.27, 28 Two patients met both missing blood sample and non-European ancestry exclusion criteria. Genotyping failed in six subjects. Three patients had a diagnosis of indeterminate colitis and were further excluded. The final dataset included 608 patients.
Among those participating, 400 (65.8%) had CD and 208 (34.2%) had UC. 298 (49%) underwent an IBD-related surgery. Those who underwent surgery were more likely to be former or current smokers (X2 = 8.6, df = 2, P = 0.01) with a greater number of years smoked (17 vs 13 years, W = 7624, P = 0.09) (Table 1) In addition, those who underwent surgery were more likely to have a diagnosis of CD (X2 = 77.4, df = 1, p < 2.2e-16), have small bowel involvement (+/- UGI and/or large bowel involvement) (X2 = 84.5, df = 2, p < 2.2e-16), have a longer disease duration (12 vs 7 years, W = 30856, p = 1.36e-12) and have more aggressive disease phenotypes (Fisher’s exact test, p < 2.2e-16). (Table 2) No significant differences in gender, CPD, FTND score, or genotype frequencies were observed between surgical and nonsurgical groups.
| Characteristic . | IBD-related Surgery (n = 298) . | No IBD-related Surgery (n = 310) . | P-value . |
|---|---|---|---|
| Race | N/A | ||
| Caucasian | 298 (100%) | 310 (100%) | |
| Gender | 0.61 | ||
| Male | 117 (39.3%) | 129 (41.6%) | |
| Female | 181 (60.7%) | 181 (58.4%) | |
| Smoking Status | 0.01 | ||
| Never | 151 (50.7%) | 190 (61.3%) | |
| Former | 105 (35.2%) | 94 (30.3%) | |
| Current | 42 (14.1%) | 26 (8.4%) | |
| CPD (n = 606) | 0.11 | ||
| 0 | 151 (50.8%) | 190 (61.5%) | |
| 1–10 | 54 (18.2%) | 46 (14.9%) | |
| 11–20 | 60 (20.2%) | 47 (15.2%) | |
| 21–30 | 11 (3.7%) | 11 (3.6%) | |
| ≥ 31 | 21 (7.1%) | 15 (4.9%) | |
| Number Years Smoked | 17 +/- 17 | 13 +/- 14.5 | 0.09** |
| Fagerström Test for Nicotine Dependence (FTND) Score | 4.5 +/- 4 | 3 +/- 4.75 | 0.13** |
| Characteristic . | IBD-related Surgery (n = 298) . | No IBD-related Surgery (n = 310) . | P-value . |
|---|---|---|---|
| Race | N/A | ||
| Caucasian | 298 (100%) | 310 (100%) | |
| Gender | 0.61 | ||
| Male | 117 (39.3%) | 129 (41.6%) | |
| Female | 181 (60.7%) | 181 (58.4%) | |
| Smoking Status | 0.01 | ||
| Never | 151 (50.7%) | 190 (61.3%) | |
| Former | 105 (35.2%) | 94 (30.3%) | |
| Current | 42 (14.1%) | 26 (8.4%) | |
| CPD (n = 606) | 0.11 | ||
| 0 | 151 (50.8%) | 190 (61.5%) | |
| 1–10 | 54 (18.2%) | 46 (14.9%) | |
| 11–20 | 60 (20.2%) | 47 (15.2%) | |
| 21–30 | 11 (3.7%) | 11 (3.6%) | |
| ≥ 31 | 21 (7.1%) | 15 (4.9%) | |
| Number Years Smoked | 17 +/- 17 | 13 +/- 14.5 | 0.09** |
| Fagerström Test for Nicotine Dependence (FTND) Score | 4.5 +/- 4 | 3 +/- 4.75 | 0.13** |
* Fisher’s exact Test
**Wilcoxon Test
| Characteristic . | IBD-related Surgery (n = 298) . | No IBD-related Surgery (n = 310) . | P-value . |
|---|---|---|---|
| Race | N/A | ||
| Caucasian | 298 (100%) | 310 (100%) | |
| Gender | 0.61 | ||
| Male | 117 (39.3%) | 129 (41.6%) | |
| Female | 181 (60.7%) | 181 (58.4%) | |
| Smoking Status | 0.01 | ||
| Never | 151 (50.7%) | 190 (61.3%) | |
| Former | 105 (35.2%) | 94 (30.3%) | |
| Current | 42 (14.1%) | 26 (8.4%) | |
| CPD (n = 606) | 0.11 | ||
| 0 | 151 (50.8%) | 190 (61.5%) | |
| 1–10 | 54 (18.2%) | 46 (14.9%) | |
| 11–20 | 60 (20.2%) | 47 (15.2%) | |
| 21–30 | 11 (3.7%) | 11 (3.6%) | |
| ≥ 31 | 21 (7.1%) | 15 (4.9%) | |
| Number Years Smoked | 17 +/- 17 | 13 +/- 14.5 | 0.09** |
| Fagerström Test for Nicotine Dependence (FTND) Score | 4.5 +/- 4 | 3 +/- 4.75 | 0.13** |
| Characteristic . | IBD-related Surgery (n = 298) . | No IBD-related Surgery (n = 310) . | P-value . |
|---|---|---|---|
| Race | N/A | ||
| Caucasian | 298 (100%) | 310 (100%) | |
| Gender | 0.61 | ||
| Male | 117 (39.3%) | 129 (41.6%) | |
| Female | 181 (60.7%) | 181 (58.4%) | |
| Smoking Status | 0.01 | ||
| Never | 151 (50.7%) | 190 (61.3%) | |
| Former | 105 (35.2%) | 94 (30.3%) | |
| Current | 42 (14.1%) | 26 (8.4%) | |
| CPD (n = 606) | 0.11 | ||
| 0 | 151 (50.8%) | 190 (61.5%) | |
| 1–10 | 54 (18.2%) | 46 (14.9%) | |
| 11–20 | 60 (20.2%) | 47 (15.2%) | |
| 21–30 | 11 (3.7%) | 11 (3.6%) | |
| ≥ 31 | 21 (7.1%) | 15 (4.9%) | |
| Number Years Smoked | 17 +/- 17 | 13 +/- 14.5 | 0.09** |
| Fagerström Test for Nicotine Dependence (FTND) Score | 4.5 +/- 4 | 3 +/- 4.75 | 0.13** |
* Fisher’s exact Test
**Wilcoxon Test
| Characteristic . | IBD-related Surgery (n = 298) . | No IBD-related Surgery (n = 310) . | P-value . |
|---|---|---|---|
| Disease Type | <2.2e-16 | ||
| CD | 248 (83.2%) | 152 (49%) | |
| UC | 50 (16.8%) | 158 (51%) | |
| Disease Duration | 12 +/- 16 | 7 +/- 10 | 1.36e-12** |
| Disease Location | <2.2e-16 | ||
| Large Bowel (including proctitis) | 72 (24.2%) | 189 (61%) | |
| Large Bowel + Small Bowel and/or Upper GI Disease | 176 (59.1%) | 90 (29%) | |
| Small Bowel (+/- Upper GI Disease) | 50 (16.8%) | 31 (10%) | |
| Disease Behavior (CD only, n = 400) | <2.2e-16* | ||
| Inflammatory | 24 (9.7%) | 105 (69.1%) | |
| Stricturing | 86 (34.7%) | 27 (17.8%) | |
| Fistulizing/Penetrating | 69 (27.8%) | 14 (9.2%) | |
| Fistulizing/Penetrating and Stricturing | 68 (27.4%) | 6 (3.9%) | |
| Fistulizing/Penetrating and Inflammatory | 1 (0.4%) | 0 | |
| CHRNA5 rs16969968 | 0.63 | ||
| AA | 36 (12.1%) | 32 (10.3%) | |
| AG | 129 (43.3%) | 145 (46.8%) | |
| GG | 133 (44.6%) | 133 (42.9%) |
| Characteristic . | IBD-related Surgery (n = 298) . | No IBD-related Surgery (n = 310) . | P-value . |
|---|---|---|---|
| Disease Type | <2.2e-16 | ||
| CD | 248 (83.2%) | 152 (49%) | |
| UC | 50 (16.8%) | 158 (51%) | |
| Disease Duration | 12 +/- 16 | 7 +/- 10 | 1.36e-12** |
| Disease Location | <2.2e-16 | ||
| Large Bowel (including proctitis) | 72 (24.2%) | 189 (61%) | |
| Large Bowel + Small Bowel and/or Upper GI Disease | 176 (59.1%) | 90 (29%) | |
| Small Bowel (+/- Upper GI Disease) | 50 (16.8%) | 31 (10%) | |
| Disease Behavior (CD only, n = 400) | <2.2e-16* | ||
| Inflammatory | 24 (9.7%) | 105 (69.1%) | |
| Stricturing | 86 (34.7%) | 27 (17.8%) | |
| Fistulizing/Penetrating | 69 (27.8%) | 14 (9.2%) | |
| Fistulizing/Penetrating and Stricturing | 68 (27.4%) | 6 (3.9%) | |
| Fistulizing/Penetrating and Inflammatory | 1 (0.4%) | 0 | |
| CHRNA5 rs16969968 | 0.63 | ||
| AA | 36 (12.1%) | 32 (10.3%) | |
| AG | 129 (43.3%) | 145 (46.8%) | |
| GG | 133 (44.6%) | 133 (42.9%) |
* Fisher’s exact Test
** Wilcoxon Test
| Characteristic . | IBD-related Surgery (n = 298) . | No IBD-related Surgery (n = 310) . | P-value . |
|---|---|---|---|
| Disease Type | <2.2e-16 | ||
| CD | 248 (83.2%) | 152 (49%) | |
| UC | 50 (16.8%) | 158 (51%) | |
| Disease Duration | 12 +/- 16 | 7 +/- 10 | 1.36e-12** |
| Disease Location | <2.2e-16 | ||
| Large Bowel (including proctitis) | 72 (24.2%) | 189 (61%) | |
| Large Bowel + Small Bowel and/or Upper GI Disease | 176 (59.1%) | 90 (29%) | |
| Small Bowel (+/- Upper GI Disease) | 50 (16.8%) | 31 (10%) | |
| Disease Behavior (CD only, n = 400) | <2.2e-16* | ||
| Inflammatory | 24 (9.7%) | 105 (69.1%) | |
| Stricturing | 86 (34.7%) | 27 (17.8%) | |
| Fistulizing/Penetrating | 69 (27.8%) | 14 (9.2%) | |
| Fistulizing/Penetrating and Stricturing | 68 (27.4%) | 6 (3.9%) | |
| Fistulizing/Penetrating and Inflammatory | 1 (0.4%) | 0 | |
| CHRNA5 rs16969968 | 0.63 | ||
| AA | 36 (12.1%) | 32 (10.3%) | |
| AG | 129 (43.3%) | 145 (46.8%) | |
| GG | 133 (44.6%) | 133 (42.9%) |
| Characteristic . | IBD-related Surgery (n = 298) . | No IBD-related Surgery (n = 310) . | P-value . |
|---|---|---|---|
| Disease Type | <2.2e-16 | ||
| CD | 248 (83.2%) | 152 (49%) | |
| UC | 50 (16.8%) | 158 (51%) | |
| Disease Duration | 12 +/- 16 | 7 +/- 10 | 1.36e-12** |
| Disease Location | <2.2e-16 | ||
| Large Bowel (including proctitis) | 72 (24.2%) | 189 (61%) | |
| Large Bowel + Small Bowel and/or Upper GI Disease | 176 (59.1%) | 90 (29%) | |
| Small Bowel (+/- Upper GI Disease) | 50 (16.8%) | 31 (10%) | |
| Disease Behavior (CD only, n = 400) | <2.2e-16* | ||
| Inflammatory | 24 (9.7%) | 105 (69.1%) | |
| Stricturing | 86 (34.7%) | 27 (17.8%) | |
| Fistulizing/Penetrating | 69 (27.8%) | 14 (9.2%) | |
| Fistulizing/Penetrating and Stricturing | 68 (27.4%) | 6 (3.9%) | |
| Fistulizing/Penetrating and Inflammatory | 1 (0.4%) | 0 | |
| CHRNA5 rs16969968 | 0.63 | ||
| AA | 36 (12.1%) | 32 (10.3%) | |
| AG | 129 (43.3%) | 145 (46.8%) | |
| GG | 133 (44.6%) | 133 (42.9%) |
* Fisher’s exact Test
** Wilcoxon Test
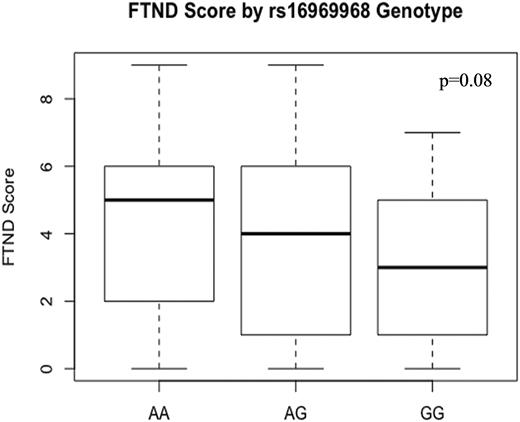
The frequency of the CHRNA5 rs16969968 A allele was 34%; A allele carriers comprised 56% of the cohort. In the entire cohort, a trend towards significance was observed between CHRNA5 rs16969968 and smoking status (X2 = 9.4, df = 4, P = 0.05) but not CPD. (Table 3) There was a trend towards nicotine dependence, as measured by the FTND, in a gene-dose dependent manner (AA with a median score of 5, AG with a median score of 4, GG with a median score of 3; F-statistic = 3.07, df = (1 & 66), P = 0.08). (Fig. 1) The relationship between genotype and smoking status was not statistically significant in each disease type (UC [Fisher’s exact test,P = 0.13], CD [Fisher’s exact test, P = 0.34]).
| Smoking History . | AA (n = 68) . | AG (n = 274) . | GG (n = 266) . | P-value . |
|---|---|---|---|---|
| Smoking Status | 0.05 | |||
| Never | 29 (8.5%) | 149 (43.7%) | 163 (47.8%) | |
| Former | 28 (14.1%) | 97 (48.7%) | 74 (37.2%) | |
| Current | 11 (16.2%) | 28 (41.2%) | 29 (42.6%) | |
| CPD (n = 606) | 0.21* | |||
| 0 | 29 (8.5%) | 149 (43.7%) | 163 (47.8%) | |
| 1–10 | 12 (12%) | 45 (45%) | 43 (43%) | |
| 11–20 | 17 (15.9%) | 50 (46.7%) | 40 (37.4%) | |
| 21–30 | 3 (13.6%) | 12 (54.5%) | 7 (31.8%) | |
| ≥ 31 | 6 (16.7%) | 18 (50%) | 12 (33.3%) |
| Smoking History . | AA (n = 68) . | AG (n = 274) . | GG (n = 266) . | P-value . |
|---|---|---|---|---|
| Smoking Status | 0.05 | |||
| Never | 29 (8.5%) | 149 (43.7%) | 163 (47.8%) | |
| Former | 28 (14.1%) | 97 (48.7%) | 74 (37.2%) | |
| Current | 11 (16.2%) | 28 (41.2%) | 29 (42.6%) | |
| CPD (n = 606) | 0.21* | |||
| 0 | 29 (8.5%) | 149 (43.7%) | 163 (47.8%) | |
| 1–10 | 12 (12%) | 45 (45%) | 43 (43%) | |
| 11–20 | 17 (15.9%) | 50 (46.7%) | 40 (37.4%) | |
| 21–30 | 3 (13.6%) | 12 (54.5%) | 7 (31.8%) | |
| ≥ 31 | 6 (16.7%) | 18 (50%) | 12 (33.3%) |
* Fisher’s exact Test
| Smoking History . | AA (n = 68) . | AG (n = 274) . | GG (n = 266) . | P-value . |
|---|---|---|---|---|
| Smoking Status | 0.05 | |||
| Never | 29 (8.5%) | 149 (43.7%) | 163 (47.8%) | |
| Former | 28 (14.1%) | 97 (48.7%) | 74 (37.2%) | |
| Current | 11 (16.2%) | 28 (41.2%) | 29 (42.6%) | |
| CPD (n = 606) | 0.21* | |||
| 0 | 29 (8.5%) | 149 (43.7%) | 163 (47.8%) | |
| 1–10 | 12 (12%) | 45 (45%) | 43 (43%) | |
| 11–20 | 17 (15.9%) | 50 (46.7%) | 40 (37.4%) | |
| 21–30 | 3 (13.6%) | 12 (54.5%) | 7 (31.8%) | |
| ≥ 31 | 6 (16.7%) | 18 (50%) | 12 (33.3%) |
| Smoking History . | AA (n = 68) . | AG (n = 274) . | GG (n = 266) . | P-value . |
|---|---|---|---|---|
| Smoking Status | 0.05 | |||
| Never | 29 (8.5%) | 149 (43.7%) | 163 (47.8%) | |
| Former | 28 (14.1%) | 97 (48.7%) | 74 (37.2%) | |
| Current | 11 (16.2%) | 28 (41.2%) | 29 (42.6%) | |
| CPD (n = 606) | 0.21* | |||
| 0 | 29 (8.5%) | 149 (43.7%) | 163 (47.8%) | |
| 1–10 | 12 (12%) | 45 (45%) | 43 (43%) | |
| 11–20 | 17 (15.9%) | 50 (46.7%) | 40 (37.4%) | |
| 21–30 | 3 (13.6%) | 12 (54.5%) | 7 (31.8%) | |
| ≥ 31 | 6 (16.7%) | 18 (50%) | 12 (33.3%) |
* Fisher’s exact Test
Logistic regression was performed to evaluate the effect of clinical and genetic predictors on IBD-related surgery, a surrogate of poorly controlled disease. CD and UC regression models each controlled for clinical variables which were considered ahead of time to have a high pre-test probability of influencing surgical outcomes.29–31 Interaction terms between genotype and smoking were included with a p value of less than 0.20 considered significant, as previously reported.25, 26
In the UC group, the main effects of age, sex, disease duration, smoking status, and rs16969968 genotype did not significantly impact the outcome of IBD-related surgery. The addition of the interaction term (CHRNA5 rs16969968 genotype * smoking status) to the model demonstrated a significant association with IBD-related surgery (OR = 2.72, 80% CI = 1.42, 5.32; P = 0.05). (Table 4)
Logistic Regression: Predictors of Abdominal Surgery in UC patients (N = 208)
| UC ONLY . | ||||
|---|---|---|---|---|
| . | Estimate . | Standard Error . | Z value . | Pr(>|z|) . |
| Age | 0.02 | 0.01 | 1.53 | 0.13 |
| Gender | -0.32 | 0.35 | -0.93 | 0.35 |
| Disease Duration | 0.01 | 0.02 | 0.53 | 0.60 |
| Smoking Status | -0.74 | 0.56 | -1.31 | 0.19 |
| CHRNA5 rs16969968 | -0.17 | 0.35 | -0.49 | 0.63 |
| CHRNA5 rs16969968 * smoking status | 1 | 0.51 | 1.94 | 0.05 |
| UC ONLY . | ||||
|---|---|---|---|---|
| . | Estimate . | Standard Error . | Z value . | Pr(>|z|) . |
| Age | 0.02 | 0.01 | 1.53 | 0.13 |
| Gender | -0.32 | 0.35 | -0.93 | 0.35 |
| Disease Duration | 0.01 | 0.02 | 0.53 | 0.60 |
| Smoking Status | -0.74 | 0.56 | -1.31 | 0.19 |
| CHRNA5 rs16969968 | -0.17 | 0.35 | -0.49 | 0.63 |
| CHRNA5 rs16969968 * smoking status | 1 | 0.51 | 1.94 | 0.05 |
AIC: 231.72. In the logistic regression without interaction between smoking and CHRNA5 genotype, the main effects of smoking and CHRNA5 genotype alone were not significant either.
Logistic Regression: Predictors of Abdominal Surgery in UC patients (N = 208)
| UC ONLY . | ||||
|---|---|---|---|---|
| . | Estimate . | Standard Error . | Z value . | Pr(>|z|) . |
| Age | 0.02 | 0.01 | 1.53 | 0.13 |
| Gender | -0.32 | 0.35 | -0.93 | 0.35 |
| Disease Duration | 0.01 | 0.02 | 0.53 | 0.60 |
| Smoking Status | -0.74 | 0.56 | -1.31 | 0.19 |
| CHRNA5 rs16969968 | -0.17 | 0.35 | -0.49 | 0.63 |
| CHRNA5 rs16969968 * smoking status | 1 | 0.51 | 1.94 | 0.05 |
| UC ONLY . | ||||
|---|---|---|---|---|
| . | Estimate . | Standard Error . | Z value . | Pr(>|z|) . |
| Age | 0.02 | 0.01 | 1.53 | 0.13 |
| Gender | -0.32 | 0.35 | -0.93 | 0.35 |
| Disease Duration | 0.01 | 0.02 | 0.53 | 0.60 |
| Smoking Status | -0.74 | 0.56 | -1.31 | 0.19 |
| CHRNA5 rs16969968 | -0.17 | 0.35 | -0.49 | 0.63 |
| CHRNA5 rs16969968 * smoking status | 1 | 0.51 | 1.94 | 0.05 |
AIC: 231.72. In the logistic regression without interaction between smoking and CHRNA5 genotype, the main effects of smoking and CHRNA5 genotype alone were not significant either.
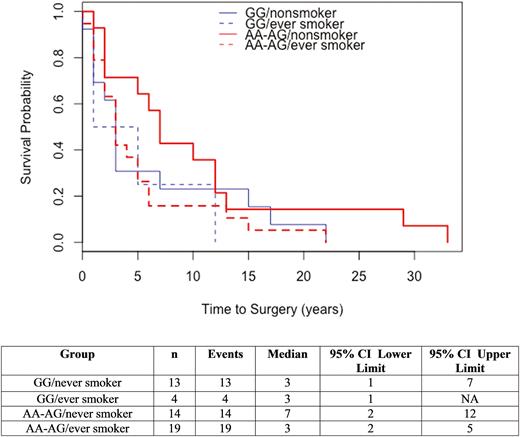
The main effects of smoking and genotype were evaluated for an effect on time to surgery. Smoking status (ever vs nonsmokers) was not significantly associated with time to surgery (Chisq = 2.1, df = 1, P = 0.14). Genotype was significantly associated with time to surgery, AA with a median of 2 years, GG with a median of 3 years, and AG with a median of 6 years (Chisq = 10, df = 2, P = 0.007). The interaction of smoking and genotype was then evaluated for effect on time to surgery and did not demonstrate a significant difference between all groups (Chisq = 3.6, df = 3, P = 0.31). (Fig. 2) Notably, those with AA or AG genotypes had an increased time to surgery if they were nonsmokers compared to ever smokers (7 versus 3 years).
In the CD group, the main effects of age, sex, disease location, smoking status, and rs16969968 genotype did not significantly impact the outcome of IBD-related surgery. Disease duration and disease behavior did significantly impact the outcome of IBD-related surgery. The addition of the interaction term to the model demonstrated an interaction effect between CHRNA5 rs16969968 and current smoking (OR = 2.88, 80% CI = 1.30, 6.64; P = 0.1). (Table 5)
Logistic Regression: Predictors of Abdominal and Perianal Surgery in CD patients (N = 400)
| CD ONLY: Abdominal and Perianal Surgery . | ||||
|---|---|---|---|---|
| . | Estimate . | Standard Error . | Z value . | Pr(>|z|) . |
| Age | -0.002 | 0.01 | -0.13 | 0.90 |
| Gender | 0.31 | 0.29 | 1.07 | 0.29 |
| Disease Duration | 0.08 | 0.02 | 4.37 | 1.27e-05 |
| Disease Behavior (Reference = inflammatory) | ||||
| Stricturing | 2.59 | 0.35 | 7.42 | 1.19e-13 |
| Fistulizing and Penetrating | 3.35 | 0.41 | 8.19 | 2.54e-16 |
| Fistulizing/Penetrating and Stricturing | 3.71 | 0.51 | 7.26 | 3.81e-13 |
| Fistulizing/Penetrating and Inflammatory (n = 1) | 14.3 | 882.7 | 0.02 | 0.99 |
| Disease Location | 0.82 | 0.43 | 1.91 | 0.06 |
| Smoking Status | ||||
| Former | 0.25 | 0.47 | 0.53 | 0.60 |
| Current | -0.48 | 0.59 | -0.83 | 0.41 |
| CHRNA5 rs16969968 | -0.31 | 0.30 | -1.02 | 0.31 |
| CHRNA5 rs16969968 * Smoking Status | ||||
| Former | -0.02 | 0.47 | -0.05 | 0.96 |
| Current | 1.06 | 0.63 | 1.67 | 0.1 |
| CD ONLY: Abdominal and Perianal Surgery . | ||||
|---|---|---|---|---|
| . | Estimate . | Standard Error . | Z value . | Pr(>|z|) . |
| Age | -0.002 | 0.01 | -0.13 | 0.90 |
| Gender | 0.31 | 0.29 | 1.07 | 0.29 |
| Disease Duration | 0.08 | 0.02 | 4.37 | 1.27e-05 |
| Disease Behavior (Reference = inflammatory) | ||||
| Stricturing | 2.59 | 0.35 | 7.42 | 1.19e-13 |
| Fistulizing and Penetrating | 3.35 | 0.41 | 8.19 | 2.54e-16 |
| Fistulizing/Penetrating and Stricturing | 3.71 | 0.51 | 7.26 | 3.81e-13 |
| Fistulizing/Penetrating and Inflammatory (n = 1) | 14.3 | 882.7 | 0.02 | 0.99 |
| Disease Location | 0.82 | 0.43 | 1.91 | 0.06 |
| Smoking Status | ||||
| Former | 0.25 | 0.47 | 0.53 | 0.60 |
| Current | -0.48 | 0.59 | -0.83 | 0.41 |
| CHRNA5 rs16969968 | -0.31 | 0.30 | -1.02 | 0.31 |
| CHRNA5 rs16969968 * Smoking Status | ||||
| Former | -0.02 | 0.47 | -0.05 | 0.96 |
| Current | 1.06 | 0.63 | 1.67 | 0.1 |
AIC: 355.69. In the logistic regression without interaction between smoking and CHRNA5 genotype, the main effects of smoking and CHRNA5 genotype alone were not significant either.
Logistic Regression: Predictors of Abdominal and Perianal Surgery in CD patients (N = 400)
| CD ONLY: Abdominal and Perianal Surgery . | ||||
|---|---|---|---|---|
| . | Estimate . | Standard Error . | Z value . | Pr(>|z|) . |
| Age | -0.002 | 0.01 | -0.13 | 0.90 |
| Gender | 0.31 | 0.29 | 1.07 | 0.29 |
| Disease Duration | 0.08 | 0.02 | 4.37 | 1.27e-05 |
| Disease Behavior (Reference = inflammatory) | ||||
| Stricturing | 2.59 | 0.35 | 7.42 | 1.19e-13 |
| Fistulizing and Penetrating | 3.35 | 0.41 | 8.19 | 2.54e-16 |
| Fistulizing/Penetrating and Stricturing | 3.71 | 0.51 | 7.26 | 3.81e-13 |
| Fistulizing/Penetrating and Inflammatory (n = 1) | 14.3 | 882.7 | 0.02 | 0.99 |
| Disease Location | 0.82 | 0.43 | 1.91 | 0.06 |
| Smoking Status | ||||
| Former | 0.25 | 0.47 | 0.53 | 0.60 |
| Current | -0.48 | 0.59 | -0.83 | 0.41 |
| CHRNA5 rs16969968 | -0.31 | 0.30 | -1.02 | 0.31 |
| CHRNA5 rs16969968 * Smoking Status | ||||
| Former | -0.02 | 0.47 | -0.05 | 0.96 |
| Current | 1.06 | 0.63 | 1.67 | 0.1 |
| CD ONLY: Abdominal and Perianal Surgery . | ||||
|---|---|---|---|---|
| . | Estimate . | Standard Error . | Z value . | Pr(>|z|) . |
| Age | -0.002 | 0.01 | -0.13 | 0.90 |
| Gender | 0.31 | 0.29 | 1.07 | 0.29 |
| Disease Duration | 0.08 | 0.02 | 4.37 | 1.27e-05 |
| Disease Behavior (Reference = inflammatory) | ||||
| Stricturing | 2.59 | 0.35 | 7.42 | 1.19e-13 |
| Fistulizing and Penetrating | 3.35 | 0.41 | 8.19 | 2.54e-16 |
| Fistulizing/Penetrating and Stricturing | 3.71 | 0.51 | 7.26 | 3.81e-13 |
| Fistulizing/Penetrating and Inflammatory (n = 1) | 14.3 | 882.7 | 0.02 | 0.99 |
| Disease Location | 0.82 | 0.43 | 1.91 | 0.06 |
| Smoking Status | ||||
| Former | 0.25 | 0.47 | 0.53 | 0.60 |
| Current | -0.48 | 0.59 | -0.83 | 0.41 |
| CHRNA5 rs16969968 | -0.31 | 0.30 | -1.02 | 0.31 |
| CHRNA5 rs16969968 * Smoking Status | ||||
| Former | -0.02 | 0.47 | -0.05 | 0.96 |
| Current | 1.06 | 0.63 | 1.67 | 0.1 |
AIC: 355.69. In the logistic regression without interaction between smoking and CHRNA5 genotype, the main effects of smoking and CHRNA5 genotype alone were not significant either.
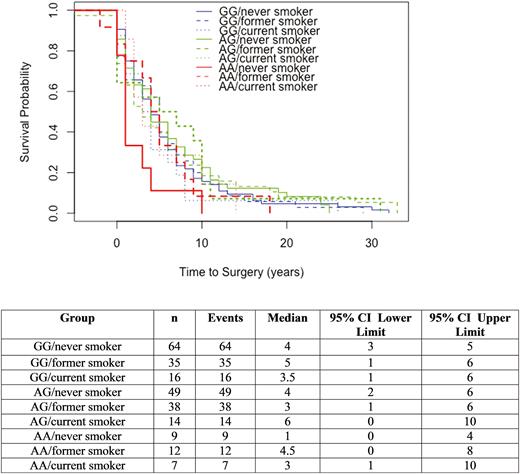
The main effects of smoking and genotype were evaluated for an effect on time to surgery. Neither smoking (never vs former vs current) nor genotype was significantly associated with time to surgery (Chisq = 0.1, df = 2, P = 0.94 and Chisq = 3, df = 2, P = 0.23, respectively). The interaction of smoking and genotype was then evaluated for effect on time to surgery and did not demonstrate a significant association (Chisq = 6.6, df = 8, P = 0.58). (Fig. 3) Notably, AA-never smokers had a reduced time to surgery compared to AA-former smokers and AA-current smokers (1 vs 3–4.5 years). One patient (AG-former smoker) had a perianal surgery 5 years prior to diagnosis of CD thus the baseline starting prior to time 0.
DISCUSSION
In this study, we evaluated the interaction between smoking status and genetic variation at CHRNA5, which has a biological relationship with both inflammation and nicotine behavior, making it an intriguing prospect for further investigation. We demonstrated an interaction between CHRNA5 rs16969968 and smoking status in influencing the risk of IBD-related surgery. We also demonstrated a trend toward higher levels of nicotine dependence in a gene-dose dependent manner. These findings add to the existing literature on gene-environment interactions in IBD, offer insight into the interaction between smoking and nicotinic receptors to alter disease course, and further highlight the importance of underlying genetic risk in nicotine dependence.
Smoking was found to be an effect modifier of CHRNA5 rs16969968 genotype in predicting need for IBD-related surgery, regardless of disease subtype. The UC group demonstrated the most significant relationship between smoking and rs16969968 in predicting surgery despite the relatively small number of cases in the regression analysis. The presence of an interaction effect suggests that the effect of one variable is dependent on the level of the other variable. In this case, the effect of CHRNA5 rs16969968 genotype on IBD-related surgery was dependent on the level of smoking. Time to surgery was not found to be significantly associated with interaction of smoking and genotype but the survival curve suggested a differential effect of smoking in those with AA and AG genotypes. Specifically, non-smokers with AA/AG genotypes went to surgery approximately 4 years later than former or current smokers with AA/AG genotypes. This could suggest the possibility of a protective effect on disease outcomes for those have AA or AG genotype and abstain from tobacco. Independent analysis of AA was limited given the small sample size of the AA group. Further study of a larger cohort could help to clarify if there is an association on time to surgery in UC patients. Univariate analysis of genotype was not significantly associated with risk of surgery but was associated with time to surgery. The lack of a trend (AG > GG > AA) makes this result difficult to interpret.
The CD cohort also demonstrated an interaction effect between current smokers and genotype. Time to surgery did not demonstrate a significant association with the interaction of smoking and genotype but inspection of the survival curve suggests that never smokers with CHRNA5 rs16969968 AA genotype had an IBD-related surgery earlier than those who were former or current smokers with an AA genotype.
The results of our study replicated, in an IBD cohort, the generalized population findings of increased nicotine dependence, as measured by the FTND score, with the CHRNA5 rs16969968 risk (A) variant. However, our results did not reach statistical significance. We suspect this was related to an inadequate sample size to detect this modest effect (OR 1.4).32 Despite not reaching statistical significance, there was an association of FTND score with CHRNA5 rs16969968 genotype in a gene-dose dependent manner (AA > AG > GG) as expected. These findings should be replicated in a larger actively smoking IBD cohort before a definitive conclusion can be drawn. If our findings hold true, IBD patients who are CHRNA5 rs16969968 A allele carriers may represent a group of high dependence smokers within IBD who would benefit from targeted smoking reduction interventions such as counseling and pharmacotherapy.
This study has numerous strengths. To our knowledge, this is the first study to assess the interaction between CHRNA5 risk variants and smoking in evaluating IBD disease outcomes. These results offer additional insight into the mechanisms by which smoking may exert its influence on IBD disease activity. Work to date has demonstrated that knockout of CHRNA5 in a mouse model leads to worsened DSS-induced colitis.23 Given the relationship between nAChRα7 and vagus-mediated inhibition of macrophage TNF release,20 it could be hypothesized that nAChRα5 works by a similar mechanism of action, specifically down regulation of macrophage inflammatory cytokine release. Replication of findings in a larger cohort and additional studies evaluating the biological relationship between smoking, CHRNA5 genetic variation, macrophage based TNF release, and luminal inflammation may help to elucidate these findings further. An additional strength of our study is that patients were given comprehensive smoking questionnaires during recruitment asking for detailed smoking histories, such as nicotine dependence and quantitative assessments of smoking. This patient-reported data likely offers more accurate assessments of smoking history than could be obtained by retrospective chart review.
There are also limitations in this study. First, this study is purely associative and offers no specific evidence into the biological mechanisms for such findings. Further studies would be needed to not only replicate our findings but to clarify the mechanisms of action. Second, analyses were only performed in Caucasian participants given the variation in allele frequency across races. Therefore, these findings may not be applicable to other racial groups. Third, the relationship between CPD and genotype was not stratified by adjunctive cessation measures such as pharmacotherapy, and this may limit interpretation. Finally, the small sample size in our analysis may have limited the ability to detect a significant difference in nicotine dependence with the rs16969968 risk variant. These findings are established in the general population literature and the inability of this study to definitively confirm these findings in an IBD population may be related to an underpowered study. The small sample size also may have also limited the ability to detect significant predictors of IBD-related surgery in the regression analysis. Therefore, independent weak or moderately predictive clinical or genetic predictors could have been missed.
In summary, we demonstrated an interaction between CHRNA5 rs16969968 and smoking status in influencing the risk of IBD-related surgery. We also demonstrated a trend toward higher levels of nicotine dependence in a gene-dose dependent manner.
ACKNOWLEDGEMENTS
KC: Research reported in this publication was supported by the NIH T32DK007130 grant and the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
YJS: partly supported by the National Institutes of Health (K25 HL121091)
SC: Support provided, in part, by the National Institutes of Health (Cresci R01 NR013396)
LSC: This research is partially supported by the National Institute on Drug Abuse R01 DA038076.
DNA Extractions: This work was supported by the Hope Center DNA/RNA Purification Core at Washington University School of Medicine
Conflicts of Interest and Source of Funding
Kelly C. Cushing: No conflicts of interest. Support provided by the T32DK007130 grant and the UL1TR000448 grant.
Adeeti Chiplunker: No conflicts of interest.
Allie Li: No conflicts of interest.
Yun Ju Sung: No conflicts of interest. Support provided, in part, by the National Institutes of Health (Sung K25 HL121091).
Taylor Geisman: No conflicts of interest.
Li-Shiun Chen: No conflicts of interest. This research is partially supported by the National Institute on Drug Abuse R01 DA038076.
Sharon Cresci: No conflicts of interest. Support provided, in part, by the National Institutes of Health (Cresci R01 NR013396).
Alexandra M Gutierrez: No conflicts of interest.



