-
PDF
- Split View
-
Views
-
Cite
Cite
Umberto Leone Roberti Maggiore, Simone Ferrero, Giorgia Mangili, Alice Bergamini, Annalisa Inversetti, Veronica Giorgione, Paola Viganò, Massimo Candiani, A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes, Human Reproduction Update, Volume 22, Issue 1, January/February 2016, Pages 70–103, https://doi.org/10.1093/humupd/dmv045
Close - Share Icon Share
Abstract
Traditionally, pregnancy was considered to have a positive effect on endometriosis and its painful symptoms due not only to blockage of ovulation preventing bleeding of endometriotic tissue but also to different metabolic, hormonal, immune and angiogenesis changes related to pregnancy. However, a growing literature is emerging on the role of endometriosis in affecting the development of pregnancy and its outcomes and also on the impact of pregnancy on endometriosis. The present article aims to underline the difficulty in diagnosing endometriotic lesions during pregnancy and discuss the options for the treatment of decidualized endometriosis in relation to imaging and symptomatology; to describe all the possible acute complications of pregnancy caused by pre-existing endometriosis and evaluate potential treatments of these complications; to assess whether endometriosis affects pregnancy outcome and hypothesize mechanisms to explain the underlying relationships.
This systematic review is based on material searched and obtained via Pubmed and Medline between January 1950 and March 2015. Peer-reviewed, English-language journal articles examining the impact of endometriosis on pregnancy and vice versa were included in this article.
Changes of the endometriotic lesions may occur during pregnancy caused by the modifications of the hormonal milieu, posing a clinical dilemma due to their atypical appearance. The management of these events is actually challenging as only few cases have been described and the review of available literature evidenced a lack of formal estimates of their incidence. Acute complications of endometriosis during pregnancy, such as spontaneous hemoperitoneum, bowel and ovarian complications, represent rare but life-threatening conditions that require, in most of the cases, surgical operations to be managed. Due to the unpredictability of these complications, no specific recommendation for additional interventions to the routinely monitoring of pregnancy of women with known history of endometriosis is advisable. Even if the results of the published studies are controversial, some evidence is suggestive of an association of endometriosis with spontaneous miscarriage, preterm birth and small for gestational age babies. A correlation of endometriosis with placenta previa (odds ratio from 1.67 to 15.1 according to various studies) has been demonstrated, possibly linked to the abnormal frequency and amplitude of uterine contractions observed in women affected. Finally, there is no evidence that prophylactic surgery would prevent the negative impact of endometriosis itself on pregnancy outcome.
Complications of endometriosis during pregnancy are rare and there is no evidence that the disease has a major detrimental effect on pregnancy outcome. Therefore, pregnant women with endometriosis can be reassured on the course of their pregnancies although the physicians should be aware of the potential increased risk of placenta previa. Current evidence does not support any modification of conventional monitoring of pregnancy in patients with endometriosis.
Introduction
Endometriosis is defined as the presence of endometrial-like tissue (stroma and glands) outside the uterus, which induces a local inflammatory response (Kennedy et al., 2005; De Nardi and Ferrari, 2011). It is an estrogen-dependent chronic condition that affects women of fertile age, and is associated with pelvic pain and infertility (Giudice, 2010). However, in recent years, evidence is emerging in support of a relevant impact of endometriosis not only in reducing fertility but also in affecting the pregnancy outcome. Different mechanisms including endocrine/inflammatory balance, bleeding from endometriotic implants, molecular and functional abnormalities of the eutopic endometrium, defective deep placentation and decidualization of the endometriotic tissue due to changes of the hormonal milieu that characterizes pregnancy are thought to be involved (Brosens et al., 2009, 2012a; Petraglia et al., 2012; Viganò et al., 2012).
Noteworthy, this rising concept is in contrast with the historical assumption that pregnancy may have a positive effect on endometriosis and its symptoms (Beecham, 1949; Kistner, 1959a, b) due to anovulation and amenorrhea preventing bleeding of endometriotic tissue but also to different metabolic, hormonal, immune and angiogenesis changes related to pregnancy (May and Becker, 2008; Taylor et al., 2009). Although the available literature is still too scanty and contradictory to draw definitive conclusions on the linkage between endometriosis and adverse pregnancy outcomes, a wide spectrum of obstetric events that originate either in the ectopic implants or in the uterus has been described.
Therefore, the aim of this paper is to review the current body of knowledge on the impact of endometriosis on the outcome of pregnancy and, in detail, to:
underline the difficulty in diagnosing endometriotic lesions during pregnancy and discuss the options for the treatment of decidualized endometriosis in relation to imaging and symptomatology;
describe all the possible acute complications of pregnancy caused by pre-existing endometriosis and evaluate the potential treatments of these complications;
assess whether endometriosis affects pregnancy outcome and hypothesize mechanisms to explain the underlying relationships.
Methods
The PRISMA statement was used for reporting the Methods, Results and Discussion sections of the current review (Moher et al., 2009). No institutional review board approval was required because only published, de-identified data were analyzed. The search strategy, inclusion and exclusion criteria were specific for each of the three main aims of this systematic review and described below.
Literature search
General criteria
A systematic computerized search of the literature, from 1950 until March 2015 (last research 31 March 2015; the search was run every month since September 2014 until March 2015) was performed in two electronic databases (PubMed and MEDLINE) in order to identify relevant articles to be included for the purpose of this systematic review. All pertinent articles were examined and their reference lists were systematically reviewed in order to identify other studies for potential inclusion in this article.
Studies were reviewed by two independent reviewers (U.L.R.M. and S.F.) and discrepancies were resolved by consensus including a third author (P.V.). The reviewers were not blinded to the names of investigators or sources of publication. First, eligibility was assessed based on titles and abstracts. Full manuscripts were obtained for all selected studies and decision for final inclusion was made after detailed examination of the papers.
Specific criteria
Three independent authors (A.I., A.B., V.G.) ran a specific literature search for each of the three main aims of this article (Fig. 1 and Supplementary Table SI). The search included the following keywords and medical subjects heading terms, alone or in combination. The first search included the terms cancer, cyst, decidualized, decidual reaction, endometrioma, endometriosis, malignancy, pregnancy, tumor; the second the terms bladder, bowel, colorectal, complication, deep infiltrative, endometriosis, extrapelvic, fallopian tube, ovarian, pregnancy, rectovaginal endometriosis, urinary tract, uterosacral, vessels, uterus; the third the terms abruptio placentae, adverse pregnancy outcome, antepartum haemorrhage, caesarean delivery, endometriosis, gestational diabetes mellitus, hypertension, intrauterine growth restriction, miscarriage, placenta praevia, post-partum hemorrhage, pre-eclampsia, preterm labor, small for gestational age.
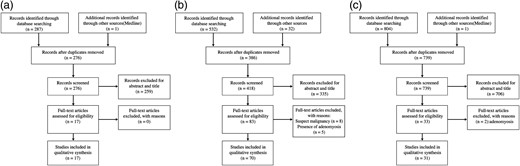
Flow chart of the search process for the purposes of the systematic review: decidualized endometriosis in the ovary and extra-ovarian sites (a), endometriosis complications during pregnancy (b), endometriosis and pregnancy outcomes (c).
Selection criteria
General criteria
Peer-reviewed, English-language journal articles that examined the impact of endometriosis on pregnancy and the impact of pregnancy on endometriosis were included in this systematic review. No limits on the age of participants, on type of pregnancy (single or multiple), on week of gestation of pregnancy or on the mode of conception (natural or assisted reproductive technology) were applied. Studies investigating the impact of adenomyosis (alone or associated with endometriosis) and of leiomyomas (associated with endometriosis) on pregnancy were excluded.
Specific criteria
For each of the three main aims of this article different type of studies were considered:
RCTs, prospective cohort studies, case–control studies, retrospective cohort studies, case series and case reports were screened where available;
case series and case reports were screened;
RCTs, prospective cohort studies, case–control studies, retrospective cohort studies and case series were screened where available.
Suspicion of malignant degeneration of decidualized endometriosis: imaging pattern and treatment issues
‘Deciduosis’ from decidualized endometriosis
The condition in which groups of decidual cells reside outside the endometrium is termed ectopic decidua or ‘deciduosis’ and is a well-known phenomenon of pregnancy. Ectopic decidua is most commonly localized in the ovary, cervix, uterine serosa and the lamina propria of the salpinx while the peritoneal localization is uncommon. More specifically, the term ‘deciduosis’ is used to indicate two different entities (i) the phenomenon of metaplasia of the sub-coelomic pluripotent mesenchymal cells under the effect of progesterone reported very frequently in the ovary of term pregnancies and regressing post-partum within 4–6 weeks and (ii) the pregnancy-associated stromal decidualization of ectopic endometrium (endometriosis) that under progesterone action increases glandular epithelial secretion, stromal vascularity and edema (Barbieri et al., 2009; Calorbe et al., 2012). Decidualized endometriosis is characterized by typical sonographic, histological and molecular patterns (Fig. 2).
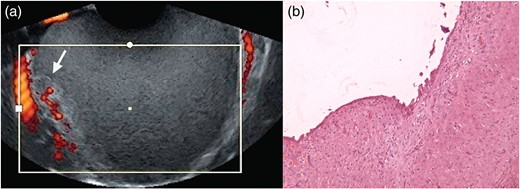
Ultrasonographic (a) and histologic (b, low-power magnification) patterns of a decidualized endometrioma (reproduced with permission from Mascilini et al., 2014 and Barbieri et al., 2009).
The sonographic pattern of decidualized ovarian endometriomas, in a proportion of cases, may mimic malignancy (Mascilini et al., 2014). As better described in depth later on, solid components can be easily recognized and the echogenicity of the cyst content is usually ground-glass or low level. The content usually consists of papillary projections with smooth rounded contour. Color Doppler analysis can detect multiple vascularization signals within the solid part with low resistance index.
Histologically, ‘deciduosis’ deriving from peritoneal metaplasia is usually found as small cell groups or single cell clusters under the mesothelium with polygonal and eosinophilic decidualized cells with various rates of vacuolar degeneration. The stroma may contain a myxoid deposit due to the vacuole rupture. Distended capillaries and numerous lymphocytes are typically found within the decidual foci (Bolat et al., 2012). Endometriotic lesions in pregnancy typically reveal a decidual reaction similar to that seen in the eutopic endometrium. The glands are usually atrophic resulting in fibrosis. Necrosis of the decidual cells, stromal myxoid change or edema and infiltration of lymphocytes may also be seen (Clement, 2007). The two entities are not easily histologically distinguishable.
The molecular aspects of decidualized endometriosis are under extensive investigation due to the potential implications for the disease development. Indeed, both eutopic and ectopic endometrial cells of women with endometriosis have compromised decidualization whose origins are probably multifactorial (Klemmt et al., 2006; Erikson et al., 2014). Main reason for this seems related to a differentiation defect in endometriotic stromal cells due to a resistance to the actions of progesterone, as progesterone is the key hormone involved in inducing the decidualization process. Progesterone resistance in endometriosis seems manifested by selective molecular abnormalities in the endometrium (Bulun et al., 2006; Burney et al., 2007). Overactivation of phosphoinositol-3 kinase (PI3K)/AKT, mitogen-activated protein kinase and epidermal growth factor receptor signaling pathways has also been hypothesized as causing aberrant decidualization of stromal cells from women with endometriosis (Erikson et al., 2014).
Amongst the interacting partners of progesterone receptor in the human endometrium are members of the forkhead box class O (FOXO) family of transcription factors. FOXO proteins, functioning downstream of the PI3K/AKT signaling pathway, are central to a diversity of cellular functions, including cell proliferation, apoptosis, differentiation and resistance to oxidative stress (Kajihara et al., 2013). While normal decidualized stromal cells in response to activation of the cAMP/PKA pathway secrete abundant amounts of prolactin and express insulin-like growth factor binding protein-1 (IGFBP-1), ectopic endometrium has a blunted expression of the decidual markers prolactin and IGFBP-1 and of their upstream transcriptional regulator FOXO1. In particular, increased activation of PI3K/AKT pathway in endometriosis would promote translocation of FOXO1 to the cytoplasm and its modification for degradation (Yin et al., 2012). Further investigations are however needed to completely clarify these mechanisms. Notably, this compromised decidualization of ectopic lesions might not only promote their proliferation and/or survival but may also reflect their limited differentiation capacity (Erikson et al., 2014).
Decidualized ovarian endometriosis in pregnancy: diagnosis
Ovarian endometrioma represents a common finding in women affected by endometriosis, with an estimated prevalence of 30–40% (Redwine, 1999; Vercellini et al., 2006; Sanchez et al., 2014). Besides corpus luteal cysts, adnexal masses are detected in 0.5–1.2% of pregnancies: of these, 11% are endometriomas, while the reported rate of ovarian cancer is 1% (Bromley and Benacerraf, 1997; Leiserowitz et al., 2006). Of the latter, a proportion of about 51% is epithelial (both invasive and borderline) and 39% are germ cell tumors, mainly dysgerminomas and malignant teratomas, in line with the young age of pregnant woman. Ovarian endometriomas in pregnancy represents a peculiar entity, still debated both concerning diagnosis and treatment. During pregnancy, changes in the dimension and in the appearance of the endometriotic cyst have been described. Ueda and colleagues observed that during pregnancy size of the cysts decreased in 52% of the cases, went unchanged in 28%, and increased in 20% (Ueda et al., 2010). A more recent study reported that the number of endometriotic cysts was unchanged in 33% of the cases, increased in 8%, reduced in 13%, and in the remaining 46% no cyst could be detected (Benaglia et al., 2013). Different possible explanations for this phenomenon have been hypothesized. The cessation of menstrual cycles may be a factor potentially involved in the different endometrioma behavior during pregnancy. In addition, the peculiar histological characteristics of each endometrioma are likely related to this variability in modification during pregnancy, since endometrioma shrinkage only occurs in selected cases. It has been suggested that only those covered by endometrium, which is more prone to decidualization, may undergo shrinkage and even ‘vanishing’ (Benaglia et al., 2013). Furthermore, as mentioned above, pregnancy-related hormonal status may effectively lead to changes in the histologic, sonographic and molecular appearance referred as ‘decidualization’, which may in some cases resemble malignant ovarian tumors, potentially leading to an unnecessary surgical intervention. Formal assessment of the frequency of this phenomenon is lacking, and on the basis of indirect evidence supporting highly variable estimations, no definitive conclusion can be drawn (Ueda et al., 2010; Benaglia et al., 2013). Benaglia and coworkers conducted a study in order to assess modifications in number and size of ovarian endometriomas before and after pregnancy in 24 women who underwent IVF procedures. Forty endometriomas were identified and no sign of decidualization of the ovarian cysts was detected (Benaglia et al., 2013). Another study aimed at clarifying the frequency of pregnancies complicated by ovarian endometriosis and to investigate the size change and outcome of ovarian endometriosis during pregnancy. Twenty-four women carrying 25 endometriomas were included in this study and signs of decidualization were seen in 3 cases (12%). However, ovarian endometriosis in pregnancy is a rare condition with an estimated frequency of about 0.05–0.5% (Bromley and Benacerraf, 1997; Leiserowitz et al., 2006; Ueda et al., 2010) and literature on decidualized ovarian endometrioma mainly consists of case reports of three or fewer patients (Table I). Some larger studies have been recently published to define its peculiar sonographic appearance (Groszmann et al., 2014; Mascilini et al., 2014). As borderline tumors and cystadenofibromas, decidualized endometriomas are difficult to classify since they show sonographic characteristics common to both malignant and benign adnexal masses. It is likely that an under-diagnosis of such a transformation should be considered in explaining the rarity of this event because the ovaries are not routinely evaluated during obstetric ultrasound. Another possible explanation is the variability in levels and response to steroid hormones among pregnant women.
Ovarian decidualized endometriosis during pregnancy: cases reported in literature.
| Author, year . | Cases (n) . | Age [range] . | History of endometriosis . | Pain . | CA125 (U/ml) . | Laterality . | Size, mm [range] . | Intracystic papillae . | Solid part, mm . | Blood flow . | Septa . | MRI . | Surgery, type . | Surgery, GA . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Miyakoshi et al. (1998) | 1 | 28 | + | − | NR | Unilateral | 85 (max diam) | + | NR | + | − | + | Oophorectomy | 20 |
| Tanaka et al. (2002) | 1 | 27 | − | − | 103 | Unilateral | 120 × 80 × 70 | + | NR | NR | + | + | Cystectomy | 12 |
| Fruscella et al. (2004) | 1 | 39 | − | − | 76 | Unilateral | 55 (max diam) | + | 8 × 10 and 5 × 5 | + | − | + | Oophorectomy | 18 |
| Sammour et al. (2005) | 1 | 28 | + | − | NR | Unilateral | 40 × 50 × 63 | + | 23 × 18 × 14 | + | − | − | Oophorectomy | 16 |
| 1 | 36 | − | − | NR | Unilateral | 37 × 27 × 25 | + | 15 × 20 × 25 | + | − | + | Oophorectomy | 15 | |
| Guerriero et al. (2005) | 1 | 38 | − | − | 109 | Unilateral | 40 × 48 | + | NR | + | − | − | Bilateral oophorectomy | 37 (CS) |
| Iwamoto et al. (2006) | 1 | 31 | + | − | 28.3 | Unilateral | 75 (max diam) | + | NR | + | − | + | Oophorectomy | 22 |
| Asch and Levine (2007) | 1 | Unilateral | NT | + | NR | + | − | − | Bilateral cystectomy | After delivery | ||||
| Poder et al. (2008) | 1 | 34 | + | − | 24 | Unilateral | 62 | + | + | + | − | + | Oophorectomy | 38 (CS) |
| Machida et al. (2008) | 3 | 32 | − | − | 119 | Unilateral | 80 × 50 | + | NR | + | − | + | Oophorectomy | 19 |
| 41 | − | − | 220 | Unilateral | 160 | + | NR | NR | + | + | Oophorectomy | 14 | ||
| 24 | − | − | 34 | Bilateral | 50 | + | NR | NR | − | + | Cystectomy | 14 | ||
| Yoshida et al. (2008) | 1 | 29 | − | − | NR | Unilateral | 85 × 53 | + | NR | − | − | + | Oophorectomy | 14 |
| 1 | 28 | − | − | NR | Unilateral | 74 × 48 | + | NR | − | − | + | Oophorectomy | 19 | |
| Takeuchi et al. (2008) | 5 | 28 [20–32] | NR | − | NR | Unilateral | 58 [40–90] | + | 20 mm (max) | NR§ | − | + | In 1 case | NR |
| Barbieri et al. (2009) | 3 | 32 | − | − | 30 | Unilateral | 66 × 44 | + | 20 × 14 | + | − | − | Cystectomy | |
| 36 | − | + | 159 | Bilateral | 85 × 63/50 × 30 | + | 27 × 14/7 × 7 | + | − | − | None | |||
| 39 | + | − | 85 | Unilateral | 38 × 14 | + | 19 × 12 | + | − | − | None | |||
| Sayasneh et al. (2012) | 1 | 35 | + | − | 89* | Unilateral | 31 × 40 × 5 × 40.5 | + | 15 | + | − | − | None | |
| Tazegül et al. (2013) | 1 | 32 | − | + (12 weeks) | 220 | Unilateral | 65 × 57 | + | 8 × 14 | + | − | − | Cystectomy | 12 |
| Proulx and Levine (2014) | 1 | 30 | NR | − | NR | Unilateral | 40 (max diam) | + | NR | + | − | + | None | |
| Mascilini et al. (2014) | 18 | 34 [20–43] | 3 (17%) | 61 [12–285]** | 3(17%) Bilateral | 66 [41–121] | +17 (94%) | +17 (94%) | +16 (94%) | +7 (39%) | − | In 13 cases (72%) | ||
| Groszmann et al. (2014) | 17 | 29 [22–43] | NR | NR | 5 (29%) Bilateral | [30–270] | NR | +14 (64%) | +12 (55%) | +8 (36%) | − | In 8 cases (47%) |
| Author, year . | Cases (n) . | Age [range] . | History of endometriosis . | Pain . | CA125 (U/ml) . | Laterality . | Size, mm [range] . | Intracystic papillae . | Solid part, mm . | Blood flow . | Septa . | MRI . | Surgery, type . | Surgery, GA . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Miyakoshi et al. (1998) | 1 | 28 | + | − | NR | Unilateral | 85 (max diam) | + | NR | + | − | + | Oophorectomy | 20 |
| Tanaka et al. (2002) | 1 | 27 | − | − | 103 | Unilateral | 120 × 80 × 70 | + | NR | NR | + | + | Cystectomy | 12 |
| Fruscella et al. (2004) | 1 | 39 | − | − | 76 | Unilateral | 55 (max diam) | + | 8 × 10 and 5 × 5 | + | − | + | Oophorectomy | 18 |
| Sammour et al. (2005) | 1 | 28 | + | − | NR | Unilateral | 40 × 50 × 63 | + | 23 × 18 × 14 | + | − | − | Oophorectomy | 16 |
| 1 | 36 | − | − | NR | Unilateral | 37 × 27 × 25 | + | 15 × 20 × 25 | + | − | + | Oophorectomy | 15 | |
| Guerriero et al. (2005) | 1 | 38 | − | − | 109 | Unilateral | 40 × 48 | + | NR | + | − | − | Bilateral oophorectomy | 37 (CS) |
| Iwamoto et al. (2006) | 1 | 31 | + | − | 28.3 | Unilateral | 75 (max diam) | + | NR | + | − | + | Oophorectomy | 22 |
| Asch and Levine (2007) | 1 | Unilateral | NT | + | NR | + | − | − | Bilateral cystectomy | After delivery | ||||
| Poder et al. (2008) | 1 | 34 | + | − | 24 | Unilateral | 62 | + | + | + | − | + | Oophorectomy | 38 (CS) |
| Machida et al. (2008) | 3 | 32 | − | − | 119 | Unilateral | 80 × 50 | + | NR | + | − | + | Oophorectomy | 19 |
| 41 | − | − | 220 | Unilateral | 160 | + | NR | NR | + | + | Oophorectomy | 14 | ||
| 24 | − | − | 34 | Bilateral | 50 | + | NR | NR | − | + | Cystectomy | 14 | ||
| Yoshida et al. (2008) | 1 | 29 | − | − | NR | Unilateral | 85 × 53 | + | NR | − | − | + | Oophorectomy | 14 |
| 1 | 28 | − | − | NR | Unilateral | 74 × 48 | + | NR | − | − | + | Oophorectomy | 19 | |
| Takeuchi et al. (2008) | 5 | 28 [20–32] | NR | − | NR | Unilateral | 58 [40–90] | + | 20 mm (max) | NR§ | − | + | In 1 case | NR |
| Barbieri et al. (2009) | 3 | 32 | − | − | 30 | Unilateral | 66 × 44 | + | 20 × 14 | + | − | − | Cystectomy | |
| 36 | − | + | 159 | Bilateral | 85 × 63/50 × 30 | + | 27 × 14/7 × 7 | + | − | − | None | |||
| 39 | + | − | 85 | Unilateral | 38 × 14 | + | 19 × 12 | + | − | − | None | |||
| Sayasneh et al. (2012) | 1 | 35 | + | − | 89* | Unilateral | 31 × 40 × 5 × 40.5 | + | 15 | + | − | − | None | |
| Tazegül et al. (2013) | 1 | 32 | − | + (12 weeks) | 220 | Unilateral | 65 × 57 | + | 8 × 14 | + | − | − | Cystectomy | 12 |
| Proulx and Levine (2014) | 1 | 30 | NR | − | NR | Unilateral | 40 (max diam) | + | NR | + | − | + | None | |
| Mascilini et al. (2014) | 18 | 34 [20–43] | 3 (17%) | 61 [12–285]** | 3(17%) Bilateral | 66 [41–121] | +17 (94%) | +17 (94%) | +16 (94%) | +7 (39%) | − | In 13 cases (72%) | ||
| Groszmann et al. (2014) | 17 | 29 [22–43] | NR | NR | 5 (29%) Bilateral | [30–270] | NR | +14 (64%) | +12 (55%) | +8 (36%) | − | In 8 cases (47%) |
NR, not reported; +, present; −, absent; CS, Cesarean section; MRI, magnetic resonance imaging; GA, gestational age.
*CA125 was measured prior to conception and not measured again.
**Available for nine women.
§Ultrasound (US) scan parameters not reported.
Ovarian decidualized endometriosis during pregnancy: cases reported in literature.
| Author, year . | Cases (n) . | Age [range] . | History of endometriosis . | Pain . | CA125 (U/ml) . | Laterality . | Size, mm [range] . | Intracystic papillae . | Solid part, mm . | Blood flow . | Septa . | MRI . | Surgery, type . | Surgery, GA . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Miyakoshi et al. (1998) | 1 | 28 | + | − | NR | Unilateral | 85 (max diam) | + | NR | + | − | + | Oophorectomy | 20 |
| Tanaka et al. (2002) | 1 | 27 | − | − | 103 | Unilateral | 120 × 80 × 70 | + | NR | NR | + | + | Cystectomy | 12 |
| Fruscella et al. (2004) | 1 | 39 | − | − | 76 | Unilateral | 55 (max diam) | + | 8 × 10 and 5 × 5 | + | − | + | Oophorectomy | 18 |
| Sammour et al. (2005) | 1 | 28 | + | − | NR | Unilateral | 40 × 50 × 63 | + | 23 × 18 × 14 | + | − | − | Oophorectomy | 16 |
| 1 | 36 | − | − | NR | Unilateral | 37 × 27 × 25 | + | 15 × 20 × 25 | + | − | + | Oophorectomy | 15 | |
| Guerriero et al. (2005) | 1 | 38 | − | − | 109 | Unilateral | 40 × 48 | + | NR | + | − | − | Bilateral oophorectomy | 37 (CS) |
| Iwamoto et al. (2006) | 1 | 31 | + | − | 28.3 | Unilateral | 75 (max diam) | + | NR | + | − | + | Oophorectomy | 22 |
| Asch and Levine (2007) | 1 | Unilateral | NT | + | NR | + | − | − | Bilateral cystectomy | After delivery | ||||
| Poder et al. (2008) | 1 | 34 | + | − | 24 | Unilateral | 62 | + | + | + | − | + | Oophorectomy | 38 (CS) |
| Machida et al. (2008) | 3 | 32 | − | − | 119 | Unilateral | 80 × 50 | + | NR | + | − | + | Oophorectomy | 19 |
| 41 | − | − | 220 | Unilateral | 160 | + | NR | NR | + | + | Oophorectomy | 14 | ||
| 24 | − | − | 34 | Bilateral | 50 | + | NR | NR | − | + | Cystectomy | 14 | ||
| Yoshida et al. (2008) | 1 | 29 | − | − | NR | Unilateral | 85 × 53 | + | NR | − | − | + | Oophorectomy | 14 |
| 1 | 28 | − | − | NR | Unilateral | 74 × 48 | + | NR | − | − | + | Oophorectomy | 19 | |
| Takeuchi et al. (2008) | 5 | 28 [20–32] | NR | − | NR | Unilateral | 58 [40–90] | + | 20 mm (max) | NR§ | − | + | In 1 case | NR |
| Barbieri et al. (2009) | 3 | 32 | − | − | 30 | Unilateral | 66 × 44 | + | 20 × 14 | + | − | − | Cystectomy | |
| 36 | − | + | 159 | Bilateral | 85 × 63/50 × 30 | + | 27 × 14/7 × 7 | + | − | − | None | |||
| 39 | + | − | 85 | Unilateral | 38 × 14 | + | 19 × 12 | + | − | − | None | |||
| Sayasneh et al. (2012) | 1 | 35 | + | − | 89* | Unilateral | 31 × 40 × 5 × 40.5 | + | 15 | + | − | − | None | |
| Tazegül et al. (2013) | 1 | 32 | − | + (12 weeks) | 220 | Unilateral | 65 × 57 | + | 8 × 14 | + | − | − | Cystectomy | 12 |
| Proulx and Levine (2014) | 1 | 30 | NR | − | NR | Unilateral | 40 (max diam) | + | NR | + | − | + | None | |
| Mascilini et al. (2014) | 18 | 34 [20–43] | 3 (17%) | 61 [12–285]** | 3(17%) Bilateral | 66 [41–121] | +17 (94%) | +17 (94%) | +16 (94%) | +7 (39%) | − | In 13 cases (72%) | ||
| Groszmann et al. (2014) | 17 | 29 [22–43] | NR | NR | 5 (29%) Bilateral | [30–270] | NR | +14 (64%) | +12 (55%) | +8 (36%) | − | In 8 cases (47%) |
| Author, year . | Cases (n) . | Age [range] . | History of endometriosis . | Pain . | CA125 (U/ml) . | Laterality . | Size, mm [range] . | Intracystic papillae . | Solid part, mm . | Blood flow . | Septa . | MRI . | Surgery, type . | Surgery, GA . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Miyakoshi et al. (1998) | 1 | 28 | + | − | NR | Unilateral | 85 (max diam) | + | NR | + | − | + | Oophorectomy | 20 |
| Tanaka et al. (2002) | 1 | 27 | − | − | 103 | Unilateral | 120 × 80 × 70 | + | NR | NR | + | + | Cystectomy | 12 |
| Fruscella et al. (2004) | 1 | 39 | − | − | 76 | Unilateral | 55 (max diam) | + | 8 × 10 and 5 × 5 | + | − | + | Oophorectomy | 18 |
| Sammour et al. (2005) | 1 | 28 | + | − | NR | Unilateral | 40 × 50 × 63 | + | 23 × 18 × 14 | + | − | − | Oophorectomy | 16 |
| 1 | 36 | − | − | NR | Unilateral | 37 × 27 × 25 | + | 15 × 20 × 25 | + | − | + | Oophorectomy | 15 | |
| Guerriero et al. (2005) | 1 | 38 | − | − | 109 | Unilateral | 40 × 48 | + | NR | + | − | − | Bilateral oophorectomy | 37 (CS) |
| Iwamoto et al. (2006) | 1 | 31 | + | − | 28.3 | Unilateral | 75 (max diam) | + | NR | + | − | + | Oophorectomy | 22 |
| Asch and Levine (2007) | 1 | Unilateral | NT | + | NR | + | − | − | Bilateral cystectomy | After delivery | ||||
| Poder et al. (2008) | 1 | 34 | + | − | 24 | Unilateral | 62 | + | + | + | − | + | Oophorectomy | 38 (CS) |
| Machida et al. (2008) | 3 | 32 | − | − | 119 | Unilateral | 80 × 50 | + | NR | + | − | + | Oophorectomy | 19 |
| 41 | − | − | 220 | Unilateral | 160 | + | NR | NR | + | + | Oophorectomy | 14 | ||
| 24 | − | − | 34 | Bilateral | 50 | + | NR | NR | − | + | Cystectomy | 14 | ||
| Yoshida et al. (2008) | 1 | 29 | − | − | NR | Unilateral | 85 × 53 | + | NR | − | − | + | Oophorectomy | 14 |
| 1 | 28 | − | − | NR | Unilateral | 74 × 48 | + | NR | − | − | + | Oophorectomy | 19 | |
| Takeuchi et al. (2008) | 5 | 28 [20–32] | NR | − | NR | Unilateral | 58 [40–90] | + | 20 mm (max) | NR§ | − | + | In 1 case | NR |
| Barbieri et al. (2009) | 3 | 32 | − | − | 30 | Unilateral | 66 × 44 | + | 20 × 14 | + | − | − | Cystectomy | |
| 36 | − | + | 159 | Bilateral | 85 × 63/50 × 30 | + | 27 × 14/7 × 7 | + | − | − | None | |||
| 39 | + | − | 85 | Unilateral | 38 × 14 | + | 19 × 12 | + | − | − | None | |||
| Sayasneh et al. (2012) | 1 | 35 | + | − | 89* | Unilateral | 31 × 40 × 5 × 40.5 | + | 15 | + | − | − | None | |
| Tazegül et al. (2013) | 1 | 32 | − | + (12 weeks) | 220 | Unilateral | 65 × 57 | + | 8 × 14 | + | − | − | Cystectomy | 12 |
| Proulx and Levine (2014) | 1 | 30 | NR | − | NR | Unilateral | 40 (max diam) | + | NR | + | − | + | None | |
| Mascilini et al. (2014) | 18 | 34 [20–43] | 3 (17%) | 61 [12–285]** | 3(17%) Bilateral | 66 [41–121] | +17 (94%) | +17 (94%) | +16 (94%) | +7 (39%) | − | In 13 cases (72%) | ||
| Groszmann et al. (2014) | 17 | 29 [22–43] | NR | NR | 5 (29%) Bilateral | [30–270] | NR | +14 (64%) | +12 (55%) | +8 (36%) | − | In 8 cases (47%) |
NR, not reported; +, present; −, absent; CS, Cesarean section; MRI, magnetic resonance imaging; GA, gestational age.
*CA125 was measured prior to conception and not measured again.
**Available for nine women.
§Ultrasound (US) scan parameters not reported.
The literature review allowed us to identify only 17 studies, reporting a total of 60 cases of ovarian decidualized endometriomas in pregnancy. Table I summarizes the main characteristics of the identified published cases.
Transvaginal sonography is the gold standard imaging method for the diagnosis of ovarian endometriomas (Barbieri et al., 2009; Mascilini et al., 2014). A typical sonographic appearance has been documented in up to 95% of cases (Patel et al., 1999; Barbieri et al., 2009), consisting of a round shaped cystic aspect, a minimum diameter of 10 mm, thick walls, regular margins, homogeneous low echogenic fluid content, scattered internal echoes and absence of papillae. However, in 5% of cases, an atypical aspect is detected, which includes anechoic content, solid appearance, and presence of punctuate echogenic foci within the cystic wall. Noteworthy is the fact that the performance of ultrasonography in terms of sensitivity and specificity is much lower during pregnancy (Alcázar et al., 2003; Barbieri et al., 2009). In addition, the potential decidualization of ovarian endometriomas leads to serious diagnostic challenges (Patel et al., 1999; Eskenazi et al., 2001; Alcázar et al., 2003) (Table I). Even considering all these studies, it is still difficult to define clear guidelines for the diagnostic management of such cases. A retrospective study including 18 pregnant patients was the first specifically aimed at describing the ultrasound characteristics of decidualized endometriomas according to the IOTA (International Ovarian Tumour Analysis) terminology (Mascilini et al., 2014). The main strength of this study was the standardized terminology used, although both the small sample size and the retrospective design have limited its value. Typically, a decidualized endometrioma appears as a uni- or multilocular cystic mass containing rounded vascularized papillary projections with smooth contour and with a ground glass or low-level echogenicity cystic content (Fig. 2). Papillations have been detected in all cases reported in literature (Table I). Their presence is relevant since papillary projections are a common sign of malignancy, present both in borderline tumors and in the malignant degeneration of endometriotic cysts (Granberg et al., 1989; Fruscella et al., 2004; Valentin et al., 2006; Testa et al., 2011). The possibility of differentiating malignant papillations from those of decidualized endometriomas would be crucial to avoid unnecessary surgery during pregnancy. As mentioned, the different round-shaped sonography appearance typically observed in benign papillations of decidualized endometriomas is the only distinguishing sign while papillary projections usually have an irregular surface in borderline malignancies.
As reported in Table I, the majority of cases showed an increased blood flow at color Doppler sonography, which therefore cannot be considered reliable in distinguishing a benign decidualized endometrioma from a malignant adnexal mass. Contrary to malignant tumors, the presence of septations was uncommon and their absence could be considered a reassuring sign. The absence of growth in these patients, followed up with serial sonographic evaluations, might be considered another reassuring sign, even if the follow-up sonographic examination throughout pregnancy was not available for all cases. In none of the cases was free pelvic fluid detected during ultrasonography. CA125 levels are not diagnostic in these patients, since it is physiologically elevated during pregnancy (Aslam et al., 2000). However, some authors have suggested a potential diagnostic role for serial CA125 measures or when levels are >1000 U/ml in the second trimester or beyond (Goh et al., 2014). Human epididymis protein 4 (HE4) levels have been found to be significantly lower in pregnant women compared with their premenopausal counterparts and rarely increased in patients with ovarian endometriotic cysts (Huhtinen et al., 2009; Moore et al., 2012). Therefore, the role of a combined assessment of CA125 and HE4 for the differential diagnosis between benign and malignant adnexal tumors in pregnancy should be further elucidated in future investigations.
Magnetic resonance imaging (MRI) without gadolinium is considered safe in pregnancy and can be useful in evaluating sonographically undetermined adnexal lesions (Adusumili et al., 2006; Goh et al., 2014; Morisawa et al., 2014). Although no well-controlled human studies have been conducted to evaluate the teratogenic effect of gadolinium in pregnant women, no harmful effects have been reported for human fetuses exposed to gadolinium in utero. Different studies have demonstrated that the fetus can excrete, swallow, and reabsorb gadolinium into the gastrointestinal tract, which persists in the amniotic fluid (Mettler et al., 2008). Therefore, in clinical practice, it is wise to consider the use of gadolinium-based contrast media in pregnant women only when the benefit to the mother overwhelmingly outweighs the theoretic risks to the fetus (Sundgren and Leander, 2011; Wang et al., 2012). MRI was performed in 35 cases, 23 of whom were included in two studies assessing the usefulness of this technique in diagnosing decidualized endometriomas during pregnancy (Takeuchi et al., 2008; Morisawa et al., 2014). These studies provided evidence that the apparent diffusion coefficient (ADC) was significantly higher for decidualized endometrial tissues as compared with malignant ovarian tumors, probably due to the edematous vascularized nature of endometrial tissue with abundant cytoplasm of stromal cells. Takeuchi et al. (2008) have evaluated the MRI features of 5 decidualized endometriomas. In 3 cases, diffusion weighted images were obtained, measuring ADC of 10 decidualized mural nodules of 3 endometriomas and these were compared with values from 7 ovarian cancers. The mean ADC of the decidualized mural nodules was 2.10 ± 0.32 × 10−3 versus 1.05 ± 0.13 × 10−3 mm2/s for the malignant ovarian cyst mural nodules (P < 0.001) (Takeuchi et al., 2008). In a more recent study by Morisawa et al. (2014), the authors retrospectively investigated the MRI findings of 18 decidualized endometriotic cysts and 24 ovarian cancers, considering height, signal intensity of the solid component on T2-diffusion weighted imaging, ADC of the solid component, size of the lesion, and signal intensity of the intracystic fluid on T1-weighted imaging. The ADC values of the intracystic decidualized solid component and of the cancer group were 1.77 × 10−3 mm2/s and 1.13 × 10−3 mm2/s, respectively (P < 0.0001). Another difference between the two entities was found in the signal of the intracystic fluid on T1-weighted imaging (higher in decidualized endometriotic cysts) as a possible result of the repeated intracystic bleeding. A lower signal intensity of the intracystic fluid during malignant transformation of the endometriotic cysts has already been described (Tanaka et al., 2000, 2010). Overall, in the presence of an endometrioma with prominent hyperintense mural nodules on T2-weighted images, the suspicion of a decidualized endometrioma should be high, but close follow-up should be provided to exclude the possibility of a malignant transformation. ADC measurement was suggested as an additional tool to help in the diagnosis (Takeuchi et al., 2008).
Decidualized ovarian endometriosis in pregnancy: treatment
The management of adnexal masses in pregnancy represents an actual dilemma between expectant management and surgical intervention. This might lead to an unnecessary removal of a benign mass on one hand, and to the conservative observation of a malignant condition on the other. Decision on surgical intervention should in any case undergo multidisciplinary discussion, balancing the level of malignant suspicion, gestational age, and fetal and maternal risks. Among all cases reported in the literature, only 19 were managed expectantly, more frequently for the most recently published cases (Table I). Probably, the increasing number of decidualized endometriomas mimicking ovarian malignancies published in these last years has contributed to moving clinicians toward a more conservative approach. All other cases underwent either cystectomy or salpingo-oophorectomy. Unfortunately, the detailed description of surgical procedures and their timing were not available for all cases.
Of note, pregnancy outcome in those patients who underwent surgery has been reported to be uneventful except for one, who suffered preterm rupture of membranes on the day of laparotomy at the 19th gestational week (Machida et al., 2008). However, surgery-related risks are reported to increase after 23 weeks' gestation, also considering that the enlarged uterus might represent a technical problem for surgeons (Whitecar et al., 1999; Usui et al., 2000; Barbieri et al., 2009). If the decision on surgical approach presents in the late third trimester, surgery should be postponed until after or at the time of delivery (Palanivelu et al., 2007).
Surprisingly, all patients underwent laparotomy (Table I) even though laparoscopy has been reported to be a safe approach during pregnancy, provided it is performed by an experienced surgeon (Palanivelu et al., 2007; Goh et al., 2014).
Decidualized endometriosis in extra-ovarian sites
Extra-ovarian endometriosis involves several sites, most commonly the peritoneum, bladder, bowel, diaphragm, pleura, lungs, breast and the skin, either intact or following surgery (scars, episiotomy). Endometriotic implants in these sites undergo changes under the influence of pregnancy-related hormones, becoming hypertrophic or gaining features of decidualization. Given its rarity, such a condition might be misdiagnosed as a malignant disease (Bergqvist, 1993; Nogales et al., 1993).
Peritoneal deciduosis in pregnancy mimicking carcinomatosis have been reported (Adhikari and Shen, 2013). Conversely, no case of peritoneal decidualized endometriosis in pregnancy has been described, despite the peritoneal surface being a common site for endometriosis localization. Tables II–IV summarizes all cases of extraovarian decidualized endometriosis (cutaneous, vesical and pulmonary) reported in the literature.
Decidualized extraovarian endometriosis of the skin, cases reported in literature.
| Author, year . | No. of cases . | Age . | Site . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Staining . | Treatment . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|
| Pellegrini (1982) | 1 | 30 | Cesarean scar | − | None | NR | NR | Excision during CS | NR |
| Nogales et al. (1993) | 1 | 25 | Cesarean scar | − | Cyclic pain and nodule starting 1 year previously. | + | Vimentin + α1 antitrypsin + Keratin − | Danazol until pregnancy Local Anti-inflammatory therapy Excision at CS | AW |
| Skidmore et al. (1996) | 1 | 40 | Umbilicus | + | Umbilical nodule 1 year previously, increasing in size Cyclic enlargement, cramping and bleeding during menstrual period in the past 5 years | − | NR | Excision during CS | Recurrence a few months after excision |
| Fair et al. (2000) | 2 | 21 | Vulvar | NR | Vulvar nodule, not noticed before pregnancy | + | Vimentin + Ki67+ PAS + | Excision | NR |
| 27 | Umbilicus | − | Umbilical nodule firstly noticed during the current pregnancy | + | NR | Excision | NR | ||
| El-Gohary et al. (2009) | 1 | 24 | Cesarean scar | NR | Lesion noted 2 years before No cyclic pain No cyclic enlargement | + | CD10+ ER − Calretinin + | NR | NR |
| Val-Bernal et al. (2011) | 1 | 36 | Cesarean scar | NR | Noted 2 years before | − | CK8+, hPL +, CD10+ Epithelial membrane antigen −, placental alkaline phosphatase −, CK 5/6 −, calretinin − | Excision | AW |
| Author, year . | No. of cases . | Age . | Site . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Staining . | Treatment . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|
| Pellegrini (1982) | 1 | 30 | Cesarean scar | − | None | NR | NR | Excision during CS | NR |
| Nogales et al. (1993) | 1 | 25 | Cesarean scar | − | Cyclic pain and nodule starting 1 year previously. | + | Vimentin + α1 antitrypsin + Keratin − | Danazol until pregnancy Local Anti-inflammatory therapy Excision at CS | AW |
| Skidmore et al. (1996) | 1 | 40 | Umbilicus | + | Umbilical nodule 1 year previously, increasing in size Cyclic enlargement, cramping and bleeding during menstrual period in the past 5 years | − | NR | Excision during CS | Recurrence a few months after excision |
| Fair et al. (2000) | 2 | 21 | Vulvar | NR | Vulvar nodule, not noticed before pregnancy | + | Vimentin + Ki67+ PAS + | Excision | NR |
| 27 | Umbilicus | − | Umbilical nodule firstly noticed during the current pregnancy | + | NR | Excision | NR | ||
| El-Gohary et al. (2009) | 1 | 24 | Cesarean scar | NR | Lesion noted 2 years before No cyclic pain No cyclic enlargement | + | CD10+ ER − Calretinin + | NR | NR |
| Val-Bernal et al. (2011) | 1 | 36 | Cesarean scar | NR | Noted 2 years before | − | CK8+, hPL +, CD10+ Epithelial membrane antigen −, placental alkaline phosphatase −, CK 5/6 −, calretinin − | Excision | AW |
AW, alive and well; CS, Cesarean section; ER, estrogen receptor; hPL, human placental lactogen; PAS, periodic acid Schiff.
Decidualized extraovarian endometriosis of the skin, cases reported in literature.
| Author, year . | No. of cases . | Age . | Site . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Staining . | Treatment . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|
| Pellegrini (1982) | 1 | 30 | Cesarean scar | − | None | NR | NR | Excision during CS | NR |
| Nogales et al. (1993) | 1 | 25 | Cesarean scar | − | Cyclic pain and nodule starting 1 year previously. | + | Vimentin + α1 antitrypsin + Keratin − | Danazol until pregnancy Local Anti-inflammatory therapy Excision at CS | AW |
| Skidmore et al. (1996) | 1 | 40 | Umbilicus | + | Umbilical nodule 1 year previously, increasing in size Cyclic enlargement, cramping and bleeding during menstrual period in the past 5 years | − | NR | Excision during CS | Recurrence a few months after excision |
| Fair et al. (2000) | 2 | 21 | Vulvar | NR | Vulvar nodule, not noticed before pregnancy | + | Vimentin + Ki67+ PAS + | Excision | NR |
| 27 | Umbilicus | − | Umbilical nodule firstly noticed during the current pregnancy | + | NR | Excision | NR | ||
| El-Gohary et al. (2009) | 1 | 24 | Cesarean scar | NR | Lesion noted 2 years before No cyclic pain No cyclic enlargement | + | CD10+ ER − Calretinin + | NR | NR |
| Val-Bernal et al. (2011) | 1 | 36 | Cesarean scar | NR | Noted 2 years before | − | CK8+, hPL +, CD10+ Epithelial membrane antigen −, placental alkaline phosphatase −, CK 5/6 −, calretinin − | Excision | AW |
| Author, year . | No. of cases . | Age . | Site . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Staining . | Treatment . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|
| Pellegrini (1982) | 1 | 30 | Cesarean scar | − | None | NR | NR | Excision during CS | NR |
| Nogales et al. (1993) | 1 | 25 | Cesarean scar | − | Cyclic pain and nodule starting 1 year previously. | + | Vimentin + α1 antitrypsin + Keratin − | Danazol until pregnancy Local Anti-inflammatory therapy Excision at CS | AW |
| Skidmore et al. (1996) | 1 | 40 | Umbilicus | + | Umbilical nodule 1 year previously, increasing in size Cyclic enlargement, cramping and bleeding during menstrual period in the past 5 years | − | NR | Excision during CS | Recurrence a few months after excision |
| Fair et al. (2000) | 2 | 21 | Vulvar | NR | Vulvar nodule, not noticed before pregnancy | + | Vimentin + Ki67+ PAS + | Excision | NR |
| 27 | Umbilicus | − | Umbilical nodule firstly noticed during the current pregnancy | + | NR | Excision | NR | ||
| El-Gohary et al. (2009) | 1 | 24 | Cesarean scar | NR | Lesion noted 2 years before No cyclic pain No cyclic enlargement | + | CD10+ ER − Calretinin + | NR | NR |
| Val-Bernal et al. (2011) | 1 | 36 | Cesarean scar | NR | Noted 2 years before | − | CK8+, hPL +, CD10+ Epithelial membrane antigen −, placental alkaline phosphatase −, CK 5/6 −, calretinin − | Excision | AW |
AW, alive and well; CS, Cesarean section; ER, estrogen receptor; hPL, human placental lactogen; PAS, periodic acid Schiff.
| Author, year . | No. of cases . | Age . | Site . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Treatment . | Histological examination . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|
| Flieder et al. (1998) | 1 | 27 | Lung, bilaterally | – | Bilateral lung nodules slowly enlarging during the previous 2 years; Right pneumothorax at 28 weeks' gestation – shortness of breath, pleuritic chest pain | + (slowly increasing during the previous 2 years) | Open lung biopsy Chest tube placement | Pulmonary deciduosis. Eosinophilic cells with granular and vacuolated eosinophilic and focally basophilic cytoplasm No endometrial glands | Unchanged after 5.5 years |
| Author, year . | No. of cases . | Age . | Site . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Treatment . | Histological examination . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|
| Flieder et al. (1998) | 1 | 27 | Lung, bilaterally | – | Bilateral lung nodules slowly enlarging during the previous 2 years; Right pneumothorax at 28 weeks' gestation – shortness of breath, pleuritic chest pain | + (slowly increasing during the previous 2 years) | Open lung biopsy Chest tube placement | Pulmonary deciduosis. Eosinophilic cells with granular and vacuolated eosinophilic and focally basophilic cytoplasm No endometrial glands | Unchanged after 5.5 years |
| Author, year . | No. of cases . | Age . | Site . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Treatment . | Histological examination . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|
| Flieder et al. (1998) | 1 | 27 | Lung, bilaterally | – | Bilateral lung nodules slowly enlarging during the previous 2 years; Right pneumothorax at 28 weeks' gestation – shortness of breath, pleuritic chest pain | + (slowly increasing during the previous 2 years) | Open lung biopsy Chest tube placement | Pulmonary deciduosis. Eosinophilic cells with granular and vacuolated eosinophilic and focally basophilic cytoplasm No endometrial glands | Unchanged after 5.5 years |
| Author, year . | No. of cases . | Age . | Site . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Treatment . | Histological examination . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|
| Flieder et al. (1998) | 1 | 27 | Lung, bilaterally | – | Bilateral lung nodules slowly enlarging during the previous 2 years; Right pneumothorax at 28 weeks' gestation – shortness of breath, pleuritic chest pain | + (slowly increasing during the previous 2 years) | Open lung biopsy Chest tube placement | Pulmonary deciduosis. Eosinophilic cells with granular and vacuolated eosinophilic and focally basophilic cytoplasm No endometrial glands | Unchanged after 5.5 years |
| Author, year . | N . | Age . | GA . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Diagnosis . | Site . | Treatment . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|
| Chertin et al. (2007) | 1 | 36 | 23 | NR | Dysuria, frequency Catamenial exacerbation | + | US Cystoscopy | Right bladder wall | Cold cup biopsy during cystoscopy | − |
| Trpkov et al. (2009) | 1 | 25 | 16 | NR | None | NR | US Cystoscopy | Anterior bladder wall | Biopsy | − |
| Szopiński et al. (2009) | 1 | 21 | NR | NR | Dysuria | + | US Cystoscopy | Posterior bladder wall | Biopsy | − |
| Lambrechts et al. (2011) | 1 | 29 | 19 | NR | Intermittent hematuria since 8 weeks | + | US MRI Cystoscopy | Junction of the urachal ligament and the bladder dome | Partial cystectomy | − |
| Faske et al. (2012) | 1 | 38 | 20 | NR | None | − | US Cystoscopy | Posterior wall of the bladder | Biopsy | − |
| Author, year . | N . | Age . | GA . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Diagnosis . | Site . | Treatment . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|
| Chertin et al. (2007) | 1 | 36 | 23 | NR | Dysuria, frequency Catamenial exacerbation | + | US Cystoscopy | Right bladder wall | Cold cup biopsy during cystoscopy | − |
| Trpkov et al. (2009) | 1 | 25 | 16 | NR | None | NR | US Cystoscopy | Anterior bladder wall | Biopsy | − |
| Szopiński et al. (2009) | 1 | 21 | NR | NR | Dysuria | + | US Cystoscopy | Posterior bladder wall | Biopsy | − |
| Lambrechts et al. (2011) | 1 | 29 | 19 | NR | Intermittent hematuria since 8 weeks | + | US MRI Cystoscopy | Junction of the urachal ligament and the bladder dome | Partial cystectomy | − |
| Faske et al. (2012) | 1 | 38 | 20 | NR | None | − | US Cystoscopy | Posterior wall of the bladder | Biopsy | − |
US, ultrasonography.
| Author, year . | N . | Age . | GA . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Diagnosis . | Site . | Treatment . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|
| Chertin et al. (2007) | 1 | 36 | 23 | NR | Dysuria, frequency Catamenial exacerbation | + | US Cystoscopy | Right bladder wall | Cold cup biopsy during cystoscopy | − |
| Trpkov et al. (2009) | 1 | 25 | 16 | NR | None | NR | US Cystoscopy | Anterior bladder wall | Biopsy | − |
| Szopiński et al. (2009) | 1 | 21 | NR | NR | Dysuria | + | US Cystoscopy | Posterior bladder wall | Biopsy | − |
| Lambrechts et al. (2011) | 1 | 29 | 19 | NR | Intermittent hematuria since 8 weeks | + | US MRI Cystoscopy | Junction of the urachal ligament and the bladder dome | Partial cystectomy | − |
| Faske et al. (2012) | 1 | 38 | 20 | NR | None | − | US Cystoscopy | Posterior wall of the bladder | Biopsy | − |
| Author, year . | N . | Age . | GA . | Abdominal endometriosis . | Symptoms . | Increased size during pregnancy . | Diagnosis . | Site . | Treatment . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|
| Chertin et al. (2007) | 1 | 36 | 23 | NR | Dysuria, frequency Catamenial exacerbation | + | US Cystoscopy | Right bladder wall | Cold cup biopsy during cystoscopy | − |
| Trpkov et al. (2009) | 1 | 25 | 16 | NR | None | NR | US Cystoscopy | Anterior bladder wall | Biopsy | − |
| Szopiński et al. (2009) | 1 | 21 | NR | NR | Dysuria | + | US Cystoscopy | Posterior bladder wall | Biopsy | − |
| Lambrechts et al. (2011) | 1 | 29 | 19 | NR | Intermittent hematuria since 8 weeks | + | US MRI Cystoscopy | Junction of the urachal ligament and the bladder dome | Partial cystectomy | − |
| Faske et al. (2012) | 1 | 38 | 20 | NR | None | − | US Cystoscopy | Posterior wall of the bladder | Biopsy | − |
US, ultrasonography.
Cutaneous decidualized endometriosis
Cutaneous decidualized endometriosis, cutaneous deciduosis, deciduoma or pseudotumoral deciduosis represents a rare, benign manifestation of endometriosis that may involve the skin or the subcutaneous tissue, both on an intact site or in relation to an abdominal surgical scar. Concurrent pelvic endometriotic implants are rarely present (Fair et al., 2000). It may represent a diagnostic challenge, since it can potentially be mistaken for malignancy due to its abnormal location and for the atypia of the decidual cells. A history of cyclical pain, the typical lesion enlargement occurring during pregnancy, the shape of the nodules with smooth rounded borders and their non-infiltrative nature may help for the diagnosis, even if a clinical-pathological analysis is required.
Decidualized endometriosis of the bladder
Bladder endometriosis is rare, reported in 1% of women with pelvic endometriosis (Shook and Nyberg, 1988). As for the other sites, the hormonally induced-decidualization of the lesion can cause its rapid growth, simulating a bladder tumor. Differential diagnoses include benign bladder polyp, bladder leiomyoma, bladder cancer and placenta percreta (Faske et al., 2012). In all cases described in the literature, the clinical assessment was performed using ultrasound and cystoscopy. In only one case, MRI was used as an additional diagnostic tool. Bladder decidualized endometriosis shares features common to both non-decidualized endometriosis and bladder malignancies. These lesions appear as a node covered by a small rim of hyperechogenic bladder wall, like benign endometriosis does. Common characteristics with bladder malignancy include the high vascularization on color Doppler analysis, feeding arterial vessels seen on MRI scans and the location most commonly found at the bladder dome. Conversely, benign endometriosis usually involves the vesicouterine pouch (Lambrechts et al., 2011). All cases reported have been treated successfully with no consequences on pregnancy outcome.
Decidualized pulmonary endometriosis
A single case of decidualized pulmonary endometriosis in pregnancy has been reported (Flieder et al., 1998) (Table III).
Complications of a pre-existing endometriosis during pregnancy
Several case reports of acute endometriosis-related complications occurring during pregnancy have been described. However, these complications are rarely reported and consequently underestimated, and they may represent life-threatening conditions for both the mother and the fetus. For this reason, physicians managing pregnancy of women with endometriosis should be aware of these insidious adverse events. Hence, in this section of the review, we offer the reader a complete overview of these complications and of their possible management (Tables V–VII).
Endometriosis-related complications involving bowel and pelvic vessels during pregnancy, cases reported in literature.
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational week) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational week at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bowel | ||||||||||||
| Intestinal perforation | Clement (1977) | 1 | 28 | − | − | NR | Lower AP | Sigmoid colon | 37 | LPT: Hartmann procedure | E+D | LB, 37 |
| Gini et al. (1981) | 1 | 23 | NR | NR | NR | Vaginal bleeding, AP | Appendix | 35 | LPT: appendectomy | E+D | LB, 35 | |
| Floberg et al. (1984) | 1 | 34 | NR | NR | NR | Lower AP | Ovary, sigmoid colon | Immediate post-partum | LPT: OC, segmental bowel resection | E+D | LB, 41 | |
| Nakatani et al. (1987) | 1 | 25 | − | − | NR | N, V, AP | Appendix | 26 | LPT: appendectomy | E+D | LB, term | |
| Loverro et al. (1999) | 1 | 28 | − | − | NR | Lower AP, hyperpyrexia | Sigmoid colon | 35 | LPT: Hartmann procedure | E+D | LB, 35 | |
| Schweitzer et al. (2006) | 1 | 32 | − | − | A | N, AP, dyspnea | Sigmoid colon | 40 | LPT: Hartmann procedure | E+D | LB, 40 | |
| Faucheron et al. (2008) | 1 | 28 | NR | NR | NR | N, AP | Appendix | 27 | LPT: appendectomy | E+D | LB, term | |
| Beamish et al. (2010) | 1 | 33 | − | − | NR | Acute AP | Cecum | 3 days post-partum | LPT: segmental bowel resection | E+D | LB, 37 | |
| Pisanu et al. (2010) | 1 | 37 | + | OC, LOA, DTC, 5 years | NR | Lower AP | Rectum | 33 | LPT: Hartmann procedure, appendectomy | E+D | LB, 33 | |
| Lebastchi et al. (2013) | 1 | 33 | − | − | NR | Upper AP | Appendix | 31 | LPT: appendectomy, ileocecetomy | E+D | LB, 31 | |
| Nishikawa et al. (2013) | 1 | 38 | + | OC, 15 years | A | Upper AP, melena | Ileum | 28 | LPT: segmental bowel resection | E+D | LB, 33 | |
| Menzlova et al. (2014) | 1 | 32 | + | OC, 3 years | NR | Asymptomatic | Rectum | Immediate post-partum | Rectum repair | NR | LB, term | |
| Setúbal et al. (2014) | 3 | 36; 35; 34 | +; −: − | −; −; − | A; S; S | AP; AP; AP | Sigmoid colon; Rectosigmoid colon; Sigmoid colon | 28; 35; 16 | EX LPT during pregnancy and LPS hysterectomy, SO, OC, segmental bowel resection post-partum; LPT: Hartman procedure, appendectomy; LPT: Hartman procedure after EX LPT | E; E+D; E+D | LB, 37; LB, 35; LB, 39 | |
| Costa et al. (2014) | 1 | 32 | − | − | NR | AP | Rectum, sigmoid colon | 25 | LPS: Hartmann procedure | E+D | LB, 41 | |
| Appendicitis | Lane (1960) | 1 | 34 | NR | NR | NR | NR | Appendix | 12 | LPT: appendectomy | E+D | NR, NR |
| Tedeschi and Masand (1971) | 1 | 30 | − | − | NR | AP | Appendix | 12 | LPT: appendectomy | E+D | NR, NR | |
| Finch and Lee (1974) | 1 | NR | NR | NR | NR | NR | Appendix | 28 | LPT: appendectomy | E+D + I | ND, 29 | |
| Nielsen et al. (1983) | 1 | NR | NR | NR | NR | AP | Appendix | Term | LPT: appendectomy | E+D + I | NR, NR | |
| Silvestrini and Marcial (1995) | 1 | 28 | NR | NR | NR | Lower AP, N, V, diarrhea | Appendix | 21 | LPT: appendectomy | E+D | NR, NR | |
| Stefanidis et al. (1999) | 1 | 27 | NR | NR | NR | Lower AP, V | Appendix | 20 | LPT: appendectomy | E | LB, 39 | |
| Perez et al. (2007) | 1 | 21 | NR | NR | NR | Lower AP, N, V | Appendix | 12 | LPT: appendectomy | E+D + I | NR, NR | |
| Utero-ovarian vessels | ||||||||||||
| Vessel rupture | Inoue et al. (1992) | 1 | 37 | NR | NR | NR | AP | Uterus | 29 | EX LPT | NR | LB, 29 |
| Mizumoto et al. (1996) | 1 | 28 | NR | NR | NR | Upper AP | Uterus | 28 | EX LPT | E+D | ND, 29 | |
| Leung et al. (1998) | 1 | 35 | − | − | NR | AP | Uterus | 33 | EX LPT | NR | IUD, 33 | |
| Ismail and Shervington (1999) | 2 | NR | NR; NR | NR | NR | AP; AP | Uterus; uterus | 33; 2 weeks post-partum | EX LPT; EX LPT | E; E | NR, 33; NR, NR | |
| Aziz et al. (2004) | 1 | 30 | − | − | NR | Lower AP | Parametrium | 20 | EX LPT: left SO | E+D | IUD, 20 | |
| O'Leary (2006) | 1 | 41 | + | DTC, 9 months | NR | Lower AP, hyperpyrexia | Parametrium | 11 days post-partum | EX LPT: subtotal hysterectomy, BSO | E+D | NR, NR | |
| Wu et al. (2007) | 1 | 31 | + | Bilateral OC, 5 years | A | Lower AP | Uterus | 33 (twins) | EX LPT | NR | LB, LB, 34 | |
| Kirkinen et al. (2007) | 1 | 37 | − | − | NR | Vaginal bleeding | Parametrium | 22 | Uterine artery embolization during pregnancy and EX LPT post-partum | E+D | LB, 24 | |
| Katorza et al. (2007) | 2 | 29; 32 | −;+ | −; OC, DTC, LOA, NR | A; A | Lower AP; lower AP | Uterus; Uterus | 25 (twins); 29 | EX LPT; EX LPT | NR; NR | LB, LB, 28; LB, 29 | |
| Passos et al. (2008) | 2 | 30; 32 | +;+ | OC, LOA, NR; LOA, 2 years | NR; NR | NR; AP | Parametrium; Parametrium and uterus | 32 (twins); 31 | EX LPT; EX LPT | NR; NR | LB, LB, 32; LB, 31 | |
| Bouet et al. (2009) | 1 | 32 | − | − | NR | Lower AP, dyspnea | Parametrium | 24 | Thoracic drainage, EX LPT: left SO | E+D | IUD, 24 | |
| Roche et al. (2008) | 1 | 43 | + | LPS, NR | A | Lower AP, hematemesis | Uterus, uteroovarian ligament | 33 (twins) | EX LPT: OC | NR | IUD, IUD, 34 | |
| Wada et al. (2009) | 1 | 31 | + | Bilateral OC, DTC, LOA, 4 months | S | Lower AP | Uterus | Immediate post-partum | EX LPT | NR | LB, 37 | |
| Zhang et al. (2009) | 2 | 38; 35 | +; + | Bilateral OC, LOA, NR; DTC, LOA, NR | A; A | AP; upper AP, hyperpyrexia | Uterus; Uterus | 29 (twins); 35 | EX LPT; EX LPT | NR; NR | IUD, IUD, 29; LB, 35 | |
| Grunewald and Jördens (2010) | 1 | 33 | − | − | NR | AP | Sacro-uterine ligament | 27 | EX LPT | E | LB, 42 | |
| Gao et al. (2010) | 1 | 29 | − | − | NR | Upper AP | Uterus | 2 days post-partum | LPS: LOA, left internal iliac artery ligation | E+D | LB, NR | |
| Urinary system | ||||||||||||
| Uroperitoneum | Chiodo et al. (2008) | 1 | 25 | + | LOA, OC, DTC, 2 years | NR | AP, hematuria | Sacro-uterine ligament with right ureter and uterine artery involvement | 31 | LPT: ligation of the right uterine artery and ureteroneocystostomy | E+D | LB, 31 |
| Leone Roberti Maggiore et al. (2015) | 1 | 30 | + | Transurethral nodule resection | A | AP | Bladder | 27 | LPT: bladder resection | E+D | LB, 27 | |
| Distorsion of renal system anatomy | Yaqub et al. (2008) | 1 | 25 | NR | NR | NR | Lower AP | Renal area | 34 | LPT: removal of cyst by blunt dissection and clamping vascular pedicle | E | LB, 34 |
| Pezzuto et al. (2009) | 1 | 34 | + | − | NR | AP | Broad ligament | 35 | Ureteral stent | NR | NR, NR | |
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational week) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational week at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bowel | ||||||||||||
| Intestinal perforation | Clement (1977) | 1 | 28 | − | − | NR | Lower AP | Sigmoid colon | 37 | LPT: Hartmann procedure | E+D | LB, 37 |
| Gini et al. (1981) | 1 | 23 | NR | NR | NR | Vaginal bleeding, AP | Appendix | 35 | LPT: appendectomy | E+D | LB, 35 | |
| Floberg et al. (1984) | 1 | 34 | NR | NR | NR | Lower AP | Ovary, sigmoid colon | Immediate post-partum | LPT: OC, segmental bowel resection | E+D | LB, 41 | |
| Nakatani et al. (1987) | 1 | 25 | − | − | NR | N, V, AP | Appendix | 26 | LPT: appendectomy | E+D | LB, term | |
| Loverro et al. (1999) | 1 | 28 | − | − | NR | Lower AP, hyperpyrexia | Sigmoid colon | 35 | LPT: Hartmann procedure | E+D | LB, 35 | |
| Schweitzer et al. (2006) | 1 | 32 | − | − | A | N, AP, dyspnea | Sigmoid colon | 40 | LPT: Hartmann procedure | E+D | LB, 40 | |
| Faucheron et al. (2008) | 1 | 28 | NR | NR | NR | N, AP | Appendix | 27 | LPT: appendectomy | E+D | LB, term | |
| Beamish et al. (2010) | 1 | 33 | − | − | NR | Acute AP | Cecum | 3 days post-partum | LPT: segmental bowel resection | E+D | LB, 37 | |
| Pisanu et al. (2010) | 1 | 37 | + | OC, LOA, DTC, 5 years | NR | Lower AP | Rectum | 33 | LPT: Hartmann procedure, appendectomy | E+D | LB, 33 | |
| Lebastchi et al. (2013) | 1 | 33 | − | − | NR | Upper AP | Appendix | 31 | LPT: appendectomy, ileocecetomy | E+D | LB, 31 | |
| Nishikawa et al. (2013) | 1 | 38 | + | OC, 15 years | A | Upper AP, melena | Ileum | 28 | LPT: segmental bowel resection | E+D | LB, 33 | |
| Menzlova et al. (2014) | 1 | 32 | + | OC, 3 years | NR | Asymptomatic | Rectum | Immediate post-partum | Rectum repair | NR | LB, term | |
| Setúbal et al. (2014) | 3 | 36; 35; 34 | +; −: − | −; −; − | A; S; S | AP; AP; AP | Sigmoid colon; Rectosigmoid colon; Sigmoid colon | 28; 35; 16 | EX LPT during pregnancy and LPS hysterectomy, SO, OC, segmental bowel resection post-partum; LPT: Hartman procedure, appendectomy; LPT: Hartman procedure after EX LPT | E; E+D; E+D | LB, 37; LB, 35; LB, 39 | |
| Costa et al. (2014) | 1 | 32 | − | − | NR | AP | Rectum, sigmoid colon | 25 | LPS: Hartmann procedure | E+D | LB, 41 | |
| Appendicitis | Lane (1960) | 1 | 34 | NR | NR | NR | NR | Appendix | 12 | LPT: appendectomy | E+D | NR, NR |
| Tedeschi and Masand (1971) | 1 | 30 | − | − | NR | AP | Appendix | 12 | LPT: appendectomy | E+D | NR, NR | |
| Finch and Lee (1974) | 1 | NR | NR | NR | NR | NR | Appendix | 28 | LPT: appendectomy | E+D + I | ND, 29 | |
| Nielsen et al. (1983) | 1 | NR | NR | NR | NR | AP | Appendix | Term | LPT: appendectomy | E+D + I | NR, NR | |
| Silvestrini and Marcial (1995) | 1 | 28 | NR | NR | NR | Lower AP, N, V, diarrhea | Appendix | 21 | LPT: appendectomy | E+D | NR, NR | |
| Stefanidis et al. (1999) | 1 | 27 | NR | NR | NR | Lower AP, V | Appendix | 20 | LPT: appendectomy | E | LB, 39 | |
| Perez et al. (2007) | 1 | 21 | NR | NR | NR | Lower AP, N, V | Appendix | 12 | LPT: appendectomy | E+D + I | NR, NR | |
| Utero-ovarian vessels | ||||||||||||
| Vessel rupture | Inoue et al. (1992) | 1 | 37 | NR | NR | NR | AP | Uterus | 29 | EX LPT | NR | LB, 29 |
| Mizumoto et al. (1996) | 1 | 28 | NR | NR | NR | Upper AP | Uterus | 28 | EX LPT | E+D | ND, 29 | |
| Leung et al. (1998) | 1 | 35 | − | − | NR | AP | Uterus | 33 | EX LPT | NR | IUD, 33 | |
| Ismail and Shervington (1999) | 2 | NR | NR; NR | NR | NR | AP; AP | Uterus; uterus | 33; 2 weeks post-partum | EX LPT; EX LPT | E; E | NR, 33; NR, NR | |
| Aziz et al. (2004) | 1 | 30 | − | − | NR | Lower AP | Parametrium | 20 | EX LPT: left SO | E+D | IUD, 20 | |
| O'Leary (2006) | 1 | 41 | + | DTC, 9 months | NR | Lower AP, hyperpyrexia | Parametrium | 11 days post-partum | EX LPT: subtotal hysterectomy, BSO | E+D | NR, NR | |
| Wu et al. (2007) | 1 | 31 | + | Bilateral OC, 5 years | A | Lower AP | Uterus | 33 (twins) | EX LPT | NR | LB, LB, 34 | |
| Kirkinen et al. (2007) | 1 | 37 | − | − | NR | Vaginal bleeding | Parametrium | 22 | Uterine artery embolization during pregnancy and EX LPT post-partum | E+D | LB, 24 | |
| Katorza et al. (2007) | 2 | 29; 32 | −;+ | −; OC, DTC, LOA, NR | A; A | Lower AP; lower AP | Uterus; Uterus | 25 (twins); 29 | EX LPT; EX LPT | NR; NR | LB, LB, 28; LB, 29 | |
| Passos et al. (2008) | 2 | 30; 32 | +;+ | OC, LOA, NR; LOA, 2 years | NR; NR | NR; AP | Parametrium; Parametrium and uterus | 32 (twins); 31 | EX LPT; EX LPT | NR; NR | LB, LB, 32; LB, 31 | |
| Bouet et al. (2009) | 1 | 32 | − | − | NR | Lower AP, dyspnea | Parametrium | 24 | Thoracic drainage, EX LPT: left SO | E+D | IUD, 24 | |
| Roche et al. (2008) | 1 | 43 | + | LPS, NR | A | Lower AP, hematemesis | Uterus, uteroovarian ligament | 33 (twins) | EX LPT: OC | NR | IUD, IUD, 34 | |
| Wada et al. (2009) | 1 | 31 | + | Bilateral OC, DTC, LOA, 4 months | S | Lower AP | Uterus | Immediate post-partum | EX LPT | NR | LB, 37 | |
| Zhang et al. (2009) | 2 | 38; 35 | +; + | Bilateral OC, LOA, NR; DTC, LOA, NR | A; A | AP; upper AP, hyperpyrexia | Uterus; Uterus | 29 (twins); 35 | EX LPT; EX LPT | NR; NR | IUD, IUD, 29; LB, 35 | |
| Grunewald and Jördens (2010) | 1 | 33 | − | − | NR | AP | Sacro-uterine ligament | 27 | EX LPT | E | LB, 42 | |
| Gao et al. (2010) | 1 | 29 | − | − | NR | Upper AP | Uterus | 2 days post-partum | LPS: LOA, left internal iliac artery ligation | E+D | LB, NR | |
| Urinary system | ||||||||||||
| Uroperitoneum | Chiodo et al. (2008) | 1 | 25 | + | LOA, OC, DTC, 2 years | NR | AP, hematuria | Sacro-uterine ligament with right ureter and uterine artery involvement | 31 | LPT: ligation of the right uterine artery and ureteroneocystostomy | E+D | LB, 31 |
| Leone Roberti Maggiore et al. (2015) | 1 | 30 | + | Transurethral nodule resection | A | AP | Bladder | 27 | LPT: bladder resection | E+D | LB, 27 | |
| Distorsion of renal system anatomy | Yaqub et al. (2008) | 1 | 25 | NR | NR | NR | Lower AP | Renal area | 34 | LPT: removal of cyst by blunt dissection and clamping vascular pedicle | E | LB, 34 |
| Pezzuto et al. (2009) | 1 | 34 | + | − | NR | AP | Broad ligament | 35 | Ureteral stent | NR | NR, NR | |
DTC, diathermocoagulation of endometriotic lesions; LOA, lysis of adhesions; OC, ovarian cystectomy; S, spontaneous; A, assisted reproductive technology (ART); AP, abdominal pain; N, nausea; V, vomiting; LPT, laparotomy; EX LPT, exploratory laparotomy; LPS, laparoscopy; BSO, bilateral salpingo-oophorectomy; SO, salpingo-oophorectomy; E, endometriosis; D, decidual change; I, inflammation; LB, live birth; IUD, intrauterine death; ND, neonatal demise.
Endometriosis-related complications involving bowel and pelvic vessels during pregnancy, cases reported in literature.
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational week) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational week at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bowel | ||||||||||||
| Intestinal perforation | Clement (1977) | 1 | 28 | − | − | NR | Lower AP | Sigmoid colon | 37 | LPT: Hartmann procedure | E+D | LB, 37 |
| Gini et al. (1981) | 1 | 23 | NR | NR | NR | Vaginal bleeding, AP | Appendix | 35 | LPT: appendectomy | E+D | LB, 35 | |
| Floberg et al. (1984) | 1 | 34 | NR | NR | NR | Lower AP | Ovary, sigmoid colon | Immediate post-partum | LPT: OC, segmental bowel resection | E+D | LB, 41 | |
| Nakatani et al. (1987) | 1 | 25 | − | − | NR | N, V, AP | Appendix | 26 | LPT: appendectomy | E+D | LB, term | |
| Loverro et al. (1999) | 1 | 28 | − | − | NR | Lower AP, hyperpyrexia | Sigmoid colon | 35 | LPT: Hartmann procedure | E+D | LB, 35 | |
| Schweitzer et al. (2006) | 1 | 32 | − | − | A | N, AP, dyspnea | Sigmoid colon | 40 | LPT: Hartmann procedure | E+D | LB, 40 | |
| Faucheron et al. (2008) | 1 | 28 | NR | NR | NR | N, AP | Appendix | 27 | LPT: appendectomy | E+D | LB, term | |
| Beamish et al. (2010) | 1 | 33 | − | − | NR | Acute AP | Cecum | 3 days post-partum | LPT: segmental bowel resection | E+D | LB, 37 | |
| Pisanu et al. (2010) | 1 | 37 | + | OC, LOA, DTC, 5 years | NR | Lower AP | Rectum | 33 | LPT: Hartmann procedure, appendectomy | E+D | LB, 33 | |
| Lebastchi et al. (2013) | 1 | 33 | − | − | NR | Upper AP | Appendix | 31 | LPT: appendectomy, ileocecetomy | E+D | LB, 31 | |
| Nishikawa et al. (2013) | 1 | 38 | + | OC, 15 years | A | Upper AP, melena | Ileum | 28 | LPT: segmental bowel resection | E+D | LB, 33 | |
| Menzlova et al. (2014) | 1 | 32 | + | OC, 3 years | NR | Asymptomatic | Rectum | Immediate post-partum | Rectum repair | NR | LB, term | |
| Setúbal et al. (2014) | 3 | 36; 35; 34 | +; −: − | −; −; − | A; S; S | AP; AP; AP | Sigmoid colon; Rectosigmoid colon; Sigmoid colon | 28; 35; 16 | EX LPT during pregnancy and LPS hysterectomy, SO, OC, segmental bowel resection post-partum; LPT: Hartman procedure, appendectomy; LPT: Hartman procedure after EX LPT | E; E+D; E+D | LB, 37; LB, 35; LB, 39 | |
| Costa et al. (2014) | 1 | 32 | − | − | NR | AP | Rectum, sigmoid colon | 25 | LPS: Hartmann procedure | E+D | LB, 41 | |
| Appendicitis | Lane (1960) | 1 | 34 | NR | NR | NR | NR | Appendix | 12 | LPT: appendectomy | E+D | NR, NR |
| Tedeschi and Masand (1971) | 1 | 30 | − | − | NR | AP | Appendix | 12 | LPT: appendectomy | E+D | NR, NR | |
| Finch and Lee (1974) | 1 | NR | NR | NR | NR | NR | Appendix | 28 | LPT: appendectomy | E+D + I | ND, 29 | |
| Nielsen et al. (1983) | 1 | NR | NR | NR | NR | AP | Appendix | Term | LPT: appendectomy | E+D + I | NR, NR | |
| Silvestrini and Marcial (1995) | 1 | 28 | NR | NR | NR | Lower AP, N, V, diarrhea | Appendix | 21 | LPT: appendectomy | E+D | NR, NR | |
| Stefanidis et al. (1999) | 1 | 27 | NR | NR | NR | Lower AP, V | Appendix | 20 | LPT: appendectomy | E | LB, 39 | |
| Perez et al. (2007) | 1 | 21 | NR | NR | NR | Lower AP, N, V | Appendix | 12 | LPT: appendectomy | E+D + I | NR, NR | |
| Utero-ovarian vessels | ||||||||||||
| Vessel rupture | Inoue et al. (1992) | 1 | 37 | NR | NR | NR | AP | Uterus | 29 | EX LPT | NR | LB, 29 |
| Mizumoto et al. (1996) | 1 | 28 | NR | NR | NR | Upper AP | Uterus | 28 | EX LPT | E+D | ND, 29 | |
| Leung et al. (1998) | 1 | 35 | − | − | NR | AP | Uterus | 33 | EX LPT | NR | IUD, 33 | |
| Ismail and Shervington (1999) | 2 | NR | NR; NR | NR | NR | AP; AP | Uterus; uterus | 33; 2 weeks post-partum | EX LPT; EX LPT | E; E | NR, 33; NR, NR | |
| Aziz et al. (2004) | 1 | 30 | − | − | NR | Lower AP | Parametrium | 20 | EX LPT: left SO | E+D | IUD, 20 | |
| O'Leary (2006) | 1 | 41 | + | DTC, 9 months | NR | Lower AP, hyperpyrexia | Parametrium | 11 days post-partum | EX LPT: subtotal hysterectomy, BSO | E+D | NR, NR | |
| Wu et al. (2007) | 1 | 31 | + | Bilateral OC, 5 years | A | Lower AP | Uterus | 33 (twins) | EX LPT | NR | LB, LB, 34 | |
| Kirkinen et al. (2007) | 1 | 37 | − | − | NR | Vaginal bleeding | Parametrium | 22 | Uterine artery embolization during pregnancy and EX LPT post-partum | E+D | LB, 24 | |
| Katorza et al. (2007) | 2 | 29; 32 | −;+ | −; OC, DTC, LOA, NR | A; A | Lower AP; lower AP | Uterus; Uterus | 25 (twins); 29 | EX LPT; EX LPT | NR; NR | LB, LB, 28; LB, 29 | |
| Passos et al. (2008) | 2 | 30; 32 | +;+ | OC, LOA, NR; LOA, 2 years | NR; NR | NR; AP | Parametrium; Parametrium and uterus | 32 (twins); 31 | EX LPT; EX LPT | NR; NR | LB, LB, 32; LB, 31 | |
| Bouet et al. (2009) | 1 | 32 | − | − | NR | Lower AP, dyspnea | Parametrium | 24 | Thoracic drainage, EX LPT: left SO | E+D | IUD, 24 | |
| Roche et al. (2008) | 1 | 43 | + | LPS, NR | A | Lower AP, hematemesis | Uterus, uteroovarian ligament | 33 (twins) | EX LPT: OC | NR | IUD, IUD, 34 | |
| Wada et al. (2009) | 1 | 31 | + | Bilateral OC, DTC, LOA, 4 months | S | Lower AP | Uterus | Immediate post-partum | EX LPT | NR | LB, 37 | |
| Zhang et al. (2009) | 2 | 38; 35 | +; + | Bilateral OC, LOA, NR; DTC, LOA, NR | A; A | AP; upper AP, hyperpyrexia | Uterus; Uterus | 29 (twins); 35 | EX LPT; EX LPT | NR; NR | IUD, IUD, 29; LB, 35 | |
| Grunewald and Jördens (2010) | 1 | 33 | − | − | NR | AP | Sacro-uterine ligament | 27 | EX LPT | E | LB, 42 | |
| Gao et al. (2010) | 1 | 29 | − | − | NR | Upper AP | Uterus | 2 days post-partum | LPS: LOA, left internal iliac artery ligation | E+D | LB, NR | |
| Urinary system | ||||||||||||
| Uroperitoneum | Chiodo et al. (2008) | 1 | 25 | + | LOA, OC, DTC, 2 years | NR | AP, hematuria | Sacro-uterine ligament with right ureter and uterine artery involvement | 31 | LPT: ligation of the right uterine artery and ureteroneocystostomy | E+D | LB, 31 |
| Leone Roberti Maggiore et al. (2015) | 1 | 30 | + | Transurethral nodule resection | A | AP | Bladder | 27 | LPT: bladder resection | E+D | LB, 27 | |
| Distorsion of renal system anatomy | Yaqub et al. (2008) | 1 | 25 | NR | NR | NR | Lower AP | Renal area | 34 | LPT: removal of cyst by blunt dissection and clamping vascular pedicle | E | LB, 34 |
| Pezzuto et al. (2009) | 1 | 34 | + | − | NR | AP | Broad ligament | 35 | Ureteral stent | NR | NR, NR | |
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational week) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational week at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bowel | ||||||||||||
| Intestinal perforation | Clement (1977) | 1 | 28 | − | − | NR | Lower AP | Sigmoid colon | 37 | LPT: Hartmann procedure | E+D | LB, 37 |
| Gini et al. (1981) | 1 | 23 | NR | NR | NR | Vaginal bleeding, AP | Appendix | 35 | LPT: appendectomy | E+D | LB, 35 | |
| Floberg et al. (1984) | 1 | 34 | NR | NR | NR | Lower AP | Ovary, sigmoid colon | Immediate post-partum | LPT: OC, segmental bowel resection | E+D | LB, 41 | |
| Nakatani et al. (1987) | 1 | 25 | − | − | NR | N, V, AP | Appendix | 26 | LPT: appendectomy | E+D | LB, term | |
| Loverro et al. (1999) | 1 | 28 | − | − | NR | Lower AP, hyperpyrexia | Sigmoid colon | 35 | LPT: Hartmann procedure | E+D | LB, 35 | |
| Schweitzer et al. (2006) | 1 | 32 | − | − | A | N, AP, dyspnea | Sigmoid colon | 40 | LPT: Hartmann procedure | E+D | LB, 40 | |
| Faucheron et al. (2008) | 1 | 28 | NR | NR | NR | N, AP | Appendix | 27 | LPT: appendectomy | E+D | LB, term | |
| Beamish et al. (2010) | 1 | 33 | − | − | NR | Acute AP | Cecum | 3 days post-partum | LPT: segmental bowel resection | E+D | LB, 37 | |
| Pisanu et al. (2010) | 1 | 37 | + | OC, LOA, DTC, 5 years | NR | Lower AP | Rectum | 33 | LPT: Hartmann procedure, appendectomy | E+D | LB, 33 | |
| Lebastchi et al. (2013) | 1 | 33 | − | − | NR | Upper AP | Appendix | 31 | LPT: appendectomy, ileocecetomy | E+D | LB, 31 | |
| Nishikawa et al. (2013) | 1 | 38 | + | OC, 15 years | A | Upper AP, melena | Ileum | 28 | LPT: segmental bowel resection | E+D | LB, 33 | |
| Menzlova et al. (2014) | 1 | 32 | + | OC, 3 years | NR | Asymptomatic | Rectum | Immediate post-partum | Rectum repair | NR | LB, term | |
| Setúbal et al. (2014) | 3 | 36; 35; 34 | +; −: − | −; −; − | A; S; S | AP; AP; AP | Sigmoid colon; Rectosigmoid colon; Sigmoid colon | 28; 35; 16 | EX LPT during pregnancy and LPS hysterectomy, SO, OC, segmental bowel resection post-partum; LPT: Hartman procedure, appendectomy; LPT: Hartman procedure after EX LPT | E; E+D; E+D | LB, 37; LB, 35; LB, 39 | |
| Costa et al. (2014) | 1 | 32 | − | − | NR | AP | Rectum, sigmoid colon | 25 | LPS: Hartmann procedure | E+D | LB, 41 | |
| Appendicitis | Lane (1960) | 1 | 34 | NR | NR | NR | NR | Appendix | 12 | LPT: appendectomy | E+D | NR, NR |
| Tedeschi and Masand (1971) | 1 | 30 | − | − | NR | AP | Appendix | 12 | LPT: appendectomy | E+D | NR, NR | |
| Finch and Lee (1974) | 1 | NR | NR | NR | NR | NR | Appendix | 28 | LPT: appendectomy | E+D + I | ND, 29 | |
| Nielsen et al. (1983) | 1 | NR | NR | NR | NR | AP | Appendix | Term | LPT: appendectomy | E+D + I | NR, NR | |
| Silvestrini and Marcial (1995) | 1 | 28 | NR | NR | NR | Lower AP, N, V, diarrhea | Appendix | 21 | LPT: appendectomy | E+D | NR, NR | |
| Stefanidis et al. (1999) | 1 | 27 | NR | NR | NR | Lower AP, V | Appendix | 20 | LPT: appendectomy | E | LB, 39 | |
| Perez et al. (2007) | 1 | 21 | NR | NR | NR | Lower AP, N, V | Appendix | 12 | LPT: appendectomy | E+D + I | NR, NR | |
| Utero-ovarian vessels | ||||||||||||
| Vessel rupture | Inoue et al. (1992) | 1 | 37 | NR | NR | NR | AP | Uterus | 29 | EX LPT | NR | LB, 29 |
| Mizumoto et al. (1996) | 1 | 28 | NR | NR | NR | Upper AP | Uterus | 28 | EX LPT | E+D | ND, 29 | |
| Leung et al. (1998) | 1 | 35 | − | − | NR | AP | Uterus | 33 | EX LPT | NR | IUD, 33 | |
| Ismail and Shervington (1999) | 2 | NR | NR; NR | NR | NR | AP; AP | Uterus; uterus | 33; 2 weeks post-partum | EX LPT; EX LPT | E; E | NR, 33; NR, NR | |
| Aziz et al. (2004) | 1 | 30 | − | − | NR | Lower AP | Parametrium | 20 | EX LPT: left SO | E+D | IUD, 20 | |
| O'Leary (2006) | 1 | 41 | + | DTC, 9 months | NR | Lower AP, hyperpyrexia | Parametrium | 11 days post-partum | EX LPT: subtotal hysterectomy, BSO | E+D | NR, NR | |
| Wu et al. (2007) | 1 | 31 | + | Bilateral OC, 5 years | A | Lower AP | Uterus | 33 (twins) | EX LPT | NR | LB, LB, 34 | |
| Kirkinen et al. (2007) | 1 | 37 | − | − | NR | Vaginal bleeding | Parametrium | 22 | Uterine artery embolization during pregnancy and EX LPT post-partum | E+D | LB, 24 | |
| Katorza et al. (2007) | 2 | 29; 32 | −;+ | −; OC, DTC, LOA, NR | A; A | Lower AP; lower AP | Uterus; Uterus | 25 (twins); 29 | EX LPT; EX LPT | NR; NR | LB, LB, 28; LB, 29 | |
| Passos et al. (2008) | 2 | 30; 32 | +;+ | OC, LOA, NR; LOA, 2 years | NR; NR | NR; AP | Parametrium; Parametrium and uterus | 32 (twins); 31 | EX LPT; EX LPT | NR; NR | LB, LB, 32; LB, 31 | |
| Bouet et al. (2009) | 1 | 32 | − | − | NR | Lower AP, dyspnea | Parametrium | 24 | Thoracic drainage, EX LPT: left SO | E+D | IUD, 24 | |
| Roche et al. (2008) | 1 | 43 | + | LPS, NR | A | Lower AP, hematemesis | Uterus, uteroovarian ligament | 33 (twins) | EX LPT: OC | NR | IUD, IUD, 34 | |
| Wada et al. (2009) | 1 | 31 | + | Bilateral OC, DTC, LOA, 4 months | S | Lower AP | Uterus | Immediate post-partum | EX LPT | NR | LB, 37 | |
| Zhang et al. (2009) | 2 | 38; 35 | +; + | Bilateral OC, LOA, NR; DTC, LOA, NR | A; A | AP; upper AP, hyperpyrexia | Uterus; Uterus | 29 (twins); 35 | EX LPT; EX LPT | NR; NR | IUD, IUD, 29; LB, 35 | |
| Grunewald and Jördens (2010) | 1 | 33 | − | − | NR | AP | Sacro-uterine ligament | 27 | EX LPT | E | LB, 42 | |
| Gao et al. (2010) | 1 | 29 | − | − | NR | Upper AP | Uterus | 2 days post-partum | LPS: LOA, left internal iliac artery ligation | E+D | LB, NR | |
| Urinary system | ||||||||||||
| Uroperitoneum | Chiodo et al. (2008) | 1 | 25 | + | LOA, OC, DTC, 2 years | NR | AP, hematuria | Sacro-uterine ligament with right ureter and uterine artery involvement | 31 | LPT: ligation of the right uterine artery and ureteroneocystostomy | E+D | LB, 31 |
| Leone Roberti Maggiore et al. (2015) | 1 | 30 | + | Transurethral nodule resection | A | AP | Bladder | 27 | LPT: bladder resection | E+D | LB, 27 | |
| Distorsion of renal system anatomy | Yaqub et al. (2008) | 1 | 25 | NR | NR | NR | Lower AP | Renal area | 34 | LPT: removal of cyst by blunt dissection and clamping vascular pedicle | E | LB, 34 |
| Pezzuto et al. (2009) | 1 | 34 | + | − | NR | AP | Broad ligament | 35 | Ureteral stent | NR | NR, NR | |
DTC, diathermocoagulation of endometriotic lesions; LOA, lysis of adhesions; OC, ovarian cystectomy; S, spontaneous; A, assisted reproductive technology (ART); AP, abdominal pain; N, nausea; V, vomiting; LPT, laparotomy; EX LPT, exploratory laparotomy; LPS, laparoscopy; BSO, bilateral salpingo-oophorectomy; SO, salpingo-oophorectomy; E, endometriosis; D, decidual change; I, inflammation; LB, live birth; IUD, intrauterine death; ND, neonatal demise.
Endometriosis-related complications involving reproductive organs during pregnancy, cases reported in literature.
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational weeks) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational weeks at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ovary | ||||||||||||
| Rupture of endometrioma | Brill et al. (1957) | 1 | 38 | NR | NR | NR | AP | Bilateral ovaries | Term | LPT: hysterectomy and BSO | E+D | LB, term |
| Steinberg (1962) | 1 | 35 | − | − | S | AP | Left ovary | 38 | LPT: OC, endometriotic nodule excision | E+D | LB, 38 | |
| Anderson and Edmond (1974) | 1 | 39 | − | − | S | AP | Left ovary | 37 | LPT: left SO | E+D | LB, 37 | |
| Rossman et al. (1983) | 1 | 25 | NR | NR | NR | AP, hyperpyrexia | Bilateral ovaries | 30 | LPT: bilateral OC | E+D | LB, 30 | |
| Johnson and Woodruff (1986) | 1 | 39 | NR | NR | NR | Lower AP | Right adnexa | 26 | LPT: hysterectomy and right SO | E | LB, 27 | |
| Vercellini et al. (1992) | 1 | 29 | − | − | S | AP | Right ovary | 35 | LPT: OC, LOA, DTC | E+D | LB, 35 | |
| Barbazan et al. (1993) | 1 | 29 | − | − | NR | Lower AP, N, V | Right ovary | 18 | LPT: right SO | E+D | LB, term | |
| Garcia-Velasco et al. (1998) | 1 | 25 | − | − | S | Lower AP | Left ovary | 9 | LPT: left SO | E+D | LB, NR | |
| Loh et al. (1998) | 1 | 25 | NR | NR | S | Lower AP | Bilateral ovaries | 6 | LPS: bilateral OC | E | LB, 39 | |
| Gregora and Higgs (1998) | 1 | 44 | + | LPS, 2 years | S | Lower AP | Bilateral ovaries | 18 | LPT: bilateral OC | E | LB, 41 | |
| Katorza et al. (2007) | 1 | 31 | + | LOA, DTC, NR | A | Lower AP | Right adnexa | 26 | LPT: right SO | E+D | TOP, 26 | |
| Ueda et al. (2010) | 1 | 35 | NR | NR | A | NR | Ovary | NR | Cyst drainage | NR | NR, preterm for placenta previa | |
| Reif et al. (2011) | 1 | 25 | + | LOA, DTC, OC 1.5 years | A | AP | Left adnexa | 27 (twin) | LPT: left salpingectomy, OC | E+D | LB, LB, 27 | |
| Williamson et al. (2011) | 1 | 37 | − | − | S | AP | Left adnexa | 37 | Conservative | NR | IUD, 37 | |
| Infected endometrioma | Phupong et al. (2004) | 1 | 35 | + | − | S | AP, N, V, diarrhea | Right ovary | 35 | LPT: OC, appendectomy | E | LB, 36 |
| Ueda et al. (2010) | 1 | 40 | + | NR | A | NR | NR | NR | Cyst drainage | NR | NR, NR | |
| Dogan et al. (2012) | 1 | 30 | NR | NR | NR | Lower AP, V, hyperpyrexia | Appendix, left adnexa | 28 | LPT: appendectomy, left salpingectomy, ovarian biopsies | E+D | LB, 28 | |
| Enlarged endometrioma | Nezhat et al. (1991) | 1 | 28 | + | OC, LOA, 3 years | S | A | Bilateral ovaries | 16 | LPS: bilateral OC, LOA | D | LB, 38 |
| Ninia (1992) | 1 | 32 | − | − | S | Lower AP | Left ovary | 16 | LPT: OC | E | LB, term | |
| Gregora and Higgs (1998) | 1 | 36 | − | − | S | A | Right ovary | 16 | LPT: cyst drainage | NR | LB, 39 | |
| Ueda et al. (2010) | 3 | 27; 31; 33 | −; −; − | NR; NR; NR | S; S; S | NR; NR; NR | NR; NR; NR | II trimester; II trimester; II trimester | SO; SO; conservative | E+D; E+D | NR, NR; NR, NR; NR, NR | |
| Fallopian tube | ||||||||||||
| Fallopian tube rupture | Aggarwal et al. (2014) | 1 | 31 | + | LOA, DTC, OC, 2 years | A | Upper AP, V | Left fallopian tube | 21 (twin) | LPT: left salpingectomy | E+D | IUD, 21 |
| Uterus | ||||||||||||
| Uterine rupture | Van De Putte et al. (1999) | 1 | 29 | + | Excision of rectovaginal nodule, 6 years | NR | A | Posterior wall of the uterus | Immediate post-partum | LPT: uterine rupture repair | NR | LB, term |
| Sholapurkar et al. (2005) | 1 | 37 | NR | NR | NR | Vaginal bleeding | Uterine scar after Cesarean section | 6 weeks post-partum | LPT: hysterectomy | E | LB, 38 | |
| Granese (2010) | 1 | 34 | + | LOA, bilateral OC, DTC, 5 years | NR | Lower AP, dyspnea | Posterior wall of the uterus | 32 | LPT: uterine rupture repair | NR | NR, 32 | |
| Chen et al. (2011) | 1 | 33 | + | Bilateral OC, 3 years; cervical cystectomy, DTC, 1 year | A | NR | Posterior wall of the uterus and cervix | 37 | LPT: uterine rupture repair | NR | LB, 37 | |
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational weeks) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational weeks at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ovary | ||||||||||||
| Rupture of endometrioma | Brill et al. (1957) | 1 | 38 | NR | NR | NR | AP | Bilateral ovaries | Term | LPT: hysterectomy and BSO | E+D | LB, term |
| Steinberg (1962) | 1 | 35 | − | − | S | AP | Left ovary | 38 | LPT: OC, endometriotic nodule excision | E+D | LB, 38 | |
| Anderson and Edmond (1974) | 1 | 39 | − | − | S | AP | Left ovary | 37 | LPT: left SO | E+D | LB, 37 | |
| Rossman et al. (1983) | 1 | 25 | NR | NR | NR | AP, hyperpyrexia | Bilateral ovaries | 30 | LPT: bilateral OC | E+D | LB, 30 | |
| Johnson and Woodruff (1986) | 1 | 39 | NR | NR | NR | Lower AP | Right adnexa | 26 | LPT: hysterectomy and right SO | E | LB, 27 | |
| Vercellini et al. (1992) | 1 | 29 | − | − | S | AP | Right ovary | 35 | LPT: OC, LOA, DTC | E+D | LB, 35 | |
| Barbazan et al. (1993) | 1 | 29 | − | − | NR | Lower AP, N, V | Right ovary | 18 | LPT: right SO | E+D | LB, term | |
| Garcia-Velasco et al. (1998) | 1 | 25 | − | − | S | Lower AP | Left ovary | 9 | LPT: left SO | E+D | LB, NR | |
| Loh et al. (1998) | 1 | 25 | NR | NR | S | Lower AP | Bilateral ovaries | 6 | LPS: bilateral OC | E | LB, 39 | |
| Gregora and Higgs (1998) | 1 | 44 | + | LPS, 2 years | S | Lower AP | Bilateral ovaries | 18 | LPT: bilateral OC | E | LB, 41 | |
| Katorza et al. (2007) | 1 | 31 | + | LOA, DTC, NR | A | Lower AP | Right adnexa | 26 | LPT: right SO | E+D | TOP, 26 | |
| Ueda et al. (2010) | 1 | 35 | NR | NR | A | NR | Ovary | NR | Cyst drainage | NR | NR, preterm for placenta previa | |
| Reif et al. (2011) | 1 | 25 | + | LOA, DTC, OC 1.5 years | A | AP | Left adnexa | 27 (twin) | LPT: left salpingectomy, OC | E+D | LB, LB, 27 | |
| Williamson et al. (2011) | 1 | 37 | − | − | S | AP | Left adnexa | 37 | Conservative | NR | IUD, 37 | |
| Infected endometrioma | Phupong et al. (2004) | 1 | 35 | + | − | S | AP, N, V, diarrhea | Right ovary | 35 | LPT: OC, appendectomy | E | LB, 36 |
| Ueda et al. (2010) | 1 | 40 | + | NR | A | NR | NR | NR | Cyst drainage | NR | NR, NR | |
| Dogan et al. (2012) | 1 | 30 | NR | NR | NR | Lower AP, V, hyperpyrexia | Appendix, left adnexa | 28 | LPT: appendectomy, left salpingectomy, ovarian biopsies | E+D | LB, 28 | |
| Enlarged endometrioma | Nezhat et al. (1991) | 1 | 28 | + | OC, LOA, 3 years | S | A | Bilateral ovaries | 16 | LPS: bilateral OC, LOA | D | LB, 38 |
| Ninia (1992) | 1 | 32 | − | − | S | Lower AP | Left ovary | 16 | LPT: OC | E | LB, term | |
| Gregora and Higgs (1998) | 1 | 36 | − | − | S | A | Right ovary | 16 | LPT: cyst drainage | NR | LB, 39 | |
| Ueda et al. (2010) | 3 | 27; 31; 33 | −; −; − | NR; NR; NR | S; S; S | NR; NR; NR | NR; NR; NR | II trimester; II trimester; II trimester | SO; SO; conservative | E+D; E+D | NR, NR; NR, NR; NR, NR | |
| Fallopian tube | ||||||||||||
| Fallopian tube rupture | Aggarwal et al. (2014) | 1 | 31 | + | LOA, DTC, OC, 2 years | A | Upper AP, V | Left fallopian tube | 21 (twin) | LPT: left salpingectomy | E+D | IUD, 21 |
| Uterus | ||||||||||||
| Uterine rupture | Van De Putte et al. (1999) | 1 | 29 | + | Excision of rectovaginal nodule, 6 years | NR | A | Posterior wall of the uterus | Immediate post-partum | LPT: uterine rupture repair | NR | LB, term |
| Sholapurkar et al. (2005) | 1 | 37 | NR | NR | NR | Vaginal bleeding | Uterine scar after Cesarean section | 6 weeks post-partum | LPT: hysterectomy | E | LB, 38 | |
| Granese (2010) | 1 | 34 | + | LOA, bilateral OC, DTC, 5 years | NR | Lower AP, dyspnea | Posterior wall of the uterus | 32 | LPT: uterine rupture repair | NR | NR, 32 | |
| Chen et al. (2011) | 1 | 33 | + | Bilateral OC, 3 years; cervical cystectomy, DTC, 1 year | A | NR | Posterior wall of the uterus and cervix | 37 | LPT: uterine rupture repair | NR | LB, 37 | |
TOP, termination of pregnancy.
Endometriosis-related complications involving reproductive organs during pregnancy, cases reported in literature.
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational weeks) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational weeks at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ovary | ||||||||||||
| Rupture of endometrioma | Brill et al. (1957) | 1 | 38 | NR | NR | NR | AP | Bilateral ovaries | Term | LPT: hysterectomy and BSO | E+D | LB, term |
| Steinberg (1962) | 1 | 35 | − | − | S | AP | Left ovary | 38 | LPT: OC, endometriotic nodule excision | E+D | LB, 38 | |
| Anderson and Edmond (1974) | 1 | 39 | − | − | S | AP | Left ovary | 37 | LPT: left SO | E+D | LB, 37 | |
| Rossman et al. (1983) | 1 | 25 | NR | NR | NR | AP, hyperpyrexia | Bilateral ovaries | 30 | LPT: bilateral OC | E+D | LB, 30 | |
| Johnson and Woodruff (1986) | 1 | 39 | NR | NR | NR | Lower AP | Right adnexa | 26 | LPT: hysterectomy and right SO | E | LB, 27 | |
| Vercellini et al. (1992) | 1 | 29 | − | − | S | AP | Right ovary | 35 | LPT: OC, LOA, DTC | E+D | LB, 35 | |
| Barbazan et al. (1993) | 1 | 29 | − | − | NR | Lower AP, N, V | Right ovary | 18 | LPT: right SO | E+D | LB, term | |
| Garcia-Velasco et al. (1998) | 1 | 25 | − | − | S | Lower AP | Left ovary | 9 | LPT: left SO | E+D | LB, NR | |
| Loh et al. (1998) | 1 | 25 | NR | NR | S | Lower AP | Bilateral ovaries | 6 | LPS: bilateral OC | E | LB, 39 | |
| Gregora and Higgs (1998) | 1 | 44 | + | LPS, 2 years | S | Lower AP | Bilateral ovaries | 18 | LPT: bilateral OC | E | LB, 41 | |
| Katorza et al. (2007) | 1 | 31 | + | LOA, DTC, NR | A | Lower AP | Right adnexa | 26 | LPT: right SO | E+D | TOP, 26 | |
| Ueda et al. (2010) | 1 | 35 | NR | NR | A | NR | Ovary | NR | Cyst drainage | NR | NR, preterm for placenta previa | |
| Reif et al. (2011) | 1 | 25 | + | LOA, DTC, OC 1.5 years | A | AP | Left adnexa | 27 (twin) | LPT: left salpingectomy, OC | E+D | LB, LB, 27 | |
| Williamson et al. (2011) | 1 | 37 | − | − | S | AP | Left adnexa | 37 | Conservative | NR | IUD, 37 | |
| Infected endometrioma | Phupong et al. (2004) | 1 | 35 | + | − | S | AP, N, V, diarrhea | Right ovary | 35 | LPT: OC, appendectomy | E | LB, 36 |
| Ueda et al. (2010) | 1 | 40 | + | NR | A | NR | NR | NR | Cyst drainage | NR | NR, NR | |
| Dogan et al. (2012) | 1 | 30 | NR | NR | NR | Lower AP, V, hyperpyrexia | Appendix, left adnexa | 28 | LPT: appendectomy, left salpingectomy, ovarian biopsies | E+D | LB, 28 | |
| Enlarged endometrioma | Nezhat et al. (1991) | 1 | 28 | + | OC, LOA, 3 years | S | A | Bilateral ovaries | 16 | LPS: bilateral OC, LOA | D | LB, 38 |
| Ninia (1992) | 1 | 32 | − | − | S | Lower AP | Left ovary | 16 | LPT: OC | E | LB, term | |
| Gregora and Higgs (1998) | 1 | 36 | − | − | S | A | Right ovary | 16 | LPT: cyst drainage | NR | LB, 39 | |
| Ueda et al. (2010) | 3 | 27; 31; 33 | −; −; − | NR; NR; NR | S; S; S | NR; NR; NR | NR; NR; NR | II trimester; II trimester; II trimester | SO; SO; conservative | E+D; E+D | NR, NR; NR, NR; NR, NR | |
| Fallopian tube | ||||||||||||
| Fallopian tube rupture | Aggarwal et al. (2014) | 1 | 31 | + | LOA, DTC, OC, 2 years | A | Upper AP, V | Left fallopian tube | 21 (twin) | LPT: left salpingectomy | E+D | IUD, 21 |
| Uterus | ||||||||||||
| Uterine rupture | Van De Putte et al. (1999) | 1 | 29 | + | Excision of rectovaginal nodule, 6 years | NR | A | Posterior wall of the uterus | Immediate post-partum | LPT: uterine rupture repair | NR | LB, term |
| Sholapurkar et al. (2005) | 1 | 37 | NR | NR | NR | Vaginal bleeding | Uterine scar after Cesarean section | 6 weeks post-partum | LPT: hysterectomy | E | LB, 38 | |
| Granese (2010) | 1 | 34 | + | LOA, bilateral OC, DTC, 5 years | NR | Lower AP, dyspnea | Posterior wall of the uterus | 32 | LPT: uterine rupture repair | NR | NR, 32 | |
| Chen et al. (2011) | 1 | 33 | + | Bilateral OC, 3 years; cervical cystectomy, DTC, 1 year | A | NR | Posterior wall of the uterus and cervix | 37 | LPT: uterine rupture repair | NR | LB, 37 | |
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational weeks) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational weeks at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ovary | ||||||||||||
| Rupture of endometrioma | Brill et al. (1957) | 1 | 38 | NR | NR | NR | AP | Bilateral ovaries | Term | LPT: hysterectomy and BSO | E+D | LB, term |
| Steinberg (1962) | 1 | 35 | − | − | S | AP | Left ovary | 38 | LPT: OC, endometriotic nodule excision | E+D | LB, 38 | |
| Anderson and Edmond (1974) | 1 | 39 | − | − | S | AP | Left ovary | 37 | LPT: left SO | E+D | LB, 37 | |
| Rossman et al. (1983) | 1 | 25 | NR | NR | NR | AP, hyperpyrexia | Bilateral ovaries | 30 | LPT: bilateral OC | E+D | LB, 30 | |
| Johnson and Woodruff (1986) | 1 | 39 | NR | NR | NR | Lower AP | Right adnexa | 26 | LPT: hysterectomy and right SO | E | LB, 27 | |
| Vercellini et al. (1992) | 1 | 29 | − | − | S | AP | Right ovary | 35 | LPT: OC, LOA, DTC | E+D | LB, 35 | |
| Barbazan et al. (1993) | 1 | 29 | − | − | NR | Lower AP, N, V | Right ovary | 18 | LPT: right SO | E+D | LB, term | |
| Garcia-Velasco et al. (1998) | 1 | 25 | − | − | S | Lower AP | Left ovary | 9 | LPT: left SO | E+D | LB, NR | |
| Loh et al. (1998) | 1 | 25 | NR | NR | S | Lower AP | Bilateral ovaries | 6 | LPS: bilateral OC | E | LB, 39 | |
| Gregora and Higgs (1998) | 1 | 44 | + | LPS, 2 years | S | Lower AP | Bilateral ovaries | 18 | LPT: bilateral OC | E | LB, 41 | |
| Katorza et al. (2007) | 1 | 31 | + | LOA, DTC, NR | A | Lower AP | Right adnexa | 26 | LPT: right SO | E+D | TOP, 26 | |
| Ueda et al. (2010) | 1 | 35 | NR | NR | A | NR | Ovary | NR | Cyst drainage | NR | NR, preterm for placenta previa | |
| Reif et al. (2011) | 1 | 25 | + | LOA, DTC, OC 1.5 years | A | AP | Left adnexa | 27 (twin) | LPT: left salpingectomy, OC | E+D | LB, LB, 27 | |
| Williamson et al. (2011) | 1 | 37 | − | − | S | AP | Left adnexa | 37 | Conservative | NR | IUD, 37 | |
| Infected endometrioma | Phupong et al. (2004) | 1 | 35 | + | − | S | AP, N, V, diarrhea | Right ovary | 35 | LPT: OC, appendectomy | E | LB, 36 |
| Ueda et al. (2010) | 1 | 40 | + | NR | A | NR | NR | NR | Cyst drainage | NR | NR, NR | |
| Dogan et al. (2012) | 1 | 30 | NR | NR | NR | Lower AP, V, hyperpyrexia | Appendix, left adnexa | 28 | LPT: appendectomy, left salpingectomy, ovarian biopsies | E+D | LB, 28 | |
| Enlarged endometrioma | Nezhat et al. (1991) | 1 | 28 | + | OC, LOA, 3 years | S | A | Bilateral ovaries | 16 | LPS: bilateral OC, LOA | D | LB, 38 |
| Ninia (1992) | 1 | 32 | − | − | S | Lower AP | Left ovary | 16 | LPT: OC | E | LB, term | |
| Gregora and Higgs (1998) | 1 | 36 | − | − | S | A | Right ovary | 16 | LPT: cyst drainage | NR | LB, 39 | |
| Ueda et al. (2010) | 3 | 27; 31; 33 | −; −; − | NR; NR; NR | S; S; S | NR; NR; NR | NR; NR; NR | II trimester; II trimester; II trimester | SO; SO; conservative | E+D; E+D | NR, NR; NR, NR; NR, NR | |
| Fallopian tube | ||||||||||||
| Fallopian tube rupture | Aggarwal et al. (2014) | 1 | 31 | + | LOA, DTC, OC, 2 years | A | Upper AP, V | Left fallopian tube | 21 (twin) | LPT: left salpingectomy | E+D | IUD, 21 |
| Uterus | ||||||||||||
| Uterine rupture | Van De Putte et al. (1999) | 1 | 29 | + | Excision of rectovaginal nodule, 6 years | NR | A | Posterior wall of the uterus | Immediate post-partum | LPT: uterine rupture repair | NR | LB, term |
| Sholapurkar et al. (2005) | 1 | 37 | NR | NR | NR | Vaginal bleeding | Uterine scar after Cesarean section | 6 weeks post-partum | LPT: hysterectomy | E | LB, 38 | |
| Granese (2010) | 1 | 34 | + | LOA, bilateral OC, DTC, 5 years | NR | Lower AP, dyspnea | Posterior wall of the uterus | 32 | LPT: uterine rupture repair | NR | NR, 32 | |
| Chen et al. (2011) | 1 | 33 | + | Bilateral OC, 3 years; cervical cystectomy, DTC, 1 year | A | NR | Posterior wall of the uterus and cervix | 37 | LPT: uterine rupture repair | NR | LB, 37 | |
TOP, termination of pregnancy.
Endometriosis-related complications involving extra-pelvic organs during pregnancy, cases reported in literature.
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational weeks) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational weeks at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | ||||||||||||
| Pneumothorax | Schoenfeld et al. (1986) | 1 | 27 | + | − | NR | Dyspnea | Lung | 24 | Thoracotomy | NR | NR, NR |
| Flieder et al. (1998) | 1 | 27 | − | − | NR | Chest pain, dyspnea | Right lung | 28 | Thoracotomy: lung biopsy | D | NR, NR | |
| Yoshioka et al. (2005) | 1 | 29 | + | Explorative thoracotomy, 3 years | S | Chest pain, dyspnea | Right lung | 8 | Thoracoscopic pleural cysts resection and pleurodesis | E | TOP, 8 | |
| Kim et al. (2010) | 1 | 34 | − | − | NR | Dyspnea | Right lung | 18 | Thoracostomy drainage and, then, at relapse, thoracoscopy biopsies | D | LB, 39 | |
| Lymph nodes | ||||||||||||
| Beavis et al. (2011) | 1 | 25 | + | Bilateral OC, NR | S | Vaginal bleeding | Para-aortic lymph nodes | 24 | Para-aortic lymph node resection, OC | E+D | LB, 26 | |
| Aorta | ||||||||||||
| Nötzold et al. (1998) | 1 | 28 | − | − | NR | Severe hypertension | Thoracic aorta | 3 weeks post-partum | Aneurysm excision and prothesis replacement | E | LB, NR | |
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational weeks) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational weeks at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | ||||||||||||
| Pneumothorax | Schoenfeld et al. (1986) | 1 | 27 | + | − | NR | Dyspnea | Lung | 24 | Thoracotomy | NR | NR, NR |
| Flieder et al. (1998) | 1 | 27 | − | − | NR | Chest pain, dyspnea | Right lung | 28 | Thoracotomy: lung biopsy | D | NR, NR | |
| Yoshioka et al. (2005) | 1 | 29 | + | Explorative thoracotomy, 3 years | S | Chest pain, dyspnea | Right lung | 8 | Thoracoscopic pleural cysts resection and pleurodesis | E | TOP, 8 | |
| Kim et al. (2010) | 1 | 34 | − | − | NR | Dyspnea | Right lung | 18 | Thoracostomy drainage and, then, at relapse, thoracoscopy biopsies | D | LB, 39 | |
| Lymph nodes | ||||||||||||
| Beavis et al. (2011) | 1 | 25 | + | Bilateral OC, NR | S | Vaginal bleeding | Para-aortic lymph nodes | 24 | Para-aortic lymph node resection, OC | E+D | LB, 26 | |
| Aorta | ||||||||||||
| Nötzold et al. (1998) | 1 | 28 | − | − | NR | Severe hypertension | Thoracic aorta | 3 weeks post-partum | Aneurysm excision and prothesis replacement | E | LB, NR | |
Endometriosis-related complications involving extra-pelvic organs during pregnancy, cases reported in literature.
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational weeks) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational weeks at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | ||||||||||||
| Pneumothorax | Schoenfeld et al. (1986) | 1 | 27 | + | − | NR | Dyspnea | Lung | 24 | Thoracotomy | NR | NR, NR |
| Flieder et al. (1998) | 1 | 27 | − | − | NR | Chest pain, dyspnea | Right lung | 28 | Thoracotomy: lung biopsy | D | NR, NR | |
| Yoshioka et al. (2005) | 1 | 29 | + | Explorative thoracotomy, 3 years | S | Chest pain, dyspnea | Right lung | 8 | Thoracoscopic pleural cysts resection and pleurodesis | E | TOP, 8 | |
| Kim et al. (2010) | 1 | 34 | − | − | NR | Dyspnea | Right lung | 18 | Thoracostomy drainage and, then, at relapse, thoracoscopy biopsies | D | LB, 39 | |
| Lymph nodes | ||||||||||||
| Beavis et al. (2011) | 1 | 25 | + | Bilateral OC, NR | S | Vaginal bleeding | Para-aortic lymph nodes | 24 | Para-aortic lymph node resection, OC | E+D | LB, 26 | |
| Aorta | ||||||||||||
| Nötzold et al. (1998) | 1 | 28 | − | − | NR | Severe hypertension | Thoracic aorta | 3 weeks post-partum | Aneurysm excision and prothesis replacement | E | LB, NR | |
| . | Authors, years . | No. of cases . | Age (years) . | History of endometriosis . | Surgery before pregnancy (type, time before) . | Conception . | Presenting symptoms . | Site of complication . | Onset of complication (gestational weeks) . | Complication management during pregnancy . | Histological examination . | Pregnancy outcome, gestational weeks at delivery . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | ||||||||||||
| Pneumothorax | Schoenfeld et al. (1986) | 1 | 27 | + | − | NR | Dyspnea | Lung | 24 | Thoracotomy | NR | NR, NR |
| Flieder et al. (1998) | 1 | 27 | − | − | NR | Chest pain, dyspnea | Right lung | 28 | Thoracotomy: lung biopsy | D | NR, NR | |
| Yoshioka et al. (2005) | 1 | 29 | + | Explorative thoracotomy, 3 years | S | Chest pain, dyspnea | Right lung | 8 | Thoracoscopic pleural cysts resection and pleurodesis | E | TOP, 8 | |
| Kim et al. (2010) | 1 | 34 | − | − | NR | Dyspnea | Right lung | 18 | Thoracostomy drainage and, then, at relapse, thoracoscopy biopsies | D | LB, 39 | |
| Lymph nodes | ||||||||||||
| Beavis et al. (2011) | 1 | 25 | + | Bilateral OC, NR | S | Vaginal bleeding | Para-aortic lymph nodes | 24 | Para-aortic lymph node resection, OC | E+D | LB, 26 | |
| Aorta | ||||||||||||
| Nötzold et al. (1998) | 1 | 28 | − | − | NR | Severe hypertension | Thoracic aorta | 3 weeks post-partum | Aneurysm excision and prothesis replacement | E | LB, NR | |
Mechanisms underlying endometriosis complications during pregnancy
Complications of endometriosis during pregnancy might be mostly attributed to the following factors:
Adhesions may create further traction on surrounding structures when the uterus is enlarged during pregnancy (Rossman et al., 1983). Adhesion formation in endometriosis may be related to the disease itself or to the surgery for the disease. The normal wound-healing process after injury to the peritoneum involves a complex inflammatory cascade of fibrin deposition, coagulation and influx of inflammatory cells. Adhesions form primarily as a result of an imbalance of fibrin deposition and fibrin breakdown. Post-surgical adhesions that originate from any abdominal/pelvic surgery, including Cesarean section, are well known to lead to a number of complications including bladder and bowel injuries (Lyell, 2011). Indeed, complications (i.e. bowel obstruction or perforation) occurring in pregnancy due to the presence of adhesions caused by previous abdominal surgery have been described also in patients without endometriosis (Kalu et al., 2006; Matsushita et al., 2011).
Endometriosis-related chronic inflammation may make tissues and vessels more friable. Indeed, while an appropriately driven and resolving inflammatory process results in successful wound healing after tissue damage, an inappropriately sustained inflammatory reaction is often related to an overactive wound-healing response leading to tissue fibrosis, which can present a threat to the maintenance of tissue structure and function. Chronically inflamed tissues, such as those involved by endometriotic lesions, are characterized by sustained, nonresolving inflammation and fibrosis leading to tissue dysfunction (Manresa et al., 2014). In this already compromised situation, the hormone saturated environment of pregnancy might be critical in the amplification of hormone-sensitive intrinsic inflammatory processes attributed to ectopic endometriotic lesions (Khan and Hay, 2015).
The intrusion of decidualized endometriotic tissue into the vessel wall and structures can increase backpressure, predisposing to tissue rupture (O'Leary, 2006). Importantly in this regard, the decidualized endometrium transforms into a well-vascularized tissue characterized by increased vascular permeability, edema, vascular remodeling and angiogenesis and an increase in luminal diameter (Plaisier, 2011). An alternative explanation to the vessel rupture due to the mechanical obstruction involves the involution of the decidualized endometrium surrounding the distended vasculature. Decidualization is dependent upon sustained progesterone signaling and progesterone withdrawal triggers involution of the decidual vessels and bleeding (Brosens et al., 2009; Erikson et al., 2014). In endometriosis, characterized by a progesterone resistance and suboptimal expression of target genes, the necrosis of foci of decidualized ectopic endometrium located in proximity to dilated utero-ovarian or parametrial vessels could lead to a dysfunctional rupture of such vessels and bleeding of unpredictable severity (Brosens et al., 2009).
Bowel
Intestinal perforation
The incidence of bowel endometriosis has been estimated at 5–12% in women affected by endometriosis (Mabrouk et al., 2012). The most frequent location is the sigmoid colon, followed by the rectum, ileum, appendix and cecum (Remorgida et al., 2007). Intestinal endometriosis may be found in every layer of the bowel wall but is most commonly found within the subserosa as superficial implants (Garg et al., 2009). Intestinal perforation linked to endometriosis is quite rare. Only twelve cases of intestinal perforation due to endometriosis not associated with pregnancy have been described in the literature (Pisanu et al., 2010).
This systematic review comprises 16 cases of bowel perforation caused by endometriosis during pregnancy or in the post-partum period (Table V). The locations of the perforations were ileum (n = 1), appendix (n = 4), cecum (n = 1), sigmoid colon (n = 8) and rectum (n = 2). Perforations occurred mostly in the third trimester (mean ± SD gestational age of 30 ± 6.3 weeks). There were only three post-partum complications. In 31% of the cases (4/13), women had a history of endometriosis before pregnancy. Three patients underwent a previous surgery for endometriosis including ovarian cystectomy, adhesiolysis and diathermocoagulation of endometriotic implants 3, 5 and 15 years before pregnancy. None of these three patients underwent bowel surgery. The last one had a diagnosis of a 3-cm rectosigmoid nodule and underwent IVF treatment.
Nonspecific symptoms (acute abdominal pain, nausea and vomiting) were experienced in 94% of the patients (15/16). Noteworthy, in two cases, pyelonephritis was suspected delaying the diagnosis and in three cases, bowel perforation was not diagnosed during the first exploratory laparotomy, thus requiring a second laparotomy (Pisanu et al., 2010; Setúbal et al., 2014). Clinical and laboratory signs of peritonitis were present in 13 patients (81%). Radiography or computed tomography (CT) demonstrated free air in the peritoneal cavity in 31% of cases (5/16). Segmental intestinal resection was performed during pregnancy in 94% of women (15/16) with the Hartmann's procedure in 46% of the cases (7/15). In the only asymptomatic patient, the injury of rectal mucosa localized 2 cm above the intact external sphincter and a second-degree perineal tear were detected and repaired immediately after the vaginal delivery (Menzlova et al., 2014). Endometriosis was confirmed histologically in all the evaluated specimens; decidualized endometriosis involving the entire intestinal wall was found in 88% of cases (14/16). Pregnancy outcome was characterized by 100% live births with a mean ± SD gestational age at delivery of 37 ± 2.9 weeks. One patient delivered at 31 weeks a viable infant who developed neonatal respiratory distress syndrome and required mechanical ventilation in a neonatal intensive care unit for few days (Lebastchi et al., 2013). In the other cases, newborns did not require any specific treatment.
Appendicitis
Endometriosis of appendix is uncommon with a prevalence of 2.8% in patients with endometriosis and 0.4% in the general population (Gustofson et al., 2006). Although it is frequently asymptomatic, it may present as acute appendicitis (Stefanidis et al., 1999), lower gastrointestinal bleeding (Shome et al., 1995), cecal intussusception (Panzer et al., 1995) and intestinal perforation, in particular during pregnancy (Gini et al., 1981; Nakatani et al., 1987; Faucheron et al., 2008, Lebastchi et al., 2013).
Acute appendicitis and endometriosis of appendix show no differences in population features (age, parity, pregnancy duration at diagnosis) and presenting signs and symptoms; therefore, it is challenging making a differential diagnosis before histological examination. On the other hand, during the third trimester, the occurrence of symptoms and complications is higher in women with appendiceal endometriosis (Perez et al., 2007).
Seven cases of appendiceal endometriosis presenting as acute appendicitis during pregnancy have been described (Table V). The age of patients ranged from 21 to 34 years with a mean ± SD of 28 ± 3.7 years. The mean ± SD gestational age at diagnosis was 20 ± 9.8 weeks and the most frequent presenting symptoms were nausea (29%), vomiting (43%) and abdominal pain (86%). The diagnosis of an acute event involving the appendix (such as acute appendicitis or bleeding of appendiceal endometriosis) is more challenging in pregnancy than in non-pregnant women because (i) the symptoms of nausea and vomiting are typical of early pregnancy, (ii) the localization of pain may be variable due to upward displacement of the appendix by the growing uterus and (iii) the white blood cell count ranging between 8000 and 20 000 cells/mm3 is normal during pregnancy. In all cases reported in the literature, the patients underwent appendectomy during pregnancy. On histological examination, some cases (43%) showed also evidence of inflammation while others (43%) revealed only decidual changes. The pregnancy outcome was unknown in most cases. One preterm labor during the third trimester with subsequent neonatal death was reported in the 1970s (Finch and Lee, 1974); more recently, a live birth at term was reported (Stefanidis et al., 1999).
Vessels: spontaneous hemoperitoneum
Spontaneous hemoperitoneum (SH) during pregnancy from ruptured utero-ovarian vessels is a rare but life-threatening complication. Sixteen publications, reporting a total of 20 cases of endometriosis-related SH in pregnancy, were reviewed (Table V). Nulliparous women represented 70% of cases of SH (14/20). Five women had twin pregnancies and six pregnancies were achieved by IVF treatment. The mean ± SD maternal age was 33 ± 4.2 years. The main presenting symptom was the sudden onset of abdominal pain with different localizations (95%) and signs of hypovolemic shock (70%). One patient complained of a lower quadrant pain associated with tachypnea because of a combined hemothorax (Bouet et al., 2009). Most of the cases were reported during the third trimester with a mean ± SD gestational age of 28.7 ± 4.3. Four cases (20%) occurred in the post-partum period. Nine women (56%) were known to have moderate to severe endometriosis diagnosed by laparoscopy prior to pregnancy (Wada et al., 2009). In 40% of the cases, the presumed preoperative diagnosis was placenta abruption with concealed hemorrhage (75%) and a uterine rupture (25%). In most cases, the diagnosis of ruptured utero-ovarian vessel was established at explorative laparotomy that was carried out in the 90% of cases for maternal reasons (hypovolemic shock and progressive anemia; 67%), for fetal reasons (fetal distress; 22%), or both (11%). Laparoscopy was performed in only one hemodynamically stable patient with extensive post-partum SH (Gao et al., 2010). There was only one case of a double uterine artery embolization during pregnancy at 22th and at 24th gestational week after the diagnosis of endometriosis from laparoscopic biopsy of a tumor mass between the cervix and the bladder revealed by ultrasonography at 14th gestational week. Two days after the last radiological procedure, a spontaneous labor started and a Cesarean delivery of a live birth baby was performed. The diagnosis was confirmed by explorative laparotomy 11 days after the delivery (Kirkinen et al., 2007). One patient underwent subtotal hysterectomy and bilateral salpingo-oophorectomy 11 days after delivery because during surgery a tumor-like mass arising from the left pelvic sidewall involving both ovaries was detected and suspected for malignancy by gynecologic oncologist (O'Leary, 2006).
At surgery, the bleeding site was the uterus in 70% of cases (14/20), the parametrium with its arteries and veins in 15% (5/20) and the uterosacral ligament in 5% (1/20). The bleeding was described as arising from varicosities on the uterine surface or vessels of parametrium in 70% of cases (14/20) and from macroscopic endometriotic lesions in 30% (6/20). Moreover, in addition to a significant amount of intra-abdominal blood (mean 2314 ml), other endometriosis localizations and pelvic adhesions were observed in 60% (12/20) and 40% (8/20) of cases, respectively. Histological examination was performed in 45% (9/20) of cases and among these specimens, decidualization of endometriosis was diagnosed in 67% (6/9) of them.
No maternal death was reported. There were seven cases of intrauterine death and one neonatal death, resulting in a perinatal mortality rate of 36% (8/22 babies). Live births were reported in the 63% of cases (14/22) and the mean ± SD gestational age of delivery was 31 ± 2.8 weeks of gestation.
In summary, when acute abdominal pain with massive hemoperitoneum occurs in pregnant nulliparous women or in the post-partum period, particularly in presence of a history of endometriosis, spontaneous rupture of utero-ovarian vessels should be considered as a possible cause of SH.
Urinary system
Two cases of pregnancy complicated by uroperitoneum due to the presence of endometriosis have been reported (Chiodo et al., 2008; Leone Roberti Maggiore et al., 2015). Distortion of renal system anatomy was observed in another two cases of endometriosis (Yaqub et al., 2008; Pezzuto et al., 2009). More details about all these cases are shown in Table V.
Adnexa
There are little data about growth dynamics of ovarian endometriosis in pregnancy. Most investigators have reported regression or cessation of growth during pregnancy (Ueda et al., 2010; Benaglia et al., 2013). As previously mentioned, Ueda et al. (2010) described retrospectively the natural history of 25 ovarian endometriotic lesions observed during pregnancy in 24 women (one case had two lesions). The size of the cyst decreased in 13 lesions (52%), was unchanged in 7 (28%), and increased in 5 (20%) with development of some complications such as decidualization, abscess and rupture.
The only case involving rupture of Fallopian tubes related to endometriosis during pregnancy is described in Table VI.
Infected endometrioma
In three cases of infected endometrioma (Phupong et al., 2004; Ueda et al., 2010, Dogan et al., 2012), symptoms and signs mimicking any cause of acute abdomen led to surgery during pregnancy with drainage of the abscess (Table VI). In one case surgery was performed at 35 weeks of gestation for the clinical suspicion of acute appendicitis (Phupong et al., 2004); in the second case, drainage of the ovarian abscess was performed in the second trimester (Ueda et al., 2010). Dogan et al. (2012) reported the case of a 30-year-old woman who underwent firstly appendectomy at 24 weeks' gestation for acute appendicitis due to decidualized endometriosis. Then, during a second laparotomy at 28 weeks' gestation, unilateral salpingectomy was performed because of a tubo-ovarian abscess arising from a decidualized ovarian endometrioma, revealed by preoperative ultrasonography and then confirmed by histology. Although an infected endometrioma is an extremely rare event, it should be included in the differential diagnosis of pelvic pain during pregnancy, especially in women with history of ovarian endometrioma.
Enlarged endometrioma
The size of endometrioma during pregnancy was reported to increase in 20% of the cases (Ueda et al., 2010). Cases of endometrioma enlargement requiring interventions during pregnancy have been reported (Table VI). In six patients (Nezhat et al., 1991; Ninia, 1992; Gregora and Higgs, 1998; Ueda et al., 2010), endometrioma increased significantly in size reaching a mean diameter ± SD of 10.3 ± 5.2 cm (range, 6–20 cm) in the second trimester (100%, 6/6). In five cases, surgery was performed to rule out malignancy and prevent surgical emergencies such as torsion, rupture and obstruction of labor (Gregora and Higgs, 1998). One patient decided to avoid surgery and the lesion regressed in the third trimester and in the post-partum period. It is of note that most of these surgical cases are quite dated and currently the indication to surgery could be different.
Rupture of endometrioma
Our work of review encompasses 14 cases of ruptured ovarian endometriosis occurring during pregnancy (Table VI). Mean maternal age ± SD was 32 ± 6.4 years (range, 44–25 years). Singleton pregnancies were predominant (13 out of 14 cases, 93%); there was only one twin pregnancy. In 3/10 (30%) cases, the event occurred in patients who underwent assisted reproductive technology (ART) procedures. There was no case of asymptomatic ruptured endometrioma. Lower abdominal pain was reported by all the patients, with no side dominance and with clinical features of acute abdomen. In three cases (Katorza et al., 2007; Reif et al., 2011; Williamson et al., 2011), a diagnosis of hemoperitoneum was made and, among these, two patients had signs of hypovolemic shock. Bilateral ovarian involvement was present in 4 cases (29%). The rupture occurred in the first trimester in only two cases (14%), in the second trimester in the 36% of cases (5/14) and more frequently in the third trimester with a mean gestational age ± SD of 32 ± 10.9 weeks (range, 6–38 weeks). Among these patients, 33% of cases had a previous history of endometriosis and surgery; in 36% of cases, the endometrioma was detected by ultrasound at the beginning of pregnancy or before conception. The diagnosis was performed at surgery in 7/14 cases (50%).
Thirteen out of fourteen cases (93%) underwent surgery during pregnancy: hysterectomy and salpingo-oophorectomy after Cesarean delivery (CD) were performed in two cases, while in the other cases the surgery was conservative [unilateral salpingo-oophorectomy (4/13), unilateral or bilateral cystectomy (6/13), and cyst drainage (1/13)]. Laparoscopy technique was used in only one case. During surgery, dense adhesions were seen in 69% of the cases while decidualized endometriosis was confirmed by histological examination in 69% of cases. In the single case that did not undergo surgery, after an initial indication of emergency CD for maternal hypotensive shock and suspicion of placenta abruption, the rapid intrauterine fetal death and the improved mother's hemodynamic conditions allowed a vaginal delivery. Post-partum imaging showed a large heterogeneous hematoma within the pelvic cavity. Four months after discharge, pelvic MRI scans revealed that the pelvic mass was caused by a ruptured left endometrioma and severe pelvic endometriosis. There were 12 cases of live birth, one intrauterine death at 37 weeks' gestation and one termination of pregnancy because post-operatively there were severe signs of intrauterine fetal asphyxia. Mean gestation at delivery of live birth ± SD was 34 ± 5.4 weeks (range, 27–38 weeks). Three cases of preterm labor occurred and the treatment with intravenous ritodrine used in two cases was not helpful (Johnson and Woodruff, 1986; Vercellini et al., 1992).
Summary
Complications deriving from ovarian endometriotic cysts, such as infected, enlarged and ruptured endometrioma, represent rare events but they should be considered in the differential diagnosis of pelvic pain during pregnancy. Conservative treatment with antibiotic therapy should represent the first-line management for infected endometrioma, although in case of severe abdominal pain and systemic involvement, drainage or surgery may be required. Changes in the size of endometrioma during pregnancy, including an increase, have been described by different authors (Ueda et al., 2010; Benaglia et al., 2013). We deem that observational management of the lesion is advisable in most of the cases; surgery may be necessary in case of acute abdomen due to torsion or rupture of the cyst. Cyst rupture is highly symptomatic (acute abdomen, hemoperitoneum, hypotension…) and surgery (preferentially laparoscopy) is required for the majority of the events.
Uterus
Uterine rupture
The reported endometriosis-related uterine acute complications in pregnancy include three cases of uterine rupture and one case of uterine hemorrhage (Van De Putte et al., 1999; Sholapurkar et al., 2005; Granese, 2010; Chen et al., 2011).
Uterine rupture represents a major obstetrical complication and more commonly involves a scarred uterus. Indeed, three patients had undergone endometriosis surgery (excision of a rectovaginal nodule, bilateral ovarian cystectomy and excision of cervical endometriosis) respectively, 6, 5 and 1 years before pregnancy. The uterine lesion was revealed in one case during manual exploration of the uterine cavity because of a retained placenta after a vaginal delivery at term. In the others, it could be detected during an emergent CD at 32 weeks for acute abdomen and at 37 weeks for fetal distress signs during labor. The rupture was localized on the posterior wall of the uterus at the lower segment level in all cases. In all cases healthy babies were born, and no maternal death was reported.
Finally, Sholapurkar et al. (2005) reported a particular case of a 37-year-old woman presenting to the emergency service for a life-threatening vaginal bleeding 6 weeks after CD performed for an arrested labor progression. A total abdominal hysterectomy was performed and the main histological abnormality was endometriosis in the Cesarean section scar involving the full thickness of isthmus from which excessive bleeding started. No other cases of development of endometriosis in the uterine scar after an interval shorter than 6 weeks from CD were found in the literature.
Extra-pelvic endometriosis
Endometriosis has also been described in virtually every location that can be reached by hematogenous, lymphatic, or direct dissemination (Veeraswamy et al., 2010).
Manifestations of endometriosis in thoracic organs are very rare. Four cases of spontaneous pneumothorax during pregnancy related to thoracic endometriosis (Schoenfeld et al., 1986; Flieder et al., 1998; Yoshioka et al., 2005; Kim et al., 2010) and a case of endometriosis involving thoracic aorta during pregnancy (Nötzold et al., 1998) have been reported (Table VII). Shortness of breath was a complaint in all four cases and two patients presented also with chest pain. The gestational age at diagnosis was 8, 18, 24 and 28 weeks. Two of them had history of catamenial pneumothorax before pregnancy. In one case treated with hormonal therapy for catamenial pneumothorax before pregnancy, after elective abortion, the patient underwent thoracoscopy to remove multiple pleural cysts and a pleurodesis was performed. In the other three cases, two thoracotomies and one thoracoscopy were performed after thoracostomy drainage. The histologic examination demonstrated presence of decidualized tissues and endometriosis.
Nötzold et al. (1998) reported a case of a patient known for a repair of an aortic coarctation developing severe and unresponsive hypertension associated with an elective CD 16 years later. A pseudoaneurysm of the descending aorta in the area of the patch repair was detected at computed tomography scan. During subsequent operation, a rupture of the suture line of the distal part of the patch was discovered and the patch and the aneurysm were excised. Histological examination revealed endometriosis between the media and the adventitial connective tissue of the vessel.
Recently, the first case of endometriosis involving para-aortic lymph nodes in a pregnant woman was published (Beavis et al., 2011). The patient with a known placenta previa and a para-aortic lymphadenopathy underwent CD at 26 weeks' gestation because of vaginal bleeding and uterine contractions persisting despite tocolytic therapy. Ovarian cystectomy for an enlarged endometrioma and a right 2.4 cm para-aortic lymph node resection were performed. Endometriosis with decidual changes in the lymph node was demonstrated at histological examination.
Endometriosis and pregnancy outcomes
Emerging clinical evidence suggests that endometriosis could negatively affect the physiological development of pregnancy. The results from epidemiological studies are contradictory and there is a lack of well-designed prospective trials to improve knowledge on the potential association between endometriosis and adverse pregnancy outcomes. In this section of the review, we summarize available literature on the impact of endometriosis on pregnancy outcome during the three trimesters of pregnancy (Tables VIII and IX). Given the extreme heterogeneity of the available epidemiological studies in terms of methodology, no meta-analysis was attempted.
Association between miscarriage and endometriosis, studies from literature.
| Author, year . | Population (cases versus controls) . | Type of study . | Diagnosis . | Association between spontaneous miscarriage and endometriosis . | Age adjusted . |
|---|---|---|---|---|---|
| Naples et al. (1981) | 65 non-ART infertile endometriosis patients who underwent conservative surgery versus 100 non-ART endometriosis (no control without endometriosis) | Retrospective cohort | Laparotomic/endoscopic | + (reduction of abortion rate in treated patients) | No |
| Olive et al. (1982) | 263 non-ART endometriosis (no controls without endometriosis) | Observational | Laparotomic/endoscopic | NR | No |
| Wheeler et al. (1983) | 39 non-ART mild endometriosis versus 9 non-ART moderate versus 23 non-ART severe (no control without endometriosis) | Retrospective cohort | Laparotomic | + (reduction of abortion rate in mild endometriosis treated patients) | No |
| Groll (1984) | Non-ART endometriosis: 27 untreated versus 28 treated with danazol versus 25 surgically treated (no control without endometriosis) | Retrospective cohort | Endometrial biopsy | + (reduction of abortion rate in treated patients) | No |
| Metzger et al. (1986) | Non-ART endometriosis: 95 who underwent conservative surgery versus 44 no surgery (no controls without endometriosis) | Prospective | Laparoscopic | − (reduction both in surgical and expectant management patients) | No |
| Fitzsimmons et al. (1987) | 52 non-ART endometriosis versus 134 non-ART secondary infertility without endometriosis | Retrospective cohort | Laparoscopic | − | No |
| Pittaway et al. (1988) | 100 non-ART endometriosis versus 150 non-ART fertile women versus 100 non-ART tubal disease | Retrospective cohort | Laparoscopic | + | No |
| Candiani et al. (1991) | 241 non-ART endometriosis versus 437 non-ART non-endometriosis | Case–control | Laparoscopic/laparotomic | − | No |
| Marcoux et al. (1997) | 172 patients who underwent operative laparoscopy versus 169 who underwent diagnostic laparoscopy (ART and non-ART) | Prospective randomized | Laparoscopic | − | No (mean age group laparoscopic surgery 31 ± 3 years; mean age group diagnostic laparoscopy 30 ± 4 years) |
| Matorras et al. (1998) | 174 endometriosis versus 174 infertile women without endometriosis (ART and non-ART) | Prospective | Laparoscopic | − | No (same mean age between groups) |
| Gruppo Italiano per lo Studio dell'Endometriosi (1999) | Non-ART endometriosis: 54 who underwent operative laparoscopy versus 47 who underwent diagnostic laparoscopy | Prospective randomized | Laparoscopic | − | No (same age between groups) |
| Omland et al. (2005) | 212 ART endometriosis versus 274 ART non-endometriosis | Retrospective cohort | Laparoscopic | + | Yes |
| Gergolet et al. (2010) | 36 non-ART endometriosis versus 143 non-ART non-endometriosis | Retrospective cohort | Laparoscopic | − | Yes |
| Vercellini et al. (2012) | 419 non-ART endometriosis | Retrospective cohort | Laparoscopic | − (+ in ovarian endometriomata) | Yes |
| Aris (2014) | 784 women with endometriosis, 30 284 without endometriosis (not specified if natural or ART) | Retrospective cohort | Laparoscopic | + | Yes |
| Barbosa et al. (2014) | 20 167 ART endometriosis versus 121 931 ART non-endometriosis | Systematic review and meta-analysis | Ultrasound/laparoscopic/both | + | Not in all the considered studies |
| Hjordt Hansen et al. (2014) | 24 667 endometriosis versus 98 668 non-endometriosis (ART and non-ART categorized) | Prospective cohort | Clinical/laparoscopic | + | No |
| Mekaru et al. (2014) | 49 non-ART endometriosis, 59 non-ART non-endometriosis | Retrospective cohort | Laparoscopic | − | Yes |
| Author, year . | Population (cases versus controls) . | Type of study . | Diagnosis . | Association between spontaneous miscarriage and endometriosis . | Age adjusted . |
|---|---|---|---|---|---|
| Naples et al. (1981) | 65 non-ART infertile endometriosis patients who underwent conservative surgery versus 100 non-ART endometriosis (no control without endometriosis) | Retrospective cohort | Laparotomic/endoscopic | + (reduction of abortion rate in treated patients) | No |
| Olive et al. (1982) | 263 non-ART endometriosis (no controls without endometriosis) | Observational | Laparotomic/endoscopic | NR | No |
| Wheeler et al. (1983) | 39 non-ART mild endometriosis versus 9 non-ART moderate versus 23 non-ART severe (no control without endometriosis) | Retrospective cohort | Laparotomic | + (reduction of abortion rate in mild endometriosis treated patients) | No |
| Groll (1984) | Non-ART endometriosis: 27 untreated versus 28 treated with danazol versus 25 surgically treated (no control without endometriosis) | Retrospective cohort | Endometrial biopsy | + (reduction of abortion rate in treated patients) | No |
| Metzger et al. (1986) | Non-ART endometriosis: 95 who underwent conservative surgery versus 44 no surgery (no controls without endometriosis) | Prospective | Laparoscopic | − (reduction both in surgical and expectant management patients) | No |
| Fitzsimmons et al. (1987) | 52 non-ART endometriosis versus 134 non-ART secondary infertility without endometriosis | Retrospective cohort | Laparoscopic | − | No |
| Pittaway et al. (1988) | 100 non-ART endometriosis versus 150 non-ART fertile women versus 100 non-ART tubal disease | Retrospective cohort | Laparoscopic | + | No |
| Candiani et al. (1991) | 241 non-ART endometriosis versus 437 non-ART non-endometriosis | Case–control | Laparoscopic/laparotomic | − | No |
| Marcoux et al. (1997) | 172 patients who underwent operative laparoscopy versus 169 who underwent diagnostic laparoscopy (ART and non-ART) | Prospective randomized | Laparoscopic | − | No (mean age group laparoscopic surgery 31 ± 3 years; mean age group diagnostic laparoscopy 30 ± 4 years) |
| Matorras et al. (1998) | 174 endometriosis versus 174 infertile women without endometriosis (ART and non-ART) | Prospective | Laparoscopic | − | No (same mean age between groups) |
| Gruppo Italiano per lo Studio dell'Endometriosi (1999) | Non-ART endometriosis: 54 who underwent operative laparoscopy versus 47 who underwent diagnostic laparoscopy | Prospective randomized | Laparoscopic | − | No (same age between groups) |
| Omland et al. (2005) | 212 ART endometriosis versus 274 ART non-endometriosis | Retrospective cohort | Laparoscopic | + | Yes |
| Gergolet et al. (2010) | 36 non-ART endometriosis versus 143 non-ART non-endometriosis | Retrospective cohort | Laparoscopic | − | Yes |
| Vercellini et al. (2012) | 419 non-ART endometriosis | Retrospective cohort | Laparoscopic | − (+ in ovarian endometriomata) | Yes |
| Aris (2014) | 784 women with endometriosis, 30 284 without endometriosis (not specified if natural or ART) | Retrospective cohort | Laparoscopic | + | Yes |
| Barbosa et al. (2014) | 20 167 ART endometriosis versus 121 931 ART non-endometriosis | Systematic review and meta-analysis | Ultrasound/laparoscopic/both | + | Not in all the considered studies |
| Hjordt Hansen et al. (2014) | 24 667 endometriosis versus 98 668 non-endometriosis (ART and non-ART categorized) | Prospective cohort | Clinical/laparoscopic | + | No |
| Mekaru et al. (2014) | 49 non-ART endometriosis, 59 non-ART non-endometriosis | Retrospective cohort | Laparoscopic | − | Yes |
Association between miscarriage and endometriosis, studies from literature.
| Author, year . | Population (cases versus controls) . | Type of study . | Diagnosis . | Association between spontaneous miscarriage and endometriosis . | Age adjusted . |
|---|---|---|---|---|---|
| Naples et al. (1981) | 65 non-ART infertile endometriosis patients who underwent conservative surgery versus 100 non-ART endometriosis (no control without endometriosis) | Retrospective cohort | Laparotomic/endoscopic | + (reduction of abortion rate in treated patients) | No |
| Olive et al. (1982) | 263 non-ART endometriosis (no controls without endometriosis) | Observational | Laparotomic/endoscopic | NR | No |
| Wheeler et al. (1983) | 39 non-ART mild endometriosis versus 9 non-ART moderate versus 23 non-ART severe (no control without endometriosis) | Retrospective cohort | Laparotomic | + (reduction of abortion rate in mild endometriosis treated patients) | No |
| Groll (1984) | Non-ART endometriosis: 27 untreated versus 28 treated with danazol versus 25 surgically treated (no control without endometriosis) | Retrospective cohort | Endometrial biopsy | + (reduction of abortion rate in treated patients) | No |
| Metzger et al. (1986) | Non-ART endometriosis: 95 who underwent conservative surgery versus 44 no surgery (no controls without endometriosis) | Prospective | Laparoscopic | − (reduction both in surgical and expectant management patients) | No |
| Fitzsimmons et al. (1987) | 52 non-ART endometriosis versus 134 non-ART secondary infertility without endometriosis | Retrospective cohort | Laparoscopic | − | No |
| Pittaway et al. (1988) | 100 non-ART endometriosis versus 150 non-ART fertile women versus 100 non-ART tubal disease | Retrospective cohort | Laparoscopic | + | No |
| Candiani et al. (1991) | 241 non-ART endometriosis versus 437 non-ART non-endometriosis | Case–control | Laparoscopic/laparotomic | − | No |
| Marcoux et al. (1997) | 172 patients who underwent operative laparoscopy versus 169 who underwent diagnostic laparoscopy (ART and non-ART) | Prospective randomized | Laparoscopic | − | No (mean age group laparoscopic surgery 31 ± 3 years; mean age group diagnostic laparoscopy 30 ± 4 years) |
| Matorras et al. (1998) | 174 endometriosis versus 174 infertile women without endometriosis (ART and non-ART) | Prospective | Laparoscopic | − | No (same mean age between groups) |
| Gruppo Italiano per lo Studio dell'Endometriosi (1999) | Non-ART endometriosis: 54 who underwent operative laparoscopy versus 47 who underwent diagnostic laparoscopy | Prospective randomized | Laparoscopic | − | No (same age between groups) |
| Omland et al. (2005) | 212 ART endometriosis versus 274 ART non-endometriosis | Retrospective cohort | Laparoscopic | + | Yes |
| Gergolet et al. (2010) | 36 non-ART endometriosis versus 143 non-ART non-endometriosis | Retrospective cohort | Laparoscopic | − | Yes |
| Vercellini et al. (2012) | 419 non-ART endometriosis | Retrospective cohort | Laparoscopic | − (+ in ovarian endometriomata) | Yes |
| Aris (2014) | 784 women with endometriosis, 30 284 without endometriosis (not specified if natural or ART) | Retrospective cohort | Laparoscopic | + | Yes |
| Barbosa et al. (2014) | 20 167 ART endometriosis versus 121 931 ART non-endometriosis | Systematic review and meta-analysis | Ultrasound/laparoscopic/both | + | Not in all the considered studies |
| Hjordt Hansen et al. (2014) | 24 667 endometriosis versus 98 668 non-endometriosis (ART and non-ART categorized) | Prospective cohort | Clinical/laparoscopic | + | No |
| Mekaru et al. (2014) | 49 non-ART endometriosis, 59 non-ART non-endometriosis | Retrospective cohort | Laparoscopic | − | Yes |
| Author, year . | Population (cases versus controls) . | Type of study . | Diagnosis . | Association between spontaneous miscarriage and endometriosis . | Age adjusted . |
|---|---|---|---|---|---|
| Naples et al. (1981) | 65 non-ART infertile endometriosis patients who underwent conservative surgery versus 100 non-ART endometriosis (no control without endometriosis) | Retrospective cohort | Laparotomic/endoscopic | + (reduction of abortion rate in treated patients) | No |
| Olive et al. (1982) | 263 non-ART endometriosis (no controls without endometriosis) | Observational | Laparotomic/endoscopic | NR | No |
| Wheeler et al. (1983) | 39 non-ART mild endometriosis versus 9 non-ART moderate versus 23 non-ART severe (no control without endometriosis) | Retrospective cohort | Laparotomic | + (reduction of abortion rate in mild endometriosis treated patients) | No |
| Groll (1984) | Non-ART endometriosis: 27 untreated versus 28 treated with danazol versus 25 surgically treated (no control without endometriosis) | Retrospective cohort | Endometrial biopsy | + (reduction of abortion rate in treated patients) | No |
| Metzger et al. (1986) | Non-ART endometriosis: 95 who underwent conservative surgery versus 44 no surgery (no controls without endometriosis) | Prospective | Laparoscopic | − (reduction both in surgical and expectant management patients) | No |
| Fitzsimmons et al. (1987) | 52 non-ART endometriosis versus 134 non-ART secondary infertility without endometriosis | Retrospective cohort | Laparoscopic | − | No |
| Pittaway et al. (1988) | 100 non-ART endometriosis versus 150 non-ART fertile women versus 100 non-ART tubal disease | Retrospective cohort | Laparoscopic | + | No |
| Candiani et al. (1991) | 241 non-ART endometriosis versus 437 non-ART non-endometriosis | Case–control | Laparoscopic/laparotomic | − | No |
| Marcoux et al. (1997) | 172 patients who underwent operative laparoscopy versus 169 who underwent diagnostic laparoscopy (ART and non-ART) | Prospective randomized | Laparoscopic | − | No (mean age group laparoscopic surgery 31 ± 3 years; mean age group diagnostic laparoscopy 30 ± 4 years) |
| Matorras et al. (1998) | 174 endometriosis versus 174 infertile women without endometriosis (ART and non-ART) | Prospective | Laparoscopic | − | No (same mean age between groups) |
| Gruppo Italiano per lo Studio dell'Endometriosi (1999) | Non-ART endometriosis: 54 who underwent operative laparoscopy versus 47 who underwent diagnostic laparoscopy | Prospective randomized | Laparoscopic | − | No (same age between groups) |
| Omland et al. (2005) | 212 ART endometriosis versus 274 ART non-endometriosis | Retrospective cohort | Laparoscopic | + | Yes |
| Gergolet et al. (2010) | 36 non-ART endometriosis versus 143 non-ART non-endometriosis | Retrospective cohort | Laparoscopic | − | Yes |
| Vercellini et al. (2012) | 419 non-ART endometriosis | Retrospective cohort | Laparoscopic | − (+ in ovarian endometriomata) | Yes |
| Aris (2014) | 784 women with endometriosis, 30 284 without endometriosis (not specified if natural or ART) | Retrospective cohort | Laparoscopic | + | Yes |
| Barbosa et al. (2014) | 20 167 ART endometriosis versus 121 931 ART non-endometriosis | Systematic review and meta-analysis | Ultrasound/laparoscopic/both | + | Not in all the considered studies |
| Hjordt Hansen et al. (2014) | 24 667 endometriosis versus 98 668 non-endometriosis (ART and non-ART categorized) | Prospective cohort | Clinical/laparoscopic | + | No |
| Mekaru et al. (2014) | 49 non-ART endometriosis, 59 non-ART non-endometriosis | Retrospective cohort | Laparoscopic | − | Yes |
Association between second and third trimesters complications and endometriosis, studies from literature.
| Author, year . | Population (cases versus controls) . | Type of study . | Type of conceivement . | Age-adjusted . | Diagnosis . | PPROM . | Preterm labor . | SGA . | Ante-partum hemorrhage . | Abruptio placentae . | Placenta praevia/accreta . | Pre-eclampsia . | Gestational diabetes . | Cesarean section . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kortelahti et al. (2003) | 137 versus 137 | Case–control | ART | Yes | Laparoscopic | NR | − | − | − | − | − | − | − | − |
| Brosens et al. (2007) | 245 versus 274 | Retrospective case control | ART and non-ART | Yes | Clinical or laparoscopic | NR | NR | NR | NR | NR | NR | − | NR | NR |
| Stephansson et al. (2009) | 13 090 versus 1 429 585 | Retrospective | (Stratification only for preterm birth) 1207 ART endometriosis, 14 688 ART non-endometriosis, 8938 non-ART endometriosis, 1 071 607 non-ART non-endometriosis | No | Clinical or laparoscopic | NR | + | − | + | NR | NR | + | NR | + |
| Hadfield et al. (2009) | 3239 versus 205 640 | Retrospective | 841 ART endometriosis, 4935 ART non-endometriosis, 2398 non-ART endometriosis, 200 705 non-ART non-endometriosis | Yes | Laparoscopic | NR | NR | NR | NR | NR | NR | − | NR | NR |
| Fernando et al. (2009) | 535 ART endometriosis, 95 ART endometrioma 1201 ART infertile, 156 subfertile women, 1260 fertile non-ART controls for all forms of endometriosis, 1140 fertile non-ART controls for ovarian endometriomata. | Retrospective cohort | 535 ART endometriosis, 95 ART endometrioma 1201 ART infertile, 156 subfertile women, 1260 fertile non-ART controls for all forms of endometriosis, 1140 fertile non-ART controls for ovarian endometriomata. | No | Clinical | NR | + | + (ovarian endometrioma ART subgroup) | NR | NR | NR | NR | NR | NR |
| Healy et al. (2010) | 1265 versus 5465 | Retrospective cohort | 1265 ART endometriosis, 5465 ART non-endometriosis | Not specified | Laparoscopic | NR | NR | NR | − | − | + | NR | NR | NR |
| Vercellini et al. (2012) | 419 cases (150 recto-vaginal, 69 ovary and peritoneum, 100 ovary, 100 peritoneum) | Retrospective cohort | Non-ART | Yes | Laparoscopic | NR | − in the ovarian endometrioma group | No significative differences between groups | NR | No significative differences between groups | + in recto-vaginal endometriosis group | No significative differences between groups | NR | + in rectovaginal endometriosis and ovarian plus peritoneal implants |
| Kuivasaari-Pirinen et al. (2012) | 49 versus 26 870 | Retrospective cohort | 49 ART endometriosis, 26 870 non-ART non-endometriosis | Yes | Laparoscopic | NR | + | − | NR | − | + | − | − | NR |
| Benaglia et al. (2012) | 78 versus 156 | Retrospective cohort | 78 ART women with endometrioma, 156 ART women without endometrioma | No | Ultrasound | NR | − | − | NR | − (grouped as severe obstetrical complications) | − (grouped as severe obstetrical complications) | − (grouped as severe obstetrical complications) | NR | − |
| Tobias et al. (2013) | 388 versus 26 451 | Prospective cohort | 388 non-ART endometriosis women, 26 451 non-ART non-endometriosis | Yes | Laparoscopic | NR | NR | NR | NR | NR | NR | NR | − | NR |
| Takemura et al. (2013) | 44 versus 305 | Retrospective cohort | 44 ART endometriosis women, 305 ART non-endometriosis | Yes | Histopathologic/laparoscopic/MRI | NR | NR | NR | NR | NR | + | NR | NR | NR |
| Aris (2014) | 784 versus 30 284 | Retrospective cohort | 784 women with endometriosis, 30 284 without endometriosis (not specified if natural or ART) | Yes | Laparoscopic | NR | − | − | NR | NR | NR | − | − | NR |
| Conti et al. (2014) | 219 versus 1331/97 versus 592 | Retrospective cohort | 219 endometriosis versus 1331 non-endometriosis nulliparous; 97 endometriosis versus 592 non-endometriosis multiparous (ART + non-ART) | Yes | Laparoscopic | + | + | + | NR | NR | NR | − | + | − |
| Mekaru et al. (2014) | 49 versus 59 | Retrospective cohort | 49 non-ART endometriosis, 59 non-ART non-endometriosis | Yes | Laparoscopic | NR | − | − | − | − | NR | − | NR | − |
| Lin et al. (2015) | 249 versus 249 | Retrospective cohort | 249 non-ART endometriosis, 249 non-ART non-endometriosis | Yes | Laparoscopic | NR | + | − | NR | − | + | − | NR | + |
| Stern et al. (2015) | 406 and 590 versus 297 987 | Retrospective | 406 endometriosis ART and 590 endometriosis non-ART versus 297 987 fertile non-ART | No | Clinical or laparoscopic | NR | − (ART group) + (non-ART group) | − (ART group) + (non-ART group) | + | NR (low numbers) | NR (low numbers) | − | − | + |
| Author, year . | Population (cases versus controls) . | Type of study . | Type of conceivement . | Age-adjusted . | Diagnosis . | PPROM . | Preterm labor . | SGA . | Ante-partum hemorrhage . | Abruptio placentae . | Placenta praevia/accreta . | Pre-eclampsia . | Gestational diabetes . | Cesarean section . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kortelahti et al. (2003) | 137 versus 137 | Case–control | ART | Yes | Laparoscopic | NR | − | − | − | − | − | − | − | − |
| Brosens et al. (2007) | 245 versus 274 | Retrospective case control | ART and non-ART | Yes | Clinical or laparoscopic | NR | NR | NR | NR | NR | NR | − | NR | NR |
| Stephansson et al. (2009) | 13 090 versus 1 429 585 | Retrospective | (Stratification only for preterm birth) 1207 ART endometriosis, 14 688 ART non-endometriosis, 8938 non-ART endometriosis, 1 071 607 non-ART non-endometriosis | No | Clinical or laparoscopic | NR | + | − | + | NR | NR | + | NR | + |
| Hadfield et al. (2009) | 3239 versus 205 640 | Retrospective | 841 ART endometriosis, 4935 ART non-endometriosis, 2398 non-ART endometriosis, 200 705 non-ART non-endometriosis | Yes | Laparoscopic | NR | NR | NR | NR | NR | NR | − | NR | NR |
| Fernando et al. (2009) | 535 ART endometriosis, 95 ART endometrioma 1201 ART infertile, 156 subfertile women, 1260 fertile non-ART controls for all forms of endometriosis, 1140 fertile non-ART controls for ovarian endometriomata. | Retrospective cohort | 535 ART endometriosis, 95 ART endometrioma 1201 ART infertile, 156 subfertile women, 1260 fertile non-ART controls for all forms of endometriosis, 1140 fertile non-ART controls for ovarian endometriomata. | No | Clinical | NR | + | + (ovarian endometrioma ART subgroup) | NR | NR | NR | NR | NR | NR |
| Healy et al. (2010) | 1265 versus 5465 | Retrospective cohort | 1265 ART endometriosis, 5465 ART non-endometriosis | Not specified | Laparoscopic | NR | NR | NR | − | − | + | NR | NR | NR |
| Vercellini et al. (2012) | 419 cases (150 recto-vaginal, 69 ovary and peritoneum, 100 ovary, 100 peritoneum) | Retrospective cohort | Non-ART | Yes | Laparoscopic | NR | − in the ovarian endometrioma group | No significative differences between groups | NR | No significative differences between groups | + in recto-vaginal endometriosis group | No significative differences between groups | NR | + in rectovaginal endometriosis and ovarian plus peritoneal implants |
| Kuivasaari-Pirinen et al. (2012) | 49 versus 26 870 | Retrospective cohort | 49 ART endometriosis, 26 870 non-ART non-endometriosis | Yes | Laparoscopic | NR | + | − | NR | − | + | − | − | NR |
| Benaglia et al. (2012) | 78 versus 156 | Retrospective cohort | 78 ART women with endometrioma, 156 ART women without endometrioma | No | Ultrasound | NR | − | − | NR | − (grouped as severe obstetrical complications) | − (grouped as severe obstetrical complications) | − (grouped as severe obstetrical complications) | NR | − |
| Tobias et al. (2013) | 388 versus 26 451 | Prospective cohort | 388 non-ART endometriosis women, 26 451 non-ART non-endometriosis | Yes | Laparoscopic | NR | NR | NR | NR | NR | NR | NR | − | NR |
| Takemura et al. (2013) | 44 versus 305 | Retrospective cohort | 44 ART endometriosis women, 305 ART non-endometriosis | Yes | Histopathologic/laparoscopic/MRI | NR | NR | NR | NR | NR | + | NR | NR | NR |
| Aris (2014) | 784 versus 30 284 | Retrospective cohort | 784 women with endometriosis, 30 284 without endometriosis (not specified if natural or ART) | Yes | Laparoscopic | NR | − | − | NR | NR | NR | − | − | NR |
| Conti et al. (2014) | 219 versus 1331/97 versus 592 | Retrospective cohort | 219 endometriosis versus 1331 non-endometriosis nulliparous; 97 endometriosis versus 592 non-endometriosis multiparous (ART + non-ART) | Yes | Laparoscopic | + | + | + | NR | NR | NR | − | + | − |
| Mekaru et al. (2014) | 49 versus 59 | Retrospective cohort | 49 non-ART endometriosis, 59 non-ART non-endometriosis | Yes | Laparoscopic | NR | − | − | − | − | NR | − | NR | − |
| Lin et al. (2015) | 249 versus 249 | Retrospective cohort | 249 non-ART endometriosis, 249 non-ART non-endometriosis | Yes | Laparoscopic | NR | + | − | NR | − | + | − | NR | + |
| Stern et al. (2015) | 406 and 590 versus 297 987 | Retrospective | 406 endometriosis ART and 590 endometriosis non-ART versus 297 987 fertile non-ART | No | Clinical or laparoscopic | NR | − (ART group) + (non-ART group) | − (ART group) + (non-ART group) | + | NR (low numbers) | NR (low numbers) | − | − | + |
PPROM, preterm premature rupture of membranes; SGA, small for gestational age.
Association between second and third trimesters complications and endometriosis, studies from literature.
| Author, year . | Population (cases versus controls) . | Type of study . | Type of conceivement . | Age-adjusted . | Diagnosis . | PPROM . | Preterm labor . | SGA . | Ante-partum hemorrhage . | Abruptio placentae . | Placenta praevia/accreta . | Pre-eclampsia . | Gestational diabetes . | Cesarean section . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kortelahti et al. (2003) | 137 versus 137 | Case–control | ART | Yes | Laparoscopic | NR | − | − | − | − | − | − | − | − |
| Brosens et al. (2007) | 245 versus 274 | Retrospective case control | ART and non-ART | Yes | Clinical or laparoscopic | NR | NR | NR | NR | NR | NR | − | NR | NR |
| Stephansson et al. (2009) | 13 090 versus 1 429 585 | Retrospective | (Stratification only for preterm birth) 1207 ART endometriosis, 14 688 ART non-endometriosis, 8938 non-ART endometriosis, 1 071 607 non-ART non-endometriosis | No | Clinical or laparoscopic | NR | + | − | + | NR | NR | + | NR | + |
| Hadfield et al. (2009) | 3239 versus 205 640 | Retrospective | 841 ART endometriosis, 4935 ART non-endometriosis, 2398 non-ART endometriosis, 200 705 non-ART non-endometriosis | Yes | Laparoscopic | NR | NR | NR | NR | NR | NR | − | NR | NR |
| Fernando et al. (2009) | 535 ART endometriosis, 95 ART endometrioma 1201 ART infertile, 156 subfertile women, 1260 fertile non-ART controls for all forms of endometriosis, 1140 fertile non-ART controls for ovarian endometriomata. | Retrospective cohort | 535 ART endometriosis, 95 ART endometrioma 1201 ART infertile, 156 subfertile women, 1260 fertile non-ART controls for all forms of endometriosis, 1140 fertile non-ART controls for ovarian endometriomata. | No | Clinical | NR | + | + (ovarian endometrioma ART subgroup) | NR | NR | NR | NR | NR | NR |
| Healy et al. (2010) | 1265 versus 5465 | Retrospective cohort | 1265 ART endometriosis, 5465 ART non-endometriosis | Not specified | Laparoscopic | NR | NR | NR | − | − | + | NR | NR | NR |
| Vercellini et al. (2012) | 419 cases (150 recto-vaginal, 69 ovary and peritoneum, 100 ovary, 100 peritoneum) | Retrospective cohort | Non-ART | Yes | Laparoscopic | NR | − in the ovarian endometrioma group | No significative differences between groups | NR | No significative differences between groups | + in recto-vaginal endometriosis group | No significative differences between groups | NR | + in rectovaginal endometriosis and ovarian plus peritoneal implants |
| Kuivasaari-Pirinen et al. (2012) | 49 versus 26 870 | Retrospective cohort | 49 ART endometriosis, 26 870 non-ART non-endometriosis | Yes | Laparoscopic | NR | + | − | NR | − | + | − | − | NR |
| Benaglia et al. (2012) | 78 versus 156 | Retrospective cohort | 78 ART women with endometrioma, 156 ART women without endometrioma | No | Ultrasound | NR | − | − | NR | − (grouped as severe obstetrical complications) | − (grouped as severe obstetrical complications) | − (grouped as severe obstetrical complications) | NR | − |
| Tobias et al. (2013) | 388 versus 26 451 | Prospective cohort | 388 non-ART endometriosis women, 26 451 non-ART non-endometriosis | Yes | Laparoscopic | NR | NR | NR | NR | NR | NR | NR | − | NR |
| Takemura et al. (2013) | 44 versus 305 | Retrospective cohort | 44 ART endometriosis women, 305 ART non-endometriosis | Yes | Histopathologic/laparoscopic/MRI | NR | NR | NR | NR | NR | + | NR | NR | NR |
| Aris (2014) | 784 versus 30 284 | Retrospective cohort | 784 women with endometriosis, 30 284 without endometriosis (not specified if natural or ART) | Yes | Laparoscopic | NR | − | − | NR | NR | NR | − | − | NR |
| Conti et al. (2014) | 219 versus 1331/97 versus 592 | Retrospective cohort | 219 endometriosis versus 1331 non-endometriosis nulliparous; 97 endometriosis versus 592 non-endometriosis multiparous (ART + non-ART) | Yes | Laparoscopic | + | + | + | NR | NR | NR | − | + | − |
| Mekaru et al. (2014) | 49 versus 59 | Retrospective cohort | 49 non-ART endometriosis, 59 non-ART non-endometriosis | Yes | Laparoscopic | NR | − | − | − | − | NR | − | NR | − |
| Lin et al. (2015) | 249 versus 249 | Retrospective cohort | 249 non-ART endometriosis, 249 non-ART non-endometriosis | Yes | Laparoscopic | NR | + | − | NR | − | + | − | NR | + |
| Stern et al. (2015) | 406 and 590 versus 297 987 | Retrospective | 406 endometriosis ART and 590 endometriosis non-ART versus 297 987 fertile non-ART | No | Clinical or laparoscopic | NR | − (ART group) + (non-ART group) | − (ART group) + (non-ART group) | + | NR (low numbers) | NR (low numbers) | − | − | + |
| Author, year . | Population (cases versus controls) . | Type of study . | Type of conceivement . | Age-adjusted . | Diagnosis . | PPROM . | Preterm labor . | SGA . | Ante-partum hemorrhage . | Abruptio placentae . | Placenta praevia/accreta . | Pre-eclampsia . | Gestational diabetes . | Cesarean section . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kortelahti et al. (2003) | 137 versus 137 | Case–control | ART | Yes | Laparoscopic | NR | − | − | − | − | − | − | − | − |
| Brosens et al. (2007) | 245 versus 274 | Retrospective case control | ART and non-ART | Yes | Clinical or laparoscopic | NR | NR | NR | NR | NR | NR | − | NR | NR |
| Stephansson et al. (2009) | 13 090 versus 1 429 585 | Retrospective | (Stratification only for preterm birth) 1207 ART endometriosis, 14 688 ART non-endometriosis, 8938 non-ART endometriosis, 1 071 607 non-ART non-endometriosis | No | Clinical or laparoscopic | NR | + | − | + | NR | NR | + | NR | + |
| Hadfield et al. (2009) | 3239 versus 205 640 | Retrospective | 841 ART endometriosis, 4935 ART non-endometriosis, 2398 non-ART endometriosis, 200 705 non-ART non-endometriosis | Yes | Laparoscopic | NR | NR | NR | NR | NR | NR | − | NR | NR |
| Fernando et al. (2009) | 535 ART endometriosis, 95 ART endometrioma 1201 ART infertile, 156 subfertile women, 1260 fertile non-ART controls for all forms of endometriosis, 1140 fertile non-ART controls for ovarian endometriomata. | Retrospective cohort | 535 ART endometriosis, 95 ART endometrioma 1201 ART infertile, 156 subfertile women, 1260 fertile non-ART controls for all forms of endometriosis, 1140 fertile non-ART controls for ovarian endometriomata. | No | Clinical | NR | + | + (ovarian endometrioma ART subgroup) | NR | NR | NR | NR | NR | NR |
| Healy et al. (2010) | 1265 versus 5465 | Retrospective cohort | 1265 ART endometriosis, 5465 ART non-endometriosis | Not specified | Laparoscopic | NR | NR | NR | − | − | + | NR | NR | NR |
| Vercellini et al. (2012) | 419 cases (150 recto-vaginal, 69 ovary and peritoneum, 100 ovary, 100 peritoneum) | Retrospective cohort | Non-ART | Yes | Laparoscopic | NR | − in the ovarian endometrioma group | No significative differences between groups | NR | No significative differences between groups | + in recto-vaginal endometriosis group | No significative differences between groups | NR | + in rectovaginal endometriosis and ovarian plus peritoneal implants |
| Kuivasaari-Pirinen et al. (2012) | 49 versus 26 870 | Retrospective cohort | 49 ART endometriosis, 26 870 non-ART non-endometriosis | Yes | Laparoscopic | NR | + | − | NR | − | + | − | − | NR |
| Benaglia et al. (2012) | 78 versus 156 | Retrospective cohort | 78 ART women with endometrioma, 156 ART women without endometrioma | No | Ultrasound | NR | − | − | NR | − (grouped as severe obstetrical complications) | − (grouped as severe obstetrical complications) | − (grouped as severe obstetrical complications) | NR | − |
| Tobias et al. (2013) | 388 versus 26 451 | Prospective cohort | 388 non-ART endometriosis women, 26 451 non-ART non-endometriosis | Yes | Laparoscopic | NR | NR | NR | NR | NR | NR | NR | − | NR |
| Takemura et al. (2013) | 44 versus 305 | Retrospective cohort | 44 ART endometriosis women, 305 ART non-endometriosis | Yes | Histopathologic/laparoscopic/MRI | NR | NR | NR | NR | NR | + | NR | NR | NR |
| Aris (2014) | 784 versus 30 284 | Retrospective cohort | 784 women with endometriosis, 30 284 without endometriosis (not specified if natural or ART) | Yes | Laparoscopic | NR | − | − | NR | NR | NR | − | − | NR |
| Conti et al. (2014) | 219 versus 1331/97 versus 592 | Retrospective cohort | 219 endometriosis versus 1331 non-endometriosis nulliparous; 97 endometriosis versus 592 non-endometriosis multiparous (ART + non-ART) | Yes | Laparoscopic | + | + | + | NR | NR | NR | − | + | − |
| Mekaru et al. (2014) | 49 versus 59 | Retrospective cohort | 49 non-ART endometriosis, 59 non-ART non-endometriosis | Yes | Laparoscopic | NR | − | − | − | − | NR | − | NR | − |
| Lin et al. (2015) | 249 versus 249 | Retrospective cohort | 249 non-ART endometriosis, 249 non-ART non-endometriosis | Yes | Laparoscopic | NR | + | − | NR | − | + | − | NR | + |
| Stern et al. (2015) | 406 and 590 versus 297 987 | Retrospective | 406 endometriosis ART and 590 endometriosis non-ART versus 297 987 fertile non-ART | No | Clinical or laparoscopic | NR | − (ART group) + (non-ART group) | − (ART group) + (non-ART group) | + | NR (low numbers) | NR (low numbers) | − | − | + |
PPROM, preterm premature rupture of membranes; SGA, small for gestational age.
Pathogenic mechanisms involved in poor pregnancy outcomes
In human pregnancy, the implantation of the blastocyst into a receptive endometrium, successful placentation and remodeling of the uterine vasculature require the integration of a number of critical stages. Implantation represents a dialogue between the mother and the fetus and signals from the embryo also direct the remodeling processes of the uterus allowing adhesion and invasion. The placenta develops into a branching villous structure with differentiation of trophoblast types that differ in function. The multinucleated syncytiotrophoblast layer provides the barrier to maternal blood, regulating oxygen and protein transport (Dey et al., 2004; Harris, 2010). Extravillous trophoblasts (EVT) colonize the decidua and remodel the uterine spiral arteries as far as the first third of the myometrium. EVT establish direct contact with maternal leukocyte populations, an increase in decidual natural killer cells (Moffett and Colucci, 2014) and recruitment of regulatory T cells (Nancy and Erlebacher, 2014) occur, creating a tolerogenic niche in which the semiallogenic fetus can develop (Arck and Hecher, 2013). As our understanding of these complex interactions increases, it is clear that dysfunctions may occur in several stages of the process: a shallow trophoblast invasion, a reduced protease expression, an altered leukocyte composition within the decidua, or spiral arteries exhibiting resistance to colonization. Pregnancy complications are thought to be dependent upon the dysregulation of these events. Lessons from studies in transgenic mice may be very informative in this regard. Transgenic mouse models moved the field toward a mechanistic understanding of the roles of many genes in uterine receptivity and implantation. Indeed, several knockout mouse models, such as mice missing the genes determinant for on-time implantation, namely Pla2g4a, Lpar3 and adrenomedullin, or with uterine deletion of Trp53 and Klf5, helped to define propagation of early defects during the course of pregnancy. Indeed, these models would suggest that defects during the peri-implantation period can perpetuate throughout pregnancy and that any adverse ripple effect in early stages may cause late-stage disturbances (Cha et al., 2012). Deferred implantation past the window of receptivity can result in misguided embryo placement and implantation resulting in placenta previa, ectopic placentation (placenta accreta) or placental insufficiency resulting in intrauterine growth restriction (IUGR) and/or pre-eclampsia. Implantation beyond the normal window can also give rise to spontaneous abortion and recurrent pregnancy loss. Premature decidual senescence can lead to preterm birth and fetal death whereas shallow trophoblast invasion into maternal deciduas and/or blood vessels can lead to pre-eclampsia (Cha et al., 2012). It should be kept in mind that there are important differences in pregnancy between mice and humans so caution should be applied when using mouse models to understand pathways of human immune adaptation to pregnancy. On the other hand, based on this model, it is not so surprising that endometriosis as a disease characterized by alterations not only in the ectopic but also in the eutopic endometrium might be associated with poor pregnancy outcomes (Fig. 3).
Potential explanations of endometriosis-related adverse pregnancy outcomes based on the model by Cha et al. (2012) implying late-stage disturbances in pregnancy following adverse ripple effect in early stages. ROS, reactive oxygen species; IUGR, intrauterine growth restriction; JZ, junctional zone.
Disturbances in women affected potentially related to defects during the peri-implantation period that may perpetuate throughout pregnancy include: Overall, these alterations of the local endometrial environment in patients with endometriosis may underlie the various mechanisms suggested as the basis of the model, implying late-stage disturbances following an adverse ripple effect in the early stages (Cha et al., 2012). Progesterone resistance and inadequate uterine contractility would be potentially involved in deferred implantation and misguided embryo placement; inflammation, activation of the free radical metabolism and alteration of the JZ might favor a shallow trophoblast invasion and preterm birth (Fig. 3).
the endometrial resistance to selective actions of progesterone, which is manifested by perturbations in a number of downstream progesterone target genes (Burney et al., 2007). Progesterone normally triggers a uterine endometrial response characterized by transformation of stromal cells into specialized decidual cells and induces an embryo receptive phenotype. As a consequence of progesterone resistance, genes critical to embryo implantation, such as prolactin FOXOA, IGF-II for decidual response or glycodelin for embryo implantation, are dysregulated in the endometrium of affected women (Vercellini et al., 2014a);
the inflammatory process whose consequences can be manifested both at endometrial (Benagiano et al., 2014) and systemic level (Gentilini et al., 2011). It should be considered that the environmental insult represented by an elevation in maternal systemic inflammation has been long proposed as the cause of pre-eclampsia or preterm birth. Studies in rodents support the systemic maternal inflammatory response and placental ischemia hypothesis as critical for pre-eclampsia development although in human pregnancy the elevation in pro-inflammatory status does not seem to precede the onset of pre-eclampsia (Ahmed and Ramma, 2015);
inadequate uterine contractility. Uterine contractions occur throughout the menstrual cycle in the non-pregnant state and throughout gestation. The contractions observed during the menstrual cycle have been termed ‘endometrial waves’ and appear to involve only the sub-endometrial layer of the myometrium. In the early follicular phase following menstruation, contractile waves occur once or twice per minute and last 10–15 s. As ovulation approaches, the frequency increases to 3–4 per minute. During the luteal phase, the frequency and amplitude decrease perhaps to facilitate implantation. In the absence of blastocyst implantation, the contraction frequency remains low but the amplitude increases dramatically producing labor-like contractions at the time of menstruation (Aguilar and Mitchell, 2010). Compared with controls, patients with endometriosis have been shown to have uterine contractions with higher frequency, amplitude and basal pressure tone;
the endometrial excessive activation of free radical metabolism. An increased release of reactive oxygen species and an increased expression of enzymes leading to the accumulation of free radicals in the cells have been demonstrated in the endometrium of women with endometriosis (Benagiano et al., 2014). Oxidative stress has been postulated as one of the central phenomena involved in maternal endothelial dysfunction with consequent negative obstetric outcomes (Sánchez-Aranguren et al., 2014);
the alteration in the uterine junctional zone (JZ) which is the highly specialized inner third of the myometrium demonstrated to be greater in thickness in women with endometriosis compared with non-affected patients (Exacoustos et al., 2013; Benagiano et al., 2014). A normal placentation process is characterized by a full conversion of the spiral arteries into large utero-placental vessels at the level of the JZ. Defective placentation is characterized by an absent or incomplete remodeling of these arteries and the primary site of the vascular abnormalities responsible for defective placentation has been suggested to lie in the JZ (Lazzarin et al., 2014);
Miscarriage
Miscarriage is defined as the spontaneous end of pregnancy prior to 20 weeks of gestation and is defined as ‘recurrent’ when at least three episodes occur (Stirrat, 1990). This paragraph focuses on past and current studies on the relation between endometriosis and miscarriage, subdividing them on the basis of their design (first, the studies that have evaluated the pre- and post-treatment incidence of spontaneous abortion during post-surgical follow-up evaluation; then, controlled and uncontrolled retrospective studies, prospective studies and a meta-analysis). From the early 1980s, great attention was devoted to try to assess a possible relationship between endometriosis and miscarriage, but the first studies had some limits (Naples et al., 1981; Olive et al., 1982; Wheeler et al., 1983; Groll, 1984). In particular, many of them were retrospective, without controls, with relatively short follow-up, enrolling a population of women with endometriosis from a pool of infertile women. Supporting the idea that medical or surgical therapy for endometriosis may reduce spontaneous abortion rate, Groll (1984) observed that while 52% of an untreated group of patients with endometriosis aborted, this figure was reduced to 12% in a surgically treated group and to 7% in patients treated with danazol. This finding was supported by other authors (Naples et al., 1981). On the contrary, Wheeler et al. (1983) observed that the preoperative to post-operative abortion rates varied significantly only for patients with mild endometriosis but not for patients with moderate and severe endometriosis (Wheeler et al., 1983). Metzger et al. (1986) compared women with endometriosis who chose an expectant management to a surgically treated group. A significant reduction of spontaneous abortion rate was found both after surgery and after the expectant management, thus suggesting that endometriosis may not be the causative factor of spontaneous abortion. In the retrospective evaluation of 350 women by Pittaway et al. (1988), group I consisted of 100 consecutive women with secondary infertility and surgically confirmed endometriosis, group 2 of 150 fertile women undergoing reversal of tubal sterilization and group 3 of 100 women with secondary infertility due to tubal disease. The frequency of spontaneous abortion was significantly higher in the endometriosis group (38%) than in both the fertile non-endometriosis group (10%) and the infertile non-endometriosis group (19%) (P-values: I versus II P < 0.001, I versus III P < 0.01, II versus III P < 0.001). When the pregnancy outcomes after treatment were examined, there was no difference between the endometriosis group and the infertile non-endometriosis group (11 versus 12%, respectively), but in the endometriosis group there was a significant decrease in the abortion rate comparing pre- and post-surgical treatment. Two prospective RCTs are available in the literature studying the effect of the laparoscopic surgical treatment on reproductive performance, including miscarriage, in patients with minimal to mild endometriosis (Marcoux et al., 1997; Gruppo Italiano per lo Studio dell'Endometriosi, 1999). Neither study was able to show a significant reduction in miscarriage rate after laparoscopic treatment.
We have identified seven retrospective studies on the relation between miscarriage and endometriosis irrespective of the treatment (Fitzsimmons et al., 1987; Candiani et al., 1991; Omland et al., 2005; Gergolet et al., 2010; Vercellini et al., 2012; Aris, 2014; Mekaru et al., 2014) and only two of them observed an association. In the studies finding an association, pregnancies by ART procedures were not excluded. More specifically, a retrospective cohort study including only IVF/ICSI pregnancies compared a group of stage I endometriosis women (n = 212) to a group of patients with tubal factor infertility (n = 540) and unexplained infertility (n = 274) as controls. When adjusted for age and BMI, the probability of first trimester miscarriage remained higher both for the endometriosis group [adjusted odds ratio (OR) = 1.96, 95% confidence interval (CI) 1.25–3.09; P = 0.004] and the tubal factor infertility group (adjusted OR = 1.88, 95% CI 1.29–2.73; P = 0.001) compared with the unexplained infertility group (Omland et al., 2005). In addition, a retrospective cohort study on both spontaneous and ART pregnant women from the Eastern Township of Canada compared women with endometriosis (n = 784) versus a non-affected population (n = 30 284). An increased rate of spontaneous abortion in women with endometriosis (2.8%) versus controls (1.5%; OR = 1.89, 95% CI 1.23–2.93; P = 0.005) was found. It is interesting to note that in this study a significant association between endometriosis and stillbirth was also reported, supporting the idea that endometriosis may represent a permanent threat throughout the whole pregnancy (Aris, 2014). Vercellini and colleagues retrospectively recruited only nulligravid women with natural conception subdividing them into four groups according to the type of endometriosis: rectovaginal (n = 150), ovarian and peritoneal (n = 69), only ovarian (n = 100) and only peritoneal (n = 100). At crude analysis, the frequency of miscarriage was significantly higher in women with ovarian endometriomas and peritoneal lesions but the difference was no longer significant at age-adjusted analysis (P = 0.08). When pooling the two groups of women with ovarian endometriomas, miscarriage was observed in 26.6% (45/169) of cases compared with 16.8% (42/250) in the remaining women (adjusted OR = 1.70, 95% CI 1.04–2.8; P < 0.05). Although the incidence observed in this cohort did not appear considerably increased compared with that in the general Italian population of corresponding age, a trend toward a higher miscarriage frequency was thus observed in women with ovarian endometriomas (Vercellini et al., 2012). The other four retrospective studies did not find any trend of association. Suggesting that the high frequency of spontaneous abortion may be a characteristic seen in populations with infertility arising for various reasons, and not to endometriosis per se, Fitzsimmons et al. (1987) used a population of secondary infertile women with and without endometriosis, laparoscopically confirmed. The spontaneous abortion rate before evaluation was similar in the two groups. Candiani et al. (1991) conducted a case–control study on 241 women with laparoscopically or laparotomically confirmed endometriosis versus 437 women in whom the disease was ruled out, as a control group. No relation emerged with a history of miscarriage [Relative ratio (RR) = 0.8, 95% CI 0.5–1.4]. The most recent study, enrolling only natural pregnancies, compared 49 laparoscopically confirmed endometriosis women versus 59 without the disease and no significant difference in the incidence of miscarriage was found (Mekaru et al., 2014). An uncommon point of view was provided by Gergolet et al. (2010) who evaluated whether endometriosis was an additional risk factor for miscarriage in 179 patients (36 cases and 143 controls) with septate uterus before and after metroplasty. Only natural pregnancies were taken in account. Before and after metroplasty, the incidence of miscarriage was not significantly different between the two groups (Gergolet et al., 2010).
Two prospective non-randomized studies were conducted on this topic (Matorras et al., 1998; Hjordt Hansen et al., 2014). Matorras et al. (1998) compared 174 infertile women with laparoscopically diagnosed endometriosis and 174 infertile women in which endometriosis was laparoscopically-ruled out in the same period of time. Abortion rate was 7.47% (13/174) in the endometriosis group and similar to the rate of 5.74% (10/174) of the infertile group without endometriosis (RR = 1.32, 95% CI 0.53–3.36). Opposite conclusions were drawn by Hjordt Hansen et al. (2014). They have compared women with endometriosis (n = 24 667) to a population of unaffected subjects without endometriosis (n = 98 668) in a Danish cohort. A higher rate of miscarriage in the case group was detected persistently through the whole study period (15 years), which is the longest follow-up done so far. In particular, overall RR for miscarriages was 1.2 (95% CI 1.2–1.29) with a 16.3% rate of miscarriage in women with endometriosis versus 13% in controls. The respective RRs were 1.21 (95% CI 1.17–1.26) for natural pregnancies and 4.34 (95% CI 3.42–5.50) for the ART subgroup (Hjordt Hansen et al., 2014).
In 2014, Barbosa et al. conducted a systematic review and meta-analysis on this topic comparing the ART outcome in women with and without endometriosis and at different stages of the disease (Barbosa et al., 2014). Ninety-two studies were included in the review and 78 in the meta-analysis: 20 167 women with endometriosis were compared with 121 931 unaffected subjects and 1703 women with Stage III/IV endometriosis were compared with 2227 women with Stage I/II endometriosis. Miscarriage RR was 1.31 (95% CI 1.07–1.59) for the comparison of women with endometriosis versus women without. Thus, a higher risk of miscarriage was observed in women with endometriosis, although it did not seem to be different in women with different stages of the disease. According to the authors, however, these findings should be interpreted with caution due to the very low quality of the available evidence. In conclusion, according to the current literature, there is insufficient evidence supporting an association between endometriosis and miscarriage.
Hypertensive disorders and pre-eclampsia
Kortelahti et al. (2003) were the first to assess the potential association between endometriosis and pre-eclampsia. No correlation was detected, but this study was conducted on a small population and the definition of pre-eclampsia reported was not in line with the definition by the American College of Obstetrician and Gynecologists (ACOG). In 2007, a retrospective case–control study set at the University of Ghent IVF centre compared the incidence of pre-eclampsia and pregnancy-induced hypertension (PIH) following the clinical and/or laparoscopic diagnosis of endometriosis-associated infertility (case group n = 245 pregnancies) or following treatment for infertility, in particular for male factor (control group n = 274 pregnancies). The incidence of pre-eclampsia was significantly lower in the group of women affected by endometriosis (0.8%) when compared with control group (5.8%) (OR = 7.5, 95% CI 1.7–33.3; P = 0.002). Analysis of the obstetric outcomes in the subgroup of patients with laparoscopic diagnosis confirmed the lower risk of pre-eclampsia in the case (1.2%) versus control (7.4%) group (OR = 6.6, 95% CI 1.2–37; P = 0.032). PIH occurred in 3.5 and 8.7% of case and control pregnancies, respectively (OR = 2.6, 95% CI 1.2–6.0; P = 0.018) (Brosens et al., 2007). A limitation of this study was that anamnestic data were collected by postal questionnaires and not by direct questions. All the subsequent studies (Hadfield et al., 2009; Kuivasaari-Pirinen et al., 2012; Vercellini et al., 2012; Aris, 2014; Conti et al., 2014; Mekaru et al., 2014; Lin et al., 2015; Stern et al., 2015) did not find an association between endometriosis and pre-eclampsia, except for a large Swedish study by Stephansson et al. (2009), who observed an increased risk for pre-eclampsia among women with endometriosis (adjusted OR = 1.13, 95% CI 1.02–1.26; P < 0.05). This nationwide study, including 1 442 675 singleton births, of whom 13 090 were from women affected by endometriosis, presented two limitations: the analysis was stratified by ART only for the ‘preterm birth’ outcome, and not testing pre-eclampsia; furthermore, the Swedish medical birth register did not allow verification of whether the endometriosis diagnosis was always histologically confirmed after surgery. The potential correlation between endometriosis and pre-eclampsia is still matter of debate (Table IX).
Placenta praevia
A higher incidence of placenta praevia has been almost consistently demonstrated in women with endometriosis by some groups, despite the very different study designs employed (Table IX). In the large Swedish nationwide cohort study by Stephansson et al. (2009) including 1 442 675 singleton births, endometriosis was shown to be associated with placental complications but no distinction has been made for the various placental abnormalities. Moreover, no stratification has been performed in relation to ART conceptions. In 2012, Vercellini and co-workers retrospectively assessed pregnancy outcomes in 419 women who achieved a first spontaneous singleton pregnancy after surgery for endometriosis and stratified the results obtained by endometriosis localization (Vercellini et al., 2012). No cases of placenta praevia were observed in patients with ovarian endometriomas only while an almost 6-fold increase in risk has been found in women with rectovaginal endometriosis compared with all women with ovarian and peritoneal lesions (OR = 5.81, 95% CI 1.53–22.03; P = 0.03).
Most of the available evidence derives, however, from results of ART procedures. In a small case–control study involving women with and without endometriosis matched for parity and ART procedures, no difference in placenta praevia incidence has been found. However, the study had probably a very limited statistical power, as the number of placental abnormalities reported was very low (Kortelahti et al., 2003). Conversely, two retrospective cohort studies comparing pregnancy outcomes of ART singleton pregnancies with those of natural pregnancies found higher rates of placenta previa in the ART groups and in the various subgroups this was particularly evident for patients with endometriosis (adjusted OR = 1.65, 95% CI 1.18–2.32 and 6.1% versus 0.6 in the general population group; P < 0.005) (Healy et al., 2010; Kuivasaari-Pirinen et al., 2012). Finally, a 4.1% incidence of placenta previa has been found in a Japanese retrospective analysis of 318 pregnancies conceived by ART but while age, parity, previous abortions, ovulatory disorders were not related with the placental abnormality, endometriosis was strongly associated (OR = 15.1, 95% CI 7.6–500; P = 0.0001) (Takemura et al., 2013).
Also a recent retrospective cohort study comparing 249 women with endometriosis and 249 women without endometriosis, all achieving singleton pregnancies naturally, showed a higher risk of placenta praevia in the subgroup of women affected by the disease (adjusted OR = 4.51, 95% CI 1.23–16.50; P = 0.023) (Lin et al., 2015).
Obstetric hemorrhages (abruptio placentae, ante- and post-partum bleeding)
The studies that have addressed the potential association between endometriosis and placental complications did consistently exclude a higher incidence of placental abruption among women affected by the disease (Kortelahti et al., 2003; Healy et al., 2010; Kuivasaari-Pirinen et al., 2012; Vercellini et al., 2012; Lin et al., 2015) (Table IX). Similarly, women with endometriosis do not seem to be at risk for developing ante-partum hemorrhages (Kortelahti et al., 2003; Healy et al., 2010) (Table IX). However, although in the large Swedish nationwide study by Stephansson et al. (2009) no distinction has been made between antepartal bleeding and placental complications, in a subsequent review one of the authors (Falconer, 2013) referred to an increased risk of antepartal bleeding in women with endometriosis (adjusted OR = 1.76, 95% CI 1.56–1.99). Further data are needed in this regard. The retrospective cohort study by Healy and co-workers was the only report addressing the risk of post-partum hemorrhage in various categories of patients undergoing ART and endometriosis was shown to be associated with an increased risk (adjusted OR = 1.28, 95% CI 1.06–1.56). Furthermore, in a retrospective study conducted on ART records in Massachusetts, USA, comparing 996 women affected by endometriosis, subdivided into 406 who achieved pregnancy by ART and 590 who did not undergo ART procedures, to 297 987 fertile women who conceived naturally, an increased rate of uterine bleeding was reported, although it was not specified if ante or post-partum bleeding (P = 0.01) (Stern et al., 2015).
Preterm birth
Preterm birth is a cause of neonatal morbidity but potentially of future adult diseases as well (Schieve et al., 2004). Different studies have investigated the possible linkage between endometriosis and preterm birth (Table IX). As previously mentioned, the pathophysiological link between endometriosis and preterm birth has been suggested to be mainly represented by increased local inflammation (Petraglia et al., 2012). Indeed, an inflammatory state associated with the presence of ectopic tissue leading to a derangement of the endometrial physiology may influence the decidua/trophoblast interaction which is influenced by the same inflammatory factors and whose imbalance is thought to be a possible pathogenic event in preterm birth.
Two main studies from Australia and Sweden, both published in 2009, demonstrated higher rates of preterm birth in women with endometriosis (Fernando et al., 2009; Stephansson et al., 2009). Fernando and coworkers (2009) conducted a large retrospective cohort study with the primary outcome of reporting preterm birth and small for gestational age (SGA) rates from ART patients with ovarian endometrioma (n = 95) compared with a control group of non-ART fertile women randomly selected from the general population (n = 1140). This study found that preterm birth was increased in the ovarian endometrioma group (adjusted OR = 1.98, 95% CI, 1.09–3.62). Another subgroup including ART women with endometriosis but without ovarian endometrioma (n = 535) was analyzed and no increased risk for preterm birth was identified (adjusted OR = 1.03, 95% CI, 0.70–1.53) (Fernando et al., 2009). The nationwide Swedish study, including 1 442 675 singleton births, assessed the association between adverse pregnancy outcome, ART and a previous diagnosis of endometriosis. The comparison between patients with (n = 8922) and without endometriosis demonstrated higher risks of preterm birth in women affected (adjusted OR = 1.33, 95% CI, 1.23–1.44). Conversely, different results were observed in a 12-year cohort study assessing the impact of endometriosis on adverse pregnancy outcomes in Eastern Townships of Canada. This study included 31 068 women who had a pregnancy between 1997 and 2008 and among these patients 6.749 (21.7%) had adverse pregnancy outcomes. Seven hundred and eighty-four women (2.5%) had endometriosis and 183 (23.3%) had both endometriosis and adverse pregnancy outcomes. The incidence of preterm birth in women with endometriosis (10.5%) was higher than in those without (9.2%); however, this trend was not statistically significant (OR = 1.15; 95% CI 0.91–1.45) (Aris, 2014). In 2015, Stern et al. conducted a retrospective study on ART records comparing 996 women affected by endometriosis (subdivided into 406 who achieved pregnancy by ART and 590 who did not undergo ART procedures) to 297 987 fertile women who conceived naturally. An increased rate of preterm labor was found for the endometriosis non-ART group (adjusted OR = 1.66, 95% CI 1.26–2.18; P < 0.05), but, surprisingly, not for the endometriosis ART group (adjusted OR = 1.22, 95% CI 0.90–1.66) (Stern et al., 2015).
Another six minor studies assessed the relationship between endometriosis and preterm birth in women achieving pregnancy naturally (Mekaru et al., 2014; Lin et al., 2015) or by ART (Kortelahti et al., 2003; Benaglia et al., 2012; Kuivasaari-Pirinen et al., 2012; Conti et al., 2014) (Table IX). A retrospective study was undertaken to evaluate pregnancy outcome in women with a primary diagnosis of endometriosis who conceived naturally. Forty-nine of the study participants had endometriosis and 59 participants did not have the disease and no statistical difference was found in the rate of preterm birth between the two groups (7.5 versus 8.3%, respectively) (Mekaru et al., 2014). On the contrary, a recent Chinese retrospective study comparing 249 women with endometriosis versus 249 controls who conceived naturally reported a higher risk of preterm labor (adjusted OR = 2.42, 95% CI 1.05–5.57; P = 0.038) (Lin et al., 2015).
No statistically significant difference was also found in a case–control study which analyzed the obstetric outcome of 137 women with endometriosis and 137 controls matched as regards to IVF procedures (adjusted OR = 0.84; 95% CI 0.38–1.88) (Kortelahti et al., 2003). Benaglia et al. (2012) reported similar findings in a multicenter retrospective cohort study that included singleton IVF pregnancies from 78 women with endometriomas at the time of IVF compared with 156 patients without endometriomas. Actually, the rate of preterm birth was lower in women with endometriomas (7%) than in those without (14%) but this difference was not statistically significant (adjusted OR = 0.47; 95% CI 0.14–1.54). A Finnish study assessed the role of etiology on IVF pregnancy outcomes in a retrospective cohort study comparing the outcomes of IVF singleton pregnancies with those of natural pregnancies in the general Finnish population. The study group consisted of 255 women with births resulting from singleton IVF pregnancies distinguished as six subgroups according to the following causes of infertility: anovulation (n = 68), endometriosis (n = 49), male factor (n = 43), tubal factor (n = 38), polycystic ovary syndrome (n = 27), and unexplained infertility (n = 30). The reference group consisted of 26 870 women who conceived naturally. Focusing on women with endometriosis, this study concluded that these patients had higher rates of preterm birth (18.6%) than the reference group (6.3%; adjusted OR = 3.25; 95% CI 1.5–7.1) (Kuivasaari-Pirinen et al., 2012). A multicenter, observational and cohort Italian study evaluated pregnancy, delivery and neonatal outcome in singleton primiparous versus multiparous women with/without endometriosis. The study group consisted of women who had a surgically confirmed history of endometriosis (n = 316), distinguishing primiparas (n = 219) and multiparas (n = 97). The control group consisted of 1923 women without endometriosis subdivided into primiparas (n = 1331) and multiparas (n = 592). The rate of preterm birth in primiparous women with endometriosis (17.8%) was higher than in those without (8.8%; adjusted OR = 2.24; 95% CI 1.46–3.44), while no difference was reported between multiparous with or without endometriosis (8.2 versus 8.8%, respectively) (Conti et al., 2014). Again, although there is some evidence suggestive of an association between endometriosis and preterm birth, it should be considered that the identified studies are characterized by marked differences in exposure categorizations, analytic approaches, disease phenotypes, choice of controls and in general methodological design. This makes it difficult to draw definitive conclusions.
SGA
A SGA baby is defined as an infant weighing less than the tenth centile on comparison of the birthweight with that expected for the same gestational age (American College of Obstetricians and Gynecologists, 2013a, b). Great interest has been devoted to the association between endometriosis and the risk of SGA babies after 2009, when Fernando et al. (2009) published the results of a retrospective cohort study on 4387 women who delivered a singleton baby (Table IX). This cohort included 535 ART patients affected by non-ovarian endometriosis, 95 ART patients with ovarian endometriomata, 1201 ART patients with other etiologies of infertility, 156 subfertile women, 1260 fertile women matched for age and baby's year of birth at a ratio of 1:2 with patients affected by all forms of endometriosis, and 1140 fertile controls matched for age and baby's year of birth at a ratio of 1:12 with patients affected by ovarian endometriotic cysts. The authors failed to document an association between endometriosis in general and SGA babies. However, a statistically significant increased risk for a SGA baby (adjusted OR = 1.95, 95% CI 1.06–3.60; P < 0.05) was found in the ovarian endometrioma ART group when compared with patients who underwent ART for non-endometriosis causes of infertility. Furthermore, the ART patients with ovarian endometrioma had a statistically increased likelihood of having a SGA baby when compared with those with other forms of endometriosis (adjusted OR = 1.99, 95% CI 1.04–33.81; P < 0.05) (Fernando et al., 2009). In 2014, Conti et al. (2014) published the results of a multicenter, cohort study including a group of Caucasian singleton pregnant women (n = 2239) studied after delivery during the post-partum hospitalization. The study group consisted of women who presented a history of histologically confirmed endometriosis (n = 316) distinguished for the first time between primiparous (n = 219) and multiparous (n = 97). Sixty percent of women were affected by an endometrioma. As control group (n = 1923) all other women were included, subdivided as well into primiparous (n = 1331) and multiparous (n = 592). Both primiparous and multiparous women with endometriosis delivered significantly more often SGA babies (OR = 2.72, 95% CI 1.46–5.06; P = 0.002 and OR = 2.93, 95% CI 1.28–6.67; P = 0.001, respectively) (Conti et al., 2014). In 2015 Stern and co-workers conducted a large retrospective study on Massachusetts, USA, records comparing 996 women affected by endometriosis (subdivided into 406 who achieved pregnancy by ART and 590 who did not undergo ART procedures) to 297 987 fertile women who conceived naturally. An increased rate of SGA babies was found in the endometriosis non-ART group (adjusted OR = 1.46, 95% CI 1.07–1.99; P < 0.05), but not in the endometriosis ART group (adjusted OR = 0.97, 95% CI 0.70–1.33) (Stern et al., 2015). Multivariate analysis did not show any influences of ART on pregnancy, delivery and neonatal outcomes but sub-analysis according to the forms of the disease was not performed.
Other retrospective analyses failed to find an association between SGA babies and endometriosis (Benaglia et al., 2012; Aris, 2014; Mekaru et al., 2014; Lin et al., 2015) (Table IX). In 2012, Benaglia and coworkers compared 78 pregnant women with endometriomas at the time of IVF and 156 patients without endometriomas who achieved pregnancy through IVF, observing a trend for a lower risk (Benaglia et al., 2012). Aris (2014) enrolled 784 women with a laparoscopic diagnosis of endometriosis versus 30 284 controls both from natural pregnancy or ART. A higher but not significant prevalence of SGA babies (2.3 versus 2.0%, respectively) and low birthweight babies (below 2500 g, 7.3 versus 6.3%, respectively) was found in endometriosis compared with unaffected women. In line with these findings, an Italian (Vercellini et al., 2012) and a large Swedish retrospective cohort study (Stephansson et al., 2009) failed to find an association between endometriosis and SGA babies. The Italian group who evaluated natural singleton pregnancies (n = 419) after surgery for endometriosis concluded that the risk of SGA and low birthweight babies, which was not significantly different among the different forms of endometriosis, was similar to the national population-based estimates. Similarly, the large Swedish nationwide study by Stephansson et al. (2009) including 1 442 675 singleton births did not find a correlation between endometriosis and the risk of SGA babies. Thus, data regarding a potential association between endometriosis and SGA baby risk are still controversial. Different study designs and different control groups may explain the underlying debate. Furthermore, all the studies addressing this topic considered only SGA babies defined according to American College of Obstetricians and Gynecologists (2013a, b), but the differentiation between SGA babies and IUGR infants, defined as an estimated fetal weight less than the third centile or less than the tenth and associated with pathological Doppler cerebro-placental ratio, umbilical artery or uterine arteries flows (Figueras and Gratacos, 2014), has never been considered.
Gestational diabetes mellitus
The association between a history of infertility and risk for gestational diabetes mellitus (GDM) was prospectively assessed among 40 773 eligible incident pregnancies in the US Nurses' Health Study II cohort from 1989 and 2001 (Tobias et al., 2013) (Table IX). Every 2 years, questionnaires were distributed in order to update information about lifestyle changes, health-related and reproductive outcomes. A history of infertility before pregnancy was reported by 5497 (20.5%) participants and was significantly associated with a 39% greater risk of GDM (adjusted RR = 1.39, 95% CI 1.24–1.57; P < 0.001). However, endometriosis (9.0%, n = 388) was not found to be associated with GDM risk (adjusted RR = 1.27, 95% CI 0.70–2.31; P = 0.43). These results were adjusted for several common risk factors, such as BMI, weight gain and lifestyle changes. This was the first study evaluating GDM in which infertility was categorized by various underlying reasons.
Nevertheless, the majority of the relevant literature about this topic derives from investigations on ART populations (Table IX). A case–control study compared 137 women with endometriosis versus 137 controls matched for IVF procedures and parity. The incidence of GDM was exactly the same in the two groups (Kortelahti et al., 2003). A retrospective cohort study compared IVF singleton pregnancies (n = 255) to natural pregnancies (n = 26 870) in the general Finnish population. No significant association between endometriosis and GDM was reported (14.3% in the endometriosis group versus 10.6% in the controls) (Kuivasaari-Pirinen et al., 2012). Furthermore, a retrospective cohort study on both natural and ART pregnant women from the Eastern Township of Canada reported similar findings. Women with endometriosis (n = 784) exhibited a lower but not significantly different incidence when compared with women without endometriosis (n = 30 284) (OR = 0.81, 95% CI 0.53–1.25) (Aris, 2014). In a retrospective study comparing 996 women affected by endometriosis, further subdivided into 406 who achieved pregnancy by ART and 590 who did not undergo ART procedures, to 297 987 fertile women who conceived naturally, an association was not found, both for ART (adjusted OR 0.93, 95% CI 0.62–1.39) and non-ART groups (adjusted OR = 1.08, 95% CI 0.75–1.57) (Stern et al., 2015). In contrast with the previous findings, the recent retrospective cohort study on both natural and ART pregnancies by Conti et al. (2014) found an association between endometriosis and GDM. A group of Caucasian singleton pregnant women (n = 2239) was studied after delivery. Primiparous pregnant women affected by endometriosis (n = 219) showed a 13.3% rate of GDM versus 6.7% in unaffected primiparous controls (n = 1331) (OR = 2.13; 95% CI 1.32–3.44; P = 0.002); however this observation could not be replicated in multiparous women (Conti et al., 2014). Therefore, except for a single study, consistency exists in the literature for a lack of an association between endometriosis and GDM (Table IX).
Cesarean delivery
In four out of the 7 studies that evaluated the association between endometriosis and CD, a significant correlation could be found (Stephansson et al., 2009; Vercellini et al., 2012; Lin et al., 2015; Stern et al., 2015) (Table IX). In particular, according to the Swedish cohort study by Stephansson et al. (2009) including 1 442 675 singleton births, CD was more common among women with endometriosis compared with women without endometriosis, and the risk was highest for pre-labor CD (adjusted OR = 1.64, 95% CI 1.54–1.75; P < 0.05), than for emergency CD (adjusted = OR 1.18, 95% CI 1.10–1.27; P < 0.05). ORs have been adjusted for maternal age, smoking, BMI, parity, years of formal education and child's year of birth, but not for ART procedures (Stephansson et al., 2009). This 2-fold increase of CD in women with endometriosis was explained by the potential increased number of episodes of ante-partum hemorrhage in these women (Falconer, 2013). In the Italian study by Vercellini et al. (2012) who retrospectively assessed pregnancy outcomes after surgery for different forms of endometriosis, a total of 212 (65.4%) women delivered vaginally and 112 (34.6%) by elective or emergency Cesarean section. The frequency of CD was significantly higher (P = 0.01) in the groups with ovarian endometriomas plus peritoneal implants (40.4%) and with rectovaginal endometriosis (42.9%) compared with the groups with ovarian (20.5%) or peritoneal endometriosis only (31.8%). In all study groups, the main indication for CD was fetal distress (24%), followed by breech presentation (13%) and dystocia (13%) (Vercellini et al., 2012). Moreover, a retrospective cohort study conducted on a Chinese population on 249 women affected by endometriosis versus 249 controls, excluding pregnancies achieved by ART procedures and adjusting for maternal age, showed an increased rate of CD (adjusted OR = 1.93, 95% CI 1.31–2.84) (Lin et al., 2015). Also a recent retrospective study by Stern and co-workers found an increased rate of primary CD in women affected by endometriosis, both conceiving by ART procedures (adjusted OR = 2.12, 95% CI 1.67–2.69; P < 0.05) or naturally (adjusted OR 1.93, 95% CI 1.60–2.33; P < 0.05), compared with a group of fertile women (Stern et al., 2015). On the contrary, a Spanish-Italian contribution that has evaluated IVF women separately for the presence (n = 61) or absence of ovarian endometriomas (n = 130) did not find a significant association with CD (adjusted OR = 1.25, 95% CI 0.63–2.50) (Benaglia et al., 2012). In line with these findings, other reports, even excluding ART babies, observed a similar rate of CD in the endometriosis group and control groups (Conti et al., 2014; Mekaru et al., 2014). In the majority of these studies, the principal indication of CD was not specified, thus we cannot exclude that other causes, such as a previous surgery, have addressed women affected by endometriosis to the choice of an elective CD. Finally, the samples size of most of the reported studies represents a limitation.
Discussion
Traditionally, endometriosis is considered as an estrogen-dependent chronic inflammatory condition causing pain symptoms that may negatively affect women's fertility. However, in the last decade, a growing number of studies have investigated the impact of this benign disease on the regular development of pregnancy and its outcome. This is the first systematic review offering to the reader an exhaustive overview of the available literature on this topic.
By definition, endometriosis is the presence of endometrial-like tissue (stroma and glands) outside the uterus; therefore, similarly to the eutopic endometrium, endometriotic tissue may be the target of the hormonal milieu that characterizes pregnancy and consequently changes its histologic, sonographic and molecular appearance as the result of a process known as ‘decidualization’. From a clinical point of view, this is a relevant issue because in some cases decidualized endometriotic tissue may mimic malignancies, potentially leading to unnecessary surgical operations. In this field, the most investigated area is represented by the diagnosis and management of ovarian decidualized endometriomas detected during pregnancy.
Pregnancy may cause extensive changes of the endometriotic cysts (i.e. rapidly growing and abundantly vascularized intraluminal vegetations), posing a clinical diagnostic dilemma. Formal estimates of the frequency of these modifications is lacking, and cross-sectional studies should be designed with the aim of assessing the true incidence of this phenomenon. Although it is generally accepted that the transformation of the endometriotic cyst is a rare event, indirect evidence shows a variable frequency between 0 and 12% (Ueda et al., 2010; Benaglia et al., 2013). It is likely that ovarian endometrioma modifications represent under-reported and underestimated events. In fact, the ultrasonographic evaluation for adnexal tumors is not routinely performed during first trimester of pregnancy, and the identification of the ovaries becomes extremely challenging during the course of pregnancy. Overall, we deem that the magnitude of ovarian endometrioma transformation in pregnancy may be more significant than indirectly suggested by the current available literature. Transvaginal ultrasound is the gold standard tool to study ovarian endometriomas during pregnancy due to its safety and high accuracy (Barbieri et al., 2009; Mascilini et al., 2014), while MRI without gadolinium is mainly used in those situations in which ultrasonography may be limited by the enlarged uterine size and by the consequent dislocation of the ovaries. Additional tools potentially useful for the differential diagnosis between benign and malignant lesions are serum markers: combined assessment of CA125 and HE4 may be helpful to guide the clinical management of decidualized endometriomas resembling malignancies detected during pregnancy. However, future studies aiming to verify this hypothesis should be performed. The clinical management of ovarian decidualized endometriomas mimicking a malignancy is also challenging. Indeed, only 60 cases have been described. On the basis of the current evidence, we deem that serial monitoring and expectant management should be considered as a first-line management. However, when a malignancy is suspected and surgery is considered necessary, a minimally invasive laparoscopic approach is recommended and it should be not performed later than 23 weeks of gestation since the risk of adverse events increases significantly due to the size of the uterus (Whitecar et al., 1999; Usui et al., 2000). In addition, considering the complexity in the management of ovarian decidualized endometriomas mimicking a malignancy, the case should be referred to a tertiary center with long experience and success in gynecology oncology, gynecologic ultrasound and endometriosis.
The complications of endometriosis during pregnancy represent the second main issue of this systematic review. These events are rare but represent life-threating conditions that require, in most of the cases, surgical operations to be managed. Acute complications of pre-existing endometriosis may be explained by three different pathogenic mechanisms: endometriosis-related chronic inflammation that makes tissues and vessels more friable (Rossman et al., 1983), adhesions which may cause increasing traction on surrounding structures when the uterus is enlarging (Manresa et al., 2014) and intrusion of decidualized endometriotic tissue into the vessel wall and structures that can increase backpressure, predisposing to tissue rupture (O'Leary, 2006). A total of 76 cases of endometriosis complications during pregnancy have been reported; SH (n = 20), bowel perforation (n = 16) and rupture of endometriomas (n = 14) are the commonest events. Despite the clinical relevance of acute complications during pregnancy leading to potentially life-threating situations for both the mother and the fetus, the rarity of such conditions, as reported in literature, should be underlined. It is also likely that the frequency of these events is underestimated because of unreported cases, giving rise to the need for large observational studies to assess the true incidence of these complications. Due to the unpredictability of these complications, no specific recommendation for additional interventions to the routine monitoring of pregnancy of women with known history of endometriosis is advisable. Currently, there is a complete lack of large epidemiological studies aimed at quantifying exactly the incidence of these complications in the population of pregnant women with endometriosis. Furthermore, there is the need to correlate the occurrence of obstetric complications with the stage of the disease in order to identify high-risk patients. For these reasons, fear about the possibility of obstetric complications is not substantiated and thus the performance of any form of prophylactic surgery to reduce the risk of such events is not justified. Another point supporting this concept is that the surgical treatment of endometriotic lesions is unlikely to represent the cure for the molecular and functional abnormalities of the eutopic endometrium (Brosens et al., 2012b), the local and systemic higher levels of inflammation (Gentilini et al., 2011; Benagiano et al., 2014), the alteration in the uterine JZ (Exacoustos et al., 2013; Benagiano et al., 2014) and inadequate uterine contractility (Aguilar and Mitchell, 2010) described in women with endometriosis. Therefore, surgery could not be considered a solution for the negative impact that endometriosis may play on the physiological development of pregnancy, which is the third theme examined in this systematic review. However, when complications occur and surgery is consequently required, operations should be performed by physicians with extensive and successful surgical experience, prefer not only by obstetricians but also by highly specialized surgeons.
Although in the last years a growing number of papers has been published on the relation between endometriosis and obstetric outcomes, final conclusions are yet to be drawn. The main criticism in the analysis of available evidence is the extreme heterogeneity in exposure categorizations, analytic approaches, disease phenotypes, method of conceiving (natural or ART), choice of controls and in general methodological design, making difficult the comparison of the results. Therefore, well-designed prospective trials should be performed selecting accurately cases and controls, stratifying the interpretation of the results according to the age of the patients, the modality of achieving the pregnancy (ideally including only natural pregnancies), the stage of endometriosis, the presence of adenomyosis, previous surgery for endometriosis, indication for modality of delivery and other known risk factors for obstetric complications of pregnancy outcome. Special attention should be given to uterine adenomyosis that is associated with endometriosis, with a prevalence between 22 and 91% (Kunz et al., 2005; Di Donato et al., 2014; Leyendecker et al., 2015), and has been correlated with miscarriage, preterm premature rupture of membranes and preterm birth (Juang et al., 2007; Vercellini et al., 2014b). However, on the basis of the available evidence no association of endometriosis with hypertensive disorders/pre-eclampsia, obstetric hemorrhages and GDM was found. Although the results of the published studies are quite controversial, some evidence is suggestive of a possible association of endometriosis with miscarriage, preterm birth and SGA babies. Most of the studies evaluating the association between endometriosis and CD found a significant correlation. However, in these studies, the principal indication of CD was often missing; despite the lack of evidence supporting the performance of a pre-labor CD in women with a history of surgery for endometriosis, it is likely that, in common clinical practice, previous surgery may have influenced the choice of an elective CD in women with endometriosis. In addition, different studies investigating this issue included women with endometriosis conceiving by ART procedures. These pregnancies may be deemed more valuable than others. Indeed, Minkoff and Berkowitz (2005) coined the term ‘precious baby’ referring to a pregnancy achieved by ART and/or at an advanced maternal age and posited that such pregnancies are managed differently than others. Thus it may be hypothesized that, compared with the general population, such babies are more frequently delivered by CD in the absence of clinical indication. Finally, it should be considered that the studies investigating the association of higher rates of CD with endometriosis found also higher rates of obstetric complications (i.e. ante-partum hemorrhage, placenta previa), thus justifying a higher performance of CD in women affected by endometriosis.
A correlation of endometriosis with placenta praevia has been demonstrated. This association may be explained by the abnormal frequency and amplitude of uterine contractions demonstrated in women with endometriosis (Kunz et al., 2000). Hence, anomalous blastocyst implantation due to uterine dysperistalsis may justify the higher incidence of placenta previa in women with endometriosis.
Conclusions
The complications of endometriosis during pregnancy are rare and there is no evidence that the disease has a major detrimental effect on pregnancy outcome. Therefore, pregnant women with endometriosis can be reassured about the course of their pregnancies although the physicians should be aware of the potential for increased risk of placenta previa. Although it seems unlikely that hormonal or surgical treatment of endometriosis influences the impact of the disease on pregnancy outcome, no study has investigated the incidence of pregnancy complications in treated and untreated patients and this should be the objective of further research.
Supplementary data
Supplementary data are available athttp://humupd.oxfordjournals.org/.
Authors' roles
U.L.R.M., S.F., A.B., A.I., V.G. and P.V. provided a substantial contribution to the review conception and drafted the article; G.M. contributed to the interpretation of the data and critically revised the manuscript; M.C. contributed to the design of the review and critically revised the paper for important intellectual content. All authors approved the final version of the article.
Funding
No funding was received to prepare this review.
Conflict of interest
The authors have no financial, personal or competing interests.



