-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher Allen, David McLernon, Sohinee Bhattacharya, Abha Maheshwari, Early pregnancy outcomes of IVF cycles using donor versus partner sperm: analysis of 1 376 454 cycles recorded by the Human Fertilisation and Embryology Authority (1991–2016), Human Reproduction, Volume 38, Issue 6, June 2023, Pages 1194–1201, https://doi.org/10.1093/humrep/dead057
Close - Share Icon Share
Abstract
Are the early pregnancy outcomes of IVF pregnancies conceived with donor sperm different to those conceived with partner sperm?
Pregnancies conceived with donor sperm have a lower odds of early pregnancy loss and ectopic pregnancy compared to pregnancies conceived with partner sperm.
The number of cycles using donor sperm has risen significantly in recent years. Adverse early pregnancy outcomes have a negative impact on women and their partners. The evidence available to date regarding early pregnancy outcomes for pregnancies conceived with IVF donor sperm is limited by low numbers and lower-quality studies.
This is a retrospective cohort study of 1 376 454 cycles conceived with either donor or partner sperm between 1991 and 2016 as recorded in the Human Fertilisation and Embryology Authority (HFEA) Register.
The HFEA has recorded data on all fertility treatments carried out in the UK from 1991 onwards, and it publishes this data in an anonymized form. This study assessed the outcomes of all pregnancies conceived with donor sperm and compared them to those conceived with partner sperm among IVF cycles recorded in the HFEA anonymized dataset from 1991 to 2016. Cycles that included intrauterine insemination, donor oocytes, preimplantation genetic testing, oocyte thaw cycles and alternative fertility treatments were excluded. The outcomes of interest were biochemical pregnancy, miscarriage, ectopic pregnancy, stillbirth and live birth. Logistic regression was used to adjust for confounding factors including age of the female partner, cause of infertility, history of previous pregnancy, fresh or frozen cycle, IVF or ICSI, number of embryos transferred, and year of treatment. Results are reported as adjusted odds ratios (aOR) and 95% CIs.
This study found reductions in the odds of biochemical pregnancy (aOR 0.82, 95% CI 0.78–0.86), miscarriage (aOR 0.93, 95% CI 0.89–0.97), and ectopic pregnancy (aOR 0.77, 95% CI 0.66–0.90) among pregnancies as a result of the use of donor sperm as opposed to partner sperm.
This study is retrospective and limited by the constraints of routinely collected data. No data were available for maternal characteristics such as BMI, smoking and partner age, which could all be potential confounders. Clustering of multiple pregnancies within women could not be accounted for as the data are reported only at the cycle level with no maternal identifiers.
This study has demonstrated that there are no increased risks of adverse pregnancy outcome with donor sperm pregnancies. The reduction in miscarriage in pregnancies using donor sperm suggests that sperm could have a role in miscarriage, as the selection process for being accepted as donor is stringent.
No external funding was sought for this study. C.A. has received funding from Ferring to attend a UK meeting for trainees in reproductive Medicine. A.M. has received funding from Ferring, Cook, Merck Serono, Geodon Ritcher, and Pharmasure for speaking at, or attending, meetings relating to reproductive medicine. She has also participated in a Ferring advisory board. S.B. has received grants from Tenovus and the UK Medical Research Council. She has also been supported with a Medical Research Scotland PhD studentship.
N/A.
Introduction
Pregnancy loss is a devastating event in the lives of women, and their partners. It has impacts both partners physically, emotionally, socially, and financially (Heazell et al., 2016, Quenby et al., 2021). In the recent past, there has been an effort to focus on reducing stillbirths (Froen et al., 2011, 2016) and miscarriage (The Lancet, 2021). Ectopic pregnancy is also a significant adverse event that has implications for future pregnancies (Bhattacharya et al., 2012). When embarking on fertility treatment, it is important for patients to be fully informed of the chances of adverse outcomes.
A recently published meta-analysis (Allen et al., 2021) assessed pregnancy outcomes after the use of donor sperm versus partner sperm. The comparison of IVF pregnancies in that systematic review identified seven studies assessing miscarriage and two studies assessing ectopic pregnancy. These studies were of variable quality showed significant statistical heterogeneity, and the pooled analyses were imprecise. None of the primary studies adjusted for potential confounding factors. It was therefore deemed that the evidence relating to chances of miscarriage and ectopic pregnancy for donor sperm compared with partner sperm pregnancies was of low quality using the GRADE criteria.
Globally use of medically assisted reproduction (MAR) is increasing year on year, and this includes the use of donor sperm. Recent publications from the USA and China have shown increasing use of donor sperm in recent years (Fang et al., 2018; Gerkowicz et al., 2018). Within the UK, the Human Fertility and Embryology Authority (HFEA) publishes information on assisted reproductive treatment trends on a regular basis. These data would suggest that, from 2007 to 2019, the number of IVF cycles using donor sperm has more than quadrupled (HFEA, 2021).
Therefore, due to the increasing use of donor sperm within IVF cycles, and the low-quality evidence relating to adverse early pregnancy outcomes, further research was warranted. The HFEA publishes anonymized data relating to all fertility treatments in the UK, including the use of donor sperm. We therefore aimed to assess the early pregnancy outcomes of IVF pregnancies conceived with donor sperm compared to those conceived with partner sperm.
Materials and methods
Study design
This retrospective cohort study involved analysis of the HFEA’s assisted reproductive technology registry, comparing donor sperm pregnancies with partner sperm pregnancies. Donor sperm pregnancies included heterosexual couples, same-sex couples, and single women. We included all IVF and ICSI cycles that took place from 1991 to 2016 that resulted in a pregnancy after the use of donor or partner sperm.
Data collection
The HFEA database is an anonymized register of all assisted reproduction cycles performed in the UK involving either IVF, with or without ICSI, or donor sperm inseminations. Clinics are legally required to submit their information to the HFEA in real time, and the HFEA has processes in place to validate the data (HFEA, 2009). The dataset undergoes regular revisions and updates, and the current dataset incorporates all cycles from 1991 to 2016. More information relating to the dataset can be found at https://www.hfea.gov.uk/about-us/our-data/. The dataset collects information regarding baseline characteristics such as female age (in age bands: 18–34, 35–37, 38–39, 40–42, 43–44, 45–50), causes of infertility (as a series of individual binary variables), whether the woman has had treatment before, and whether any pregnancies or births have occurred as a result. It also records information such as the treatment carried out, number of oocytes recovered, whether the oocytes were thawed, whether ICSI was used, the number of embryos created, whether it was a fresh or a frozen cycle, and treatment outcomes. It does not record an indication for treatment and nor is it mandatory to have an infertility diagnosis recorded. With specific reference to donor sperm cycles, the anonymized dataset does not provide information on each woman’s relationship status.
Exclusion criteria
We excluded those cycles that involved donor insemination (as the dataset does not include cycles of intrauterine insemination with partner sperm), gamete/zygote intra-fallopian transfer (GIFT/ZIFT), subzonal insemination (SUZI), and cycles that used the additional measures of preimplantation genetic testing, donor oocytes, or donor embryos. Cycles that used thawed oocytes had no embryos transferred, that used both donor and partner sperm, or that were lost to follow-up were also excluded, as were cycles where no embryos were transferred (as a small number of pregnancies are reported from cycles where zero embryos were recorded as being transferred, due to a likely data entry error).
Outcomes
Outcomes in the HFEA anonymized dataset are reported by the individual clinics, and therefore, the clinics are responsible for deciding on how they define different outcomes. For the purpose of this study, we have defined biochemical pregnancy as any pregnancy that only achieved the outcome of biochemical pregnancy. Miscarriage is not directly defined in the dataset, but in the UK, from 1990, the age of viability was set at 24 weeks of completed gestation. Therefore, any pregnancy progressing beyond a biochemical pregnancy but lost before 24 weeks of gestation would be considered a miscarriage. The dataset reports outcomes at two time points: early outcomes (identified at an early pregnancy scan usually done at around 7 weeks of gestation, following a positive pregnancy test) and final outcomes for any ongoing pregnancies. There are seven possible early pregnancy outcomes with each cycle having up to three recorded: none, biochemical pregnancy, miscarriage, ectopic, heterotopic, molar, and observed intrauterine foetal pulsation. A single final outcome is reported for each of up to four foetuses and these could be: miscarriage, ectopic/heterotopic pregnancy, termination, embryo reduction, still birth, or live birth. This information was collated and therefore each cycle can have multiple outcomes. Ectopic and heterotopic pregnancies were considered a single outcome for the purposes of this study. Biochemical pregnancy was only considered an outcome if it was the only outcome recorded for a cycle.
Therefore, the final list of outcomes available for assessment in this study was: biochemical pregnancy, miscarriage, ectopic/heterotopic pregnancy, molar pregnancy, termination of pregnancy, embryo reduction, still birth, and live birth (where a live birth is defined as the live birth of one or more babies from one pregnancy). Primary outcomes for this study were: biochemical pregnancy only, miscarriage, ectopic pregnancy, molar pregnancy, termination, and embryo reduction. Still birth and livebirth were secondary outcomes and included only to provide a rounded picture of the outcomes of the ongoing pregnancies. Obstetric and perinatal outcomes were not considered as they have been reported in another publication (Allen et al., 2021).
Baseline characteristics
We included the baseline characteristics below in the analysis to identify any potential confounders: maternal age (in bands: 18–34, 35–37, 38–39, 40–42, 43–44, 45–50), causes of infertility (unexplained, tubal factor, ovulatory disorder, male factor, endometriosis, which are recorded in the dataset as binary variables, therefore each woman can have zero or one or more causes of infertility), previous pregnancy history, IVF or ICSI cycle, number of embryos transferred, and fresh or frozen cycle. Previous pregnancy history was a calculated variable based on the HFEA variables ‘number of previous pregnancies from treatment’ and the ‘number of previous live births from treatment’.
Statistical analysis
Pearson’s chi-squared test was used to test for an association of baseline characteristics between the donor and partner sperm groups. The primary analysis compared outcomes of all donor sperm pregnancies (IVF/ICSI) with all recorded partner sperm pregnancies (IVF/ICSI). Crude odds ratios (ORs) were calculated with 95% CIs and then binomial logistic regression was used to calculate adjusted odds ratios (aORs) for donor versus partner sperm for each outcome. We adjusted for age of the female partner, history of previous pregnancy with or without live birth, number of embryos transferred, whether ICSI was used, fresh or frozen cycle, cause of infertility (unexplained, tubal factor, ovulatory disorder, male factor, endometriosis), and year of treatment.
The HFEA anonymized dataset reports cycle level data and not women level data meaning that it was not possible to account for the clustering effect of multiple pregnancies per woman. A sensitivity analysis was performed to include only first pregnancies to remove this unmeasurable clustering effect within the dataset.
Data were analysed using IBM SPSS statistics (version 27, IBM Corp Armonk, NY, USA).
Ethics
This study used a pseudo-anonymized publicly available dataset with no patient identifying information included. Therefore, ethical or Caldicott Guardian approval was not required.
Results
The HFEA dataset recorded a total of 1 376 454 cycles between 1991 and 2016. Following exclusion of cycles based on unsuitable treatments or lack of essential information, 893 899 cycles were potentially eligible for inclusion. Of the 893 899 cycles, 45 341 used donor sperm and 848 558 used partner sperm, and pregnancy rates were 39.5% and 37.0% for donor and partner sperm, respectively (these percentages are prior to exclusion of pregnancies lost to follow-up, and include biochemical pregnancies). There were a total of 326 554 pregnancies with outcome data recorded and no losses to follow-up. This provided 17 634 donor sperm IVF and 308 920 partner sperm IVF pregnancies for analysis (Fig. 1).
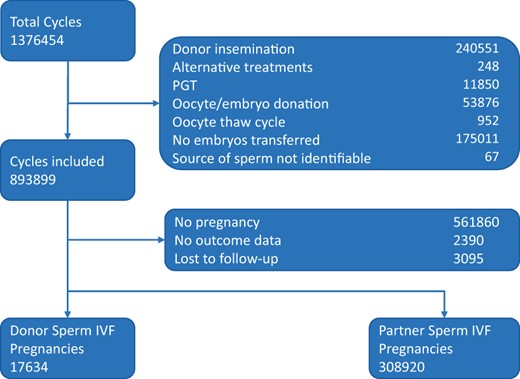
Flow chart of cycle selection. This flow chart describes the inclusion and exclusion of cycles assessed in this study.
Table I shows the baseline characteristics for the two groups. All comparisons were statistically significant due to the large numbers (P < 0.001 for all). However, a few clinically important differences are worthy of note. Women in the donor sperm group were slightly older, were more likely to have had a previous pregnancy (22.9% versus 16.2%) and had slightly more pregnancies that did not result in a live birth (7.8% versus 5.1%). There was a higher proportion of couples having male factor infertility (59.2% versus 45.4%) in the donor sperm group, while the other causes of infertility were more frequent in the partner sperm group. There was also a significantly higher proportion of women with no infertility diagnosis in the donor sperm group (34.6% versus 4.5%), likely reflecting the fact that the donor sperm group contains same-sex couples and single women.
Baseline characteristics of pregnant women following donor and partner sperm IVF/ICSI.
| . | Donor sperm . | Partner sperm . | |||
|---|---|---|---|---|---|
| Total = 17 634 . | Total = 308 920 . | ||||
| n . | % . | n . | % . | ||
| Patient age at treatment | 18-34 | 8649 | 49.0 | 165 934 | 53.7 |
| 35-37 | 4074 | 23.1 | 76 705 | 24.8 | |
| 38-39 | 2426 | 13.8 | 38 472 | 12.5 | |
| 40-42 | 2074 | 11.8 | 24 126 | 7.8 | |
| 43-44 | 351 | 2.0 | 3167 | 1.0 | |
| 45-50 | 60 | 0.3 | 516 | 0.2 | |
| Pregnancy history** | No previous pregnancy | 13 597 | 77.1 | 258 921 | 83.8 |
| Previous pregnancies, no live births | 1018 | 5.8 | 12 761 | 4.1 | |
| Previous pregnancies, mixed outcomes: unsuccessful pregnancies and live births | 355 | 2.0 | 3170 | 1.0 | |
| Previous pregnancies, all live births | 2664 | 15.1 | 34 068 | 11.0 | |
| Cause of infertility* | Tubal disease | 910 | 5.2 | 62 324 | 20.2 |
| Ovulatory Disorder | 914 | 5.2 | 42 696 | 13.8 | |
| Male Factor | 10 448 | 59.2 | 140 119 | 45.4 | |
| Patient Unexplained | 2559 | 14.5 | 94 885 | 30.7 | |
| Endometriosis | 464 | 2.6 | 21 369 | 6.9 | |
| Fresh versus frozen | Fresh | 13 961 | 79.2 | 250 578 | 81.1 |
| Frozen | 3673 | 20.8 | 58 342 | 18.9 | |
| IVF versus ICSI | IVF | 13 239 | 75.1 | 177 600 | 57.5 |
| ICSI | 4395 | 24.9 | 131 320 | 42.5 | |
| . | Donor sperm . | Partner sperm . | |||
|---|---|---|---|---|---|
| Total = 17 634 . | Total = 308 920 . | ||||
| n . | % . | n . | % . | ||
| Patient age at treatment | 18-34 | 8649 | 49.0 | 165 934 | 53.7 |
| 35-37 | 4074 | 23.1 | 76 705 | 24.8 | |
| 38-39 | 2426 | 13.8 | 38 472 | 12.5 | |
| 40-42 | 2074 | 11.8 | 24 126 | 7.8 | |
| 43-44 | 351 | 2.0 | 3167 | 1.0 | |
| 45-50 | 60 | 0.3 | 516 | 0.2 | |
| Pregnancy history** | No previous pregnancy | 13 597 | 77.1 | 258 921 | 83.8 |
| Previous pregnancies, no live births | 1018 | 5.8 | 12 761 | 4.1 | |
| Previous pregnancies, mixed outcomes: unsuccessful pregnancies and live births | 355 | 2.0 | 3170 | 1.0 | |
| Previous pregnancies, all live births | 2664 | 15.1 | 34 068 | 11.0 | |
| Cause of infertility* | Tubal disease | 910 | 5.2 | 62 324 | 20.2 |
| Ovulatory Disorder | 914 | 5.2 | 42 696 | 13.8 | |
| Male Factor | 10 448 | 59.2 | 140 119 | 45.4 | |
| Patient Unexplained | 2559 | 14.5 | 94 885 | 30.7 | |
| Endometriosis | 464 | 2.6 | 21 369 | 6.9 | |
| Fresh versus frozen | Fresh | 13 961 | 79.2 | 250 578 | 81.1 |
| Frozen | 3673 | 20.8 | 58 342 | 18.9 | |
| IVF versus ICSI | IVF | 13 239 | 75.1 | 177 600 | 57.5 |
| ICSI | 4395 | 24.9 | 131 320 | 42.5 | |
All baseline characteristics were significantly different between donor sperm and partner sperm groups. P < 0.001 (Pearson’s chi-squared test) for all tests of association between baseline characteristics and donor versus partner sperm.
This is a composite variable: the dataset provides the number of previous pregnancies and the number of previous live birth events; we combined these two variables with a total of four values.
Causes of infertility are dichotomous variables and each woman may have more than one cause of infertility.
Baseline characteristics of pregnant women following donor and partner sperm IVF/ICSI.
| . | Donor sperm . | Partner sperm . | |||
|---|---|---|---|---|---|
| Total = 17 634 . | Total = 308 920 . | ||||
| n . | % . | n . | % . | ||
| Patient age at treatment | 18-34 | 8649 | 49.0 | 165 934 | 53.7 |
| 35-37 | 4074 | 23.1 | 76 705 | 24.8 | |
| 38-39 | 2426 | 13.8 | 38 472 | 12.5 | |
| 40-42 | 2074 | 11.8 | 24 126 | 7.8 | |
| 43-44 | 351 | 2.0 | 3167 | 1.0 | |
| 45-50 | 60 | 0.3 | 516 | 0.2 | |
| Pregnancy history** | No previous pregnancy | 13 597 | 77.1 | 258 921 | 83.8 |
| Previous pregnancies, no live births | 1018 | 5.8 | 12 761 | 4.1 | |
| Previous pregnancies, mixed outcomes: unsuccessful pregnancies and live births | 355 | 2.0 | 3170 | 1.0 | |
| Previous pregnancies, all live births | 2664 | 15.1 | 34 068 | 11.0 | |
| Cause of infertility* | Tubal disease | 910 | 5.2 | 62 324 | 20.2 |
| Ovulatory Disorder | 914 | 5.2 | 42 696 | 13.8 | |
| Male Factor | 10 448 | 59.2 | 140 119 | 45.4 | |
| Patient Unexplained | 2559 | 14.5 | 94 885 | 30.7 | |
| Endometriosis | 464 | 2.6 | 21 369 | 6.9 | |
| Fresh versus frozen | Fresh | 13 961 | 79.2 | 250 578 | 81.1 |
| Frozen | 3673 | 20.8 | 58 342 | 18.9 | |
| IVF versus ICSI | IVF | 13 239 | 75.1 | 177 600 | 57.5 |
| ICSI | 4395 | 24.9 | 131 320 | 42.5 | |
| . | Donor sperm . | Partner sperm . | |||
|---|---|---|---|---|---|
| Total = 17 634 . | Total = 308 920 . | ||||
| n . | % . | n . | % . | ||
| Patient age at treatment | 18-34 | 8649 | 49.0 | 165 934 | 53.7 |
| 35-37 | 4074 | 23.1 | 76 705 | 24.8 | |
| 38-39 | 2426 | 13.8 | 38 472 | 12.5 | |
| 40-42 | 2074 | 11.8 | 24 126 | 7.8 | |
| 43-44 | 351 | 2.0 | 3167 | 1.0 | |
| 45-50 | 60 | 0.3 | 516 | 0.2 | |
| Pregnancy history** | No previous pregnancy | 13 597 | 77.1 | 258 921 | 83.8 |
| Previous pregnancies, no live births | 1018 | 5.8 | 12 761 | 4.1 | |
| Previous pregnancies, mixed outcomes: unsuccessful pregnancies and live births | 355 | 2.0 | 3170 | 1.0 | |
| Previous pregnancies, all live births | 2664 | 15.1 | 34 068 | 11.0 | |
| Cause of infertility* | Tubal disease | 910 | 5.2 | 62 324 | 20.2 |
| Ovulatory Disorder | 914 | 5.2 | 42 696 | 13.8 | |
| Male Factor | 10 448 | 59.2 | 140 119 | 45.4 | |
| Patient Unexplained | 2559 | 14.5 | 94 885 | 30.7 | |
| Endometriosis | 464 | 2.6 | 21 369 | 6.9 | |
| Fresh versus frozen | Fresh | 13 961 | 79.2 | 250 578 | 81.1 |
| Frozen | 3673 | 20.8 | 58 342 | 18.9 | |
| IVF versus ICSI | IVF | 13 239 | 75.1 | 177 600 | 57.5 |
| ICSI | 4395 | 24.9 | 131 320 | 42.5 | |
All baseline characteristics were significantly different between donor sperm and partner sperm groups. P < 0.001 (Pearson’s chi-squared test) for all tests of association between baseline characteristics and donor versus partner sperm.
This is a composite variable: the dataset provides the number of previous pregnancies and the number of previous live birth events; we combined these two variables with a total of four values.
Causes of infertility are dichotomous variables and each woman may have more than one cause of infertility.
Pregnancies conceived with donor sperm were less likely to be biochemical pregnancies (aOR 0.82, 95% CI 0.78–0.86), miscarriages (aOR 0.93, 95% CI 0.89–0.97), or ectopic pregnancies (aOR 0.77, 95% CI 0.66–0.90) than pregnancies conceived using partner sperm. The odds of live birth were greater with the use of donor sperm compared with partner sperm (aOR 1.17, 95% CI 1.13–1.21). There was no demonstrable difference in the odds of terminations, embryo reductions, and still births between the two groups. For these three outcomes, the confidence intervals were wide and, therefore, it was not possible to draw firm conclusions. Molar pregnancies could not be analysed as there were no occurrences in the donor sperm group (Table II).
| Outcome . | Donor sperm IVF/ICSI N = 17 634 . | Partner sperm IVF/ICSI N = 308 920 . | Donor versus partner Unadjusted OR (95% CI) . | Donor versus partner Adjusted OR (95% CI) . | ||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | . | . | |
| Biochemical Pregnancy | 2057 | 11.7 | 40 342 | 13.1 | 0.88 (0.84–0.92) | 0.82 (0.78–0.86) |
| Miscarriage | 2778 | 15.8 | 49 499 | 16.0 | 0.98 (0.94–1.02) | 0.93 (0.89–0.97) |
| Ectopic pregnancy | 177 | 1.0 | 4580 | 1.5 | 0.67 (0.58–0.78) | 0.77 (0.66–0.90) |
| Molar pregnancy | 0 | 0.0 | 68 | 0.0 | – | – |
| Termination | 122 | 0.7 | 1873 | 0.6 | 1.14 (0.95–1.37) | 1.01 (0.84–1.23) |
| Embryo reduction | 26 | 0.1 | 296 | 0.1 | 1.54 (1.03–2.30) | 1.31 (0.86–1.99) |
| Still birth | 92 | 0.5 | 1581 | 0.5 | 1.02 (0.83–1.26) | 1.02 (0.82–1.27) |
| Live birth | 12 828 | 72.7 | 218 896 | 70.9 | 1.10 (1.06–1.14) | 1.17 (1.13–1.21) |
| Outcome . | Donor sperm IVF/ICSI N = 17 634 . | Partner sperm IVF/ICSI N = 308 920 . | Donor versus partner Unadjusted OR (95% CI) . | Donor versus partner Adjusted OR (95% CI) . | ||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | . | . | |
| Biochemical Pregnancy | 2057 | 11.7 | 40 342 | 13.1 | 0.88 (0.84–0.92) | 0.82 (0.78–0.86) |
| Miscarriage | 2778 | 15.8 | 49 499 | 16.0 | 0.98 (0.94–1.02) | 0.93 (0.89–0.97) |
| Ectopic pregnancy | 177 | 1.0 | 4580 | 1.5 | 0.67 (0.58–0.78) | 0.77 (0.66–0.90) |
| Molar pregnancy | 0 | 0.0 | 68 | 0.0 | – | – |
| Termination | 122 | 0.7 | 1873 | 0.6 | 1.14 (0.95–1.37) | 1.01 (0.84–1.23) |
| Embryo reduction | 26 | 0.1 | 296 | 0.1 | 1.54 (1.03–2.30) | 1.31 (0.86–1.99) |
| Still birth | 92 | 0.5 | 1581 | 0.5 | 1.02 (0.83–1.26) | 1.02 (0.82–1.27) |
| Live birth | 12 828 | 72.7 | 218 896 | 70.9 | 1.10 (1.06–1.14) | 1.17 (1.13–1.21) |
Adjusted for maternal age, pregnancy history, number of embryos transferred, IVF or ICSI, fresh or frozen cycle, and causes of infertility (unexplained, tubal factor, ovulatory disorder, male factor, endometriosis) and year of treatment.
OR: odds ratio.
| Outcome . | Donor sperm IVF/ICSI N = 17 634 . | Partner sperm IVF/ICSI N = 308 920 . | Donor versus partner Unadjusted OR (95% CI) . | Donor versus partner Adjusted OR (95% CI) . | ||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | . | . | |
| Biochemical Pregnancy | 2057 | 11.7 | 40 342 | 13.1 | 0.88 (0.84–0.92) | 0.82 (0.78–0.86) |
| Miscarriage | 2778 | 15.8 | 49 499 | 16.0 | 0.98 (0.94–1.02) | 0.93 (0.89–0.97) |
| Ectopic pregnancy | 177 | 1.0 | 4580 | 1.5 | 0.67 (0.58–0.78) | 0.77 (0.66–0.90) |
| Molar pregnancy | 0 | 0.0 | 68 | 0.0 | – | – |
| Termination | 122 | 0.7 | 1873 | 0.6 | 1.14 (0.95–1.37) | 1.01 (0.84–1.23) |
| Embryo reduction | 26 | 0.1 | 296 | 0.1 | 1.54 (1.03–2.30) | 1.31 (0.86–1.99) |
| Still birth | 92 | 0.5 | 1581 | 0.5 | 1.02 (0.83–1.26) | 1.02 (0.82–1.27) |
| Live birth | 12 828 | 72.7 | 218 896 | 70.9 | 1.10 (1.06–1.14) | 1.17 (1.13–1.21) |
| Outcome . | Donor sperm IVF/ICSI N = 17 634 . | Partner sperm IVF/ICSI N = 308 920 . | Donor versus partner Unadjusted OR (95% CI) . | Donor versus partner Adjusted OR (95% CI) . | ||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | . | . | |
| Biochemical Pregnancy | 2057 | 11.7 | 40 342 | 13.1 | 0.88 (0.84–0.92) | 0.82 (0.78–0.86) |
| Miscarriage | 2778 | 15.8 | 49 499 | 16.0 | 0.98 (0.94–1.02) | 0.93 (0.89–0.97) |
| Ectopic pregnancy | 177 | 1.0 | 4580 | 1.5 | 0.67 (0.58–0.78) | 0.77 (0.66–0.90) |
| Molar pregnancy | 0 | 0.0 | 68 | 0.0 | – | – |
| Termination | 122 | 0.7 | 1873 | 0.6 | 1.14 (0.95–1.37) | 1.01 (0.84–1.23) |
| Embryo reduction | 26 | 0.1 | 296 | 0.1 | 1.54 (1.03–2.30) | 1.31 (0.86–1.99) |
| Still birth | 92 | 0.5 | 1581 | 0.5 | 1.02 (0.83–1.26) | 1.02 (0.82–1.27) |
| Live birth | 12 828 | 72.7 | 218 896 | 70.9 | 1.10 (1.06–1.14) | 1.17 (1.13–1.21) |
Adjusted for maternal age, pregnancy history, number of embryos transferred, IVF or ICSI, fresh or frozen cycle, and causes of infertility (unexplained, tubal factor, ovulatory disorder, male factor, endometriosis) and year of treatment.
OR: odds ratio.
To allow for the inability to control for clustering of cycles within women, we performed a sensitivity analysis excluding cycles where there was a history of previous pregnancy. The results of this analysis were similar to the primary analysis with regard to biochemical pregnancy, miscarriage, ectopic pregnancy and live birth (Table III).
Outcomes of donor sperm versus partner sperm IVF/ICSI pregnancies in first pregnancies only.
| Outcome . | Donor sperm IVF/ICSI N = 13 597 . | Partner sperm IVF/ICSI N = 258 921 . | Donor versus partner Unadjusted OR (95% CI) . | Donor versus partner Adjusted OR (95% CI) . | ||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | . | . | |
| Biochemical pregnancy | 1564 | 11.5 | 32 745 | 12.6 | 0.90 (0.85–0.95) | 0.82 (0.77–0.86) |
| Miscarriage | 2075 | 15.3 | 40 605 | 15.7 | 0.97 (0.92–1.02) | 0.91 (0.86–0.96) |
| Ectopic | 140 | 1.0 | 3892 | 1.5 | 0.68 (0.58–0.81) | 0.76 (0.64–0.91) |
| Live birth | 9971 | 73.3 | 184 815 | 71.4 | 1.10 (1.06–1.15) | 1.19 (1.14–1.24) |
| Outcome . | Donor sperm IVF/ICSI N = 13 597 . | Partner sperm IVF/ICSI N = 258 921 . | Donor versus partner Unadjusted OR (95% CI) . | Donor versus partner Adjusted OR (95% CI) . | ||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | . | . | |
| Biochemical pregnancy | 1564 | 11.5 | 32 745 | 12.6 | 0.90 (0.85–0.95) | 0.82 (0.77–0.86) |
| Miscarriage | 2075 | 15.3 | 40 605 | 15.7 | 0.97 (0.92–1.02) | 0.91 (0.86–0.96) |
| Ectopic | 140 | 1.0 | 3892 | 1.5 | 0.68 (0.58–0.81) | 0.76 (0.64–0.91) |
| Live birth | 9971 | 73.3 | 184 815 | 71.4 | 1.10 (1.06–1.15) | 1.19 (1.14–1.24) |
Adjusted for maternal age, number of embryos transferred, IVF or ICSI, fresh or frozen cycle, causes of infertility (unexplained, tubal factor, ovulatory disorder, male factor, endometriosis), and year of treatment.
OR: odds ratio.
Outcomes of donor sperm versus partner sperm IVF/ICSI pregnancies in first pregnancies only.
| Outcome . | Donor sperm IVF/ICSI N = 13 597 . | Partner sperm IVF/ICSI N = 258 921 . | Donor versus partner Unadjusted OR (95% CI) . | Donor versus partner Adjusted OR (95% CI) . | ||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | . | . | |
| Biochemical pregnancy | 1564 | 11.5 | 32 745 | 12.6 | 0.90 (0.85–0.95) | 0.82 (0.77–0.86) |
| Miscarriage | 2075 | 15.3 | 40 605 | 15.7 | 0.97 (0.92–1.02) | 0.91 (0.86–0.96) |
| Ectopic | 140 | 1.0 | 3892 | 1.5 | 0.68 (0.58–0.81) | 0.76 (0.64–0.91) |
| Live birth | 9971 | 73.3 | 184 815 | 71.4 | 1.10 (1.06–1.15) | 1.19 (1.14–1.24) |
| Outcome . | Donor sperm IVF/ICSI N = 13 597 . | Partner sperm IVF/ICSI N = 258 921 . | Donor versus partner Unadjusted OR (95% CI) . | Donor versus partner Adjusted OR (95% CI) . | ||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | . | . | |
| Biochemical pregnancy | 1564 | 11.5 | 32 745 | 12.6 | 0.90 (0.85–0.95) | 0.82 (0.77–0.86) |
| Miscarriage | 2075 | 15.3 | 40 605 | 15.7 | 0.97 (0.92–1.02) | 0.91 (0.86–0.96) |
| Ectopic | 140 | 1.0 | 3892 | 1.5 | 0.68 (0.58–0.81) | 0.76 (0.64–0.91) |
| Live birth | 9971 | 73.3 | 184 815 | 71.4 | 1.10 (1.06–1.15) | 1.19 (1.14–1.24) |
Adjusted for maternal age, number of embryos transferred, IVF or ICSI, fresh or frozen cycle, causes of infertility (unexplained, tubal factor, ovulatory disorder, male factor, endometriosis), and year of treatment.
OR: odds ratio.
Discussion
Summary of findings
This study has shown that pregnancies conceived with donor sperm have a lower chance of early pregnancy loss compared with pregnancies conceived using partner sperm. Consequently, this leads to an increase in the odds of live birth as there was no difference in the odds of still birth in pregnancies conceived with donor sperm.
Strengths and limitations
It should be noted that our denominator in this study is pregnancies. We feel this is appropriate as the outcomes of interest are only possible once a pregnancy has occurred. Hence it is a clinically relevant denominator. Other denominators could have been chosen, such as per embryo transfer procedure, per embryo transferred or per cycle started, and appropriate denominators have been debated previously (Chiu et al., 2020; Snowden et al., 2020; Wilkinson et al., 2021) with no consensus. Per cycle started was not appropriate for this study as the dataset does not specify those cycles that planned for an embryo transfer but failed to achieve it and those that planned a freeze-all approach from the outset. Therefore, it was not possible to isolate the cycles where embryo transfer was intended even if it was not achieved. Per embryo transferred or per transfer procedure would have been a valid alternative. However, that does not answer the question proposed in this study.
This study uses data from a large national, prospectively collected population-based IVF registry from the UK which is regularly validated by the HFEA. This large published dataset has more than five times the number of pregnancies reported from all other studies and additionally presents all the available outcome data for the included pregnancies. As it is based on national data, it is more generalizable to similar populations across the world.
However, the dataset lacks key information on smoking status (Pineles et al., 2014), body mass index (Maheshwari et al., 2007) and detailed previous pregnancy information (Magnus et al., 2019), which are associated with adverse pregnancy outcomes; therefore, the analysis could not be adjusted for these potential confounders. While in the dataset the number of previous pregnancies and the number of previous live births from previous treatments are available for each cycle, there was no information about pregnancies which did not result in live birth as to whether those were miscarriages, ectopic, terminations, or stillbirths. We have accounted for unsuccessful pregnancy in the analysis, but this assessment may be incomplete as the dataset only reported on previous treatment-related pregnancies. Importantly, the dataset also does not record the age of the partner and, therefore, we were unable to adjust for the age of the sperm provider. There is evidence that increasing male age is associated with an increased risk of miscarriage (Du Fosse et al., 2020). Therefore, since the maximum age of sperm donors in the UK is limited to 45 by the HFEA (HFEA, 2019), the donor sperm conceived IVF pregnancies may have a different risk profile compared with partner sperm IVF pregnancies. There were a number of other factors that were not adjusted for in our analysis including gender of the pregnancy, blastocyst versus cleavage stage embryo transfer (which may impact on the ectopic pregnancy rate (Li et al., 2015)), and whether or not frozen embryo replacement cycles were done in a natural cycle or in a programmed cycle. It was not possible to adjust for these factors because gender cannot be determined for all early pregnancies, the dataset does not differentiate between blastocyst and cleavage stage embryos in frozen embryo transfer cycles, and the dataset does not report the nature of each frozen replacement cycle. This study also includes all pregnancies, both singletons and multiple pregnancies. It is not clinically possible to determine whether a pregnancy is a singleton or a multiple for all pregnancies, specifically for biochemical and ectopic pregnancies; therefore, it was not appropriate to adjust for this in the statistical analysis.
In addition, the data are reported at the cycle level with no maternal identifiers, and therefore, we could not adjust for clustering of multiple pregnancies within each woman. However, since our sensitivity analysis only included first IVF pregnancies and did not demonstrate any significant changes to the findings, we feel that the lack of hierarchical adjustment would not have changed our findings and that our conclusions are valid.
The donor sperm recipient population is inherently a selected population and distinct from the infertile population. For over 50% of donor sperm cycles in the UK, there is no male partner, while for others it will be obligated because of a lack of partner sperm. The aim of this study has not been to suggest that donor sperm is a treatment to improve outcomes for patients suffering from adverse pregnancy outcomes, but rather to establish whether those pregnancies conceived with donor sperm could have been at an increased risk of adverse outcomes and therefore could have needed additional counselling and follow-up. The finding of a lower miscarriage rate with donor sperm compared with partner sperm reinforces the concept of a male contribution to miscarriage and should add weight to the need for further research into this area.
Explanation of findings
UK sperm donors are required by the regulator, the HFEA, to be screened for adverse medical histories and familial medical conditions (Clarke et al., 2021). In addition, they have basic screening for chromosomal abnormalities and focused testing for specific genetic conditions based on their ethnicity, for example, Tay-Sachs disease in Jews of Eastern European descent. They will all have a basic semen analysis and a test thaw to ensure that their sperm is of sufficient quality to be used for donation. Therefore, sperm donors represent a highly screened population of healthy males with normal semen parameters, compared to the general infertility population who may have a semen abnormality in association with male factor infertility, underlying health conditions or undiagnosed chromosomal abnormalities.
This can explain the lower risk of miscarriage as partners of men with balanced translocations are known to have a higher risk of miscarriage due to unbalanced translocations within their gametes (Sugiura-Ogasawara et al., 2004). Reciprocal chromosomal translocations are present in the general population in 1/712 (0.14%) people (Nielsen and Wohlert, 1991) compared to the infertile male population where it ranges from 1.6% to 6.65% (Ching et al., 2012). Therefore, in absolute terms, donors are at significantly lower risk of having gametes with unbalanced translocations due to their mandatory screening, and hence a lower chance of biochemical pregnancy and miscarriage. There is also evidence that sperm DNA damage is associated with infertility (Cho and Agarwal, 2018) and with miscarriage (Zhao et al., 2014). This, therefore, represents another possible factor in the reduced rate of miscarriage in the donor sperm group, as there is evidence from at least two studies showing that they have lower rates of sperm DNA damage than infertile men (Simon et al., 2011; Tvrdá et al., 2018).
When we consider ectopic pregnancy, we know that tubal factor infertility affects approximately 24–29% of couples with infertility (Miller et al., 1999). As per NICE guidance, tubal patency testing should be offered but is not mandatory (NICE, 2017), and therefore, given that the rates of tubal factor infertility in our study are lower, it is possible that there is undiagnosed tubal factor infertility within the partner sperm group. Conversely, the donor sperm group will include single women and women from same-sex relationships who are likely to be comparable to the general population, which may explain the lower proportion of tubal factor infertility for this group in our study. Therefore, this relative disparity in the rates of tubal disease may explain the higher rate of ectopic pregnancy in the partner sperm group.
With the lower rates of early pregnancy loss and ectopic pregnancy, there was an expected increase in the rate of live births in the donor sperm group, with no significant impact on the numbers of still births.
Comparisons with other studies
Our recent meta-analysis (Allen et al., 2021) did not demonstrate any difference in the rate of miscarriage for pregnancies conceived with donor sperm IVF when compared with those conceived with partner sperm IVF (OR 0.81, 95% CI 0.56–1.18). The lack of a significant difference may reflect the lower numbers in the meta-analysis, and hence a lower precision, as the total number of donor sperm miscarriages included was 739 in that review, compared with 2778 miscarriages and 2057 biochemical pregnancies. However, the meta-analysis did find a reduction in the odds of having an ectopic pregnancy in donor sperm pregnancies (OR 0.64, 95% CI 0.19–0.84), in keeping with this current study. Since the publication of the meta-analysis, an analysis of 418 donor sperm cycles compared with 337 partner sperm cycles using ICSI has been published (Mignini Renzini et al., 2021). They found that there was an increase in the live birth rate with the use of donor sperm for women aged 37 years and above (aOR 1.87, 95% CI 1.08–3.07), although not in the women under 37 years (this was reported per cycle rather than per pregnancy). They also reported an approximate 45% reduction in the miscarriage rate per clinical pregnancy when donor sperm was used (P = 0.021). While this study had a relatively small sample size and used slightly different denominators, the results are consistent with our study.
Implications for clinical practice
The results of this study demonstrate that no additional monitoring should be needed for donor sperm IVF pregnancies in the early stages of pregnancy when compared with women having IVF with their partner’s sperm. Women can be reassured that there is no higher risk of early pregnancy loss or ectopic pregnancy beyond that of their background risk.
Implications for research
A significant limitation of this study is the lack of information with regards to the age of the male partner. Further research should be carried out to assess whether the reduction in miscarriage rates remain after male age is accounted for. Further studies should attempt to elucidate the mechanism through which the male may contribute to miscarriage and possible treatments to manage this.
Conclusion
Women mandated to have donor sperm for conception can be reassured that they are at no greater risk of early pregnancy complications than their counterparts using partner sperm. Clinicians, service providers, and commissioners can be reassured that once a pregnancy is achieved, there is a higher rate of live birth with a lower rate of early pregnancy loss and ectopic pregnancy, meaning that donor sperm pregnancies will put no additional strain on early pregnancy services compared with partner sperm pregnancies.
Data availability
The data underlying this article are available freely at HFEA website: https://www.hfea.gov.uk/about-us/data-research/#:~:text=The%20HFEA%20holds%20information%20on%20fertility%20treatments%2C%20patients%2C,running%20database%20of%20its%20kind%20in%20the%20world.
Authors' roles
C.A. designed and carried out the protocol, performed the analysis, and wrote the manuscript. D.M. advised on the statistical analysis, gave input to the protocol and manuscript, and approved the final version. S.B. helped to design the protocol, gave input to the analysis and manuscript, and approved the final version. A.M. conceived the project, helped to design the protocol, gave input to the analysis and manuscript, and approved the final version.
Funding
No external funding was sought for this study.
Conflicts of interest
C.A. has received funding from Ferring to attend a UK meeting for trainees in reproductive Medicine. A.M. has received funding from Ferring, Cook, Merck Serono, Geodon Ritcher, and Pharmasure for speaking at, or attending, meetings relating to reproductive medicine. She has also participated in a Ferring advisory board. S.B. has received grants from Tenovus and the UK Medical Research Council. She has also been supported with a Medical Research Scotland PhD studentship.



