-
PDF
- Split View
-
Views
-
Cite
Cite
Shih-Chieh Lin, Wan-Ning Li, Shin-Chih Lin, Haun-Tzu Hou, Ya-Chuan Tsai, Tin-Chien Lin, Meng-Hsing Wu, Shaw-Jenq Tsai, Targeting YAP1 ameliorates progesterone resistance in endometriosis, Human Reproduction, Volume 38, Issue 6, June 2023, Pages 1124–1134, https://doi.org/10.1093/humrep/dead071
Close - Share Icon Share
Abstract
Does YAP1 inhibition alleviate progesterone resistance in endometriosis?
YAP1 inhibition reduces progesterone resistance in vitro and in vivo.
Progesterone resistance not only causes treatment failure for endometriosis but also inhibits eutopic endometrial cell proliferation, dysregulates decidualization, and reduces the success rates of pregnancy. Hippo/yes-associated protein 1 (YAP1) signaling pathway plays an important role in the pathogenesis of endometriosis.
Paraffin-embedded tissues containing paired endometriotic and endometrial specimens (n = 42) and serum samples isolated from normal controls (n = 15) or endometriotic patients with (n = 25) or without (n = 21) prior dienogest treatment were analyzed. A mouse model of endometriosis was also used to evaluate the effects of YAP1 inhibition on progesterone resistance.
Primary endometriotic and endometrial stromal cells treated with YAP1 inhibitor or miR-21 mimic/inhibitor were used for the in vitro studies including decidualization induction, chromatin immunoprecipitation (ChIP), and RNA immunoprecipitation. Tissue specimens and serum from human and mouse were used for immunohistochemistry staining, exosome isolation, and microRNA (miRNA) quantification, respectively.
Herein, we report, by using ChIP-PCR and RNA-IP, that YAP1 inhibits progesterone receptor (PGR) expression through upregulation of miR-21-5p. Upregulation of miR-21-5p not only reduces PGR expression but also inhibits endometrial stromal cell decidualization. Indeed, levels of YAP1 and miR-21-5p are inversely correlated with the level of PGR in human endometrial samples. In contrast, knockdown of YAP1 or treatment with verteporfin (VP), a YAP1 inhibitor, reduces miR-21-5p expression, thus leading to an increase in PGR expression in ectopic endometriotic stromal cells. In the mouse model of endometriosis, treatment with VP increases PGR expression and enhances decidualization. More importantly, VP synergistically increases the treatment effect of progestin in causing the regression of endometriotic lesions and improves the decidualization capability of the endometrium. Interestingly, treatment with dienogest, a synthetic progestin, reduces YAP1 and miR-21-5p expression in human cells and in the mouse model of endometriosis. Patients who received dienogest treatment for 6 months show a significant decrease in serum extracellular vesicle-associated miR-21-5p level.
A public dataset (GSE51981) containing a large cohort of endometriotic tissues is available from the Gene Expression Omnibus (GEO).
A large cohort of clinical samples is needed to verify the current diagnostic value of miR-21-5p in future studies.
The reciprocal regulation of YAP1 and PGR suggests that combined YAP1 inhibitor and progestin may be a better therapeutic approach for treating endometriosis.
This study was supported by the Ministry of Science and Technology, Taiwan (MOST-111-2636-B-006-012, MOST-111-2314-B-006-075-MY3, and MOST-106-2320-B-006-072-MY3). The authors have no conflict of interest to disclose.
Introduction
Endometriosis is considered a hormone-dependent disease. Therefore, most current medicinal treatments for endometriosis focus on depleting systemic hormones, especially estrogen concentrations. Progestins are synthetic progesterone receptor agonists and are widely used hormonal therapeutic compounds, which induce anti-estrogenic, pro-apoptotic, anti-inflammatory, and anti-neurogenic effects (Gezer and Oral, 2015). As a result, it allows pain relief and interruption of pathogenic mechanisms within the endometriotic lesions. However, some patients only partially or do not respond to progestin therapy at all (Barra et al., 2019). There are several possible reasons for the treatment failure, but a reduction in progesterone receptor B (PGR-B), the functional variant of PGR, in endometriotic lesions seems to be an important one.
The mechanism of causing PGR-B reduction in endometriotic lesions is not clearly characterized. It has been reported that the promoter of PGR-B is hypermethylated, leading to the downregulation of its expression (Wu et al., 2006). However, this DNA hypermethylation theory only explains a minute amount of PGR-B downregulation as it only partially affects the promoter of PGR-B in epithelial cells but not in stromal cells (Wu et al., 2008). Thus, there must be some other mechanisms that cause the downregulation of PGR-B in endometriotic cells.
Our previous series of studies demonstrated that hypoxia is an important regulator in controlling endometriosis development and progression (see Wu et al., 2019 for review). By closely re-examining these data, we reason that hypoxia may also regulate the expression of PGR-B in endometriotic lesions. First of all, the expression of estrogen receptor (ER)-β is significantly increased in ectopic endometriotic tissue by hypoxia (Wu et al., 2012). It has been shown that increased ER-β leads to the suppression of ER-α and PGR expression and disturbs progesterone signaling (Burney et al., 2007; Reis et al., 2020). Second, yes-associated protein 1 (YAP1), a master regulator downstream of HIF-1α and an effector of the Hippo pathway, is overexpressed and activated in endometriotic cells due to hypoxia-mediated transcriptional repression of its upstream inhibitor, LAST1 (Lin et al., 2017). In addition, we have shown that hypoxia induces anthrax toxin receptor 2 (ANTXR2) upregulation via histone modification of the ANTXR2 promoter (Lin et al., 2019). Activation of ANTXR2 signaling further increases YAP1 nuclear activity. Treatment with a YAP1 inhibitor, verteporfin (VP), to block YAP1 activity can effectively inhibit estradiol biosynthesis (Lin et al., 2017), suggesting that Hippo/YAP1 pathway may play a role in steroid responsiveness such as progesterone insensitivity.
Based on these observations and the current concepts of endometriosis, we hypothesized that the Hippo/YAP1 pathway, which is activated by hypoxia, plays an important role in endometriosis treatment failure via the downregulation of PGR-B. Since PGR is critical in endometrial decidualization and embryo implantation, downregulation of PGR-B by the Hippo/YAP1 pathway may also contribute to the infertility of patients with endometriosis. Herein, we report that activation of YAP1 signaling suppresses PGR expression and subsequently reduces progesterone sensitivity and decidualization. Blocking YAP1 activity by its inhibitor improves progesterone sensitivity and enhances the decidualization capability in human endometriotic cells and in a mouse model of endometriosis.
Materials and methods
Collection of human endometriotic specimens
Specimens such as sera and tissues were collected from the endometriosis-free (defined as control) and endometriotic patients in the Department of Obstetrics and Gynecology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University. This study was approved by the Institutional Review Board of the National Cheng Kung University Medical Center and informed consent was obtained from each patient. Patient information was shown in our previous study (Li et al., 2020).
Isolation and culture of primary eutopic and ectopic stromal cells
Detail procedures for the isolation of primary eutopic and ectopic stromal cells were described in our previous studies (Lin et al., 2019). Both eutopic and ectopic stromal cells were cultured in Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F-12 HAM (DMEM/F12) medium with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 µg/ml). Culture media were changed every 2 days. In addition, mycoplasma contamination was routinely checked by using DAPI staining for cells and PCR method for the culture medium.
In vitro decidualization
In vitro decidualization (IVD) was performed according to the published procedure (Michalski et al., 2018) with modifications. Briefly, eutopic or ectopic stromal cells (2 × 105) were treated with 10 nM estradiol (E2), 1 μM medroxyprogesterone acetate, and 50 μM cyclic adenosine monophosphate (cAMP) in DMEM/F12 medium (EPA media) containing 2% charcoal-stripped FBS (CS-FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml), for 6 days (EPA media were changed every 2 days).
Drug, siRNA, and miR-21 mimic/inhibitor treatments
Eutopic stromal cells (2 × 105) were cultured in 1% CS-FBS DMEM/F12 media for 48 h and then treated with different doses of Dienogest (Bayer, Germany) for another 24 h. For gene intervention study, cells were transfected with siRNA against YAP1 or PGR, or with miR-21 mimic or inhibitor (Thermo Fisher Scientific, Waltham, MA, USA) for 24 h and subjected to subsequent assessments. The concentration of siRNA used was 40 nM and the concentration of miR-21 inhibitor/mimic used was 50 nM.
RNA isolation and quantitative RT-PCR
Total RNA and microRNA from primary stromal cells of human/mice specimens were isolated by using TRIzol reagent (Invitrogen, Thermo Fisher Scientific) according to the manufacturer's instructions. RNA concentration was determined by Nanodrop spectrophotometer (ND-1000, NanoDrop, Wilmington, DE, USA). Reverse transcription was performed by random hexamer (Genomics, New Taipei City, Taiwan) at 42°C for 90 min followed by 95°C for 10 min. In contrast, miR-21- and U6 snRNA- (an internal control) specific reverse transcription was performed by using TaqMan MicroRNA Assays (Invitrogen, Thermo Fisher Scientific). Expression levels of mRNA and miR-21 were quantified by using StepOnePlus real-time PCR system (Applied Biosystem, Foster City, CA, USA) with SYBR Green (Applied Biosystems, Thermo Fisher Scientific) or TaqMan (Applied Biosystems, Thermo Fisher Scientific) dye. Primer sequences are listed in Supplementary Table SI.
AGO2-RNA immunoprecipitation assay
miR-21-mimic-treated eutopic stromal cells were exposed to UV light (254 nm) for crosslinking of AGO2 protein and interacting RNA. Cell pellets were collected and lysed by RIPA buffer containing RNasin® Ribonuclease Inhibitor (Promega, Madison, WI, USA). Control or AGO2 antibody (10 µg) was individually added to samples with 500 µg of protein at 4°C overnight. Next, RNA immunoprecipitation (RIP) samples were incubated with Dynabeads magnetic beads (Thermo Fisher Scientific) for 2 h followed by washing with Diethyl pyrocarbonate (DEPC)-treated PBS 12 times (5 min/time). Finally, TRIzol reagents were added into the RIP samples containing magnetic beads for RNA isolation.
Chromatin immunoprecipitation assay
PGR-knocked down eutopic or YAP1-knocked down ectopic stromal cells (2 × 106) were fixed by 1% formaldehyde for 10 min at room temperature and then fragmented by a sonicator. Next, PGR or YAP1 antibodies (10 µg) were added into the samples for pulling down PGR- or YAP1-DNA complexes and incubated at 4°C overnight. Then, DNA was isolated by washing, reverse-crosslinking by treating with protease K (0.25 µg/ml) for 40 min at 57°C and purifying via phenol/chloroform method. Finally, the precipitated DNA was amplified by primers for verifying the PGR- or YAP1-interacting DNA region. Primer sequences and antibody information are listed in Supplementary Tables SI and SII, respectively.
Immunohistochemistry
Paraffin-embedded normal endometrial and endometriotic tissues were cut into 5-μm-thick sections and incubated with the first antibody at 4°C overnight after dewaxing, antigen retrieval, and blocking. Antigen retrieval was performed by incubating tissue slices in citric acid buffer (pH 6.0) at 95°C for 20 min. Samples were washed and incubated with a second antibody at room temperature for 1 h. The immunohistochemistry signal was detected by AEC substrate buffer (Bio SB, Inc., Santa Barbara, CA, USA) and counter-stained by hematoxylin (Bio SB, Inc.). All the staining results were scanned and quantified by the TissueFaxs scanning system in the core facility of National Cheng-Kung University.
Mouse model of endometriosis
To set up the mouse model of endometriosis, 8- to 10-week-old female (C57BL/6NCrj) mice were purchased from the Animal Center at the College of Medicine, National Cheng Kung University. One side of the uterus was removed from the female mouse. Next, endometrial tissue was scraped from the removed uterus and cut into four pieces of the same size. Then, endometria were sealed in the peritoneal cavity of the same mouse and incubated for 1 month. VP (10 mg/kg body weight), dienogest (2 mg/kg body weight), or a combination of the two drugs was individually given to the mice three times a week for one more month via intraperitoneal injection. Lesion and sera were collected at the time mice were sacrificed. All animal procedures were approved by the Institutional Animal Care and Use Committee in Laboratory Animal Center, College of Medicine, National Cheng Kung University.
Extracellular vesicles’ extraction
Serum-free conditioned media from eutopic and ectopic stromal cells or sera from endometriosis patients and mice were centrifuged at 3000g for 10 min to remove debris. Next, extracellular vesicles (EVs) from the above samples were isolated by using a size-exclusion chromatography column (HansaBioMed, Tallinn, Estonia) as previously described (Li et al., 2020).
Bioinformatics analyses
For Gene Set Enrichment Analysis (GSEA), YAP1 and miR-21 signatures from the molecular signatures database in GSEA were analyzed from GSE7700 and GSE52674 datasets, respectively. These gene signatures were further analyzed by GSEA using another set of data that contains expression levels of genes in ectopic endometriotic stromal cells treated with or without decidualization reagents (GSE75423). For whole genome correlation analysis, levels of YAP1, miR-21-5p, PGR, and its downstream targets from the endometriosis dataset (GSE51981) were analyzed by Pearson’s correlation analysis and presented as a heat map.
Statistical analysis
The results were presented as means ± standard error of the mean. Differences between the two groups were analyzed by using Student’s t-test. Furthermore, differences over three groups were analyzed by using one-way analysis of variance followed by a post hoc analysis using Tukey’s multiple analysis test. All the statistical analyses were performed by using a commercial statistical software (GraphPad Prism 9, GraphPad Software, and San Diego, CA, USA). Statistical significance was set at P < 0.05.
Results
Progesterone receptor is negatively regulated by YAP1 via miR-21-5p
To explore the effects of YAP1 in endometriosis treatment failure, specifically in progesterone resistance, we evaluated the expression level of PGR in endometriotic stromal cells with YAP1 knockdown. The results showed that levels of PGR total form and PGR-B, the functional isoform of PGR, were markedly elevated after YAP1 knockdown in endometriotic stromal cells (Fig. 1A). To further investigate the underlying mechanism of how YAP1 regulates PGR expression in endometriosis, we analyzed YAP1 chromatin immunoprecipitation (ChIP)-Seq data from ChIP-atlas (https://chip-atlas.org/) and identified miR-21-5p as a downstream target of YAP1 (Supplementary Fig. S1A). In addition, in silico analysis of potential miRNAs that target PGR predicted miR-21-5p as a potential miRNA to target PGR (Supplementary Fig. S1B). These two lines of evidence suggest that YAP1 may regulate PGR expression through miR-21-5p-dependent actions in endometriosis. Indeed, the ChIP-PCR data confirmed the binding of YAP1 to the promoter of miR-21-5p, and this was inhibited by treatment with the YAP1 inhibitor, VP (Fig. 1B). Knockdown of YAP1 causes miR-21-5p downregulation (Fig. 1C) while overexpression of miR-21-5p in normal endometrial stromal cells reduced the levels of PGR and PGR-B (Fig. 1D). RNA-IP assay further demonstrated the binding of miR-21-5p/Ago2 complex to the predicted sequence of PGR (Fig. 1E). Taken together, these data reveal that PGR is negatively regulated by YAP1 via the function of miR-21-5p in endometriosis.
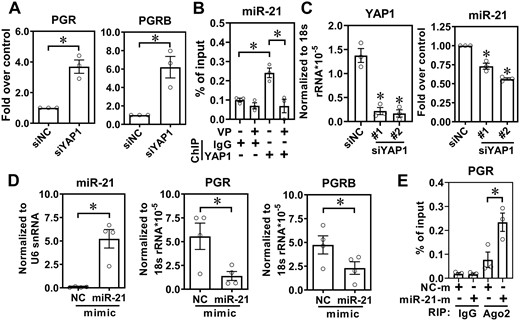
Levels of progesterone receptor (PGR) are suppressed by yes-associated protein 1 (YAP1) via miR-21-5p. (A) PGR and PGR-B levels were quantified in human ectopic endometriotic stromal cells with or without YAP1 knockdown for 48 h (n = 3). siNC: knockdown control. (B) Chromatin immunoprecipitation (ChIP)-PCR assay shows the binding of YAP1 to the miR-21-5p promoter. Ectopic endometriotic stromal cells were treated with vehicle or verteporfin (VP) for 24 h and the ChIP was performed by control IgG or anti-YAP1 antibody. The level of YAP1 binding to the gene locus was analyzed by real-time PCR quantification (n = 3). (C) YAP1 and miR-21-5p expression levels were determined in human endometriotic stromal cells with or without YAP1 knockdown for 48 h (n = 3). (D) Control mimic (NC) or miR-21-5p mimic (50 nM) was transfected into normal endometrial stromal cells for 48 h. Levels of miR-21-5p (left panel), PGR (middle panel), and PGR-B (right panel) were determined by RT-qPCR (n = 4). (E) Normal endometrial stromal cells were transfected with control RNA (NC-m) or miR21 mimics (miR21-m) for 48 h. The binding of miR-21/Ago2 complex to the PGR 3′UTR was immunoprecipitated with control IgG or anti-Ago2 antibody followed by RT-qPCR (n = 3). Asterisks (*) indicate P < 0.05. Results were presented as mean ± SEM.
Abnormal expression of YAP1/miR-21-5p/PGR in endometriotic tissues
To verify the clinical expression profiles of YAP1, miR-21-5p, and PGR in endometriosis, RNA levels were quantified in paired eutopic endometrial and ectopic endometriotic tissues by RT-qPCR. The results showed that the miR-21-5p level was elevated while PGR and PGR-B levels were reduced in the ectopic endometriotic tissues as compared to their eutopic counterparts (Fig. 2A and B). Immunohistochemistry (IHC) staining demonstrated that PGR was mainly expressed in the stromal region of endometrial tissue and its level was decreased in the ectopic endometriotic tissue (Fig. 2C and D). Examination of serial sections of endometriotic tissues revealed that the level of PGR is negatively correlated with that of YAP1 in the same regions (Fig. 2E). Quantification of RNA from paired eutopic and ectopic samples showed that levels of YAP1 and miR-21-5p are positively correlated while PGR is negatively correlated with both YAP1 and miR-21-5p (Fig. 2F). Analysis of a public dataset containing a large cohort of clinical specimens of endometriosis also showed similar results (Fig. 2G). Taken together, these data demonstrate that the elevation of YAP1 suppresses PGR expression in endometriotic samples.
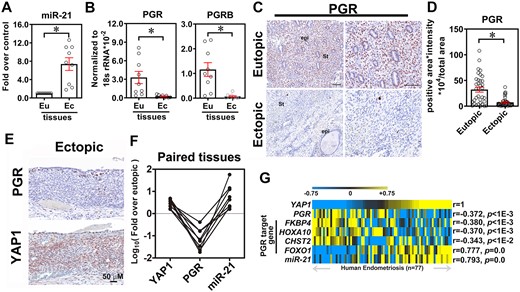
Expression of yes-associated protein 1 (YAP1), miR-21-5p, and progesterone receptor (PGR) in the clinical specimens of endometriosis. Expression levels of miR-21-5p (A) and PGR and PGR-B (B) were quantified in the paired endometrial and endometriotic tissues by qRT-PCR (n = 9 pairs of eutopic (Eu) and ectopic (Ec) samples). Asterisks (*) indicate P < 0.05. Results were presented as mean ± SEM. Representative images (C) and quantified results (D) of PGR in paired Eu endometrial and Ec endometriotic tissues (n = 42). St: stromal cells, Epi: glandular epithelial cells. Asterisk indicates p < 0.05. (E) Representative immunohistochemistry images show the inverse correlation of PGR (upper panel) and YAP1 (lower panel) in Ec endometriotic tissues. (F) Expression levels of YAP1, miR-21-5p, and PGR from the same paired endometriotic specimens were quantified by RT-qPCR. Data were presented as fold over Eu (n = 7). (G) Heat map showed the correlation analyses of YAP1, miR-21-5p, PGR, and its downstream targets in the public dataset (GSE51981) containing a large cohort of endometriotic tissues. Statistical test was performed by using Pearson's correlation test.
YAP1-induced miR-21-5p represses decidualization of eutopic endometrial stromal cells
To test whether elevated YAP1 and miR-21-5p impair the decidualization of endometrial stromal cells, we first performed GSEA in the dataset of ectopic endometriotic stromal cells treated with decidualization reagents (GSE75423). The results showed that both YAP1 and miR-21-5p signatures were enriched with the phenotype of decidualization (Fig. 3A), suggesting that both of them may play roles in the decidualization process. The levels of prolactin (PRL) and insulin-like growth factor binding protein 1 (IGFBP1), two decidualization markers, in normal endometrial stromal cells were markedly induced by EPA media, which induces decidualization, and these markers were repressed by overexpression of miR-21-5p (Fig. 3B). In contrast, ectopic endometriotic stromal cells responded poorly to EPA media, but the response was significantly enhanced when cells were pre-transfected with miR-21-5p inhibitor (Fig. 3C) or treated with VP (Fig. 3D).
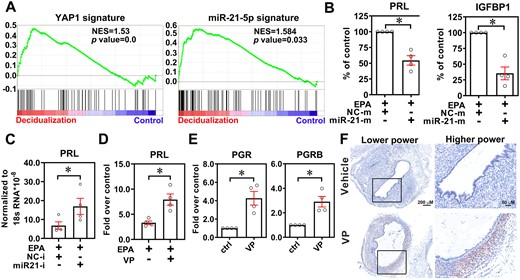
Inhibition of yes-associated protein 1 (YAP1) restores progesterone receptor (PGR) expression and progesterone response. (A) YAP1 and miR-21-5p gene signatures were generated from GSE7700 and GSE52674, respectively. Next, the gene signatures were cross-referenced by the GSEA tool with a dataset of ectopic endometriotic stromal cells treated with or without decidualization reagents (GSE75423). NES: normalized enrichment score. (B) Prolactin and IGFBP1 expression levels were analyzed in normal endometrial stromal cells treated with or without miR-21-5p mimic (50 nM) in the presence of decidualization reagents (EPA) for 6 days (n = 4). Asterisks (*) indicate P < 0.05. (C) Levels of prolactin mRNA in ectopic endometriotic stromal cells treated with EPA with or without control inhibitor (NC-i) or miR-21-5p inhibitor (miR21-i, 50 nM) transfection. Asterisk (*) indicates P < 0.05. (D) Expression of prolactin in ectopic endometriotic stromal cells treated with control or decidualization reagents (10 nM estradiol, 1 μM medroxyprogesterone acetate, and 50 μM cAMP, EPA) for 6 days (n = 4) with or without verteporfin (VP, 61.25 nM) pretreatment. Asterisk (*) indicates P < 0.05. (E) PGR and PGR-B mRNA levels in ectopic endometriotic stromal cells treated with or without VP (61.25 nM) for 6 days (n = 4). Asterisks (*) indicate P < 0.05. (F) Representative immunohistochemistry images show the expression of PGR in ectopic endometriotic lesions derived from mice treated with vehicle or VP (25 mg/kg body weight) for 1 month.
Since blockage of YAP1 function by VP promotes PGR expression in vitro (Fig. 3E), we hypothesized that treatment with VP may increase progesterone sensitivity. First, we evaluated the expression of PGR in the mouse model of endometriosis and found that the level of PGR in the endometriotic lesion was very low or even undetectable (Fig. 3F). Treatment with VP markedly induced PGR, mostly PGRB expression. Immunohistochemistry staining revealed that the major cell type with PGR upregulation was the stromal cell (Fig. 3F). Taken together, these findings suggest that targeting YAP1 may improve progesterone sensitivity via blocking miR-21-5p-mediated PGR downregulation.
Progesterone agonist combined with YAP1 inhibitor improves the therapeutic efficacy in the mouse model of endometriosis
Since dienogest was used as a therapeutic drug for endometriosis patients, we treated ectopic endometriotic stromal cells with different doses of dienogest and unexpectedly found that the expression levels of YAP1 and miR-21-5p were decreased in a dose-dependent manner (Fig. 4A and B). To confirm this finding, PGR was knocked down in eutopic endometrial stromal cells and the levels of YAP1 and miR-21-5p were quantified. The results show that the knockdown of PGR significantly upregulated YAP1 and miR-21-5p (Fig. 4C and D and Supplementary Fig. S2A). The PGR-binding element (PRE) in the YAP1 promoter and miR-21-5p promoter was identified by bioinformatic prediction (Supplementary Fig. S2B and C) and ChIP assay confirmed that PGR binds to the PREs in YAP1 and miR-21-5p, respectively (Fig. 4E and F).
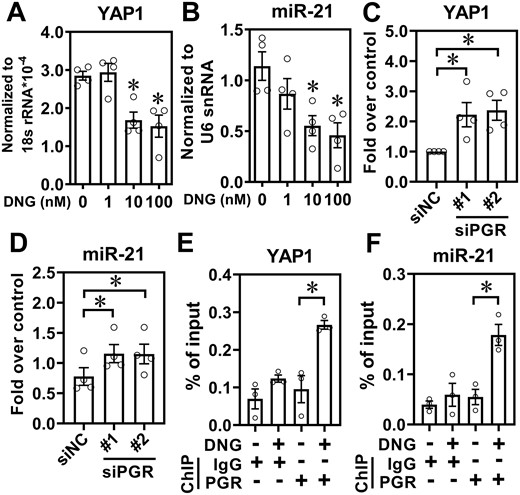
Inhibition of yes-associated protein 1 (YAP1) and miR-21-5p by progesterone. Normal endometrial stromal cells were treated with different doses of dienogest (DNG, 1–100 nM) for 24 h and levels of YAP1 (A) and miR-21-5p (B) were quantified by RT-qPCR (n = 3). (C and D) Knockdown of progesterone receptor de-repressed YAP1 and miR-21-5p. Normal endometrial stromal cells were transfected with scramble siRNA (siNC) or siRNA targeting progesterone receptor (PGR) (siPGR) for 48 h and the levels of YAP1 (C) and miR-21-5p (D) were quantified (n = 4). Normal endometrial stromal cells were treated with vehicle or DNG (10 nM) for 24 h and chromatin immunoprecipitation (ChIP)-PCR was performed to determine the binding of PGR to the promoters of YAP1 (E) and miR-21-5p (F), respectively (n = 3). Asterisks indicate P < 0.05.
Next, we evaluated whether dienogest can improve the decidualization capacity of endometriotic stromal cells. Our results showed that treatment with dienogest failed to induce PRL expression (Fig. 5A), suggesting that dienogest alone was not able to trigger decidualization. In contrast, treatment with VP alone significantly increased PRL expression, and the combined treatment with dienogest and VP compound had synergistic effects on PRL expression in ectopic endometriotic stromal cells (Fig. 5A). Finally, a mouse model of endometriosis was used to verify the treatment efficacy of the combined therapies of dienogest and VP (Fig. 5B). Treatment with dienogest failed to ameliorate endometriotic lesions (Fig. 5C). VP alone reduced the mouse endometriotic lesion size and markedly reduced the lesion size when combined with dienogest (Fig. 5C). Importantly, VP alone or in combination with dienogest did not affect the intact uterus (Fig. 5D and Supplementary Fig. S3). Taken together, these results suggest that dienogest combined with VP might has greater treatment efficacy for endometriosis patients who are progesterone resistant.
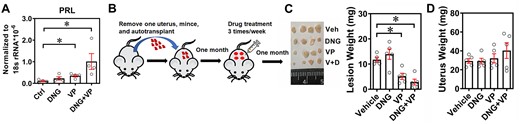
Dienogest (DNG) and verteporfin synergistically improve the endometriosis treatment efficacy. (A) Ectopic endometriotic stromal cells were treated with vehicle, verteporfin (VP, 61.25 nM) alone, DNG alone (10−9 M), and combined treatments of VP (61.25 nM) and DNG (10−9 M) for 3 days. Levels of PRL were quantified by RT-qPCR (n = 4). Asterisks (*) indicate P < 0.05. (B) Schematic drawing of autograft and drug treatment protocol. (C) Representative picture (left panel) and quantitative results (right panel) of lesions derived from mice treated with vehicle (Veh), DNG (2 mg/kg body weight), VP (10 mg/kg body weight), and DNG plus VP (V + D). Asterisks (*) indicate P < 0.05. (D) Quantitative results of intact uterine weight from different treatment groups.
Exosomal miR-21-5p has clinical applications for endometriosis
The unexpected finding that dienogest treatment leads to downregulation of YAP1 and miR-21-5p in ectopic endometriotic stromal cells prompted us to investigate whether YAP1 and miR-21-5p can serve as biomarkers to predict the status and treatment outcomes of endometriosis. Since non-invasive or minimal invasive markers are the first priority of choice, we aimed to detect the presence of YAP1 and miR-21-5p in liquid biopsies. EVs were isolated from eutopic endometrial and ectopic endometriotic stromal cells and subjected to YAP1, miR-21-5p, and PGR measurement. Among them, only miR-21-5p was detected in the EVs isolated from eutopic endometrial and ectopic endometriotic stromal cells and showed an increasing pattern in the EVs derived from ectopic endometriotic stromal cells (Fig. 6A).
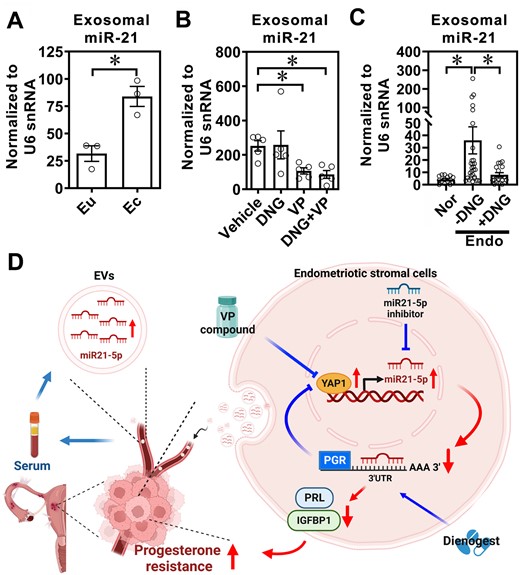
Extracellular vesicle-miR-21-5p as a serum biomarker for endometriosis. (A) Levels of miR-21-5p were quantified in EVs isolated from eutopic (Eu) endometrial stromal cells and ectopic (Ec) endometriotic stromal cells (n = 3/each). (B) Sera EV-miR-21-5p in endometriosis-like mice treated with vehicle, dienogest (DNG), verteporfin (VP), or both (DNG + VP) for 1 month. Extracellular vesicles were isolated by using a size-exclusion chromatographic column and EV-miR-21-5p was quantified using real-time RT-qPCR. (C) Levels of miR-21-5p were quantified in EVs isolated from sera of normal patients (Nor, n = 15), endometriosis (Endo) patients without DNG treatment (−DNG, n = 25), and endometriosis patients who had received DNG treatment for 6 months (+DNG, n = 21). The levels of miR-21-5p were measured by RT-qPCR. Asterisks (*) indicate P < 0.05. (D) Schematic drawing of working model.
We next quantified the levels of miR-21-5p in EVs isolated from sera of mice treated with vehicle, dienogest, VP or VP plus dienogest. The results showed that EV-miR-21-5p levels were low in control mouse sera (data not shown), elevated in sera derived from mice with endometriosis-like lesions, and low again in VP- and VP plus dienogest-treated mice sera (Fig. 6B). Finally, we analyzed miR-21-5p levels in the EVs isolated from sera of normal controls or endometriosis patients with or without dienogest treatments. The results showed that miR-21-5p levels were elevated in the EVs isolated from sera of endometriosis patients while treatment with dienogest for 6 months markedly reduced the miR-21-5p levels (Fig. 6C).
Discussion
It is well known that endometriosis is an estrogen-dependent gynecological disease in women of reproductive age (Wang et al., 2020); thus, progesterone-based and antiestrogen-based therapies are the current treatment strategies for endometriosis patients. Synthetic progestins that specifically bind to progesterone receptors with an antiandrogenic effect are recommended as the first line long-term medical treatment of endometriosis. However, not all endometriosis patients respond to progesterone-based therapy. Therefore, overcoming progesterone resistance is a crucial issue in clinical practice. Herein, we report that YAP1 inhibits PGR-B via transcriptionally upregulating miR-21-5p. miR-21-5p binds to PGR mRNA and causes its degradation, which results in reducing the progesterone responsiveness. Treatment with VP inhibits YAP1 activity, enhances PGR-B expression, and restores progesterone responsiveness. Interestingly, dienogest suppresses YAP-1 and miR-21-5p expression, which may synergize the overall treatment effect (Fig. 6D). Taken together, our data suggest that targeting YAP1 may be a potential approach to improve progesterone responsiveness for treating endometriosis patients.
One of the potential factors causing the attenuation of progesterone response in endometriosis is reduced expression of PGR in endometriotic cells. The PGR gene has been known to encode PGR-A and PGR-B isoforms (Kastner et al., 1990). Several studies have reported that loss of total PGR and PGR-B isoforms is observed in endometriotic lesions (Bulun et al., 2012; Bedaiwy et al., 2015). Although DNA hypermethylation of the PGR-B promoter (Wu et al., 2006) has been shown to correlate with PGR-B downregulation, the same group has reported that this only causes partial DNA methylation and occurs in glandular epithelial cells but not in endometrial stromal cells (Wu et al., 2008). Another study reported that overexpression of miR-194-3p might be the reason for PGR downregulation in eutopic endometrium of women with endometriosis; however, this conclusion is questionable because of inconsistent or even conflicting data presented in the paper (Pei et al., 2018). Therefore, we reason that there are unrevealed mechanisms responsible for PGR-B downregulation in the eutopic endometrium of women with endometriosis. In this study, we have demonstrated that YAP1 activation and miR-21-5p overexpression cause PGR, especially PGR-B, downregulation. In paired human samples, levels of YAP1 and miR-21-5p are positively correlated with each other but inversely correlated with PGR. Forced expression of miR-21-5p reduces PGR-B expression in human endometrial stromal cells. In contrast, knockdown of YAP1 or treatment with VP to block YAP1 activity results in PGR upregulation in human ectopic endometriotic stromal cells and in a mouse model of endometriosis, respectively. These data provide solid evidence that PGR is downregulated by YAP1 via miR-21-5p in endometriotic lesions.
To address whether YAP1-inhibited PGR expression results in progesterone resistance, we evaluate the decidualizing capacity of stromal cells since it has been known that progesterone is crucial for decidualization (Large and DeMayo, 2012). A previous study has shown that both endometriotic and endometrial stromal cells isolated from women with endometriosis display impaired capacities for decidualization, by comparing to stromal cells isolated from healthy endometrium (Klemmt et al., 2006). A recent study revealed that overexpression of miR-196a-5p represses PGR and the decidualization process in the eutopic endometrium of women with endometriosis (Zhou et al., 2016). However, the miR-196a-5p effect is only temporary since there is no difference between the control and miR-196a mimic groups at 72 h after treatment (Zhou et al., 2016). Our data show that forced expression of miR-21-5p reduced the expression of PRL and IGFBP1, two decidualization markers, even 6 days after transfection, indicating that the effect is persistent. More importantly, we showed that treatment with a YAP1 inhibitor enhanced the decidualizing capacity in vitro and in vivo. These data provide evidence to support that reduced PGR due to YAP1 and miR-21-5p overexpression in ectopic endometriotic cells is likely an important factor in causing progesterone resistance.
The main purpose of endometriosis treatment is to relieve the pain and prevent repeated surgery. In the past decades, nonsteroidal anti-inflammatory drugs, GnRH agonists, oral contraceptives, and oral progestin have mainly been used in clinical practice for endometriosis patients (Vercellini et al., 2014; Falcone and Flyckt, 2018). However, some of those drugs will cause unfavorable side effects after long-term use. For example, long-term use of GnRH agonists such as leuprolide results in menopausal symptoms and loss of bone density (Jeng et al., 2014). Recently, dienogest (2 mg/day) is a new medical treatment widely used for endometriosis patients because it is safe and significantly effective in the reduction of chronic pelvic pain and in improving quality of life when compared to oral contraceptive pills (El Taha et al., 2021; Piacenti et al., 2021). However, there are some unresolved issues such as the observations that not all endometriosis patients respond to dienogest treatment (Uludag et al., 2021), that endometriotic lesions do not totally disappear after long-term dienogest treatment (Andres et al., 2015; Vignali et al., 2020; Uludag et al., 2021), and that dienogest-treated endometriosis patients over 10 months have been reported to have a higher endometriosis reoperation rates than control patients (Seo et al., 2021). These pieces of clinical evidence indicate that improvements in the response rate and treatment efficacy of dienogest for endometriosis patients are crucial issues in clinical practice. Since reduced PGR levels have been observed in endometriotic lesions (Bulun et al., 2012; Bedaiwy et al., 2015), elevation of the level of PGR in endometriotic stromal cells might be a feasible way to enhance the treatment efficacy of dienogest. Herein, we have demonstrated that treatment with YAP1 inhibitor increases PGR levels and improves dienogest response rate in terms of increasing decidualization and reducing lesion sizes. Our data provide a proof of concept that inhibiting the Hippo/YAP1 pathway is a plausible strategy to improve treatment efficacy for endometriosis patients, especially for those who are progesterone resistant.
Another important issue that remains unresolved in endometriosis is the lack of reliable non-invasive biomarkers for diagnosis and prognosis. A previous study aimed to identify plasma miRNAs as biomarkers and found that three sets of miRNA signatures might be useful to predict the presence of endometriosis. However, further studies showed that the specificity was only 37% in a separate validation cohort (Vanhie et al., 2019). We reason that this might be due to the fact that they directly measured the free miRNA levels in blood samples, which contain many confounding factors influencing the detection of miRNA levels. It is known that cargos carried by EVs are devoid of mechanical and enzymatical degradations and are relatively stable. Indeed, our previous study showed that EV-VEGF-C has better sensitivity and specificity in differentiating women with or without endometriosis (Li et al., 2020). In this study, we have shown that EV-miR-21-5p is a reliable biomarker for diagnosis and prognosis. EV-miR-21-5p is low in endometriosis-free women and elevated in women with endometriosis. More importantly, patients who had received dienogest treatment for 6 months show a significant reduction in plasma EV-miR-21-5p. This reciprocal regulation suggests that miR-21-5p not only is a good biomarker but also an ideal molecular target. Suppression of miR-21-5p will enhance PGR expression and thus increase progestin treatment efficacy, which will further reduce miR-21-5p levels and may result in better treatment outcomes.
Conclusion
Progesterone resistance is a critical issue in endometriosis treatment. We now provide scientific insight to unravel the underlying mechanisms responsible for the decreased progesterone sensitivity. Restoration of PGR in ectopic endometriotic lesions by inhibiting YAP1 signaling represents a logical strategy for increasing progesterone-based treatment efficiency and warrants further investigation by initiating a clinical trial.
Data availability
The data presented in the study are available from the Gene Expression Omnibus (GEO) repository and accession numbers are GSE51981, GSE7700, GSE52674, and GSE75423.
Acknowledgements
We are grateful for the technical support provided by Yi-Huan Yeh, Yen-Yu Lai, Yi-Tseng Tang, and Mei-Feng Huang, for their contributions to IHC staining, bioinformatic analysis, animal handling and surgery, and isolation of primary endometrial stromal cells, respectively.
Authors’ roles
Shih-Chieh Lin, W.-N.L., Shin-Chih Lin, H.-T.H., Y.-C.T., and T.-C.L. performed experiments and analyzed data. Shih-Chieh Lin, M.-H.W., and S.-J.T. conceived the project and wrote the article. All authors agreed on the final version of the article.
Funding
The Ministry of Science and Technology, Taiwan (MOST-111-2636-B-006-012 to Shih-Chieh Lin, MOST-111-2314-B-006-075-MY3 to M.-H.W., and MOST-106-2320-B-006-072-MY3 to S.-J.T.).
Conflict of interest
All authors declare no competing financial interests.



