-
PDF
- Split View
-
Views
-
Cite
Cite
Lea Lykke Harrits Lunddorf, Linn Håkonsen Arendt, Andreas Ernst, Nis Brix, Ulla Brent Knudsen, Jørn Olsen, Cecilia Høst Ramlau-Hansen, Maternal polycystic ovarian syndrome and pubertal development in daughters and sons: a population-based cohort study, Human Reproduction, Volume 37, Issue 11, November 2022, Pages 2623–2634, https://doi.org/10.1093/humrep/deac197
Close - Share Icon Share
Abstract
Does maternal polycystic ovarian syndrome (PCOS) affect the timing of pubertal development in daughters and sons?
Maternal PCOS was associated with earlier adrenarche in daughters.
Female adolescents with PCOS often experience earlier adrenarche compared to adolescents without PCOS, due to hyperandrogenism. Likewise, they usually have hyperandrogenism during pregnancy, which might potentially affect the development of the foetus, including its future reproductive health.
In this population-based cohort study, we included 15 596 mothers–child pairs from the Danish National Birth Cohort (DNBC) Puberty Cohort, who were followed from foetal life until full sexual maturation or 18 years of age.
Using register-based and self-reported information on maternal PCOS and menstrual irregularities, collected during pregnancy, we categorized the mothers as having PCOS (n = 251), oligomenorhoea (n = 134), ‘other menstrual irregularities’ (n = 2411) or no menstrual abnormalities (reference group, n = 12 800). The children provided self-reported information on pubertal development every 6 months from the age of 11 years. The main outcome measures were adjusted mean age differences (in months) at attaining several individual pubertal milestones using an interval-censored regression model, as well as the average difference in age at attaining all pubertal milestones combined into a single estimate using Huber–White robust variance estimation.
We found that maternal PCOS was associated with an accelerated pubertal development in daughters with an overall average difference of −3.3 (95% CI: −6.3; −0.4) months based on all pubertal milestones compared to the reference group. When further looking into the average difference for adrenarche only (pubarche, axillary hair and acne), the average difference was −5.4 (95% CI: −8.7; −2.1) months compared to the reference group; whereas thelarche and menarche did not occur earlier in daughters of mothers with PCOS (average difference: −0.8 (95% CI: −3.9; 2.4) months). Oligomenorrhoea and ‘other menstrual irregularities’ were not associated with pubertal development in daughters. Neither PCOS, oligomenorrhoea nor ‘other menstrual irregularities’ were associated with pubertal development in sons.
We expect some degree of non-differential misclassification of maternal PCOS and menstrual irregularities as well as pubertal development in the children.
Maternal PCOS might accelerate adrenarche in daughters. Whether this is due to genetics, epigenetics or prenatal programming by hyperandrogenism in foetal life remains unsolved. The results from the present study can be generalized to Caucasian populations.
The study is funded by the Faculty of Health at Aarhus University. The authors have no financial relationships or competing interests to disclose.
N/A.
Introduction
Polycystic ovarian syndrome (PCOS) is the most common endocrine pathology of women of reproductive age with an observed prevalence varying from 6% to 20% (Yildiz et al., 2012). Generally, PCOS is underdiagnosed due to its complexity and pronounced heterogeneity. Rather vague symptoms, such as prolonged or irregular menstrual cycles, might indicate PCOS, but might not necessarily lead to a diagnosis.
The major endocrine characteristic of PCOS is excess androgens (Ehrmann, 2005), primarily from the ovaries (Franks and Hardy, 2018), which usually persists during pregnancy (Sir-Petermann et al., 2002). Additionally, women with PCOS have high levels of GnRH, LH and anti-Müllerian hormone (AMH), as well as high ratio of LH/FSH (Sir-Petermann et al., 2002; Tata et al., 2018). Although testosterone is lipophilic and can cross the placental barrier, high placental aromatase activity will normally convert androgens to oestrogen. Nonetheless, maternal PCOS is associated with foetal hyperandrogenism perhaps due to altered aromatase activity, placental androgen overproduction (Maliqueo et al., 2013) or the effect of high AMH levels (Tata et al., 2018).
The origin of PCOS is relatively uncertain, but genetics, epigenetics and foetal programming through prenatal hyperandrogenism have been recognized as important potential aetiological factors of PCOS; thereby making PCOS a hereditary disorder (Filippou and Homburg, 2017; Sanchez-Garrido and Tena-Sempere, 2020; Stener-Victorin and Deng, 2021).
Studies have previously found that maternal PCOS increases the risk of pregnancy complications (Palomba et al., 2015) and adverse birth outcomes (Doherty et al., 2015), and that children of women with PCOS are at higher risk of congenital malformations (Doherty et al., 2015), overweight (Zhang et al., 2022), metabolic and neuropsychiatric disorders (Doherty et al., 2015; Dubey et al., 2021; Stener-Victorin and Deng, 2021), as well as altered hormonal profiles and inherited PCOS in daughters (Barry et al., 2010; Torchen et al., 2019). The causal link between maternal PCOS and pubertal development in the children is still unknown.
Puberty involves the adrenarche and the gonadarche. The adrenarche is the onset of androgen secretion from the adrenal cortex, which phenotypically results in pubarche, axillary hair growth, as well as differentiation and activation of apocrine and sebaceous glands in the skin in both sexes. In boys, these physiological changes will be accompanied by development of the genitals and vocal changes due to both adrenarche and gonadarche (activation of the hypothalamus–pituitary–gonads (HPG) axis). In girls, gonadarche results in development of the oestrogen-dependent pubertal milestones, thelarche and menarche.
In this study, we investigate whether the timing of pubertal development is altered in children exposed to maternal PCOS in foetal life compared to unexposed children. As studies have found premature adrenarche to be an initial sign in girls with PCOS (Voutilainen and Jääskeläinen, 2015), we hypothesize that daughters of mothers with PCOS have earlier adrenarche due to prenatal hyperandrogenism or inherited PCOS. Additionally, we hypothesize that prenatal hyperandrogenism induced by maternal PCOS can lead to earlier pubertal development in sons as well.
Materials and methods
Data source and study population
We used the Danish National Birth Cohort (DNBC) and the DNBC Puberty Cohort to define our study population of mother–child pairs (Fig. 1).
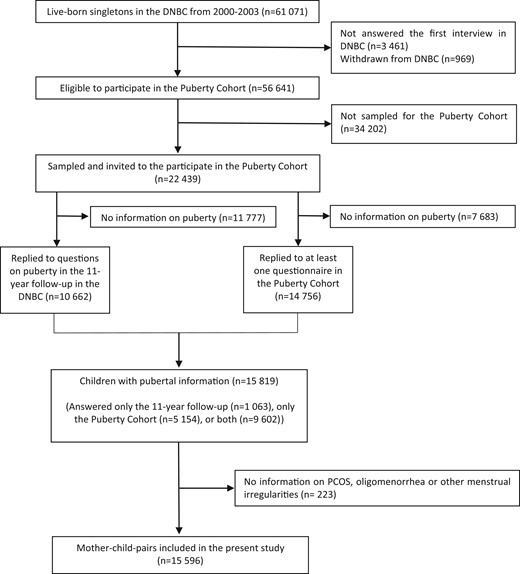
Flowchart of participation in the DNBC Puberty Cohort, Denmark, 2000–2020. DNBC, Danish National Birth Cohort; PCOS, polycystic ovary syndrome.
In the DNBC, pregnant women were enrolled at their first antenatal visit at their general practitioner (around gestational weeks 6–12) from 1997 to 2002 (Olsen et al., 2001). Overall, 91 661 women with 101 042 pregnancies were included during the enrolment period (participation rate: 60%). The women completed a computer-assisted telephone interview around gestational week 17 and 32, as well as 6 months postpartum. Additionally, children and parents were asked to fill out web-based questionnaires at 7 years (participation rate: 58%) and 11 years (participation rate: 60%). The 11-year questionnaire included, among others, questions on the children’s current pubertal development identical to those asked in the DNBC Puberty Cohort (see below).
In the DNBC Puberty Cohort, all live-born, singletons born between 2000 and 2003, whose mothers had participated in the first interview during pregnancy and had not withdrawn from the cohort, were eligible for participation (n = 56 641) (Ernst et al., 2020). Based on 28 sampling frames within 15 prenatal exposures potentially of interest for pubertal timing, we sampled children for invitation to participate. In addition, we invited a random sample of 8000 children (Brix et al., 2019b). Overall, we sampled and invited 22 439 children to participate in the DNBC Puberty Cohort (Fig. 1). We asked them to report their current stage of pubertal development every 6 months from the age of 11.5 years and onwards. We followed the individual child until full sexual maturation (Tanner stage 5; Marshall and Tanner, 1969; Marshall and Tanner, 1970) or until the child turned 18 years of age, whichever occurred first. Overall, 15 819 (7696 sons and 8123 daughters (participation rate: 70%)) responded to at least one pubertal questionnaire either in the DNBC 11-year follow-up only (n = 1063), in the DNBC Puberty Cohort only (n = 5154) or in both (n = 9602) (Fig. 1).
Information on PCOS and menstrual irregularities were missing for 223 (1.4%) of the study population. Thereby, the final study-population consists of 15 596 mother–child pairs (Fig. 1).
Assessment of maternal PCOS, oligomenorrhoea and irregular menstrual cycle
As PCOS is underdiagnosed, we combined information from the Danish National Patient Registry (DNPR) with self-reported information from the DNBC and constructed the exposure groups as follows: (i) PCOS (n = 251), (ii) oligomenorrhoea (n = 134), (iii) ‘other menstrual irregularities’ (n = 2411) and (iv) no menstrual abnormalities (reference group) (n = 12 800). The exposure groups were constructed as follows.
Firstly, we obtained register-based diagnostic codes on PCOS, oligomenorrhoea and ‘other menstrual irregularities’ at any time before, during or after pregnancy between 1991 and 2016 (mean years from birth of the index child to first diagnosis: 2.2 (standard deviation (SD): 5.6) years) (Supplementary Data File S1). The DNPR covers all diagnostic codes from in- and outpatient clinic visits, including date of diagnosis, classified according to the International Classification of Diseases version 10 (ICD-10) (Andersen et al., 1999). In total, 251 of the mothers had a diagnostic codes of PCOS, 66 had a diagnostic codes of oligomenorrhoea and 152 had a diagnostic codes of ‘other menstrual irregularities’ (Supplementary Data File S1).
Secondly, we included the self-reported information from the DNBC in order to further limit the number of unacknowledged and undiagnosed cases of PCOS in the reference group. In the first interview, the mothers answered the following questions regarding menstrual cycle characteristics: (i) ‘Are your periods regular, i.e. can you predict, within a week, when you will have your next period?’. If yes, they were asked: (ii) ‘How many days pass from 1st day in one period to 1st day in the next period?’. If they were not registered with any diagnostic code, we categorized mothers as having oligomenorrhoea if they reported >35 days between their periods (n = 71), and we categorized mothers as having ‘other menstrual irregularities’ if they reported that they could not predict their period (n = 2284). Unfortunately, the mothers were not asked about PCOS diagnosis, hyperandrogenism, nor ultrasound scans; thus, the PCOS exposure group was solely compounded of register-based information.
Assessment of pubertal development
The children provided information on pubertal development every 6 months using a web-based questionnaire assisted by a short descriptive text and drawn pictures of each Tanner stage (Marshall and Tanner, 1969; Marshall and Tanner, 1970). We asked the sons to state their current Tanner stage 1–5 (pubic hair and genital development). Moreover, we asked whether they had had their first ejaculation (if yes, they were asked to state the exact age in years and months), voice break (sometimes changed, completely changed, not changed), axillary hair development (yes/no) and acne occurrence (yes/no). We asked the daughters to state their current Tanner stage 1–5 (pubic hair and breast development). Further, we asked if they had had their first menstrual bleeding (if yes, they were asked to state the exact age in years and months), axillary hair development (yes/no) and acne occurrence (yes/no).
Co-variates
We identified potential confounders by using directed acyclic graphs (Pearl, 2004) and existing literature. We adjusted the main analysis for maternal smoking in first trimester, maternal pre-pregnancy body mass index (BMI) (restricted cubic splines with five knots), highest social economic group of the parents based on education and occupational level and maternal age at menarche (all from the baseline interviews from DNBC), as well as maternal age (restricted cubic splines with four knots) from the Danish Medical Birth Register (DMBR) (Table I) (Bliddal et al., 2018).
Maternal and child characteristics according to maternal polycystic ovary syndrome (PCOS), oligomenorrhoea or ‘other menstrual irregularities’ among 15 596 sons and daughters in the DNBC Puberty Cohort, Denmark, 2000–2020.
| . | Reference group . | PCOS . | Oligomenorrhoea . | ‘Other menstrual irregularities’ . | Missing . |
|---|---|---|---|---|---|
| 12 800 (80.9) | 251 (1.6) | 134 (0.9) | 2411 (15.2) | ||
| Sex of child | 0 (0.0) | ||||
| Sons | 6212 (48.5) | 119 (47.4) | 67 (50.0) | 1194 (49.5) | |
| Daughters | 6588 (51.5) | 132 (52.6) | 67 (50.0) | 1217 (50.5) | |
| Maternal pre-pregnancy BMI | 209 (1.3) | ||||
| Mean (95% CI) | 23.8 (23.7; 23.8) | 26.1 (25.4; 26.8) | 24.2 (23.4; 25.0) | 23.9 (23.7; 24.1) | |
| Socio-economic group of parents | 0 (0.0) | ||||
| High-grade professional | 3048 (23.8) | 50 (19.9) | 26 (19.4) | 527 (21.9) | |
| Low-grade professional | 4199 (32.8) | 79 (31.5) | 45 (33.6) | 803 (33.3) | |
| Skilled worker | 3514 (27.5) | 79 (31.5) | 36 (26.9) | 656 (27.2) | |
| Unskilled worker | 2039 (15.9) | 43 (17.1) | 27 (20.2) | 425 (17.6) | |
| Maternal age at menarche | ∼120 (∼0.8)a | ||||
| Earlier than peers | 3284 (25.7) | 72 (28.7) | 29 (21.6) | 576 (23.9) | |
| Same time as peers | 7371 (57.6) | ∼110 (∼43.8)a | 72 (53.7) | 1294 (53.7) | |
| Later than peers | 2054 (16.1) | 64 (25.5) | 33 (24.6) | 516 (21.4) | |
| Maternal age at delivery in years | 6 (0.0) | ||||
| Mean (95% CI) | 30.8 (30.7; 30.9) | 29.6 (29.1; 30.2) | 29.5 (28.9; 30.2) | 29.9 (29.8; 30.1) | |
| Maternal smoking in first trimester | ∼55 (∼0.4)a | ||||
| No | 9188 (71.8) | ∼195 (∼77.7)a | 94 (70.2) | 1723 (71.5) | |
| 1–10 cigarettes per day | 2831 (22.1) | 48 (19.1) | 33 (24.6) | 541 (22.4) | |
| >10 cigarettes per day | 736 (5.8) | 8 (3.2) | 7 (5.2) | 140 (5.8) | |
| Child BMI at 7 years | 4702 (30.1) | ||||
| Mean (95% CI) | 15.6 (15.6; 15.7) | 15.8 (15.4; 16.1) | 15.9 (15.5; 16.4) | 15.7 (15.6; 15.8) | |
| Maternal diabetes mellitus type 2 or gestational diabetes mellitus | 0 (0.0) | ||||
| No | 12 527 (97.9) | 227 (90.4) | 128 (95.5) | 2342 (97.1) | |
| Yes | 273 (2.1) | 24 (9.6) | 6 (4.5) | 69 (2.9) | |
| Maternal hypertensive disorders | 0 (0.0) | ||||
| No | 11 818 (92.3) | 214 (85.3) | 121 (90.3) | 2216 (91.9) | |
| Yes | 982 (7.7) | 37 (14.7) | 13 (9.7) | 195 (8.1) | |
| . | Reference group . | PCOS . | Oligomenorrhoea . | ‘Other menstrual irregularities’ . | Missing . |
|---|---|---|---|---|---|
| 12 800 (80.9) | 251 (1.6) | 134 (0.9) | 2411 (15.2) | ||
| Sex of child | 0 (0.0) | ||||
| Sons | 6212 (48.5) | 119 (47.4) | 67 (50.0) | 1194 (49.5) | |
| Daughters | 6588 (51.5) | 132 (52.6) | 67 (50.0) | 1217 (50.5) | |
| Maternal pre-pregnancy BMI | 209 (1.3) | ||||
| Mean (95% CI) | 23.8 (23.7; 23.8) | 26.1 (25.4; 26.8) | 24.2 (23.4; 25.0) | 23.9 (23.7; 24.1) | |
| Socio-economic group of parents | 0 (0.0) | ||||
| High-grade professional | 3048 (23.8) | 50 (19.9) | 26 (19.4) | 527 (21.9) | |
| Low-grade professional | 4199 (32.8) | 79 (31.5) | 45 (33.6) | 803 (33.3) | |
| Skilled worker | 3514 (27.5) | 79 (31.5) | 36 (26.9) | 656 (27.2) | |
| Unskilled worker | 2039 (15.9) | 43 (17.1) | 27 (20.2) | 425 (17.6) | |
| Maternal age at menarche | ∼120 (∼0.8)a | ||||
| Earlier than peers | 3284 (25.7) | 72 (28.7) | 29 (21.6) | 576 (23.9) | |
| Same time as peers | 7371 (57.6) | ∼110 (∼43.8)a | 72 (53.7) | 1294 (53.7) | |
| Later than peers | 2054 (16.1) | 64 (25.5) | 33 (24.6) | 516 (21.4) | |
| Maternal age at delivery in years | 6 (0.0) | ||||
| Mean (95% CI) | 30.8 (30.7; 30.9) | 29.6 (29.1; 30.2) | 29.5 (28.9; 30.2) | 29.9 (29.8; 30.1) | |
| Maternal smoking in first trimester | ∼55 (∼0.4)a | ||||
| No | 9188 (71.8) | ∼195 (∼77.7)a | 94 (70.2) | 1723 (71.5) | |
| 1–10 cigarettes per day | 2831 (22.1) | 48 (19.1) | 33 (24.6) | 541 (22.4) | |
| >10 cigarettes per day | 736 (5.8) | 8 (3.2) | 7 (5.2) | 140 (5.8) | |
| Child BMI at 7 years | 4702 (30.1) | ||||
| Mean (95% CI) | 15.6 (15.6; 15.7) | 15.8 (15.4; 16.1) | 15.9 (15.5; 16.4) | 15.7 (15.6; 15.8) | |
| Maternal diabetes mellitus type 2 or gestational diabetes mellitus | 0 (0.0) | ||||
| No | 12 527 (97.9) | 227 (90.4) | 128 (95.5) | 2342 (97.1) | |
| Yes | 273 (2.1) | 24 (9.6) | 6 (4.5) | 69 (2.9) | |
| Maternal hypertensive disorders | 0 (0.0) | ||||
| No | 11 818 (92.3) | 214 (85.3) | 121 (90.3) | 2216 (91.9) | |
| Yes | 982 (7.7) | 37 (14.7) | 13 (9.7) | 195 (8.1) | |
Values are n (%) unless stated otherwise.
Rounded up or down in closest fifth because of missing <5, due to the General Data Protection Regulation and the Danish Data Protection Act.
Maternal and child characteristics according to maternal polycystic ovary syndrome (PCOS), oligomenorrhoea or ‘other menstrual irregularities’ among 15 596 sons and daughters in the DNBC Puberty Cohort, Denmark, 2000–2020.
| . | Reference group . | PCOS . | Oligomenorrhoea . | ‘Other menstrual irregularities’ . | Missing . |
|---|---|---|---|---|---|
| 12 800 (80.9) | 251 (1.6) | 134 (0.9) | 2411 (15.2) | ||
| Sex of child | 0 (0.0) | ||||
| Sons | 6212 (48.5) | 119 (47.4) | 67 (50.0) | 1194 (49.5) | |
| Daughters | 6588 (51.5) | 132 (52.6) | 67 (50.0) | 1217 (50.5) | |
| Maternal pre-pregnancy BMI | 209 (1.3) | ||||
| Mean (95% CI) | 23.8 (23.7; 23.8) | 26.1 (25.4; 26.8) | 24.2 (23.4; 25.0) | 23.9 (23.7; 24.1) | |
| Socio-economic group of parents | 0 (0.0) | ||||
| High-grade professional | 3048 (23.8) | 50 (19.9) | 26 (19.4) | 527 (21.9) | |
| Low-grade professional | 4199 (32.8) | 79 (31.5) | 45 (33.6) | 803 (33.3) | |
| Skilled worker | 3514 (27.5) | 79 (31.5) | 36 (26.9) | 656 (27.2) | |
| Unskilled worker | 2039 (15.9) | 43 (17.1) | 27 (20.2) | 425 (17.6) | |
| Maternal age at menarche | ∼120 (∼0.8)a | ||||
| Earlier than peers | 3284 (25.7) | 72 (28.7) | 29 (21.6) | 576 (23.9) | |
| Same time as peers | 7371 (57.6) | ∼110 (∼43.8)a | 72 (53.7) | 1294 (53.7) | |
| Later than peers | 2054 (16.1) | 64 (25.5) | 33 (24.6) | 516 (21.4) | |
| Maternal age at delivery in years | 6 (0.0) | ||||
| Mean (95% CI) | 30.8 (30.7; 30.9) | 29.6 (29.1; 30.2) | 29.5 (28.9; 30.2) | 29.9 (29.8; 30.1) | |
| Maternal smoking in first trimester | ∼55 (∼0.4)a | ||||
| No | 9188 (71.8) | ∼195 (∼77.7)a | 94 (70.2) | 1723 (71.5) | |
| 1–10 cigarettes per day | 2831 (22.1) | 48 (19.1) | 33 (24.6) | 541 (22.4) | |
| >10 cigarettes per day | 736 (5.8) | 8 (3.2) | 7 (5.2) | 140 (5.8) | |
| Child BMI at 7 years | 4702 (30.1) | ||||
| Mean (95% CI) | 15.6 (15.6; 15.7) | 15.8 (15.4; 16.1) | 15.9 (15.5; 16.4) | 15.7 (15.6; 15.8) | |
| Maternal diabetes mellitus type 2 or gestational diabetes mellitus | 0 (0.0) | ||||
| No | 12 527 (97.9) | 227 (90.4) | 128 (95.5) | 2342 (97.1) | |
| Yes | 273 (2.1) | 24 (9.6) | 6 (4.5) | 69 (2.9) | |
| Maternal hypertensive disorders | 0 (0.0) | ||||
| No | 11 818 (92.3) | 214 (85.3) | 121 (90.3) | 2216 (91.9) | |
| Yes | 982 (7.7) | 37 (14.7) | 13 (9.7) | 195 (8.1) | |
| . | Reference group . | PCOS . | Oligomenorrhoea . | ‘Other menstrual irregularities’ . | Missing . |
|---|---|---|---|---|---|
| 12 800 (80.9) | 251 (1.6) | 134 (0.9) | 2411 (15.2) | ||
| Sex of child | 0 (0.0) | ||||
| Sons | 6212 (48.5) | 119 (47.4) | 67 (50.0) | 1194 (49.5) | |
| Daughters | 6588 (51.5) | 132 (52.6) | 67 (50.0) | 1217 (50.5) | |
| Maternal pre-pregnancy BMI | 209 (1.3) | ||||
| Mean (95% CI) | 23.8 (23.7; 23.8) | 26.1 (25.4; 26.8) | 24.2 (23.4; 25.0) | 23.9 (23.7; 24.1) | |
| Socio-economic group of parents | 0 (0.0) | ||||
| High-grade professional | 3048 (23.8) | 50 (19.9) | 26 (19.4) | 527 (21.9) | |
| Low-grade professional | 4199 (32.8) | 79 (31.5) | 45 (33.6) | 803 (33.3) | |
| Skilled worker | 3514 (27.5) | 79 (31.5) | 36 (26.9) | 656 (27.2) | |
| Unskilled worker | 2039 (15.9) | 43 (17.1) | 27 (20.2) | 425 (17.6) | |
| Maternal age at menarche | ∼120 (∼0.8)a | ||||
| Earlier than peers | 3284 (25.7) | 72 (28.7) | 29 (21.6) | 576 (23.9) | |
| Same time as peers | 7371 (57.6) | ∼110 (∼43.8)a | 72 (53.7) | 1294 (53.7) | |
| Later than peers | 2054 (16.1) | 64 (25.5) | 33 (24.6) | 516 (21.4) | |
| Maternal age at delivery in years | 6 (0.0) | ||||
| Mean (95% CI) | 30.8 (30.7; 30.9) | 29.6 (29.1; 30.2) | 29.5 (28.9; 30.2) | 29.9 (29.8; 30.1) | |
| Maternal smoking in first trimester | ∼55 (∼0.4)a | ||||
| No | 9188 (71.8) | ∼195 (∼77.7)a | 94 (70.2) | 1723 (71.5) | |
| 1–10 cigarettes per day | 2831 (22.1) | 48 (19.1) | 33 (24.6) | 541 (22.4) | |
| >10 cigarettes per day | 736 (5.8) | 8 (3.2) | 7 (5.2) | 140 (5.8) | |
| Child BMI at 7 years | 4702 (30.1) | ||||
| Mean (95% CI) | 15.6 (15.6; 15.7) | 15.8 (15.4; 16.1) | 15.9 (15.5; 16.4) | 15.7 (15.6; 15.8) | |
| Maternal diabetes mellitus type 2 or gestational diabetes mellitus | 0 (0.0) | ||||
| No | 12 527 (97.9) | 227 (90.4) | 128 (95.5) | 2342 (97.1) | |
| Yes | 273 (2.1) | 24 (9.6) | 6 (4.5) | 69 (2.9) | |
| Maternal hypertensive disorders | 0 (0.0) | ||||
| No | 11 818 (92.3) | 214 (85.3) | 121 (90.3) | 2216 (91.9) | |
| Yes | 982 (7.7) | 37 (14.7) | 13 (9.7) | 195 (8.1) | |
Values are n (%) unless stated otherwise.
Rounded up or down in closest fifth because of missing <5, due to the General Data Protection Regulation and the Danish Data Protection Act.
Data analyses
To compare pubertal development in children prenatally exposed to PCOS, oligomenorrhoea or ‘other menstrual irregularities’ to the reference group, we estimated the crude and adjusted mean age difference (in months) with 95% CIs at attaining each of the different pubertal milestones.
As the children provided information on their current stage of pubertal development every 6 months, the data on pubertal milestones were either left-, interval- or right-censored. Data were left-censored if the pubertal milestones were attained before the first questionnaire, interval-censored if attained between two questionnaires, and right-censored if still not attained at the time of the last questionnaire. Thus, age at achieving each pubertal milestone was bounded within an age interval with a lower and an upper limit; allowing inclusion of all individuals regardless of the number of questionnaires returned (one or more) (Ernst et al., 2019). In order to account for the data structure, we used a regression model for normally distributed, censored data fitted by maximum likelihood estimation (using the -intreg- command in Stata 16.0 software (Stata Corporation, College Station, TX, USA)) (Sun, 2006). We inspected the assumption of normally distributed residuals by plotting the non-parametric cumulative incidence function based on the Turnbull Estimator against the normal distribution (using the icenreg package in R studio (ver. 1.0.136)). We stratified the plots by levels of explanatory variables and visually checked the assumption of independent variance, which could not be refuted.
Using Huber–White robust variance estimation (Huber, 1967; White, 1980), we generated a single estimate for the average difference in age at attaining all pubertal milestones for each sex. This accounts for the correlations between multiple outcomes (Ernst et al., 2019). Moreover, in daughters we estimated the average difference in age at attaining adrenarche only (pubarche, axillary hair and acne) and the average difference in age at attaining thelarche and menarche.
To account for potential selection bias due to non-participation in the DNBC Puberty Cohort, we generated selection weights using a multivariable logistic regression of the inverse probability of participation based on a priori specified factors related to participation (Hernan et al., 2004); making the 15 596 included children representative of the 22 439 invited if correctly specified. To account for the sampling regime in the DNBC Puberty Cohort, we generated sampling weights as the inverse probability of being sampled. Thereby, we reweighted the 22 439 invited children to be representative to the 56 641 eligible children as described previously (Brix et al., 2019b). We multiplied the sampling- and selection weights and added them to all analyses. Lastly, we used robust standard errors to account for the use of weights and the clustering of siblings in the cohort.
Sub-analyses
We performed three mediation analyses as sub-analyses using G-computation (VanderWeele, 2016). If an overall association was found in the average difference in the main analysis, we estimated the proportion of the association that was mediated trough the specified potential mediators (natural indirect effect, NIE), and through all other pathways (natural direct effect, NDE). We used non-parametric bootstrap with 1000 replications to obtain 95% CIs. In order to estimate the overall NIE, we estimated the average NIE of the NIHs of each milestone in each mediation analysis.
In the first mediation analysis, we investigated whether BMI of the children at 7 years of age (median age: 7.1 (25th–75th percentile: 7.0–7.2) years) could have mediated the association (Brix et al., 2020b; Zhang et al., 2022). We calculated the BMI based on self-reported height (cm) and weight (kg) of the children provided by the mothers in the 7-year follow-up of the DNBC (continual variable). To explore potential selection bias due to missing information on childhood BMI, we restricted the main analysis to participants with information on childhood BMI (data not shown) and compared the estimates for the total effects from the mediation analysis to these.
In the second mediation analysis, we investigated whether maternal type 2 diabetes mellitus (T2DM) or gestational diabetes mellitus (GDM) mediated the association (Yu et al., 2016; Lauridsen et al., 2018). We gathered the information on maternal T2DM before or during pregnancy and GDM during pregnancy using both diagnostic codes and self-reported information from the first three interviews of DNBC, as previously described in details (Lauridsen et al., 2018). Then, we categorized the mothers as either having T2DM/GDM (n = 372) or not (n = 15 224).
In the third mediation analysis, we investigated whether maternal hypertensive disorders mediated the association (Palomba et al., 2015; Lunddorf et al., 2020). As described in a previous paper, we obtained information on maternal hypertensive disorders using both diagnostic codes and self-reported information from the first three interviews of DNBC (Lunddorf et al., 2020). In total, 1243 mothers had a hypertensive disorder during pregnancy (hypertension (gestational/pre-gestational), preeclampsia, eclampsia, HELLP Syndrome or unspecific hypertensive disorders), whereas 14 353 had no hypertensive disorders.
Results
When compared with the reference group, mothers with PCOS had on average higher BMI, were of slightly lower socio-economic class, had less often had their first menstrual bleeding at the same time as peers and more often later than peers, were less often smokers, and had more often T2DM/GDM and hypertensive disorders (Table I). Mothers with oligomenorrhoea or ‘other menstrual irregularities’ were on average of slightly lower socio-economic class, had more often had their first menstrual bleeding later than peers and were slightly older compared to the reference group (Table I).
Pubertal development in daughters
Maternal PCOS was associated with earlier pubertal development in daughters (adjusted average difference of all pubertal milestones: −3.3 (95% CI: −6.3; −0.4) months) (Table II). This was mainly driven by earlier age at pubic hair development (TPH2: −3.9 (95% CI: −7.1; −0.6) months; TPH3: −3.7 (95% CI: −6.4; −0.9) months; TPH4: −6.8 (95% CI: −10.5; −3.1) months; TPH5: −6.7 (−13.0; −0.5) months), earlier axillary hair (−6.8 (95% CI: −11.0; −2.5) months) and tendencies of earlier acne (−4.0 (95% CI: −8.6; 0.7) months). Consequently, the average difference of adrenarche was −5.4 (95% CI: −8.7; −2.1) months earlier compared to the reference group. The average difference of thelarche and menarche was not significantly different from the reference group (−0.8 (95% CI: −3.9; 2.4) months) (Table II).
Mean age differences (in months) at pubertal development according to maternal polycystic ovary syndrome (PCOS), oligomenorrhoea or ‘other menstrual irregularities’ among 15 596 daughters and sons in the Puberty Cohort, 2000–2020.
| Daughters . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Puberty outcomes . | Na . | Ref. (n = 6591) . | PCOS (n = 132) . | Oligomenorrhoea (n = 67) . | ‘Other menstrual irregularities’ (n = 1217) . | ||||
| Crude mean age (years) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | |||
| Tanner stage | |||||||||
| Pubic hair | 2 | 7790 | 11.3 | −3.2 | −3.9 (−7.1; −0.6) | −0.7 | −1.5 (−4.8; 1.9) | −0.1 | −0.4 (−1.5; 0.6) |
| 3 | 7790 | 12.5 | −3.3 | −3.7 (−6.4; −0.9) | −1.2 | −1.8 (−4.8; 1.1) | −0.4 | −0.7 (−1.7; 0.3) | |
| 4 | 7790 | 13.5 | −6.3 | −6.8 (−10.5; −3.1) | −3.0 | −3.9 (−7.9, 0.1) | −1.4 | −1.8 (−3.1; −0.5) | |
| 5 | 7790 | 15.7 | −6.9 | −6.7 (−13.0; −0.3) | −2.2 | −2.7 (−9.4; 4.0) | −2.6 | −2.7 (−4.7; −0.8) | |
| Breast | 2 | 7789 | 9.9 | 1.8 | 1.5 (−4.2; 7.2) | 3.0 | 2.6 (−3.0; 8.1) | −0.2 | −0.9 (−2.8; 1.0) |
| 3 | 7789 | 11.6 | −0.7 | −0.8 (−4.4; 2.8) | −1.1 | −1.7 (−5.8; 2.4) | 0.8 | 0.5 (−0.7; 1.6) | |
| 4 | 7789 | 13.1 | −1.8 | −1.9 (−5.5; 1.7) | 1.1 | 0.5 (−3.9; 4.9) | 0.6 | 0.2 (−1.1; 1.4) | |
| 5 | 7789 | 16.1 | −1.7 | −1.3 (−7.6; 5.0) | 0.3 | −1.1 (−10.2; 8.0) | 1.0 | 0.4 (−1.9; 2.8) | |
| Menstruation | 7788 | 13.0 | −1.6 | −1.6 (−4.4; 1.1) | 1.7 | 1.1 (−1.8; 4.1) | 1.0 | 0.7 (−0.3; 1.6) | |
| Axillary hair | 7795 | 12.0 | −6.7 | −6.8 (−11.0; −2.5) | 2.2 | 1.8 (−2.7; 6.3) | −0.8 | −1.0 (−2.3; 0.4) | |
| Acne | 7795 | 11.4 | −4.1 | −4.0 (−8.6; 0.7) | −2.7 | −2.9 (−9.0; 3.1) | −0.9 | −1.1 (−2.6; 0.5) | |
| Average difference (all pubertal milestones) | 7778 | – | −3.2 | −3.3 (−6.3; −0.4) | −0.4 | −1.1 (−4.5; 2.4) | −0.2 | −0.5 (−1.5; 0.4) | |
| Average difference (androgen milestones)d | 7.780 | – | −5.2 | −5.4 (−8.7; −2.1) | −1.2 | −1.8 (−5.4; 1.7) | −0.9 | −1.2 (−2.3; −0.1) | |
| Average difference (oestrogen milestones)e | 7.778 | – | −0.7 | −0.8 (−3.9; 2.4) | 0.6 | −0.1 (−3.8; 3.6) | 0.8 | 0.4 (−0.6; 1.4) | |
| Daughters . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Puberty outcomes . | Na . | Ref. (n = 6591) . | PCOS (n = 132) . | Oligomenorrhoea (n = 67) . | ‘Other menstrual irregularities’ (n = 1217) . | ||||
| Crude mean age (years) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | |||
| Tanner stage | |||||||||
| Pubic hair | 2 | 7790 | 11.3 | −3.2 | −3.9 (−7.1; −0.6) | −0.7 | −1.5 (−4.8; 1.9) | −0.1 | −0.4 (−1.5; 0.6) |
| 3 | 7790 | 12.5 | −3.3 | −3.7 (−6.4; −0.9) | −1.2 | −1.8 (−4.8; 1.1) | −0.4 | −0.7 (−1.7; 0.3) | |
| 4 | 7790 | 13.5 | −6.3 | −6.8 (−10.5; −3.1) | −3.0 | −3.9 (−7.9, 0.1) | −1.4 | −1.8 (−3.1; −0.5) | |
| 5 | 7790 | 15.7 | −6.9 | −6.7 (−13.0; −0.3) | −2.2 | −2.7 (−9.4; 4.0) | −2.6 | −2.7 (−4.7; −0.8) | |
| Breast | 2 | 7789 | 9.9 | 1.8 | 1.5 (−4.2; 7.2) | 3.0 | 2.6 (−3.0; 8.1) | −0.2 | −0.9 (−2.8; 1.0) |
| 3 | 7789 | 11.6 | −0.7 | −0.8 (−4.4; 2.8) | −1.1 | −1.7 (−5.8; 2.4) | 0.8 | 0.5 (−0.7; 1.6) | |
| 4 | 7789 | 13.1 | −1.8 | −1.9 (−5.5; 1.7) | 1.1 | 0.5 (−3.9; 4.9) | 0.6 | 0.2 (−1.1; 1.4) | |
| 5 | 7789 | 16.1 | −1.7 | −1.3 (−7.6; 5.0) | 0.3 | −1.1 (−10.2; 8.0) | 1.0 | 0.4 (−1.9; 2.8) | |
| Menstruation | 7788 | 13.0 | −1.6 | −1.6 (−4.4; 1.1) | 1.7 | 1.1 (−1.8; 4.1) | 1.0 | 0.7 (−0.3; 1.6) | |
| Axillary hair | 7795 | 12.0 | −6.7 | −6.8 (−11.0; −2.5) | 2.2 | 1.8 (−2.7; 6.3) | −0.8 | −1.0 (−2.3; 0.4) | |
| Acne | 7795 | 11.4 | −4.1 | −4.0 (−8.6; 0.7) | −2.7 | −2.9 (−9.0; 3.1) | −0.9 | −1.1 (−2.6; 0.5) | |
| Average difference (all pubertal milestones) | 7778 | – | −3.2 | −3.3 (−6.3; −0.4) | −0.4 | −1.1 (−4.5; 2.4) | −0.2 | −0.5 (−1.5; 0.4) | |
| Average difference (androgen milestones)d | 7.780 | – | −5.2 | −5.4 (−8.7; −2.1) | −1.2 | −1.8 (−5.4; 1.7) | −0.9 | −1.2 (−2.3; −0.1) | |
| Average difference (oestrogen milestones)e | 7.778 | – | −0.7 | −0.8 (−3.9; 2.4) | 0.6 | −0.1 (−3.8; 3.6) | 0.8 | 0.4 (−0.6; 1.4) | |
| Sons . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Puberty outcomes . | Na . | Ref. (n = 6215) . | PCOS (n = 119) . | Oligomenorrhoea (n = 67) . | ‘Other menstrual irregularities’ (n = 1194) . | ||||
| Crude mean age (years) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | |||
| Tanner stage | |||||||||
| Pubic hair | 2 | 7394 | 11.3 | −0.1 | 0.0 (−3.7; 3.7) | 0.4 | 0.3 (−4.6; 5.2) | 0.8 | 0.7 (−0.6; 2.0) |
| 3 | 7394 | 12.7 | −1.7 | −1.3 (−4.9; 2.4) | −1.1 | −1.1 (−5.8; 3.6) | 0.2 | 0.2 (−1.0; 1.3) | |
| 4 | 7394 | 13.5 | −1.2 | −0.8 (−3.9; 2.4) | 0.3 | 0.2 (−3.2; 3.6) | −0.1 | −0.1 (−1.2; 1.0) | |
| 5 | 7394 | 14.8 | −1.4 | −0.7 (−4.3; 3.0) | 0.6 | 0.7 (−5.9; 7.2) | −0.4 | −0.3 (−1.8; 1.3) | |
| Genitals | 2 | 7390 | 10.9 | −1.0 | −0.8 (−4.8; 3.2) | 1.3 | 1.3 (−3.6; 6.2) | 1.6 | 1.6 (0.3; 3.0) |
| 3 | 7390 | 12.5 | −1.0 | −0.7 (−4.6; 3.2) | 0.5 | 0.4 (−4.5; 5.4) | 1.0 | 1.1 (−0.3; 2.4) | |
| 4 | 7390 | 13.7 | −0.3 | 0.0 (−3.3; 3.2) | 1.5 | 1.4 (−3.7; 6.5) | 0.2 | 0.2 (−1.1; 1.5) | |
| 5 | 7390 | 15.8 | −0.4 | −0.3 (−6.4; 5.8) | 0.1 | 0.0 (−7.7; 7.8) | 0.4 | 0.4 (−1.8; 2.7) | |
| Ejaculation | 7386 | 13.3 | 2.3 | 2.5 (−1.2; 6.1) | −3.9 | −4.1 (−8.2; −0.1) | 0.6 | 0.6 (−0.7; 1.8) | |
| Voice break | 7199 | 13.0 | −2.0 | −1.3 (−5.3; 2.7) | 4.0 | 4.1 (−1.9; 10.0) | −1.6 | −1.5 (−2.8; −0.1) | |
| Axillary hair | 7399 | 13.3 | −2.8 | −2.0 (−6.2; 2.2) | −4.6 | −4.7 (−9.5; 0.1) | −0.7 | −0.7 (−2.2; 0.7) | |
| Acne | 7399 | 12.3 | −2.4 | −1.5 (−5.6; 2.6) | 0.7 | 0.8 (−3.6; 5.2) | −0.8 | −0.7 (−2.0; 0.6) | |
| Average difference | 7179 | – | −0.9 | −0.5 (−3.1; 2.1) | 0.0 | 0.0 (−3.6; 3.5) | −0.1 | 0.0 (−0.9; 0.8) | |
| Sons . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Puberty outcomes . | Na . | Ref. (n = 6215) . | PCOS (n = 119) . | Oligomenorrhoea (n = 67) . | ‘Other menstrual irregularities’ (n = 1194) . | ||||
| Crude mean age (years) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | |||
| Tanner stage | |||||||||
| Pubic hair | 2 | 7394 | 11.3 | −0.1 | 0.0 (−3.7; 3.7) | 0.4 | 0.3 (−4.6; 5.2) | 0.8 | 0.7 (−0.6; 2.0) |
| 3 | 7394 | 12.7 | −1.7 | −1.3 (−4.9; 2.4) | −1.1 | −1.1 (−5.8; 3.6) | 0.2 | 0.2 (−1.0; 1.3) | |
| 4 | 7394 | 13.5 | −1.2 | −0.8 (−3.9; 2.4) | 0.3 | 0.2 (−3.2; 3.6) | −0.1 | −0.1 (−1.2; 1.0) | |
| 5 | 7394 | 14.8 | −1.4 | −0.7 (−4.3; 3.0) | 0.6 | 0.7 (−5.9; 7.2) | −0.4 | −0.3 (−1.8; 1.3) | |
| Genitals | 2 | 7390 | 10.9 | −1.0 | −0.8 (−4.8; 3.2) | 1.3 | 1.3 (−3.6; 6.2) | 1.6 | 1.6 (0.3; 3.0) |
| 3 | 7390 | 12.5 | −1.0 | −0.7 (−4.6; 3.2) | 0.5 | 0.4 (−4.5; 5.4) | 1.0 | 1.1 (−0.3; 2.4) | |
| 4 | 7390 | 13.7 | −0.3 | 0.0 (−3.3; 3.2) | 1.5 | 1.4 (−3.7; 6.5) | 0.2 | 0.2 (−1.1; 1.5) | |
| 5 | 7390 | 15.8 | −0.4 | −0.3 (−6.4; 5.8) | 0.1 | 0.0 (−7.7; 7.8) | 0.4 | 0.4 (−1.8; 2.7) | |
| Ejaculation | 7386 | 13.3 | 2.3 | 2.5 (−1.2; 6.1) | −3.9 | −4.1 (−8.2; −0.1) | 0.6 | 0.6 (−0.7; 1.8) | |
| Voice break | 7199 | 13.0 | −2.0 | −1.3 (−5.3; 2.7) | 4.0 | 4.1 (−1.9; 10.0) | −1.6 | −1.5 (−2.8; −0.1) | |
| Axillary hair | 7399 | 13.3 | −2.8 | −2.0 (−6.2; 2.2) | −4.6 | −4.7 (−9.5; 0.1) | −0.7 | −0.7 (−2.2; 0.7) | |
| Acne | 7399 | 12.3 | −2.4 | −1.5 (−5.6; 2.6) | 0.7 | 0.8 (−3.6; 5.2) | −0.8 | −0.7 (−2.0; 0.6) | |
| Average difference | 7179 | – | −0.9 | −0.5 (−3.1; 2.1) | 0.0 | 0.0 (−3.6; 3.5) | −0.1 | 0.0 (−0.9; 0.8) | |
Numbers in analyses vary, as not all children gave information on all pubertal milestones.
Mean monthly difference in age at tatting each pubertal milestone compared to the reference group.
Adjusted for maternal pre-pregnancy BMI, social-economic status, maternal age at menarche, maternal age at birth and maternal smoking during first trimester of pregnancy.
Androgen-dependent pubertal milestones: Pubic hair Tanner stage 2–5, axillary hair and acne.
Oestrogen-dependent pubertal milestones: Breast Tanner stage 2–5 and menarche.
Mean age differences (in months) at pubertal development according to maternal polycystic ovary syndrome (PCOS), oligomenorrhoea or ‘other menstrual irregularities’ among 15 596 daughters and sons in the Puberty Cohort, 2000–2020.
| Daughters . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Puberty outcomes . | Na . | Ref. (n = 6591) . | PCOS (n = 132) . | Oligomenorrhoea (n = 67) . | ‘Other menstrual irregularities’ (n = 1217) . | ||||
| Crude mean age (years) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | |||
| Tanner stage | |||||||||
| Pubic hair | 2 | 7790 | 11.3 | −3.2 | −3.9 (−7.1; −0.6) | −0.7 | −1.5 (−4.8; 1.9) | −0.1 | −0.4 (−1.5; 0.6) |
| 3 | 7790 | 12.5 | −3.3 | −3.7 (−6.4; −0.9) | −1.2 | −1.8 (−4.8; 1.1) | −0.4 | −0.7 (−1.7; 0.3) | |
| 4 | 7790 | 13.5 | −6.3 | −6.8 (−10.5; −3.1) | −3.0 | −3.9 (−7.9, 0.1) | −1.4 | −1.8 (−3.1; −0.5) | |
| 5 | 7790 | 15.7 | −6.9 | −6.7 (−13.0; −0.3) | −2.2 | −2.7 (−9.4; 4.0) | −2.6 | −2.7 (−4.7; −0.8) | |
| Breast | 2 | 7789 | 9.9 | 1.8 | 1.5 (−4.2; 7.2) | 3.0 | 2.6 (−3.0; 8.1) | −0.2 | −0.9 (−2.8; 1.0) |
| 3 | 7789 | 11.6 | −0.7 | −0.8 (−4.4; 2.8) | −1.1 | −1.7 (−5.8; 2.4) | 0.8 | 0.5 (−0.7; 1.6) | |
| 4 | 7789 | 13.1 | −1.8 | −1.9 (−5.5; 1.7) | 1.1 | 0.5 (−3.9; 4.9) | 0.6 | 0.2 (−1.1; 1.4) | |
| 5 | 7789 | 16.1 | −1.7 | −1.3 (−7.6; 5.0) | 0.3 | −1.1 (−10.2; 8.0) | 1.0 | 0.4 (−1.9; 2.8) | |
| Menstruation | 7788 | 13.0 | −1.6 | −1.6 (−4.4; 1.1) | 1.7 | 1.1 (−1.8; 4.1) | 1.0 | 0.7 (−0.3; 1.6) | |
| Axillary hair | 7795 | 12.0 | −6.7 | −6.8 (−11.0; −2.5) | 2.2 | 1.8 (−2.7; 6.3) | −0.8 | −1.0 (−2.3; 0.4) | |
| Acne | 7795 | 11.4 | −4.1 | −4.0 (−8.6; 0.7) | −2.7 | −2.9 (−9.0; 3.1) | −0.9 | −1.1 (−2.6; 0.5) | |
| Average difference (all pubertal milestones) | 7778 | – | −3.2 | −3.3 (−6.3; −0.4) | −0.4 | −1.1 (−4.5; 2.4) | −0.2 | −0.5 (−1.5; 0.4) | |
| Average difference (androgen milestones)d | 7.780 | – | −5.2 | −5.4 (−8.7; −2.1) | −1.2 | −1.8 (−5.4; 1.7) | −0.9 | −1.2 (−2.3; −0.1) | |
| Average difference (oestrogen milestones)e | 7.778 | – | −0.7 | −0.8 (−3.9; 2.4) | 0.6 | −0.1 (−3.8; 3.6) | 0.8 | 0.4 (−0.6; 1.4) | |
| Daughters . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Puberty outcomes . | Na . | Ref. (n = 6591) . | PCOS (n = 132) . | Oligomenorrhoea (n = 67) . | ‘Other menstrual irregularities’ (n = 1217) . | ||||
| Crude mean age (years) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | |||
| Tanner stage | |||||||||
| Pubic hair | 2 | 7790 | 11.3 | −3.2 | −3.9 (−7.1; −0.6) | −0.7 | −1.5 (−4.8; 1.9) | −0.1 | −0.4 (−1.5; 0.6) |
| 3 | 7790 | 12.5 | −3.3 | −3.7 (−6.4; −0.9) | −1.2 | −1.8 (−4.8; 1.1) | −0.4 | −0.7 (−1.7; 0.3) | |
| 4 | 7790 | 13.5 | −6.3 | −6.8 (−10.5; −3.1) | −3.0 | −3.9 (−7.9, 0.1) | −1.4 | −1.8 (−3.1; −0.5) | |
| 5 | 7790 | 15.7 | −6.9 | −6.7 (−13.0; −0.3) | −2.2 | −2.7 (−9.4; 4.0) | −2.6 | −2.7 (−4.7; −0.8) | |
| Breast | 2 | 7789 | 9.9 | 1.8 | 1.5 (−4.2; 7.2) | 3.0 | 2.6 (−3.0; 8.1) | −0.2 | −0.9 (−2.8; 1.0) |
| 3 | 7789 | 11.6 | −0.7 | −0.8 (−4.4; 2.8) | −1.1 | −1.7 (−5.8; 2.4) | 0.8 | 0.5 (−0.7; 1.6) | |
| 4 | 7789 | 13.1 | −1.8 | −1.9 (−5.5; 1.7) | 1.1 | 0.5 (−3.9; 4.9) | 0.6 | 0.2 (−1.1; 1.4) | |
| 5 | 7789 | 16.1 | −1.7 | −1.3 (−7.6; 5.0) | 0.3 | −1.1 (−10.2; 8.0) | 1.0 | 0.4 (−1.9; 2.8) | |
| Menstruation | 7788 | 13.0 | −1.6 | −1.6 (−4.4; 1.1) | 1.7 | 1.1 (−1.8; 4.1) | 1.0 | 0.7 (−0.3; 1.6) | |
| Axillary hair | 7795 | 12.0 | −6.7 | −6.8 (−11.0; −2.5) | 2.2 | 1.8 (−2.7; 6.3) | −0.8 | −1.0 (−2.3; 0.4) | |
| Acne | 7795 | 11.4 | −4.1 | −4.0 (−8.6; 0.7) | −2.7 | −2.9 (−9.0; 3.1) | −0.9 | −1.1 (−2.6; 0.5) | |
| Average difference (all pubertal milestones) | 7778 | – | −3.2 | −3.3 (−6.3; −0.4) | −0.4 | −1.1 (−4.5; 2.4) | −0.2 | −0.5 (−1.5; 0.4) | |
| Average difference (androgen milestones)d | 7.780 | – | −5.2 | −5.4 (−8.7; −2.1) | −1.2 | −1.8 (−5.4; 1.7) | −0.9 | −1.2 (−2.3; −0.1) | |
| Average difference (oestrogen milestones)e | 7.778 | – | −0.7 | −0.8 (−3.9; 2.4) | 0.6 | −0.1 (−3.8; 3.6) | 0.8 | 0.4 (−0.6; 1.4) | |
| Sons . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Puberty outcomes . | Na . | Ref. (n = 6215) . | PCOS (n = 119) . | Oligomenorrhoea (n = 67) . | ‘Other menstrual irregularities’ (n = 1194) . | ||||
| Crude mean age (years) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | |||
| Tanner stage | |||||||||
| Pubic hair | 2 | 7394 | 11.3 | −0.1 | 0.0 (−3.7; 3.7) | 0.4 | 0.3 (−4.6; 5.2) | 0.8 | 0.7 (−0.6; 2.0) |
| 3 | 7394 | 12.7 | −1.7 | −1.3 (−4.9; 2.4) | −1.1 | −1.1 (−5.8; 3.6) | 0.2 | 0.2 (−1.0; 1.3) | |
| 4 | 7394 | 13.5 | −1.2 | −0.8 (−3.9; 2.4) | 0.3 | 0.2 (−3.2; 3.6) | −0.1 | −0.1 (−1.2; 1.0) | |
| 5 | 7394 | 14.8 | −1.4 | −0.7 (−4.3; 3.0) | 0.6 | 0.7 (−5.9; 7.2) | −0.4 | −0.3 (−1.8; 1.3) | |
| Genitals | 2 | 7390 | 10.9 | −1.0 | −0.8 (−4.8; 3.2) | 1.3 | 1.3 (−3.6; 6.2) | 1.6 | 1.6 (0.3; 3.0) |
| 3 | 7390 | 12.5 | −1.0 | −0.7 (−4.6; 3.2) | 0.5 | 0.4 (−4.5; 5.4) | 1.0 | 1.1 (−0.3; 2.4) | |
| 4 | 7390 | 13.7 | −0.3 | 0.0 (−3.3; 3.2) | 1.5 | 1.4 (−3.7; 6.5) | 0.2 | 0.2 (−1.1; 1.5) | |
| 5 | 7390 | 15.8 | −0.4 | −0.3 (−6.4; 5.8) | 0.1 | 0.0 (−7.7; 7.8) | 0.4 | 0.4 (−1.8; 2.7) | |
| Ejaculation | 7386 | 13.3 | 2.3 | 2.5 (−1.2; 6.1) | −3.9 | −4.1 (−8.2; −0.1) | 0.6 | 0.6 (−0.7; 1.8) | |
| Voice break | 7199 | 13.0 | −2.0 | −1.3 (−5.3; 2.7) | 4.0 | 4.1 (−1.9; 10.0) | −1.6 | −1.5 (−2.8; −0.1) | |
| Axillary hair | 7399 | 13.3 | −2.8 | −2.0 (−6.2; 2.2) | −4.6 | −4.7 (−9.5; 0.1) | −0.7 | −0.7 (−2.2; 0.7) | |
| Acne | 7399 | 12.3 | −2.4 | −1.5 (−5.6; 2.6) | 0.7 | 0.8 (−3.6; 5.2) | −0.8 | −0.7 (−2.0; 0.6) | |
| Average difference | 7179 | – | −0.9 | −0.5 (−3.1; 2.1) | 0.0 | 0.0 (−3.6; 3.5) | −0.1 | 0.0 (−0.9; 0.8) | |
| Sons . | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Puberty outcomes . | Na . | Ref. (n = 6215) . | PCOS (n = 119) . | Oligomenorrhoea (n = 67) . | ‘Other menstrual irregularities’ (n = 1194) . | ||||
| Crude mean age (years) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | Crudeb . | Adjustedb,c (95% CI) . | |||
| Tanner stage | |||||||||
| Pubic hair | 2 | 7394 | 11.3 | −0.1 | 0.0 (−3.7; 3.7) | 0.4 | 0.3 (−4.6; 5.2) | 0.8 | 0.7 (−0.6; 2.0) |
| 3 | 7394 | 12.7 | −1.7 | −1.3 (−4.9; 2.4) | −1.1 | −1.1 (−5.8; 3.6) | 0.2 | 0.2 (−1.0; 1.3) | |
| 4 | 7394 | 13.5 | −1.2 | −0.8 (−3.9; 2.4) | 0.3 | 0.2 (−3.2; 3.6) | −0.1 | −0.1 (−1.2; 1.0) | |
| 5 | 7394 | 14.8 | −1.4 | −0.7 (−4.3; 3.0) | 0.6 | 0.7 (−5.9; 7.2) | −0.4 | −0.3 (−1.8; 1.3) | |
| Genitals | 2 | 7390 | 10.9 | −1.0 | −0.8 (−4.8; 3.2) | 1.3 | 1.3 (−3.6; 6.2) | 1.6 | 1.6 (0.3; 3.0) |
| 3 | 7390 | 12.5 | −1.0 | −0.7 (−4.6; 3.2) | 0.5 | 0.4 (−4.5; 5.4) | 1.0 | 1.1 (−0.3; 2.4) | |
| 4 | 7390 | 13.7 | −0.3 | 0.0 (−3.3; 3.2) | 1.5 | 1.4 (−3.7; 6.5) | 0.2 | 0.2 (−1.1; 1.5) | |
| 5 | 7390 | 15.8 | −0.4 | −0.3 (−6.4; 5.8) | 0.1 | 0.0 (−7.7; 7.8) | 0.4 | 0.4 (−1.8; 2.7) | |
| Ejaculation | 7386 | 13.3 | 2.3 | 2.5 (−1.2; 6.1) | −3.9 | −4.1 (−8.2; −0.1) | 0.6 | 0.6 (−0.7; 1.8) | |
| Voice break | 7199 | 13.0 | −2.0 | −1.3 (−5.3; 2.7) | 4.0 | 4.1 (−1.9; 10.0) | −1.6 | −1.5 (−2.8; −0.1) | |
| Axillary hair | 7399 | 13.3 | −2.8 | −2.0 (−6.2; 2.2) | −4.6 | −4.7 (−9.5; 0.1) | −0.7 | −0.7 (−2.2; 0.7) | |
| Acne | 7399 | 12.3 | −2.4 | −1.5 (−5.6; 2.6) | 0.7 | 0.8 (−3.6; 5.2) | −0.8 | −0.7 (−2.0; 0.6) | |
| Average difference | 7179 | – | −0.9 | −0.5 (−3.1; 2.1) | 0.0 | 0.0 (−3.6; 3.5) | −0.1 | 0.0 (−0.9; 0.8) | |
Numbers in analyses vary, as not all children gave information on all pubertal milestones.
Mean monthly difference in age at tatting each pubertal milestone compared to the reference group.
Adjusted for maternal pre-pregnancy BMI, social-economic status, maternal age at menarche, maternal age at birth and maternal smoking during first trimester of pregnancy.
Androgen-dependent pubertal milestones: Pubic hair Tanner stage 2–5, axillary hair and acne.
Oestrogen-dependent pubertal milestones: Breast Tanner stage 2–5 and menarche.
Overall, oligomenorrhoea and ‘other menstrual irregularities’ were not associated with pubertal development in daughters (average difference of all pubertal milestones: oligomenorrhoea: −1.1 (95% CI: −4.5; 2.4) months and ‘other menstrual irregularities’: −0.5 (95% CI: −1.4; 0.5) months) (Table II). Though, adrenarche tended to occur slightly earlier in daughters of mothers with ‘other menstrual irregularities’ (average difference of adrenarche: −1.2 (95% CI: −2.3; −0.1) months) (Table II).
Pubertal development in sons
Maternal PCOS, oligomenorrhoea or ‘other menstrual irregularities’ were not associated with timing of pubertal development in sons (average difference of all pubertal milestones: PCOS: −0.5 (95% CI: −3.1; 2.1), oligomenorrhoea: 0.0 (95% CI: −3.6; 3.5) or ‘other menstrual irregularities’: 0.0 (95% CI: −0.9; 0.8) months) (Table II).
Sub-analyses
We found that the association between maternal PCOS and earlier adrenarche in daughters was not mediated through childhood BMI (average NIE: −0.2 months, Table II). Some of the association was mediated through maternal T2DM/GDM (average NIE: −1.6 months, Table III). Overall, none of the association was mediated through maternal hypertensive disorders (average NIE: 0.2 months, Table III).
Exploring the potential mediating effect of ‘7 year BMI (continuous)’ and ‘type 2 diabetes mellitus (T2DM) or gestational diabetes mellitus (GDM, dichotomized)’ showing the mean age differences (in months) at pubertal development in daughters of mothers with polycystic ovary syndrome (PCOS) divided into natural direct effect (NDE) and natural indirect effect (NIE).
| . | Daughters exposed to PCOS (n = 128) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Mean age difference (months) (95% CI) divided into NDE and NIEa,b . | ||||||
| . | Potential mediator: . | ||||||
| . | 7 year BMIc . | Maternal T2DM/GDM . | Maternal hypertensive disorders . | ||||
| Pubertal milestone . | NDE . | NIE . | NDE . | NIE . | NDE . | NIE . | |
| Tanner stage | |||||||
| Pubic hair | 2 | −5.2 (−8.4; −2.1) | 0.1 (−0.8; 1.0) | −3.4 (−6.6; −0.2) | −5.4 (−9.9; −0.8) | −4.0 (−7.6; −0.5) | 0.1 (−0.4; 0.6) |
| 3 | −4.4 (−7.2; −1.5) | −0.1 (−0.9; 0.7) | −3.1 (−6.0; −0.1) | −0.6 (−1.3; 0.2) | −4.1 (−6.9; −1.3) | 0.2 (−0.4; 0.8) | |
| 4 | −7.1 (−11.6; −2.5) | −0.3 (−1.6; 1.0) | −5.9 (−9.8; −2.0) | −0.7 (−1.9; 0.4) | −7.0 (−11.0; −3.1) | 0.2 (−0.4; 0.7) | |
| 5 | −5.1 (−13.1; 2.9) | −1.2 (−3.8; 1.4) | −4.8 (−11.6; 2.0) | −1.5 (−3.3; 0.3) | −7.4 (−13.9; −0.8) | 0.4 (−0.7; 1.4) | |
| Axillary hair | −11.6 (−16.7; −6.5) | 1.1 (−0.3; 2.4) | −6.7 (−11.5; −1.8) | −0.1 (−1.1; 0.8) | −7.1 (−11.7; −2.5) | 0.1 (−0.5; 0.8) | |
| Acne | −4.1 (−9.8; 1.6) | −0.1 (−1.8; 1.5) | −3.1 (−8.2; 1.9) | −0.6 (−2.4; 1.2) | −3.5 (−8.3; 1.3) | −0.2 (−1.3; 1.0) | |
| Average NIE | −0.1 | −1.5 | 0.1 | ||||
| . | Daughters exposed to PCOS (n = 128) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Mean age difference (months) (95% CI) divided into NDE and NIEa,b . | ||||||
| . | Potential mediator: . | ||||||
| . | 7 year BMIc . | Maternal T2DM/GDM . | Maternal hypertensive disorders . | ||||
| Pubertal milestone . | NDE . | NIE . | NDE . | NIE . | NDE . | NIE . | |
| Tanner stage | |||||||
| Pubic hair | 2 | −5.2 (−8.4; −2.1) | 0.1 (−0.8; 1.0) | −3.4 (−6.6; −0.2) | −5.4 (−9.9; −0.8) | −4.0 (−7.6; −0.5) | 0.1 (−0.4; 0.6) |
| 3 | −4.4 (−7.2; −1.5) | −0.1 (−0.9; 0.7) | −3.1 (−6.0; −0.1) | −0.6 (−1.3; 0.2) | −4.1 (−6.9; −1.3) | 0.2 (−0.4; 0.8) | |
| 4 | −7.1 (−11.6; −2.5) | −0.3 (−1.6; 1.0) | −5.9 (−9.8; −2.0) | −0.7 (−1.9; 0.4) | −7.0 (−11.0; −3.1) | 0.2 (−0.4; 0.7) | |
| 5 | −5.1 (−13.1; 2.9) | −1.2 (−3.8; 1.4) | −4.8 (−11.6; 2.0) | −1.5 (−3.3; 0.3) | −7.4 (−13.9; −0.8) | 0.4 (−0.7; 1.4) | |
| Axillary hair | −11.6 (−16.7; −6.5) | 1.1 (−0.3; 2.4) | −6.7 (−11.5; −1.8) | −0.1 (−1.1; 0.8) | −7.1 (−11.7; −2.5) | 0.1 (−0.5; 0.8) | |
| Acne | −4.1 (−9.8; 1.6) | −0.1 (−1.8; 1.5) | −3.1 (−8.2; 1.9) | −0.6 (−2.4; 1.2) | −3.5 (−8.3; 1.3) | −0.2 (−1.3; 1.0) | |
| Average NIE | −0.1 | −1.5 | 0.1 | ||||
Mean monthly difference in age at tatting each pubertal milestone compared to the reference group.
Adjusted for maternal pre-pregnancy BMI, social-economic status, maternal age at menarche, maternal age at birth and maternal smoking during first trimester of pregnancy.
Number of missing information on 7 year BMI: n = 4773. Total direct effect (NDE+NIH) is comparable to the results obtained in the main analysis when restring to those with information on 7 year BMI (data not shown).
Exploring the potential mediating effect of ‘7 year BMI (continuous)’ and ‘type 2 diabetes mellitus (T2DM) or gestational diabetes mellitus (GDM, dichotomized)’ showing the mean age differences (in months) at pubertal development in daughters of mothers with polycystic ovary syndrome (PCOS) divided into natural direct effect (NDE) and natural indirect effect (NIE).
| . | Daughters exposed to PCOS (n = 128) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Mean age difference (months) (95% CI) divided into NDE and NIEa,b . | ||||||
| . | Potential mediator: . | ||||||
| . | 7 year BMIc . | Maternal T2DM/GDM . | Maternal hypertensive disorders . | ||||
| Pubertal milestone . | NDE . | NIE . | NDE . | NIE . | NDE . | NIE . | |
| Tanner stage | |||||||
| Pubic hair | 2 | −5.2 (−8.4; −2.1) | 0.1 (−0.8; 1.0) | −3.4 (−6.6; −0.2) | −5.4 (−9.9; −0.8) | −4.0 (−7.6; −0.5) | 0.1 (−0.4; 0.6) |
| 3 | −4.4 (−7.2; −1.5) | −0.1 (−0.9; 0.7) | −3.1 (−6.0; −0.1) | −0.6 (−1.3; 0.2) | −4.1 (−6.9; −1.3) | 0.2 (−0.4; 0.8) | |
| 4 | −7.1 (−11.6; −2.5) | −0.3 (−1.6; 1.0) | −5.9 (−9.8; −2.0) | −0.7 (−1.9; 0.4) | −7.0 (−11.0; −3.1) | 0.2 (−0.4; 0.7) | |
| 5 | −5.1 (−13.1; 2.9) | −1.2 (−3.8; 1.4) | −4.8 (−11.6; 2.0) | −1.5 (−3.3; 0.3) | −7.4 (−13.9; −0.8) | 0.4 (−0.7; 1.4) | |
| Axillary hair | −11.6 (−16.7; −6.5) | 1.1 (−0.3; 2.4) | −6.7 (−11.5; −1.8) | −0.1 (−1.1; 0.8) | −7.1 (−11.7; −2.5) | 0.1 (−0.5; 0.8) | |
| Acne | −4.1 (−9.8; 1.6) | −0.1 (−1.8; 1.5) | −3.1 (−8.2; 1.9) | −0.6 (−2.4; 1.2) | −3.5 (−8.3; 1.3) | −0.2 (−1.3; 1.0) | |
| Average NIE | −0.1 | −1.5 | 0.1 | ||||
| . | Daughters exposed to PCOS (n = 128) . | ||||||
|---|---|---|---|---|---|---|---|
| . | Mean age difference (months) (95% CI) divided into NDE and NIEa,b . | ||||||
| . | Potential mediator: . | ||||||
| . | 7 year BMIc . | Maternal T2DM/GDM . | Maternal hypertensive disorders . | ||||
| Pubertal milestone . | NDE . | NIE . | NDE . | NIE . | NDE . | NIE . | |
| Tanner stage | |||||||
| Pubic hair | 2 | −5.2 (−8.4; −2.1) | 0.1 (−0.8; 1.0) | −3.4 (−6.6; −0.2) | −5.4 (−9.9; −0.8) | −4.0 (−7.6; −0.5) | 0.1 (−0.4; 0.6) |
| 3 | −4.4 (−7.2; −1.5) | −0.1 (−0.9; 0.7) | −3.1 (−6.0; −0.1) | −0.6 (−1.3; 0.2) | −4.1 (−6.9; −1.3) | 0.2 (−0.4; 0.8) | |
| 4 | −7.1 (−11.6; −2.5) | −0.3 (−1.6; 1.0) | −5.9 (−9.8; −2.0) | −0.7 (−1.9; 0.4) | −7.0 (−11.0; −3.1) | 0.2 (−0.4; 0.7) | |
| 5 | −5.1 (−13.1; 2.9) | −1.2 (−3.8; 1.4) | −4.8 (−11.6; 2.0) | −1.5 (−3.3; 0.3) | −7.4 (−13.9; −0.8) | 0.4 (−0.7; 1.4) | |
| Axillary hair | −11.6 (−16.7; −6.5) | 1.1 (−0.3; 2.4) | −6.7 (−11.5; −1.8) | −0.1 (−1.1; 0.8) | −7.1 (−11.7; −2.5) | 0.1 (−0.5; 0.8) | |
| Acne | −4.1 (−9.8; 1.6) | −0.1 (−1.8; 1.5) | −3.1 (−8.2; 1.9) | −0.6 (−2.4; 1.2) | −3.5 (−8.3; 1.3) | −0.2 (−1.3; 1.0) | |
| Average NIE | −0.1 | −1.5 | 0.1 | ||||
Mean monthly difference in age at tatting each pubertal milestone compared to the reference group.
Adjusted for maternal pre-pregnancy BMI, social-economic status, maternal age at menarche, maternal age at birth and maternal smoking during first trimester of pregnancy.
Number of missing information on 7 year BMI: n = 4773. Total direct effect (NDE+NIH) is comparable to the results obtained in the main analysis when restring to those with information on 7 year BMI (data not shown).
Discussion
Principal findings
We found that maternal PCOS was associated with an accelerated adrenarche (pubarche, axillary hair and acne) in daughters, whereas thelarche and menarche occurred at similar time as the reference group. Oligomenorrhoea and ‘other menstrual irregularities’ were not associated with altered timing of pubertal development.
PCOS, oligomenorrhoea or ‘other menstrual irregularities’ were not associated with timing of pubertal development in sons.
Potential underlying mechanisms
Genetics, epigenetics and foetal programming
Observational studies have established the inherited nature of PCOS (Risal et al., 2019). However, the specific role of genetics, epigenetics and foetal programming in the pathogenesis of PCOS remain unclear. Family and twin studies have found an autosomal dominant hereditary pattern of PCOS with an estimated heritability of PCOS of around 70% (Legro et al., 1998; Govind et al., 1999; Vink et al., 2006). However, genome-wide association studies (GWAS) have only identified genetic loci associated with PCOS that accounts for 10% of the heritability (Stener-Victorin and Deng, 2021). Additionally, emerging evidence suggest that epigenetic modifications are strongly associated with the susceptibility to PCOS (Qu et al., 2012; Wu et al., 2014; Yu et al., 2015; Kokosar et al., 2016; Nilsson et al., 2018).
We only observed earlier adrenarche in the daughters of mothers with PCOS. As premature adrenarche can be an initial sign of PCOS (Voutilainen and Jääskeläinen, 2015), we speculate whether the observed association is due to not yet diagnosed PCOS in the daughters inherited from the mother (Sanchez-Garrido and Tena-Sempere, 2020). Studies have shown higher levels of androgens in the umbilical venous blood in daughters of mothers with PCOS (Barry et al., 2010). Additionally, daughters of women with PCOS have higher LH, androgen and AMH levels, as well as pancreatic β-cell dysfunction, hyperinsulinaemia and increased ovarian volume during pre- and peri-pubertal years compared to daughters of mothers without PCOS (Sir-Petermann et al., 2009; Torchen et al., 2014; Crisosto et al., 2019; Torchen et al., 2019). One study has found that girls with premature adrenarche, especially those whose mothers had PCOS, had a higher risk of developing PCOS later in life because of high AMH levels during adolescence (Efthymiadou et al., 2019).
Today, the participants in the DNBC Puberty Cohort are between 19 and 22 years of age, and studying the incidence of PCOS among daughters is not possible yet. The HPG axis is generally considered immature during the peri-pubertal years (Mansfield and Emans, 1984), and diagnosing PCOS before the age of 18 years is challenging and incautious due to normal features such as irregular menses, mild hyperandrogenism and multi-follicular ovaries (Kristensen et al., 2010; Peña et al., 2020).
In sons of mothers with PCOS, one study found higher androgen, FSH, total cholesterol and LDL levels, as well as higher waist-to-hip ratio (Crisosto et al., 2017). In fathers and brothers of women with PCOS, others found increased levels of AMH, FSH and LH (Torchen et al., 2016). We did not observe indications of altered reproductive functions in the form of puberty in the present study.
Overweight, insulin resistance and hypertension
Obesity, hyperinsulinaemia and insulin resistance are integrated phenotypic characteristics of PCOS (Dunaif et al., 1989; Mannerås-Holm et al., 2011). Moreover, PCOS is an independent risk factor for GDM and hypertensive disorders during pregnancy (Mills et al., 2020), and pregnant women with PCOS more often experience excessive gestational weight gain (Kent et al., 2018). Using Mendelian randomization, researchers have found that high BMI can lead to PCOS perhaps due to an underlying cause, whereas PCOS does not elevate BMI in itself (Brower et al., 2019). This might already be evident during adolescence, as high BMI at 15 year is associated with persistent oligomenorrhoea, one of the features of PCOS, at age 18 years (van Hooff et al., 2004). Additionally, weight loss often leads to decreased insulin and androgens levels, as well as improved ovarian function (Kiddy et al., 1992). Wherefore, weight loss is a key factor in the treatment of women with PCOS (Tarlatzis et al., 2008). Moreover, a meta-analysis using cross-trait analysis suggest a shared genetic basis underlying obesity and PCOS (Liu et al., 2022), and using Mendelian randomization, a genetic link underlying T2DM, glycaemic traits and PCOS is established independently of BMI (Liu et al., 2022).
We adjusted all analyses for maternal pre-pregnancy BMI as this has been identified as a risk factor of earlier pubertal development in daughters and sons (Brix et al., 2019a,b). Further, maternal GDM and hypertensive disorders have been found to be associated with earlier pubertal development especially in daughters (Lauridsen et al., 2018; Lunddorf et al., 2020); thus, we investigated the causal pathways further in sub-analyses. We found that maternal T2DM/GDM likely accounts for some, but not all, of the association between maternal PCOS and adrenarche in daughters. However, this is mainly driven by the NIE of TPH4 (Table III), which might be a change finding. Additionally, the results did not indicate any mediation through maternal hypertensive disorders (Table III).
Furthermore, studies have proposed an association between maternal PCOS and higher BMI in the children (Zhang et al., 2022), which further is associated with earlier pubertal development (Brix et al., 2020b). We investigated whether the observed association between maternal PCOS and adrenarche in daughters was due to this causal path, even when adjusting for maternal pre-pregnancy BMI. The sub-analysis did not indicate mediation through BMI at 7 years of age (Table III).
Strengths and limitations of the study
We included a large study-population of both daughters and sons from a nationwide, ethnic homogenous birth-cohort, whom we followed from foetal life until young adulthood. Herein, we have detailed information on pubertal development collected every 6 months throughout adolescence, which limits the risk of misclassification and recall problems regarding the outcomes. To enhance the children’s ability to self-assess their current pubertal stage, we accompanied the questionnaires with pictures and a short descriptive text for each Tanner stage. As the children were unaware of the current hypothesis of the present study, the self-reported pubertal development is unlikely to differentiate across levels of the exposure, and we presume any misclassification would be non-differential; biasing the results towards the null. We have previously performed a validation study in the DNBC Puberty Cohort investigating the inter-rater agreement between self-assessed Tanner stages and clinical examination and the intra-individual agreement of self-assessed information on various pubertal milestones (Ernst et al., 2018). We found fair to moderate inter-rater agreement and moderate to good intra-individual agreement, which makes room for use of the self-reported information in large, aetiological studies such as the present (Ernst et al., 2018).
As the data collection started when participants were 11 years of age, some–especially girls—had started their pubertal development already. Additionally, some had not attained all the pubertal milestones within the follow-up period. The statistical method accounts for both of these situations adequately if the assumption of normally distributed residuals is correct.
As PCOS is highly underdiagnosed, we strived to identify unacknowledged and undiagnosed cases of PCOS using both diagnostic codes and self-reported information from the DNBC; thereby, limiting the number of exposed in the reference group. The self-reported information on menstrual cycle was provided by the mothers during pregnancy, which limits the risk of recall problems of the exposure. Moreover, the self-reported information on menstrual patterns was provided during pregnancy with no knowledge of future studies on pubertal development of the children, which reduces the risk of differential misclassification. The diagnostic codes were drawn from Danish health registers independently of the present study.
We had a low prevalence of mothers with PCOS compared to previous studies, which indicates misclassification of mothers with PCOS into other exposure groups and the reference group. In order to limit the misclassification and have a clean reference group, we constructed the exposure groups ‘oligomenorrhea’ and ‘other menstrual irregularities’. Included in these exposure groups were mothers who did not have a diagnostic code of PCOS, but either had self-reported or register-based information indicating that they might have an underlying and undiagnosed PCOS. Nonetheless, we acknowledge that a number of these will have other causes of their menstrual abnormalities besides PCOS; though, we were unable to identify what. Unfortunately, we did not have information on ultrasound scans nor hyperandrogenism, e.g. modified Ferriman–Gallwey (m-FG) scores, by which sub-division into different PCOS phenotypes was not possible.
The majority of women are diagnosed during their fertile years, which was also evident in the present study (mean time from birth of index child to diagnosis: 2.2 (SD: 5.6) years). Thus, the mothers were on average diagnosed 18.8 years ago (SD: 5.6 years) by August 2022. The Rotterdam Criteria were introduced in 2003 in Denmark. As the prevalence of PCOS is generally higher when applying the Rotterdam criteria (Yildiz et al., 2012), we would expect a larger number of diagnosed if the data collection was replicated today.
In order to limit the risk of selection bias, we included selection weight in all analyses. Furthermore, we found that 18.8% of non-participants and 17.9% of participants were exposed to PCOS, oligomenorrhoea or ‘other menstrual irregularities’. That was likewise the case when looking into the different date sources of information on puberty. Among those only participating in the 11-year follow-up of the DNBC, 17.8% were exposed; 19.0% when looking at those only participating in the DNBC Puberty Cohort; and 17.3% when looking at those participating in both. Thereby, we conclude that participation in the present study was not strongly associated with maternal PCOS. Additionally, we have previously performed a study investigated whether participation in the study was associated with pubertal development (Brix et al., 2020a). We found that the two were not associated. Overall, the risk of selection bias is considered limited.
Conclusion
Maternal PCOS was associated with earlier adrenarche in daughters, but not with earlier thelarche and menarche. Maternal PCOS was not associated with earlier timing of male pubertal development. Whether the observed association in daughters is due to genetics, epigenetics or prenatal programming by hyperandrogenism intrauterine remain unsolved, and this should be explored in future research.
Data Availability
Data available on request to the DNBC and the DNBC Puberty Cohort.
Authors’ roles
All authors conceptualized and designed the study. C.H.R.-H. and J.O. acquired the data. L.L.H.L. analysed the data. All authors helped interpret the data. L.L.H.L. drafted the article. All authors reviewed the article critically for the intellectual content, approved the final version to be published and agree to be accountable for all aspects of the work.
Funding
The DNBC was established with a significant grant from the Danish National Research Foundation. Additional support was obtained from the Danish Regional Committees, the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Health Foundation and other minor grants. The DNBC Biobank has been supported by the Novo Nordisk Foundation and the Lundbeck Foundation. Follow-up of mothers and children have been supported by the Danish Medical Research Council (SSVF 0646, 271-08-0839/06-066023, O602-01042B, 0602-02738B), the Lundbeck Foundation (195/04, R100-A9193), The Innovation Fund Denmark 0603-00294B (09-067124), the Nordea Foundation (02-2013-2014), Aarhus Ideas (AU R9-A959-13-S804), University of Copenhagen Strategic Grant (IFSV 2012) and the Danish Council for Independent Research (DFF-4183-00594 and DFF-4183-00152). The DNBC Puberty Cohort was established with support from the Danish Council for Independent Research (DFF 4183-00594) and Aarhus Ideas (AU R9-A959-13-S804). The current study was supported by the Faculty of Health at Aarhus University. The authors have no other financial relations to disclose.
Conflict of interest
The authors have no conflict of interest to disclose.



