-
PDF
- Split View
-
Views
-
Cite
Cite
S G Kristensen, Q Liu, L S Mamsen, T Greve, S E Pors, A B Bjørn, E Ernst, K T Macklon, C Y Andersen, A simple method to quantify follicle survival in cryopreserved human ovarian tissue, Human Reproduction, Volume 33, Issue 12, December 2018, Pages 2276–2284, https://doi.org/10.1093/humrep/dey318
Close - Share Icon Share
Abstract
Can follicle survival in frozen–thawed human ovarian tissue be quantified in situ using the dye Neutral Red (NR) to stain viable follicles specifically?
A follicle survival rate within ovarian tissue can be calculated using NR followed by histological evaluation and evidence for a consistently high follicle survival in a series of ovarian tissue from 25 Danish girls and women undergoing ovarian tissue cryopreservation (OTC) was obtained.
Securing follicle survival in cryopreserved ovarian tissue is crucial for proper quality control when centers wish to implement OTC. The only established technique for validation of follicle survival is xenografting of thawed ovarian tissue to immunodeficient mice. However, this functional test is expensive, time consuming, requires animal facilities and only provides a qualitative—not quantitative—measure for follicle survival.
Quantification of follicle survival in human ovarian tissue donated from 30 girls and women having tissue cryopreserved for fertility preservation from 2000 to 2015 at the Laboratory of Reproductive Biology in Copenhagen, Denmark.
Cryopreserved ovarian cortex was donated from 25 girls and young women aged 10–36 years (mean age: 25 years) and the average storage time in liquid nitrogen was 9.1 ± 5.6 years, ranging from 1.6 to 17.9 years. In 12 of the cases, the ovarian tissue was collected from the local hospital and in the other 13 cases the ovarian tissue was transported on ice up to 6 h prior to freezing. Donated fresh ovarian surplus tissue was obtained from five women aged 23–34 years (mean age: 27 years). Ovarian tissues were chopped into small fragments and incubated in culture medium containing 50 mg/ml NR for 3–4 h. Fragments of ovarian tissue containing clearly NR-stained follicles were selected for counting, encapsulated in 4% agar and were processed for histology to calculate a follicular survival rate.
The mean follicle survival rate in the 25 patients after freezing and thawing was 84% ± 11 (mean ±SD), ranging from 50% to 98%. The high follicle survival rate in this clinical series of patients reflects a constant high-quality service performed in our center and confirms the robustness of the slow freezing protocol. No significant association between follicle survival rates and storage time was found using linear regression analysis, suggesting that storage in liquid nitrogen does not affect viability of the tissue. No significant association in follicle survival rates was found between ovarian tissues collected at the local hospital compared to tissues transported on ice prior to freezing, supporting that prolonged cooling does not seem to greatly affect the follicle survival. For the fresh ovarian tissue, the average follicle survival rate was 91% ± 5 (mean ± SD) in five patients, ranging from 81% to 95%.
Even though the NR staining requires active incorporation of the dye, the test is merely a short in situ test that cannot completely replace the functional value of xenografting studies in which the integrity and developmental potential of the ovarian follicles are assessed.
OTC is now being employed around the world but to date it has been difficult for centers to evaluate the effectiveness of their program and perform proper quality control. NR staining combined with histological evaluation is the first quantitative method to provide a survival rate for follicles in frozen–thawed human ovarian tissue and offer a valuable and easily applicable tool to validate the cryopreservation procedure when implementing OTC or as routine quality control for the overall freezing performance within tissue banking facilities.
The Research Pools of Rigshospitalet, the Danish Cancer Foundation, Dagmar Marshalls Foundation, and the Novo Nordic Foundation are thanked for having funded this study. The authors have no conflicts of interest.
Introduction
Ovarian tissue cryopreservation (OTC) is the only fertility preserving option for young girls, who do not yet produce mature oocytes, and young women in urgent need of potentially sterilizing cancer treatment. Subsequent transplantation of cryostored ovarian tissue has so far resulted in restoration of hormonal cyclicity in 95% of the >300 transplanted women worldwide, the birth of more than 100 children and induction of puberty in 2 young girls (Poirot et al., 2012; Ernst et al., 2013; Donnez and Dolmans, 2017; Gellert et al., 2018). Numerous fertility clinics worldwide are now starting to implement the technique, however, the procurement and setup of OTC differ profoundly in many aspects from conventional ART. The success and efficacy of OTC is difficult to measure as it usually takes several years before the patients return for transplantation of the stored tissue, and in the case of children, sometimes decades (Andersen, 2015). Thorough validation of the freezing and thawing procedures to secure surviving follicles in the stored tissue are therefore required (Andersen et al., 2018).
To date, the golden standard for validation of follicle survival in cryostored human ovarian tissue has been xenografting of thawed ovarian tissue to immunodeficient mice, which provides a functional qualitative test of the follicles’ ability to survive in vivo (Gook et al., 2005; Amorim et al., 2011; Rosendahl et al., 2011). However, the xenografting model has several drawbacks; (i) it does not provide quantitative measures for follicle survival, (ii) it takes around 2 months to obtain the results and (iii) it is costly and requires skills and equipment which is usually not readily available at most fertility centers. Several in vitro models have been applied to evaluate follicle survival after freezing and thawing of ovarian tissue including morphological evaluation of follicle integrity and stromal cell density by means of classical histological analysis (Keros et al., 2009; Chang et al., 2011; Klocke et al., 2015; Sanfilippo et al., 2015; Fabbri et al., 2016), culture of ovarian fragments or isolated preantral follicles with hormone secretion measurements (Isachenko et al., 2009a; Klocke et al., 2015), and evaluation of DNA fragmentation in primordial follicles by TUNEL assay (Chang et al., 2011; Herraiz et al., 2014; Sanfilippo et al., 2015), whereas these methods have been useful tools to indicate a level of potential follicle survival within the stored tissue, they have not provided a quantitative measure of follicle survival rate for clinical use.
Neutral Red (NR) is a vital dye that readily passes the cell membrane in situ and concentrates in lysosomes of viable cells (Allison and Young, 1964). In 2010, Chambers and colleagues showed that NR concentrates in follicular structures expressing a distinct red color within weakly stained stromal tissue, and that NR predicts viable follicle density in situ in both ovine and human cortical ovarian tissues (Chambers et al., 2010). Recently, NR staining has been used to evaluate different thawing procedures for human ovarian tissue (Morewood et al., 2017), the effect of prolonged cooling on feline ovarian tissue prior to cryopreservation (Martins et al., 2018), the effectiveness of different digestion enzymes for human ovarian tissue (Schmidt et al., 2018) and the comparison between vitrification and slow freezing techniques on whole rat ovaries (Milenkovic et al., 2012).
The aim of the current study was to develop a simple in situ test for quantification of follicle survival in cryopreserved ovarian tissue, which would be applicable to most clinics. Using NR staining, the number of viable follicles within the frozen–thawed ovarian tissue was evaluated and subsequent histological processing provided the total number of follicles, which enabled the calculation of a follicle survival rate for the cryostored tissue.
Material and Methods
Human ovarian tissue and patient characteristics
Human ovarian cortical tissue was donated by patients who had undergone unilateral oophorectomy and OTC at the Laboratory of Reproductive Biology (LRB) in Copenhagen, Denmark from year 2000–2015.
Donated frozen cortical tissue was obtained from 25 girls and young women aged 10–36 years (mean age: 25 years). The mean ovarian volume per ovary was 6.8 ± 3.1 ml (range: 1.3–13.0 ml, n = 20), and the diagnosis at the time of cryopreservation included breast cancer (n = 11), Morbus(Mb)/non-Hodgkin lymphoma (n = 6), sarcomas (n = 2), leukemia (n = 1), various other cancers (n = 3), adrenal gland disorder (n = 1) and Mola (n = 1). Average storage time for the ovarian tissue in liquid nitrogen (from the time of cryopreservation to thawing) was 9.1 ± 5.6 years (mean ±SD), ranging from 1.6 to 17.9 years. The ovarian tissue was either transported directly to the laboratory in 37°C media from the operating theater at the local hospital or transported on crushed ice for 2–6 h from hospitals in Aarhus, Odense or Malmø (Sweden). In the current study, 12 of the cases were collected from the local hospital (transport time around 10 min) and in the other 13 cases the ovarian tissue was received on ice from primarily Aarhus in which the transport time was around 4–6 h.
Donated fresh ovarian surplus tissue was obtained from five women aged 23–34 years (mean age: 27 years). Diagnosis included Mb.Hodgkin (n = 2), breast cancer (n = 1), ovarian cancer (n = 1) and Aplastic anemia (n = 1). In three out of the five cases, the ovarian tissue was collected at the local hospital, whereas the tissue in the other two cases was transported on ice.
NR staining of human ovarian tissue
In the cases of donated frozen ovarian cortex, 2–3 pieces of ovarian cortical tissue (5×5 mm) from each patient were thawed according to clinical procedure (Rosendahl et al., 2011). In cases with fresh ovarian surplus tissue, the tissue pieces were collected directly from the dissection dishes after the preparation of the cortical tissue for OTC.
The critical step in the staining procedure is chopping of the tissue to a thickness <100 μm in order to visualize clearly the NR-stained follicles within the tissue (Fig. 1A). The thawed cortical tissue pieces were chopped into small fragments using the McIIwain Tissue Chopper (Mickle Laboratory, Guildford, UK), adjusted to 0.1 mm. The cutting procedure was repeated at least 10–12 times until the ovarian tissue pieces had been completely homogenized and the majority of fragments were <100 μm thick (Fig. 2A–C). During the chopping procedure, it is important to keep the tissue fragments moist by adding thawing or culture media. Tissue fragments were transferred into a 60-mm culture dish (NunclonTM Dish, Nunc A/S, Roskilde, Denmark) containing 8 ml preheated 37°C McCoy’s 5a culture medium containing 26 mM sodium bicarbonate and 25 mM HEPES (Invitrogen, GIBCO), supplemented with 0.1% v/v human serum albumin (HSA; CSL Behring 20%, Marburg, Germany), 2 mM glutamax (Invitrogen, GIBCO), 0.05 mg/ml penicillin/streptomycin (Invitrogen, GIBCO®), 5.5 mg/ml transferrin, 6 ng/ml selenium, 10 mg/ml insulin (Invitrogen Corporation, GIBCOTM) and 50 μg/ml NR solution (N2889, Sigma Aldrich Logistik GmbH, Schnelldorf, Germany). Tissue fragments were incubated with NR in situ for 3–4 h at 37°C and 5% CO2 in air (Fig. 2D and E).

(A) Frozen/thawed human ovarian cortical tissue (5 × 5 mm) sectioned at 100 μm using a Vibratome tissue slicer. NR-stained follicles present within the tissue after 3–4 h in situ incubation. (B) The number of NR-stained follicles in relation to the number of follicles found at the corresponding histological evaluation of small fragments of fresh surplus ovarian tissue from five patients. NR, Neutral Red.
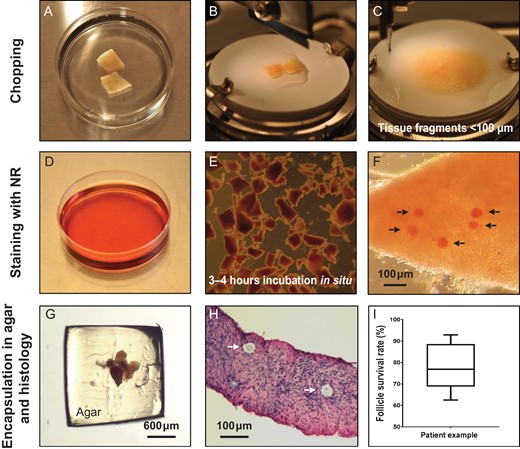
Practical steps. (A) Two pieces of thawed human ovarian tissue. (B) Ovarian tissue pieces placed on the cutting board in the McIIwain Tissue Chopper adjusted to 0.1 mm. (C) Tissue pieces were cut into smaller fragments <100 μm by repeated chopping (10–12 times). (D) Culture dish containing culture medium with 50 μg/ml NR solution (15 μl of NR per 1 ml of culture media). (E) Ovarian tissue fragments were incubated in NR containing culture media for 3–4 h in situ. (F) Selected tissue fragments were evaluated for the presence of NR-stained follicles and the exact number was recorded. Arrows point to NR-stained follicles within the tissue fragment. (G) Tissue fragments were encapsulated in a 4% w/v agar gel. (H) The total number of follicles in each tissue fragment was recorded during histological evaluation. Arrows point to follicles within the tissue fragment. (I) Box-plot (median, quartiles,range) depicts the follicle survival rate for Patient 16, and the exact follicle numbers and survival rates are presented in Supplementary Table SI. NR, Neutral Red.
Tissue fragments containing NR-stained follicles were identified under a stereomicroscope (Leica MZ12, Microsystems Ltd) (Fig. 1A and 2F). Small tissue fragments <100 μm were selected and clearly visible NR-stained follicles (Fig. 2F) were counted by turning the fragment around with a 23-G needle. If the tissue fragment is too thick, there is a high risk that NR-stained follicles in the middle of the tissue are not visible in the stereomicroscope, which will result in an inaccurate calculation of follicle survival. An NR-stained follicle count was recorded for each tissue fragment, and each fragment was subsequently encapsulated in 4% agar and processed for histology.
Encapsulation of tissue fragments in agar
In order to process the tiny tissue fragments for histology, they were encapsulated in 4% w/v agar before fixation. A 4% agar solution (BactoTM Agar, cat#214050, Becton, Dickinson and Company, France) was boiled and left in the heating cabinet until a temperature of around 50°C was obtained. The tissue fragment was placed in a 6-mm culture dish and excess media were removed but care was taken to avoid the tissue fragment drying out completely by monitoring it under the stereomicroscope. Around 600 μl of agar solution was aspirated and kept in a pipette tip for a short period of time—just enough for it not to solidify in the pipette—and then one big drop of agar was placed on the top of the ovarian tissue fragment. The agar was allowed to solidify and the agar drop with the tissue fragment was flipped over with a scalpel, and another drop of agar solution was placed at the bottom of the agar drop to seal the tissue encapsulation. Using a scalpel, the edges of the agar drop were removed by cutting around the encapsulated tissue fragment (Fig. 2G). In the cases where the follicle density was low, 2–6 ovarian fragments were pooled in one agar drop. The agar-embedded tissue fragments were immediately fixed in Bouin’s fixative for a least 3 h or overnight.
Histological analysis
Histological processing was performed using standard methods. The entire tissue fragment was cut into serial sections of 5 μm in thickness and stained with Periodic Acid–Schiff (PAS). Follicles were visualized by light microscopy using a Zeiss Axiophot microscope (Broch and Michelsen Instrument AS, Zeiss, West Germany) and captured with standard Leica DFC420C software (Leica Microsystems Ltd., Heerbrugg, Germany) (Fig. 2H). The total number of follicles within each tissue fragment was counted and recorded, and a follicle survival rate in percentage was calculated for each ovarian fragment by the following equation; ‘Number of NR-stained follicles’ divided by ‘Total number of follicles found by histology’ multiplied by 100. Between 2–8 agar-embedded tissue fragment clots were evaluated per patient irrespective of whether the tissue was fresh or frozen. Thus, an average follicle survival rate in the frozen/thawed ovarian tissue could be calculated for each patient (Supplementary Table SI and Fig. 2I; box-plot showing a patient example).
Xenografting
Two 8-week-old female immunodeficient mice (NMRI-nu/nu; Taconic, Denmark) were used to perform in vivo validation of follicle survival in frozen/thawed ovarian tissue samples in two clinical cases. Mice were kept in the regular animal house with free access to food and water. Two weeks prior to transplantation with human ovarian tissue the mice were ovariectomized. One piece of frozen/thawed ovarian tissue was transplanted to a subcutaneously prepared pocket onto the flank of the mouse, and after 4 weeks the ovarian grafts were recovered and fixed in Bouin’s solution overnight before histological processing and evaluation.
Ethical approval
The use of donated human ovarian tissue for research was approved by the Minister of Health in Denmark and by the ethical committee of the municipalities of Copenhagen and Frederiksberg (H-2-2011-044).
The use of animals was approved by the Animal Experiments Inspectorate (2015-15-0201–00505).
Statistical analyses
Statistical analysis was performed using GraphPad Prism 7.02 program (GraphPad Software, Inc., CA, USA). Significance level was defined as a probability lower than 0.05 (P < 0.05). A linear regression model was used to test potential correlations. For the 25 patients with frozen–thawed tissue, the model assumption was checked using residual plots (Supplementary Fig. S1) and one outlier (Patient 4) with a notable lower follicle survival rate was confirmed using Grubb’s test (alpha = 0.05). An unpaired non-parametric Mann–Whitney U test was used to analyze differences in survival rates between tissues which had been transported or not.
Results
Follicle survival rate in fresh ovarian tissue
Fresh ovarian surplus tissue was obtained from five patients and stained with NR followed by histological evaluation of selected tissue fragments. On average 10 ± 5 (mean ±SD) NR-stained follicles were counted per agar-embedded tissue fragment clot, ranging from 4 to 21 follicles. A significant positive association was found between the number of NR-stained follicles in the fresh ovarian tissue in relation to the total number of follicles found following histological evaluation (Fig. 1B).
In total, an average of 49 NR-stained follicles (ranging from 27 to 71) were counted per patient in 3–7 agar-embedded tissue fragment clots, resulting in an overall follicle survival rate of 91 ± 5% (mean ± SD) in fresh ovarian tissue from the five patients, ranging from 81% to 95% (Table I).
Follicle survival rates (mean ± SD) in fresh ovarian surplus tissue from five patients having ovarian tissue cryopreserved for fertility preservation.
| Patient no. | Transport on ice prior to freezing | Number of tissue fragments evaluated | Number of NR-stained follicles | Total number of follicles (histology) | Follicle survival rate (%) |
| 1 | Yes | 6 | 71 | 76 | 92 ± 8.5 |
| 2 | Yes | 3 | 32 | 37 | 81 ± 13.1 |
| 3 | No | 5 | 47 | 51 | 93 ± 6.2 |
| 4 | No | 7 | 69 | 75 | 95 ± 6.8 |
| 5 | No | 3 | 27 | 28 | 96 ± 5.9 |
| Mean | 91.4 ± 5.0 |
| Patient no. | Transport on ice prior to freezing | Number of tissue fragments evaluated | Number of NR-stained follicles | Total number of follicles (histology) | Follicle survival rate (%) |
| 1 | Yes | 6 | 71 | 76 | 92 ± 8.5 |
| 2 | Yes | 3 | 32 | 37 | 81 ± 13.1 |
| 3 | No | 5 | 47 | 51 | 93 ± 6.2 |
| 4 | No | 7 | 69 | 75 | 95 ± 6.8 |
| 5 | No | 3 | 27 | 28 | 96 ± 5.9 |
| Mean | 91.4 ± 5.0 |
NR, Neutral Red.
Follicle survival rates (mean ± SD) in fresh ovarian surplus tissue from five patients having ovarian tissue cryopreserved for fertility preservation.
| Patient no. | Transport on ice prior to freezing | Number of tissue fragments evaluated | Number of NR-stained follicles | Total number of follicles (histology) | Follicle survival rate (%) |
| 1 | Yes | 6 | 71 | 76 | 92 ± 8.5 |
| 2 | Yes | 3 | 32 | 37 | 81 ± 13.1 |
| 3 | No | 5 | 47 | 51 | 93 ± 6.2 |
| 4 | No | 7 | 69 | 75 | 95 ± 6.8 |
| 5 | No | 3 | 27 | 28 | 96 ± 5.9 |
| Mean | 91.4 ± 5.0 |
| Patient no. | Transport on ice prior to freezing | Number of tissue fragments evaluated | Number of NR-stained follicles | Total number of follicles (histology) | Follicle survival rate (%) |
| 1 | Yes | 6 | 71 | 76 | 92 ± 8.5 |
| 2 | Yes | 3 | 32 | 37 | 81 ± 13.1 |
| 3 | No | 5 | 47 | 51 | 93 ± 6.2 |
| 4 | No | 7 | 69 | 75 | 95 ± 6.8 |
| 5 | No | 3 | 27 | 28 | 96 ± 5.9 |
| Mean | 91.4 ± 5.0 |
NR, Neutral Red.
Follicle survival rate in frozen/thawed ovarian tissue
Frozen–thawed ovarian cortical tissue was obtained from 25 patients and stained with NR followed by histological evaluation of selected tissue fragments. On average 16 ± 5 (mean ± SD) NR-stained follicles were counted per agar-embedded tissue fragment clot, ranging from 7 to 38 follicles. In total, an average of 65 NR-stained follicles (ranging from 28 to 132) were counted per patient in 2–8 agar-embedded tissue fragment clots, resulting in an overall follicle survival rate of 84 ± 11% (mean ± SD) in frozen–thawed ovarian tissue from 25 patients, ranging from 50% to 98% (Table II).
Follicle survival rates (mean ± SD) in frozen/thawed ovarian tissue from 25 patients undergoing OTC for fertility preservation from 2000 to 2015.
| Patient no Storagetime (in years) . | Transport on ice prior to freezing . | Number of tissue fragments evaluated . | Number of NR-stained follicles . | Total number of follicles (histology) . | Follicle survival rate (%) . | |
|---|---|---|---|---|---|---|
| 1 | 1.6 | Yes | 3 | 28 | 37 | 76 ± 4.6 |
| 2 | 2.0 | Yes | 3 | 35 | 39 | 91 ± 7.7 |
| 3 | 2.2 | Yes | 8 | 132 | 172 | 75.9 ± 11.0 |
| 4 | 2.6 | No | 4 | 48 | 99 | 50 ± 8.2 |
| 5 | 2.9 | Yes | 4 | 69 | 96 | 72 ± 8.8 |
| 6 | 3.0 | Yes | 7 | 120 | 177 | 68.3 ± 10.1 |
| 7 | 3.4 | Yes | 2 | 42 | 48 | 87 ± 4.7 |
| 8 | 4.8 | No | 5 | 81 | 92 | 89 ± 11.9 |
| 9 | 5.5 | Yes | 3 | 59 | 67 | 88 ± 6.8 |
| 10 | 5.9 | No | 5 | 79 | 82 | 97 ± 2.8 |
| 11 | 6.4 | Yes | 5 | 85 | 99 | 87 ± 14.5 |
| 12 | 7.8 | Yes | 4 | 40 | 53 | 77 ± 10.9 |
| 13 | 8.3 | No | 5 | 66 | 72 | 92 ± 1.9 |
| 14 | 9.8 | Yes | 4 | 67 | 80 | 84 ± 6.6 |
| 15 | 11.3 | Yes | 4 | 69 | 77 | 91 ± 9.9 |
| 16 | 11.6 | Yes | 5 | 64 | 82 | 78 ± 10.1 |
| 17 | 11.6 | Yes | 4 | 64 | 74 | 90 ± 9.7 |
| 18 | 13.6 | No | 3 | 53 | 59 | 92 ± 6.6 |
| 19 | 13.6 | No | 4 | 76 | 109 | 70.2 ± 4.3 |
| 20 | 14.4 | No | 4 | 58 | 73 | 85 ± 15.8 |
| 21 | 16.1 | No | 3 | 52 | 60 | 88 ± 9.1 |
| 22 | 16.2 | No | 3 | 39 | 43 | 91 ± 2.4 |
| 23 | 16.7 | No | 4 | 43 | 47 | 92 ± 5.9 |
| 24 | 17.2 | No | 4 | 85 | 102 | 83.1 ± 9.5 |
| 25 | 17.9 | No | 4 | 74 | 76 | 98 ± 3.9 |
| Mean | 83.6 ± 10.7 | |||||
| Patient no Storagetime (in years) . | Transport on ice prior to freezing . | Number of tissue fragments evaluated . | Number of NR-stained follicles . | Total number of follicles (histology) . | Follicle survival rate (%) . | |
|---|---|---|---|---|---|---|
| 1 | 1.6 | Yes | 3 | 28 | 37 | 76 ± 4.6 |
| 2 | 2.0 | Yes | 3 | 35 | 39 | 91 ± 7.7 |
| 3 | 2.2 | Yes | 8 | 132 | 172 | 75.9 ± 11.0 |
| 4 | 2.6 | No | 4 | 48 | 99 | 50 ± 8.2 |
| 5 | 2.9 | Yes | 4 | 69 | 96 | 72 ± 8.8 |
| 6 | 3.0 | Yes | 7 | 120 | 177 | 68.3 ± 10.1 |
| 7 | 3.4 | Yes | 2 | 42 | 48 | 87 ± 4.7 |
| 8 | 4.8 | No | 5 | 81 | 92 | 89 ± 11.9 |
| 9 | 5.5 | Yes | 3 | 59 | 67 | 88 ± 6.8 |
| 10 | 5.9 | No | 5 | 79 | 82 | 97 ± 2.8 |
| 11 | 6.4 | Yes | 5 | 85 | 99 | 87 ± 14.5 |
| 12 | 7.8 | Yes | 4 | 40 | 53 | 77 ± 10.9 |
| 13 | 8.3 | No | 5 | 66 | 72 | 92 ± 1.9 |
| 14 | 9.8 | Yes | 4 | 67 | 80 | 84 ± 6.6 |
| 15 | 11.3 | Yes | 4 | 69 | 77 | 91 ± 9.9 |
| 16 | 11.6 | Yes | 5 | 64 | 82 | 78 ± 10.1 |
| 17 | 11.6 | Yes | 4 | 64 | 74 | 90 ± 9.7 |
| 18 | 13.6 | No | 3 | 53 | 59 | 92 ± 6.6 |
| 19 | 13.6 | No | 4 | 76 | 109 | 70.2 ± 4.3 |
| 20 | 14.4 | No | 4 | 58 | 73 | 85 ± 15.8 |
| 21 | 16.1 | No | 3 | 52 | 60 | 88 ± 9.1 |
| 22 | 16.2 | No | 3 | 39 | 43 | 91 ± 2.4 |
| 23 | 16.7 | No | 4 | 43 | 47 | 92 ± 5.9 |
| 24 | 17.2 | No | 4 | 85 | 102 | 83.1 ± 9.5 |
| 25 | 17.9 | No | 4 | 74 | 76 | 98 ± 3.9 |
| Mean | 83.6 ± 10.7 | |||||
OTC, ovarian tissue cryopreservation.
Follicle survival rates (mean ± SD) in frozen/thawed ovarian tissue from 25 patients undergoing OTC for fertility preservation from 2000 to 2015.
| Patient no Storagetime (in years) . | Transport on ice prior to freezing . | Number of tissue fragments evaluated . | Number of NR-stained follicles . | Total number of follicles (histology) . | Follicle survival rate (%) . | |
|---|---|---|---|---|---|---|
| 1 | 1.6 | Yes | 3 | 28 | 37 | 76 ± 4.6 |
| 2 | 2.0 | Yes | 3 | 35 | 39 | 91 ± 7.7 |
| 3 | 2.2 | Yes | 8 | 132 | 172 | 75.9 ± 11.0 |
| 4 | 2.6 | No | 4 | 48 | 99 | 50 ± 8.2 |
| 5 | 2.9 | Yes | 4 | 69 | 96 | 72 ± 8.8 |
| 6 | 3.0 | Yes | 7 | 120 | 177 | 68.3 ± 10.1 |
| 7 | 3.4 | Yes | 2 | 42 | 48 | 87 ± 4.7 |
| 8 | 4.8 | No | 5 | 81 | 92 | 89 ± 11.9 |
| 9 | 5.5 | Yes | 3 | 59 | 67 | 88 ± 6.8 |
| 10 | 5.9 | No | 5 | 79 | 82 | 97 ± 2.8 |
| 11 | 6.4 | Yes | 5 | 85 | 99 | 87 ± 14.5 |
| 12 | 7.8 | Yes | 4 | 40 | 53 | 77 ± 10.9 |
| 13 | 8.3 | No | 5 | 66 | 72 | 92 ± 1.9 |
| 14 | 9.8 | Yes | 4 | 67 | 80 | 84 ± 6.6 |
| 15 | 11.3 | Yes | 4 | 69 | 77 | 91 ± 9.9 |
| 16 | 11.6 | Yes | 5 | 64 | 82 | 78 ± 10.1 |
| 17 | 11.6 | Yes | 4 | 64 | 74 | 90 ± 9.7 |
| 18 | 13.6 | No | 3 | 53 | 59 | 92 ± 6.6 |
| 19 | 13.6 | No | 4 | 76 | 109 | 70.2 ± 4.3 |
| 20 | 14.4 | No | 4 | 58 | 73 | 85 ± 15.8 |
| 21 | 16.1 | No | 3 | 52 | 60 | 88 ± 9.1 |
| 22 | 16.2 | No | 3 | 39 | 43 | 91 ± 2.4 |
| 23 | 16.7 | No | 4 | 43 | 47 | 92 ± 5.9 |
| 24 | 17.2 | No | 4 | 85 | 102 | 83.1 ± 9.5 |
| 25 | 17.9 | No | 4 | 74 | 76 | 98 ± 3.9 |
| Mean | 83.6 ± 10.7 | |||||
| Patient no Storagetime (in years) . | Transport on ice prior to freezing . | Number of tissue fragments evaluated . | Number of NR-stained follicles . | Total number of follicles (histology) . | Follicle survival rate (%) . | |
|---|---|---|---|---|---|---|
| 1 | 1.6 | Yes | 3 | 28 | 37 | 76 ± 4.6 |
| 2 | 2.0 | Yes | 3 | 35 | 39 | 91 ± 7.7 |
| 3 | 2.2 | Yes | 8 | 132 | 172 | 75.9 ± 11.0 |
| 4 | 2.6 | No | 4 | 48 | 99 | 50 ± 8.2 |
| 5 | 2.9 | Yes | 4 | 69 | 96 | 72 ± 8.8 |
| 6 | 3.0 | Yes | 7 | 120 | 177 | 68.3 ± 10.1 |
| 7 | 3.4 | Yes | 2 | 42 | 48 | 87 ± 4.7 |
| 8 | 4.8 | No | 5 | 81 | 92 | 89 ± 11.9 |
| 9 | 5.5 | Yes | 3 | 59 | 67 | 88 ± 6.8 |
| 10 | 5.9 | No | 5 | 79 | 82 | 97 ± 2.8 |
| 11 | 6.4 | Yes | 5 | 85 | 99 | 87 ± 14.5 |
| 12 | 7.8 | Yes | 4 | 40 | 53 | 77 ± 10.9 |
| 13 | 8.3 | No | 5 | 66 | 72 | 92 ± 1.9 |
| 14 | 9.8 | Yes | 4 | 67 | 80 | 84 ± 6.6 |
| 15 | 11.3 | Yes | 4 | 69 | 77 | 91 ± 9.9 |
| 16 | 11.6 | Yes | 5 | 64 | 82 | 78 ± 10.1 |
| 17 | 11.6 | Yes | 4 | 64 | 74 | 90 ± 9.7 |
| 18 | 13.6 | No | 3 | 53 | 59 | 92 ± 6.6 |
| 19 | 13.6 | No | 4 | 76 | 109 | 70.2 ± 4.3 |
| 20 | 14.4 | No | 4 | 58 | 73 | 85 ± 15.8 |
| 21 | 16.1 | No | 3 | 52 | 60 | 88 ± 9.1 |
| 22 | 16.2 | No | 3 | 39 | 43 | 91 ± 2.4 |
| 23 | 16.7 | No | 4 | 43 | 47 | 92 ± 5.9 |
| 24 | 17.2 | No | 4 | 85 | 102 | 83.1 ± 9.5 |
| 25 | 17.9 | No | 4 | 74 | 76 | 98 ± 3.9 |
| Mean | 83.6 ± 10.7 | |||||
OTC, ovarian tissue cryopreservation.
Initially a significant positive association between storage time and mean follicle survival rate was found, however, this association was solely caused by one influential outlier observation from Patient 4, and the association disappeared once Patient 4 was excluded from the analysis. Thus, no significant association was found between follicle survival rate and storage time (Fig. 3), and neither age of the patient or ovarian volume was correlated with mean follicle survival rate.
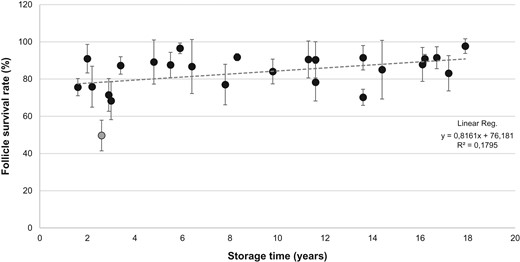
Follicle survival rate (percentage) in relation to storage time. Graph depicts calculated follicle survival rates (mean ± SD) following NR staining in donated frozen/thawed cortical tissue from 25 patients undergoing ovarian tissue cryopreservation for fertility preservation from 2000 to 2015. Linear regression analysis showed no significant correlation between follicle survival rate and storage time. Gray circle shows the identified outlier (Patient 4) which was excluded from the statistical analysis. NR, Neutral Red.
Regarding transportation of the ovarian tissue prior to freezing, no significant association was observed in the follicle survival rates between tissue collected at the local hospital and tissue transported on ice for up to 6 h prior to freezing (Fig. 4).
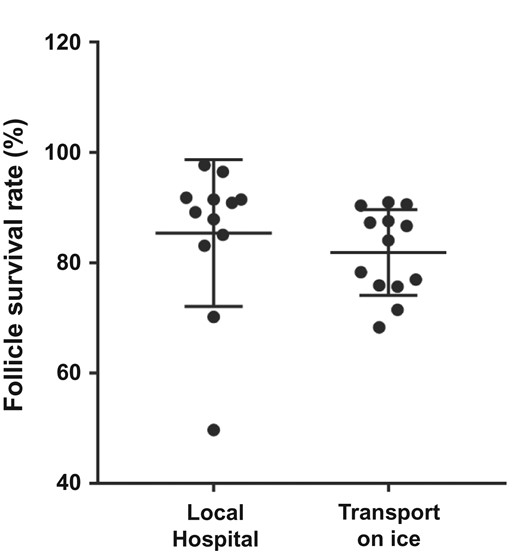
Average follicle survival rate (percentage) in frozen/thawed ovarian tissue collected at the local hospital in preheated culture media (n = 12 cases) compared to ovarian tissue transported on ice for up to 6 h prior to freezing (n = 13 cases). No significant difference in follicle survival rate was found between the two groups.
Validating OTC in clinical cases
In two clinical cases, NR staining and xenografting studies were used to validate follicle survival in frozen ovarian tissue. In the first case, two samples of frozen ovarian tissue from a 28-year-old woman with colorectal cancer were received from an external source for validation. NR staining of one of the thawed tissue samples (Fig. 5A) resulted in an overall identification of around 20 NR-stained follicles within the tissue fragments (Fig. 5B). Six tissue fragments containing a total of nine NR-stained follicles were selected for histological analysis and embedded in agar. Histological evaluation showed that the six tissue fragments contained a total number of 32 follicles (Fig. 5C), resulting in a follicle survival rate of 28%. Xenografting studies in which the remaining piece of thawed ovarian tissue had been grafted in an immunodeficient mouse for 4 weeks showed good vascularization of the graft (Fig 5D) and the presence of surviving follicles with good morphology within the grafted tissue (Fig. 5E).
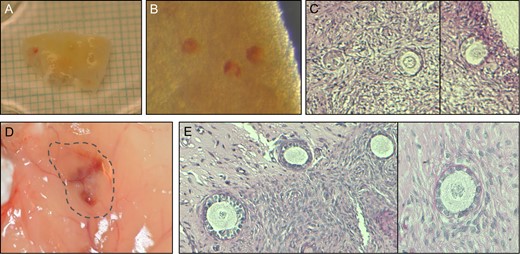
Validating follicle survival in cryopreserved ovarian tissue from a Polish patient by NR staining and xenografting. (A) A piece of frozen/thawed ovarian tissue. (B) NR-stained follicles within the tissue. (C) Histological evaluation of tissue fragments with NR-stained follicles. (D) Following 4 weeks xenografting, revascularization was observed in the graft. (E) Histological evaluation of the xenografted tissue. NR, Neutral Red.
In the second case, the Planer Freezer performed an incomplete run in which the freezing of the ovarian tissue samples stopped at –31°C and an emergency procedure was initiated. NR staining of one piece of frozen/thawed ovarian tissue resulted in a total count of 66 NR-stained follicles within three agar-embedded tissue fragment clots and a total count of 69 follicles after histological evaluation, resulting in a follicle survival rate of 96% ± 4 (mean ± SD). Xenografting studies in which one piece of thawed ovarian tissue had been grafted in an immunodeficient mouse for 4 weeks showed the presence of surviving follicles with good morphology within the grafted tissue (Supplementary Fig. S2).
Discussion
In this study, we present a simple and reliable method to quantify follicle survival for OTC. For the first time, NR staining combined with histological evaluation provides a quantitative method to calculate a survival rate for follicles in ovarian tissue. The method cannot replace the xenografting model, but it can be used as a validation tool to assess freezing protocols when clinics start a program for OTC and as a routine quality control for the overall freezing performance within the tissue banking facility.
The advantages of NR over other in situ dyes like Calcein AM and rhodamine 123 are that NR can be reconstituted in a physiological medium, it is non-toxic for the follicles and can be visualized with a standard light microscopy (Nemes et al., 1979; Picton et al., 1999; Chambers et al., 2010). Collectively, these features enable an easy implementation of the NR staining procedure in most fertility clinics as it does not require special microscopes or safety measures. In addition, the NR dye is cheap and easily available.
The NR dye only stain viable follicles within human ovarian tissue which was confirmed with the fluorescent dye CFDA-SE in our previous study (Kristensen et al. 2011), whereas non-viable follicles will show the absence of staining. Thus, the number of NR-stained follicles in relation to the total number of follicles found in the histological evaluation equals the follicle survival rate. As only tissue fragments with clearly visible NR-stained follicles were evaluated and not all tissue fragments potentially containing non-stained dead follicles, a slight overestimate of follicular survival is a potential limitation to this method.
In the current study, we calculated follicle survival rates in frozen–thawed ovarian tissues in a series of 25 patients aged 10–36 years having ovarian tissue cryopreserved for fertility in our center from 2000 to 2015. On average, 84% of the follicles survived the freezing and thawing and this consistently high follicle survival rate in the vast majority of patients reflects a robust and effective service. In Denmark, OTC has been performed since 1999 and this is the first study to evaluate clinically preserved ovarian tissue after a period of 18 years of storage. We found no significant association between follicle survival rates and storage time which demonstrates that storage in liquid nitrogen does not affect viability of the tissue. Moreover, these data confirm the robustness of the slow freezing protocol with the use of low concentrations of cryoprotectants and its consistent high level of performance. Since 1999 OTC has been centralized to just one center in Denmark. The ovarian tissue is removed at the local hospital near the patient and transported to our central laboratory where cryopreservation and storage are performed. The impact of transportation and prolonged cooling remains an area which is poorly explored, and the final endpoints with pregnancies and live births from the clinical programs are years ahead of us. However, so far, the clinical outcomes support transportation, as live births have been reported in both the Danish and German cohorts (Rosendahl et al., 2011; Jensen et al., 2015; Van der Ven et al., 2016). Moreover, one study has shown that human cortical tissue exposed to temperatures of 0–4°C for 0–26 h prior to freezing showed the development of preantral follicles comparable to controls during subsequent in vitro culture (Isachenko et al., 2009b). Animal studies have also confirmed that follicle viability and morphology were comparable to unfrozen controls after the ovarian tissue had been kept at 4°C for 18–24 h prior to freezing (Lucci et al., 2004; Martins et al., 2018). Importantly, when storage of the ovarian tissue was performed at 20°C for 18 h prior to freezing, the percentage of morphologically normal follicles was significantly reduced compared to controls (Lucci et al., 2004). This highlights the crucial aspect of cooling the tissue and thereby lowering cellular metabolism in the transport model for OTC. In the current study, the ovarian tissue was transported on ice for up to 6 h in 13 of the cases, whereas the tissue was picked up in preheated media at the local hospital in the other 12 cases. We found no significant association in the follicle survival rates in frozen–thawed ovarian tissues collected at the local hospital compared to tissues transported on ice prior to freezing which supports that prolonged cooling does not seem to greatly affect the follicle survival.
In the current study, we also found a follicle survival of 91% in fresh ovarian tissue. Compared to the follicle survival in frozen–thawed ovarian tissue (84%), the freezing and thawing procedure appeared to reduce the follicle survival, supporting the concept that sublethal damage in a proportion of the frozen–thawed follicles and oocytes cannot be completely avoided (Hovatta et al., 1996; Baird et al., 1999; Keros et al., 2009; Isachenko et al., 2009a). In an ovine study from 1999, Baird and colleagues suggested that the follicle survival after xenografting to immunodeficient mice for 4 weeks was 35% for fresh ovarian tissue and 28% for frozen–thawed ovarian tissue (Baird et al., 1999). Thus, the impact of the freezing–thawing process only comprised 7%, whereas the transplantation procedure caused the majority, up to 70%, of the follicle loss. In our current study, we also found a 7% reduction in the average follicle survival rates in frozen–thawed tissues compared to fresh tissues which supports that only a minor portion of follicles are lost due to the cryopreservation procedure itself.
In selected cases, we have performed the validation of the cryopreservation procedure for other institutions. In one case, two pieces of frozen ovarian tissue from a 28-year-old woman with colorectal cancer was evaluated. NR staining showed a follicle survival rate of 28% and xenografted tissue showed the presence of surviving follicles. Our validation report concluded that the freezing procedure was not optimal, but follicles had survived in vivo. It was decided to transplant 59% of the cryostored tissue to the patient who had postmenopausal hormone levels and 24 weeks later FSH was reduced to normal and follicular development in the transplanted ovarian tissue was observed (Radwan et al., 2016). Thus, the patient regained ovarian function and hormone production, however, she did not conceive. This could indicate that the reduced follicle survival observed with NR staining only secured enough surviving follicles to provide hormone production, but not fertility.
Finally, if an error occurs during a clinical case of OTC and the follicle viability is potentially compromised, NR staining can be used to test and quantify follicle survival. In one case, our Planer Freezer performed an incomplete run in which an emergency procedure was performed. NR staining and xenografting showed a follicle survival rate of 96% and the presence of morphological normal follicles in the xenografted tissue. Thus, in this case the error occurred quite late during the freezing and the samples had probably just barely passed the critical temperature in which the tissue had actually attained a vitrified stage. The emergency procedure sufficiently completed the final freezing, hereby securing a high level of follicle survival. This case highlights the necessity of proper quality control and emergency procedures in connection with OTC.
Conclusion
In conclusion, we report the first clinical series in which follicle survival has been quantified in frozen–thawed ovarian tissue in 25 girls and young women undergoing OTC for fertility preservation in Denmark from 2000 to 2015. Using NR staining in combination with histological evaluation, a follicle survival rate was calculated for each patient, resulting in the confirmation of a consistently high-quality cryopreservation performance in our center and validation of long-term cryostorage and transport of the ovarian tissue prior to freezing.
NR staining is a simple and reliable method in which follicle viability in ovarian tissue can be assessed for initial validation and provide a quantitative measure for freezing performance. The staining should optimally be used in combination with xenografting studies for the validation of newly implemented OTC protocols and could be a valuable tool for quality control in many ovarian tissue banking facilities.
Acknowledgements
The authors wish to thank biomedical laboratory scientist Marianne Sguazzino for her excellent help in processing the ovarian tissue for histology. We also greatly appreciate the generosity of the women who donated their ovarian tissue for the research.
Authors’ roles
SGK designed the project, wrote the paper, performed NR staining and histological analysis on frozen–thawed ovarian tissue, performed xenografting studies and produced figures and tables. QL performed NR staining and histological analysis on frozen–thawed ovarian tissue. LSM performed the statistical analysis and produced figures. TG performed NR staining and histological analysis on fresh ovarian tissue. SEP performed xenografting studies. ABB, EE and KTM consulted and recruited the patients for OTC. CYA designed the project and wrote the paper. All authors approved the final manuscript.
Conflict of interest
None declared.
Funding
The Research Pools of Rigshospitalet, the Danish Cancer Foundation, Dagmar Marshalls Foundation and the Novo Nordic Foundation.



