-
PDF
- Split View
-
Views
-
Cite
Cite
Erin A Taylor, Dmitry Khodyakov, Zachary Predmore, Christine Buttorff, Alice Kim, Assessing the feasibility and likelihood of policy options to lower specialty drug costs, Health Affairs Scholar, Volume 2, Issue 10, October 2024, qxae118, https://doi.org/10.1093/haschl/qxae118
Close - Share Icon Share
Abstract
Specialty drugs are high-cost medications often used to treat complex chronic conditions. Even with insurance coverage, patients may face very high out-of-pocket costs, which in turn may restrict access. While the Inflation Reduction Act of 2022 included policies designed to reduce specialty drug costs, relatively few policies have been enacted during the past decade. In 2022-2023, we conducted a scoping literature review to identify a range of policy options and selected a set of 9 that have been regularly discussed or recently considered to present to an expert stakeholder panel to seek consensus on (1) the feasibility of implementing each policy and (2) its likely impact on drug costs. Experts rated only 1 policy highly on both feasibility and impact: grouping originator biologics and biosimilars under the same Medicare Part B reimbursement code. They rated 3 policies focused on setting payment limits as likely to have positive (downward) impact on costs but of uncertain feasibility. They considered 4 policies as uncertain on both criteria. Experts rated capping monthly out-of-pocket costs as feasible but unlikely to reduce specialty drug costs. Based on these results, we offer 4 recommendations to policymakers considering ways to reduce specialty drug costs.
Introduction
Over the past decade, rising prescription drug costs have garnered substantial attention from policymakers, payers, patients, and other stakeholders.1 Policymakers have debated a range of legislative and other policy proposals designed to rein in rising prescription drug costs. With the notable exception of the Inflation Reduction Act (IRA) of 2022,2 little meaningful movement at the federal level has occurred in terms of implementing policies designed to reduce costs. One potential reason for this lack of action is that key stakeholders have different interests, priorities, and concerns when weighing different policy options. For example, patients are interested in having access to affordable cutting-edge prescription drugs;3,4 insurers are concerned about the high costs of covering new expensive medications due to limited information about their efficacy;5 and manufacturers seek to protect their ability to price new medications based on the anticipated market and efficacy of the drug.6,7 Policymakers must balance these competing concerns while considering overall healthcare system costs, drug efficacy, political priorities, and the feasibility of a given policy.8 Such competing interests and priorities complicate the process of identifying and implementing effective policy solutions.
Specialty drugs are high-cost medications that are typically prescribed by specialists.9 They are often derived from biologic, as opposed to chemical, processes and used to treat complex chronic conditions, such as cancer or rheumatoid arthritis.10 Although they represent the minority of dispensed prescriptions, specialty drugs account for the majority of drug spending in the United States.11,12 These medications therefore represent an important focus for policymakers seeking options to reduce drug costs.
While insurance coverage can help patients access specialty drugs at lower prices, high out-of-pocket costs can still be a substantial barrier to access.13,14 Previous research has found that between 22% and 50% of Medicare Part D prescriptions for specialty drugs are not filled15 and that higher out-of-pocket costs were associated with a higher likelihood of prescription abandonment or delayed initiation of cancer medications.16,17 These delays or non-initiation of treatment can lead to adverse health outcomes, including increased likelihood of hospitalizations or emergency department visits.18 In addition, the overall cost of these medications can be substantial, which can put pressure on payer budgets and increase overall health system costs.19,20
To provide policymakers and other stakeholders with actionable information on the feasibility of implementing a set of policies, along with their likely impact on drug costs, we sought input from a range of stakeholders using an online expert panel designed to elicit meaningful feedback on policy options. To accomplish this, we conducted a scoping literature review to identify policy options that have been proposed over the past decade and convened an online expert panel to explore the existence of consensus among a diverse group of experts, including researchers, clinicians, drug manufacturers, payers, and patient advocates, on the extent to which selected policy options were both feasible to implement and likely to help address high specialty drug costs. In the discussion section, we offer a series of considerations for policymakers debating different policies designed to reduce specialty drug costs.
Data and methods
Scoping review
We conducted a scoping review in Summer 2022 of selected high-impact peer-reviewed health policy journals, gray literature, government reports, and newspaper and trade publications with the goal of identifying proposed and implemented policy options that addressed high specialty drug costs in the US health system. The inclusion of newspapers, trade journals, and other public-facing commentary allowed us to incorporate not only academic research but also proposals from policymakers and those arising from public sentiment. We used a broad set of search terms to capture articles about specialty drugs, as well as a set of terms to exclude policies aimed at decreasing the use of illegal drugs. We also used a broad set of terms to capture references to drug costs and prices.
Two researchers (A.K. and Z.P.) screened the titles and abstracts using DistillerSR.21 To be included, the title, abstract, or first paragraph of each news article needed to mention specialty drugs (defined as those with high costs, complex dosing or administration regimens, requiring monitoring as a part of administration, or treating rare diseases), high-cost drugs, or biologic drugs. They must also have included discussion of a policy option that could be enacted or implemented by policymakers. Finally, the policy option must have focused on lowering the price or cost of the drugs to either a payer or patients. Articles were excluded if they were about policies that had been enacted in non-US countries.
We identified and screened titles and abstracts of 1737 potentially relevant publications, reviewed full texts of 502 publications, and 4 researchers (A.K., C.B., E.A.T., and Z.P.) abstracted information from 165 publications that described specific policy options. Each reviewed and abstracted the same set of 20 full text articles, met, and discussed disagreements. The inclusion and exclusion criteria were the same as during the title and abstract screen. Two researchers (C.B. and E.A.T.) then reviewed and abstracted ∼25% each of the full texts, while 1 researcher (Z.P.) reviewed ∼50%. We grouped descriptions of similar policies together, identifying 57 unique policy options. The policies fell into 4 categories reflecting the mechanisms by which the policies would lower specialty drug costs: payment limits, drug reimbursement, transparency, and drug benefit design.
We selected the most frequently mentioned or particularly innovative policies that had yet to be implemented at the federal level for consideration by experts on our panel. We also asked participants on our expert panel (described below) to suggest additional policies for consideration as part of the initial intake survey. We selected the following policies as part of the payment limits category: expanding Medicare drug price negotiations, using cost-effectiveness to make coverage decisions, establishing prices for all payers based on health technology assessments (HTAs), and using international reference pricing to set maximum prices. We selected the following policies as part of the drug reimbursement category: grouping biologics and biosimilars together for reimbursement by Medicare Part B, applying rebates at the point of sale, and implementing value-based contracting. We selected 1 policy in the transparency category, which focused on increasing transparency in pricing through the pharmaceutical supply chain. Finally, we selected as part of the drug benefit design category the policy that would cap out-of-pocket costs for specialty drugs at $50 per month. The supplemental file provides additional detail on the policies included in this study.
Expert panel
To explore the likely impact and feasibility of the selected policy options, we conducted an online modified-Delphi expert panel. We sought to identify about 60 experts representing a range of stakeholders, including researchers, clinicians, manufacturers, payers, and patient advocates. Previous research recommends that multi-stakeholder online modified-Delphi panels include more than 40 participants to allow for representation of diverse perspectives while accounting for attrition, which is common in Delphi studies.22 The experts were identified based on appearance as the first and/or senior authors of select publications included in our literature review, those who were representatives of key professional organizations such as the Pharmaceutical Care Management Association, AARP, and the National Patient Advocate Foundation, and those who were members of our professional networks. To cast the widest possible net and ensure representation of different stakeholder groups, we also posted an announcement about our study on LinkedIn.
Those interested in participating completed a registration form that included close-ended demographic questions, such as gender, ethnicity, and stakeholder group, as well as open-ended questions about their relevant expertise. We reviewed the information shared by the registrants to determine whether they had the expertise to participate and to ensure inclusion of experts representing all 5 stakeholder groups. These efforts resulted in recruiting 61 experts who met our eligibility criteria and who were invited to participate in the panel.
Between January 25 and March 23, 2023, we conducted a 3-round panel using ExpertLensTM—an online modified-Delphi platform for expert and stakeholder panels which has been used in more than 3 dozen studies.23,24 In Round 1, experts considered 9 policy options. For each policy option, we provided a brief description; a summary of evidence of potential policy impacts, negative impacts, and feasibility as discussed in the literature; and up to 5 references that informed our evidence description (see Supplemental File for more information). We asked experts to use 9-point Likert scales to rate the feasibility (extent to which each policy option could reasonably be implemented in the United States) and potential impact (likelihood that each policy option could reduce specialty drug prices once implemented) and to explain their ratings in open-text boxes below each question.
In Round 2, experts saw how their own ratings compared with those of other participants, whether the panel achieved consensus, and what participants said when they rated different policy options (Figure 1). Experts were invited to discuss Round 1 results using an asynchronous, anonymous, and moderated discussion board.
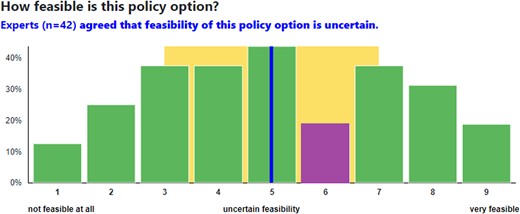
Round 2 ratings example. Source: RAND. Notes: The chart represents an example distribution of experts' responses. Each bar shows the percent of participants choosing each response. The purple bar indicates the participant's own response. The blue line indicates the group median, whereas the yellow area indicates an interquartile range. The statement presented above the chart informs participants about the group decision.
Before the start of Round 3, we revised the wording of 1 policy option, deleted 3 policy options, and added 3 new ones based on the feedback received in previous rounds (the Supplemental File shows the policy options asked in each round). We asked participants to use the same 2 rating scales to provide their final policy ratings and explain the reasons behind them.
Data analysis
To determine whether experts reached consensus on the feasibility and impact of each policy option in Rounds 1 and 3, we used a 2-step analytic approach described in the RAND/UCLA Appropriateness Method's Manual. We first examined whether experts disagreed with each other, defined as more than a third of experts choosing an answer ≤3 and another third choosing an answer ≥7 on a 9-point scale. If there was no disagreement, we looked at the panel's median. If the median was ≥6.5, the panel considered a policy to be feasible and/or likely to reduce drug costs. If the median was <6.5 but >3, we considered a policy to be of uncertain feasibility and/or likelihood to reduce drug costs. If the median was ≤3, we considered a policy to be not feasible and/or unlikely to reduce drug costs. To develop a final prioritized list of policy options, we used Round 3 data and rank-ordered policy options that experts agreed on using the median rating on the impact and feasibility scales.
To better explain the likely impact and feasibility of policy options, we thematically analyzed rationale and discussion comments. Following standard practices for analyzing qualitative ExpertLens data,25,26 we grouped all rationale comments for a given policy based on the numeric ratings; we also grouped all discussion comments by policy. A team of 3 coders reviewed and coded all qualitative comments inductively. They identified main reasons why a particular policy was rated high or low on each rating criterion. D.K. reviewed all coding results for consistency; E.A.T., an expert on drug costs, reviewed coding to ensure the correct interpretation of expert comments. Researchers discussed any coding disagreements until they reached consensus. We include illustrative quotes from experts to better explain their perspective.
Results
Expert panel participants
Of the 61 invited experts, 45 participated in at least 1 panel round. Three participants asked to be removed due to competing demands, and the remaining 13 did not complete any round. Of the 45 participants, 42 answered Round 1 and 29 answered Round 3 questions.
The panel was diverse in terms of participants' stakeholder groups and demographic characteristics (Table 1). More than half were researchers; the remaining 44% was split fairly evenly among clinicians, payers, manufacturers, and patient advocates. Forty percent of participants were female, and 76% were White, with the remaining experts identifying as Asian. The majority were younger than 50 years old (65%), and most (53%) had 10 or more years of experience with specialty drug issues.
| Characteristic . | Number . | Percent . |
|---|---|---|
| Stakeholder group | ||
| Researcher | 25 | 56 |
| Other | 20 | 44 |
| Gender | ||
| Female | 18 | 40 |
| Male | 25 | 56 |
| N/A | 2 | 4 |
| Race | ||
| Asian | 9 | 20 |
| White | 34 | 76 |
| Age | ||
| 30-39 years | 15 | 33 |
| 40-49 years | 14 | 31 |
| 50-59 years | 8 | 18 |
| 60+ years | 6 | 13 |
| N/A | 2 | 4 |
| Years of experience with topic | ||
| <5 | 3 | 7 |
| 5-9 | 15 | 33 |
| 10-14 | 11 | 24 |
| 15-19 | 3 | 7 |
| 20 or more | 10 | 22 |
| N/A | 3 | 7 |
| Characteristic . | Number . | Percent . |
|---|---|---|
| Stakeholder group | ||
| Researcher | 25 | 56 |
| Other | 20 | 44 |
| Gender | ||
| Female | 18 | 40 |
| Male | 25 | 56 |
| N/A | 2 | 4 |
| Race | ||
| Asian | 9 | 20 |
| White | 34 | 76 |
| Age | ||
| 30-39 years | 15 | 33 |
| 40-49 years | 14 | 31 |
| 50-59 years | 8 | 18 |
| 60+ years | 6 | 13 |
| N/A | 2 | 4 |
| Years of experience with topic | ||
| <5 | 3 | 7 |
| 5-9 | 15 | 33 |
| 10-14 | 11 | 24 |
| 15-19 | 3 | 7 |
| 20 or more | 10 | 22 |
| N/A | 3 | 7 |
Authors' analysis of expert panel responses.
N/A indicates the respondent did not answer that question.
| Characteristic . | Number . | Percent . |
|---|---|---|
| Stakeholder group | ||
| Researcher | 25 | 56 |
| Other | 20 | 44 |
| Gender | ||
| Female | 18 | 40 |
| Male | 25 | 56 |
| N/A | 2 | 4 |
| Race | ||
| Asian | 9 | 20 |
| White | 34 | 76 |
| Age | ||
| 30-39 years | 15 | 33 |
| 40-49 years | 14 | 31 |
| 50-59 years | 8 | 18 |
| 60+ years | 6 | 13 |
| N/A | 2 | 4 |
| Years of experience with topic | ||
| <5 | 3 | 7 |
| 5-9 | 15 | 33 |
| 10-14 | 11 | 24 |
| 15-19 | 3 | 7 |
| 20 or more | 10 | 22 |
| N/A | 3 | 7 |
| Characteristic . | Number . | Percent . |
|---|---|---|
| Stakeholder group | ||
| Researcher | 25 | 56 |
| Other | 20 | 44 |
| Gender | ||
| Female | 18 | 40 |
| Male | 25 | 56 |
| N/A | 2 | 4 |
| Race | ||
| Asian | 9 | 20 |
| White | 34 | 76 |
| Age | ||
| 30-39 years | 15 | 33 |
| 40-49 years | 14 | 31 |
| 50-59 years | 8 | 18 |
| 60+ years | 6 | 13 |
| N/A | 2 | 4 |
| Years of experience with topic | ||
| <5 | 3 | 7 |
| 5-9 | 15 | 33 |
| 10-14 | 11 | 24 |
| 15-19 | 3 | 7 |
| 20 or more | 10 | 22 |
| N/A | 3 | 7 |
Authors' analysis of expert panel responses.
N/A indicates the respondent did not answer that question.
Policy impact and feasibility rankings
Table 2 presents the rankings and decisions for the impact of each option on specialty drug costs and the feasibility of each option at the end of Round 3. Experts generally agreed on the impact and feasibility of all 9 policy options. They considered 4 policies to be of high impact and 2 policies feasible to implement. They deemed 4 policies to be of uncertain impact and 7 of uncertain feasibility. Experts rated 1 policy as unlikely to have an impact. No policy was deemed unfeasible to implement.
| Policy category . | Policy description . | Impact . | Feasibility . | ||
|---|---|---|---|---|---|
| Median and IQR . | Decision . | Median and IQR . | Decision . | ||
| Payment limits | Expand number of Medicare price negotiation drugs and enable private insurers to use prices | 8 (7-8) | + | 5 (3-6) | u |
| Drug reimbursement | Group biologics and biosimilars in Part B | 7 (6-8) | + | 7 (7-8) | + |
| Payment limits | Use cost-effectiveness to make coverage decisions | 7 (7-8) | + | 5 (3.5-6) | u |
| Payment limits | Use health technology assessment to set maximum prices | 7 (6-8) | + | 5 (3-7) | u |
| Drug reimbursement | Apply rebates at the point of sale | 5 (3-5) | u | 5 (4-6) | u |
| Payment limits | Use international reference pricing to set maximum prices | 5 (3-7) | u | 4 (3-6) | u |
| Transparency | Require price transparency throughout the supply chain | 4 (2-6) | u | 6 (5-7) | u |
| Drug reimbursement | Implement value-based contracting | 4 (3-5) | u | 5 (3-7) | u |
| Drug benefit design | Cap OOP at $50 per month | 3 (1-5) | − | 7 (5-7) | + |
| Policy category . | Policy description . | Impact . | Feasibility . | ||
|---|---|---|---|---|---|
| Median and IQR . | Decision . | Median and IQR . | Decision . | ||
| Payment limits | Expand number of Medicare price negotiation drugs and enable private insurers to use prices | 8 (7-8) | + | 5 (3-6) | u |
| Drug reimbursement | Group biologics and biosimilars in Part B | 7 (6-8) | + | 7 (7-8) | + |
| Payment limits | Use cost-effectiveness to make coverage decisions | 7 (7-8) | + | 5 (3.5-6) | u |
| Payment limits | Use health technology assessment to set maximum prices | 7 (6-8) | + | 5 (3-7) | u |
| Drug reimbursement | Apply rebates at the point of sale | 5 (3-5) | u | 5 (4-6) | u |
| Payment limits | Use international reference pricing to set maximum prices | 5 (3-7) | u | 4 (3-6) | u |
| Transparency | Require price transparency throughout the supply chain | 4 (2-6) | u | 6 (5-7) | u |
| Drug reimbursement | Implement value-based contracting | 4 (3-5) | u | 5 (3-7) | u |
| Drug benefit design | Cap OOP at $50 per month | 3 (1-5) | − | 7 (5-7) | + |
Authors' analysis of expert panel responses.
Median ranking and interquartile range (IQR) presented.
“+” denotes a positive decision, meaning that experts considered a given policy to be likely to reduce specialty drug prices or feasible to implement (a median score of 6.5-9, without disagreement).
“u” denotes an uncertain decision, meaning that experts considered a given policy to be of uncertain impact or feasibility (a median score of 3.5-6, without disagreement).
“−” denotes a negative decision, meaning that experts considered a given policy to be not likely to reduce specialty drug price or not feasible to implement (a median score of 1-3, without disagreement).
| Policy category . | Policy description . | Impact . | Feasibility . | ||
|---|---|---|---|---|---|
| Median and IQR . | Decision . | Median and IQR . | Decision . | ||
| Payment limits | Expand number of Medicare price negotiation drugs and enable private insurers to use prices | 8 (7-8) | + | 5 (3-6) | u |
| Drug reimbursement | Group biologics and biosimilars in Part B | 7 (6-8) | + | 7 (7-8) | + |
| Payment limits | Use cost-effectiveness to make coverage decisions | 7 (7-8) | + | 5 (3.5-6) | u |
| Payment limits | Use health technology assessment to set maximum prices | 7 (6-8) | + | 5 (3-7) | u |
| Drug reimbursement | Apply rebates at the point of sale | 5 (3-5) | u | 5 (4-6) | u |
| Payment limits | Use international reference pricing to set maximum prices | 5 (3-7) | u | 4 (3-6) | u |
| Transparency | Require price transparency throughout the supply chain | 4 (2-6) | u | 6 (5-7) | u |
| Drug reimbursement | Implement value-based contracting | 4 (3-5) | u | 5 (3-7) | u |
| Drug benefit design | Cap OOP at $50 per month | 3 (1-5) | − | 7 (5-7) | + |
| Policy category . | Policy description . | Impact . | Feasibility . | ||
|---|---|---|---|---|---|
| Median and IQR . | Decision . | Median and IQR . | Decision . | ||
| Payment limits | Expand number of Medicare price negotiation drugs and enable private insurers to use prices | 8 (7-8) | + | 5 (3-6) | u |
| Drug reimbursement | Group biologics and biosimilars in Part B | 7 (6-8) | + | 7 (7-8) | + |
| Payment limits | Use cost-effectiveness to make coverage decisions | 7 (7-8) | + | 5 (3.5-6) | u |
| Payment limits | Use health technology assessment to set maximum prices | 7 (6-8) | + | 5 (3-7) | u |
| Drug reimbursement | Apply rebates at the point of sale | 5 (3-5) | u | 5 (4-6) | u |
| Payment limits | Use international reference pricing to set maximum prices | 5 (3-7) | u | 4 (3-6) | u |
| Transparency | Require price transparency throughout the supply chain | 4 (2-6) | u | 6 (5-7) | u |
| Drug reimbursement | Implement value-based contracting | 4 (3-5) | u | 5 (3-7) | u |
| Drug benefit design | Cap OOP at $50 per month | 3 (1-5) | − | 7 (5-7) | + |
Authors' analysis of expert panel responses.
Median ranking and interquartile range (IQR) presented.
“+” denotes a positive decision, meaning that experts considered a given policy to be likely to reduce specialty drug prices or feasible to implement (a median score of 6.5-9, without disagreement).
“u” denotes an uncertain decision, meaning that experts considered a given policy to be of uncertain impact or feasibility (a median score of 3.5-6, without disagreement).
“−” denotes a negative decision, meaning that experts considered a given policy to be not likely to reduce specialty drug price or not feasible to implement (a median score of 1-3, without disagreement).
Policies from the payment limits and drug reimbursement categories were considered as likely to have a positive impact, while the price transparency policy was rated as of uncertain impact, and the $50 OOP cap policy was rated as likely to have a negative impact. The 2 feasible policies came from the drug reimbursement and drug benefit design categories.
When considering panel ratings on both criteria at the same time, only 1 policy—grouping originator biologics and their biosimilars for the calculation of prices paid by Medicare Part B for biologics—was deemed both feasible and likely to have an impact on reducing costs. Panelists indicated it was likely to reduce costs because the reimbursement rate would decrease once biosimilars were included in the calculation, and providers would purchase the lower-cost versions to retain the margin between their costs and the reimbursement rate. In describing the feasibility of this policy, one expert stated: “This policy is already in place for generic drugs under Medicare Part B and could be easily implemented for biosimilars” (Expert 34). Others noted that this policy could be implemented without congressional approval as the reason for high feasibility rating.
The only other policy deemed feasible called for limiting out-of-pocket costs for specialty drugs to $50 per 30-day supply. Experts thought this policy was politically feasible to implement because it “benefits from concentrated benefits and dispersed costs; the individuals who benefit from the copay cap are likely to be more vocal than those who experience a small increase in health insurance premiums” (Expert 57). However, experts were concerned about the impact of this policy because it would lower patient costs but did not include incentives to reduce overall drug pricing and reimbursement, and because lower cost sharing would likely lead to increased drug utilization and thus increased overall drug spending.
Experts considered 3 options as likely to reduce drug costs, but they were uncertain about their feasibility: (1) expanding the Medicare drug price provisions of the IRA to include more drugs and mandate these negotiated prices be used by private insurers; (2) using HTA to set maximum limits on the amount manufacturers can charge for specialty drugs; and (3) mandating the use of cost-effectiveness analysis to make coverage decisions for high-cost prescription drugs.
The first of these policies was considered very likely to reduce costs, especially in the commercial market. As Expert 08 put it, “The much larger impact would be in the commercial market, where at least for injection and infusion (Part B) drugs, reimbursement far exceeds Medicare reimbursement rates.” The policy was considered to have uncertain feasibility, however, because it is still a new authority for CMS and thus the impacts are unknown. Experts noted that it would be difficult to convince lawmakers to extend the negotiation authority to commercial payers. In contrast, those rating this policy as very feasible explained that since some small authority for CMS to negotiate has already been enacted, it should be easier to extend the authority.
Experts rated the remaining 2 policies as likely to have positive impact because they felt that the results of HTAs would provide additional information to payers that could help them negotiate better coverage and reimbursement terms with drug manufacturers. “If this type of information were made available, in particular from multiple sources and evaluated over time in the real world,” said Expert 35, “it would give payers more leverage to negotiate price discounts.” Nonetheless, several experts flagged that the Institute for Clinical and Economic Review is already providing this information in the United States. The feasibility of both options was deemed uncertain due to concerns over the use of quality-adjusted life years (QALY) as an outcome, even though this metric is used in other countries. “Anything using a QALY is going to be a lightning rod for accusations of discrimination. It is possible to set up an HTA that doesn’t utilize QALYS (Germany) and that would improve the feasibility of this option,” said Expert 17.
Four policies were rated as uncertain on both criteria. Experts raised concerns about political and implementation feasibility of these policies. For applying rebates at the point of sale, experts cited the implementation challenge of determining the rebate because “rebates are often calculated based on volume of sales” (Expert 08) and “the same drug may have different rebates on different formularies” (Expert 01). On the use of international reference pricing to set maximum prices, experts highlighted the challenges in obtaining international pricing data, ensuring their accuracy, and comparing the United States to other countries. According to Expert 59, “there are different economic realities in each country that would require contextualizing within the benchmarking process.” As for requiring price transparency throughout the supply chain, one expert mentioned the challenges of calculating and disentangling costs and defining what needs to be disclosed. This policy would require clearly structured data to be reported in a uniform way, which could be costly and administratively burdensome to implement. Regarding the implementation of value-based contracting, experts noted challenges of measuring the effectiveness of drugs and the lack of data systems to track patient outcomes.
A common theme in experts' perspectives on the impact of all 4 of these policies was the concern of pushback from the pharmaceutical industry and potential for negative unintended consequences. Experts noted that applying rebates at the point of sale may cause manufacturers to be less willing to grant rebates and to increase prices elsewhere to recoup lost profits. If maximum prices were set using international reference pricing, experts contended that manufacturers may seek to increase benchmark prices: “The most obvious way that they might do this would be to try to raise the launch price for their drugs in the countries that are serving as the reference basket. The impact of this kind of skullduggery could be limited by selecting countries that themselves establish price ceilings (eg by using cost/QALY)” (Expert 57). Regarding price transparency throughout the supply chain, experts emphasized that supply chain entities would strongly oppose regulations mandating disclosure, since the industry benefits from being able to charge different prices to different payers. Furthermore, data can be difficult to interpret and easily manipulated. Finally, experts argued that manufacturers may increase drug prices in response to value-based contracting, with the knowledge that profits will be higher for drugs with better outcomes.
Discussion
Previous studies have used similar methods to identify paths forward to constraining drug prices;27,28 our study adds to this literature by conducting a modified-Delphi panel that encouraged engagement by different stakeholders to identify consensus. This consensus was achieved using an online tool that enabled masking of each participant's identity and stakeholder affiliation to collect and weight opinions equally. Four important considerations for policymakers arise from these results.
First, if the policy goal is to lower overall specialty drug costs, policies establishing payment limits or focusing on reimbursement changes are most likely to achieve the desired impact. Indeed, all 4 policies deemed impactful by the experts came from these 2 policy categories. The Medicare Part B HCPCS code policy, which would group biosimilars together with originator biologics in determining reimbursement, was deemed both feasible and likely to have an impact on overall costs because it would affect the final reimbursement payment to be made by the Medicare Program. All 3 payment limit policies rated highly on impact, including expanding the number of Medicare price negotiation drugs and enabling commercial insurers to access those prices, and using cost-effectiveness analyses to make coverage and pricing decisions, act directly to reduce the net price paid for specialty drugs.
The 1 policy option targeting costs paid by patients for their specialty drugs was deemed less likely than other included policies to have an impact on the final total cost for the drug. While the cap on specialty drug out-of-pocket costs of $50 has been enacted by several states,29,30 experts rated this policy as not likely to have a positive impact on specialty drug costs. This is because it only targeted patient costs and not any of the costs paid by payers, pharmacies, and other stakeholders through the process of acquiring and dispensing the drug. Policies that target out-of-pocket costs may increase access to expensive medications for patients but are unlikely to have an impact on reducing total costs for the targeted medications.
Second, policymakers should consider ways to address feasibility when debating policy options, because all but 1 policy were rated as having uncertain feasibility. Experts often distinguished between technical feasibility (eg, whether insurers or other affected stakeholders could logistically implement the policy) and political feasibility (eg, whether state or federal lawmakers could pass relevant legislation, or whether a policy could be enacted via a regulation or rule change without additional legislation). Addressing questions of technical feasibility during a policy debate may make the policy either more or less attractive to policymakers and may help stakeholders better understand the intended approach to implementing the policy.
Political feasibility presents a different potential barrier to implementation; when policymakers are unwilling to consider options that might reduce specialty drug costs due to political reasons, the field of potential options becomes narrower. Bundling different policies together or narrowing the focus of a policy may improve feasibility via political negotiations, as policymakers can consider whether adding policy options might alter the overall reception to the proposed legislation. Additionally, whether policies are enacted at the state or federal level can impact their political feasibility. Federal legislation may be more difficult to enact as it may be subject to the filibuster; many policies not enacted at the federal level have been enacted at the state level.31 Policy that can be enacted through the promulgation of rules or regulations also will likely be more politically feasible to enact than policy that requires legislation.
Third, policymakers may benefit from revisiting old policy options that have been discussed and previously rejected due to political infeasibility. Several of the policy options considered as part of this study rely on cost-effectiveness analysis, HTAs, or otherwise measuring the value of a drug to establish prices or adjust reimbursement rates. A number of these policies were rated as likely to have a positive impact on specialty drug costs, but uncertain feasibility. Two of these policies could be considered as very similar to each other—making coverage decisions vs establishing maximum prices using HTAs. Experts noted that there is a marked disagreement in the United States over the methods that could reasonably be used to make coverage or reimbursement decisions, especially related to the use of QALYs to determine whether a given drug meets a cost-effectiveness threshold. While QALYs offer a measure of value and can simplify a difficult process of determining coverage and reimbursement, “value” may be defined differently for different patient populations;32 these differences could be considered in designing a new approach to evaluate cost-effectiveness. Reconsidering policy options that have been debated previously, but from a new perspective or by incorporating different approaches, may help to make a potentially impactful policy more politically feasible. In addition, the Medicare drug price negotiation provisions included in the IRA had been debated and rejected many times before finally being included in enacted legislation. Therefore, being open to reconsidering options with modifications to appeal to policymakers could result in successful enactment of policies that could open the door to meaningful changes.
Finally, policymakers should identify ways to address pushback from the pharmaceutical industry and the potential for any unintended consequences. Policies rated by experts as uncertain in both impact and feasibility were more likely to have these types of concerns flagged, as compared to other policies. The IRA was enacted despite pushback from the pharmaceutical industry, though recent lawsuits33,34 filed by manufacturers suggest that there is still uncertainty regarding the drug price negotiation provisions. The IRA and other drug pricing policies are also often predicted to have negative effects on pharmaceutical industry innovation;35 policies to lower drug prices could be paired with policies that encourage innovation. Considering the anticipated response from the industry and preparing meaningful responses to arguments regarding unintended consequences of a given policy may help increase both the impact and feasibility of a policy.
Although we engaged a large and diverse pool of experts on drug costs, our panel has limitations. First, to reduce participant burden, we asked experts to comment on a small number of policy options, which we described briefly. Although participants seemed to have been pleased with the amount of information provided, additional data and analyses might have better informed experts' ratings. In addition, some policy options were not considered but may be worth further consideration by policymakers, including domestic reference pricing, caps on overall OOP costs in drug benefits, and policies targeting PBM practices that disfavor low list price medications to reduce biosimilar uptake. Second, we asked experts to rate each policy on impact and feasibility. In rating feasibility, some focused on technical feasibility, while others focused on political feasibility. Responses may have differed if we had asked the experts to focus on 1 type of feasibility instead of both, though the comments provided as part of the panel did provide insights into which type of feasibility was considered in the rating. Future panels could include more policymakers or experts closely involved in policymaking to provide more input on feasibility. Third, although not all experts provided final Round 3 ratings, as is standard in Delphi panels, the 64.4% participation rate in this final round was higher than in many other studies.36 Finally, although diverse in terms of stakeholder composition, half of our panel consisted of researchers, whose perspectives might have been weighted more heavily in the final panel ratings.
Conclusion
Based on feedback from an expert panel, we identified 1 policy option considered likely to be both impactful and feasible. We recommend this option, which was to group originator biologics and their biosimilars together for Medicare reimbursement, be considered first. Other policies discussed were either rated as impactful but uncertain feasibility, or unlikely to be impactful but highly feasible. Four policies were rated as uncertain across both decisions. Policies rated as positive impact targeted payment limits on overall drug costs as opposed to only patient out-of-pocket costs. We offer 4 considerations for policymakers debating the merits of these policies.
Acknowledgments
The authors are grateful to the ExpertLens panel participants for their insightful contributions. The authors would like to acknowledge Dr. Bellinda King-Kallimanis, Dr. Kelly Anderson, Dr. Erin Weeda, Dr. Dhaval Gosalia, Mrs. Julie Brown-Georgi, Dr. Jakub Hlavka, Dr. Sujith Ramachandran, Dr. Jingyan Yang, Dr. David Vanness, Mr. Sean Dickson, Ms. Kirsten Axelsen, Dr. Christine Leopold, Dr. Joseph Ross, Dr. Daniel Ollendorf, Prof. Adrian Towse, Dr. John Gray, Mr. MacKay Jimeso, Mr. Greg Baker, Dr. Molly Jeffery, Ms. Linda Cahn, Dr. Pramod John, Dr. Surish P. Shanmugam, Dr. Jeromie Ballreich, Ms. Jennifer Chumbley Hogue, Dr. Andrew Babb, Dr. Surachat Ngorsuraches, Dr. Ankur Pandya, and Dr. Nirosha Lederer, among others, for their participation in the panel.
Supplementary material
Supplementary material is available at Health Affairs Scholar online.
Funding
This work was funded by the National Institute for Health Care Reform as part of contract AWD-00001673 with RAND.
Conflicts of interest
None of the authors have any relevant conflicts of interest. D.K. is the creator of ExpertLens which was used to collect data for this study.
Please see ICMJE form(s) for author conflicts of interest. These have been provided as supplementary materials.
Data availability
Data available upon reasonable request from the corresponding author.
Notes
AARP Report: average specialty drug price reached $84,442 in 2020, rising more than three times faster than the prices of other goods and services [press release]. Accessed September 23, 2024. https://press.aarp.org/2021-9-28-Average-Specialty-Drug-Price-Reached-84,442-2020-Rising-Three-Times-Faster-Prices-Other-Goods-Services.



