-
PDF
- Split View
-
Views
-
Cite
Cite
Rob Van Houdt, Michael Givskov, Chris W. Michiels, Quorum sensing in Serratia, FEMS Microbiology Reviews, Volume 31, Issue 4, July 2007, Pages 407–424, https://doi.org/10.1111/j.1574-6976.2007.00071.x
Close - Share Icon Share
Abstract
Many bacteria use cell–cell communication to monitor their population density, synchronize their behaviour and socially interact. This communication results in a coordinated gene regulation and is generally called quorum sensing. In gram-negative bacteria, the most common quorum signal molecules are acylated homoserine lactones (AHLs), although other low-molecular-mass signalling molecules have been described such as Autoinducer-2 (AI-2). The phenotypes that are regulated in Serratia species by means of AHLs are remarkably diverse and of profound biological and ecological significance, and often interconnected with other global regulators. Furthermore, AHL- and AI-2-mediated systems (less profoundly studied) are continuously being discovered and explored in Serratia spp., many having interesting twists on the basic theme. Therefore, this review will highlight the current known quorum sensing systems in Serratia spp., including the important nosocomial pathogen Serratia marcescens .
Introduction
In the past few decades, research groups throughout the world have discovered that bacteria are not loners but that the behaviour of an individual, a population or community is influenced by cell–cell communication. Small molecules produced and released by bacteria are ‘words’ which can reach other cells and elicit ‘answers’. The language used by these bacteria is chemical in nature and generally designated as quorum sensing.
This cell–cell communication relies on the principle that when a single bacterium produces low-molecular-mass signalling molecules, the extracellular concentration is below a certain threshold. However, when the cell density increases, a critical concentration can be reached that allows the signalling molecule to be sensed and enables the bacteria to respond. Different chemical classes of bacterial signalling molecules have been identified. Most gram-negative quorum sensors utilize N -acyl-homoserine lactones (AHLs) as signalling molecules ( Lazdunski et al. , 2004 ). In addition, some gram-negative bacteria produce other signalling molecules such as 2-heptyl-3-hydroxy-4-quinolone (PQS) and diketopiperazines in Pseudomonas aeruginosa ( Holden et al. , 2000 ), and 3OH palmitic acid methyl ester (3OH PAME) in the plant pathogen Ralstonia solanacearum ( Flavier et al. , 1997 ). Gram-positive bacteria commonly utilize amino acids and short posttranslationally processed peptides for cell-density-dependent gene regulation ( Sturme et al. , 2002 ). In addition, Actinomycetes utilize γ-butyrolactones that are structurally similar to AHLs ( Horinouchi & Beppu, 1992 ). Autoinducer-2 (AI-2), first discovered in Vibrio harveyi ( Bassler et al. , 1993 ), has recently been suggested as a new type of language used by both gram-negative and gram-positive bacteria ( Schauder & Bassler, 2001 ).
These quorum sensing signalling systems control diverse physiological functions in gram-negative and gram-positive bacteria; a few examples are biofilm formation in Pseudomonas aeruginosa and Streptococcus gordonii ( Davies et al. , 1998 ; McNab et al. , 2003 ), Ti plasmid conjugation in Agrobacterium tumefaciens ( Zhang et al. , 1993 ; Piper et al. , 1999 ), production of antibiotics in Erwinia carotova ssp. carotova , Photorhabdus luminescens and Streptomyces coelicolor ( Bainton et al. , 1992 ; Takano et al. , 2000 ; Derzelle et al. , 2002 ), and competence in Streptococcus pneumoniae ( Havarstein et al. , 1995 ). This review will focus on the wealth of information on quorum sensing in Serratia spp., including the important nosocomial pathogen Serratia marcescens , and will illustrate the diversity of quorum sensing signalling molecules and target genes and the complexity that underlies quorum sensing in Serratia strains.
The genus Serratia
A miraculous bloody discolouration in polenta (corn mush), which gave rise to many strange ideas and fears in 1819, prompted Bartolomeo Bizio, a pharmacist from Padua in Italy, to study this phenomenem. In 1823, he wrote a letter to the most eminent priest Angelo Bellani, in which he explained all information regarding the phenomenon and of the special characteristics of the related organism (translated by Merlino et al. , 1924 ). Bizio identified a microorganism as the cause and named it Serratia marcescens : Serratia in honour of an Italian physicist named Serafino Serrati, who he believed had a prior claim over ‘a foreigner’ as inventor of the steamboat, and marcescens as from the Latin word for decaying because the blood pigment was found to deteriorate quickly.
The genus Serratia comprises gram-negative rods c . 0.9–2 μm long and 0.5–0.8 μm in diameter, and is part of the family Enterobacteriaceae and at the time of writing consists of the following recognized species: Serratia entomophila , S. ficaria , S. fonticola , S. grimesii , S. liquefaciens , S. marcescens , S. odorifera , S. plymuthica , S. proteamaculans , S. quinivorans , S. rubidaea and S. ureilytica ( Euzéby, 1997 ). Most Serratia spp. are motile by peritrichous flagella and are facultative anaerobic, chemoorganotrophic bacteria with both a respiratory and a fermentative type of metabolism. Being ubiquitous inhabitants of soil, water and plant surfaces, Serratia spp. are commonly associated with raw food materials and cause spoilage of various foods. In addition, they are capable of colonizing a wide variety of surfaces in the digestive tracts of rodents, insects, fish and humans ( Grimont & Grimont, 1978 ; Daschner et al. , 1980 ), and may pose a food-borne health hazard as opportunistic pathogens. All species except S. entomophila have been frequently isolated from clinical samples. Serratia marcescens , in particular, represents an important nosocomial pathogen capable of causing pneumonia, intravenous catheter-associated infections, urinary tract infections, osteomyelitis and endocarditis ( Eisenstein et al. , 1990 ) that can be exacerbated by multiple-antibiotic resistance ( Arakawa et al. , 2000 ; Knowles et al. , 2000 ; Traub et al. , 2000 ). Swimming and swarming motility and extracellular enzyme activities, i.e. nuclease, protease, lipase and haemolysin, are other traits that may contribute to pathogenesis ( Hejazi & Falkiner, 1997 ). Serratia strains other than of S. marcescens have also been implicated in a variety of infections. For example, S. odorifera caused pneumonia and sepsis ( Cook & Lopez, 1998 ; Lee et al. , 2006 ); S. ficaria caused endophthalmitis and sepsis ( Darbas et al. , 1994 ; Badenoch et al. , 2002 ); S. quinivorans caused pneumonia ( Bollet et al. , 1993 ); S. fonticola was isolated from a leg abscess ( Bollet et al. , 1991 ); invasive properties have been attributed to S. rubidaea ( Ursua et al. , 1996 ); S. liquefaciens caused transfusion-related sepsis ( Roth et al. , 2000 ), and outbreaks have been reported in a critical care unit and in a neurosurgery department ( Harnett et al. , 2001 ; Dubouix et al. , 2005); and S. plymuthica is regarded as a significant opportunistic pathogen ( Berg et al. , 2000 ) to which a variety of infections including peritonitis, pneumonia, sepsis and wound infections have been attributed ( Clark & Janda, 1985 ; Reina et al. , 1992 ; Domingo et al. , 1994 ; Carrero et al. , 1995 ; Nouh & Bhandari, 2000 ). The increasing number of documented infections caused by such strains and the difficult identification of these bacteria by commercial systems urges for a more detailed investigation of the physiology, virulence and taxonomy of this genus ( Stock et al. , 2003 ).
The N -acyl- l -homoserine lactones
One of the first bacterial communication systems was described in the marine symbiotic bacterium Vibrio fischeri ( Nealson & Hastings, 1979 ), which has two lifestyle modes: it can grow as free-living cells in the sea to a low population density; alternatively, it forms a symbiotic association with fish and squid species. Only under the latter conditions the bacteria are luminescent, and this has been linked to an AHL-dependent quorum sensing system. This system has become a paradigm of quorum sensing in gram-negative bacteria, and the two major proteins involved, the I protein homologous to LuxI of V. fischeri , and the R protein homologous to LuxR of V. fischeri , are briefly discussed below. The I protein catalyses the synthesis of the N -acyl- l -homoserine lactone (AHL) signalling molecules, whereas the R protein is a transcriptional activator that, upon activation, triggers the responses. At low cell density the AHLs are synthesized at a basal level and newly synthesized AHLs leave the cell either by diffusion across the cell envelope or by active transport by antibiotic export pumps ( Evans et al. , 1998 ; Kohler et al. , 2001 ). As the population grows and the extracellular AHL concentration increases, there will also be an increasing AHL influx and thus an increasing intracellular AHL concentration. The binding of AHL to the transcriptional regulatory R protein then results in its activation and the complex binds to specific DNA promoter region sequences, resulting in expression of downstream target genes. In many systems the I gene is a target gene for the R protein, and its transcription is upregulated once the AHL concentration reaches a certain threshold, resulting in autoinduction of AHL production.
LuxI-type AHL synthases and concurrent AHLs
AHLs are synthesized from the substrates S -adenosyl- l -methionine (SAM) and acylated acyl carrier protein (acyl-ACP) ( More et al. , 1996 ; Val & Cronan, 1998 ; Parsek et al. , 1999 ) and the reaction is proposed to proceed via a two-substrate, three-product mechanism ( Parsek et al. , 1999 ). The AHL synthase first binds to SAM and subsequently to acyl-ACP, the acyl group from acyl-ACP is then donated to the amine of SAM, releasing ACP. Next, the AHL molecule is released, followed by the release of methylthioadenosine (MTA). As acyl-ACP is normally used in lipid biosynthesis and SAM as a methyl donor, both acyl-ACP and SAM are used in novel ways in these reactions ( More et al. , 1996 ). The I proteins from different bacterial species produce AHLs that vary over a broad range in acyl chain length from 4 to 18 carbons, as well as in the oxidation state at the C3 position and in saturation ( Kuo et al. , 1994 ; Fuqua & Eberhard, 1999 ). A range of AHLs and AHL production profiles have been described in a number of Serratia spp., showing the specificity and diversity of quorum sensing signal molecules and regulation in this genus ( Wei & Lai, 2006 ). Table 1 summarizes the AHLs identified in different Serratia spp. either by MS or by specific bioassays based on thin-layer chromatography (TLC) analysis coupled to an agar overlay seeded with a reporter bacterium in which the proper production of AHL molecules has been blocked by mutation but which contain an AHL-responsive reporter gene. N -hexanoyl- l -homoserine lactone (C6-HSL) is the most common compound, identified in Serratia sp. ATCC 39006, S. marcescens strains MG1, 12 and SS-1, S. plymuthica RVH1 and S. proteamaculans B5a. Also widespread are N -butanoyl- l -homoserine lactone (C4-HSL) and N -3-oxo-hexanoyl- l -homoserine lactone (3-oxo-C6-HSL), produced respectively by Serratia sp. ATCC 39006, S. marcescens strain MG1 and 12, and S. plymuthica RVH1, and by S. marcescens SS-1, S. plymuthica RVH1 and S. proteamaculans B5a.
| Species | Strain | AHLs | LuxI/LuxR | Regulated phenotypes | Reference |
| Serratia sp. | ATCC 39006 | C4-HSL , C6-HSL | SmaI/SmaR | Production of carbapenem, prodigiosin, pectate lyase and cellulase | Thomson et al. , (2000) |
| Serratia marcescens | MG1 | C4-HSL , C6-HSL | SwrI/SwrR | Swarming motility; production of serrawattin, protease and S-layer protein; biofilm formation; butanediol fermentation | Eberl et al. , (1996a , b) |
| SS-1 | C6-HSL , 3-oxo-C6-HSL , C7-HSL , C8-HSL | SpnI/SpnR | Sliding motility; production of biosurfactant, prodigiosin and nuclease | Horng et al. , (2002) | |
| Strain 12 | C4-HSL , C6-HSL | SmaI/SmaR | Swarming motility; haemolytic activity; production of caseinase and chitinase; biofilm formation | Coulthurst et al. , (2006) | |
| Serratia plymuthica | IC1270 | 3-hydroxy-C6-HSL , 3-hydroxy-C8-HSL | SplI/SplR | Ovadis et al. , (2004) | |
| RVH1 | C4-HSL , C6-HSL , 3-oxo-C6-HSL | SplI/SplR | Production of nuclease, chitinase, protease and antibacterial compound; butanediol fermentation | Van Houdt et al. , (2007) | |
| Serratia proteamaculans | B5a | C6-HSL , 3-oxo-C6-HSL | SprI/SprR | Production of lipase, protease and chitinase | Christensen et al. , (2003) |
| Species | Strain | AHLs | LuxI/LuxR | Regulated phenotypes | Reference |
| Serratia sp. | ATCC 39006 | C4-HSL , C6-HSL | SmaI/SmaR | Production of carbapenem, prodigiosin, pectate lyase and cellulase | Thomson et al. , (2000) |
| Serratia marcescens | MG1 | C4-HSL , C6-HSL | SwrI/SwrR | Swarming motility; production of serrawattin, protease and S-layer protein; biofilm formation; butanediol fermentation | Eberl et al. , (1996a , b) |
| SS-1 | C6-HSL , 3-oxo-C6-HSL , C7-HSL , C8-HSL | SpnI/SpnR | Sliding motility; production of biosurfactant, prodigiosin and nuclease | Horng et al. , (2002) | |
| Strain 12 | C4-HSL , C6-HSL | SmaI/SmaR | Swarming motility; haemolytic activity; production of caseinase and chitinase; biofilm formation | Coulthurst et al. , (2006) | |
| Serratia plymuthica | IC1270 | 3-hydroxy-C6-HSL , 3-hydroxy-C8-HSL | SplI/SplR | Ovadis et al. , (2004) | |
| RVH1 | C4-HSL , C6-HSL , 3-oxo-C6-HSL | SplI/SplR | Production of nuclease, chitinase, protease and antibacterial compound; butanediol fermentation | Van Houdt et al. , (2007) | |
| Serratia proteamaculans | B5a | C6-HSL , 3-oxo-C6-HSL | SprI/SprR | Production of lipase, protease and chitinase | Christensen et al. , (2003) |
AHLs were identified by specific bio-assays ( * ) or by MS ( † ).
Serratia liquefaciens MG1 was recently identified as Serratia marcesens MG1 ( Rice et al. , 2005 ).
| Species | Strain | AHLs | LuxI/LuxR | Regulated phenotypes | Reference |
| Serratia sp. | ATCC 39006 | C4-HSL , C6-HSL | SmaI/SmaR | Production of carbapenem, prodigiosin, pectate lyase and cellulase | Thomson et al. , (2000) |
| Serratia marcescens | MG1 | C4-HSL , C6-HSL | SwrI/SwrR | Swarming motility; production of serrawattin, protease and S-layer protein; biofilm formation; butanediol fermentation | Eberl et al. , (1996a , b) |
| SS-1 | C6-HSL , 3-oxo-C6-HSL , C7-HSL , C8-HSL | SpnI/SpnR | Sliding motility; production of biosurfactant, prodigiosin and nuclease | Horng et al. , (2002) | |
| Strain 12 | C4-HSL , C6-HSL | SmaI/SmaR | Swarming motility; haemolytic activity; production of caseinase and chitinase; biofilm formation | Coulthurst et al. , (2006) | |
| Serratia plymuthica | IC1270 | 3-hydroxy-C6-HSL , 3-hydroxy-C8-HSL | SplI/SplR | Ovadis et al. , (2004) | |
| RVH1 | C4-HSL , C6-HSL , 3-oxo-C6-HSL | SplI/SplR | Production of nuclease, chitinase, protease and antibacterial compound; butanediol fermentation | Van Houdt et al. , (2007) | |
| Serratia proteamaculans | B5a | C6-HSL , 3-oxo-C6-HSL | SprI/SprR | Production of lipase, protease and chitinase | Christensen et al. , (2003) |
| Species | Strain | AHLs | LuxI/LuxR | Regulated phenotypes | Reference |
| Serratia sp. | ATCC 39006 | C4-HSL , C6-HSL | SmaI/SmaR | Production of carbapenem, prodigiosin, pectate lyase and cellulase | Thomson et al. , (2000) |
| Serratia marcescens | MG1 | C4-HSL , C6-HSL | SwrI/SwrR | Swarming motility; production of serrawattin, protease and S-layer protein; biofilm formation; butanediol fermentation | Eberl et al. , (1996a , b) |
| SS-1 | C6-HSL , 3-oxo-C6-HSL , C7-HSL , C8-HSL | SpnI/SpnR | Sliding motility; production of biosurfactant, prodigiosin and nuclease | Horng et al. , (2002) | |
| Strain 12 | C4-HSL , C6-HSL | SmaI/SmaR | Swarming motility; haemolytic activity; production of caseinase and chitinase; biofilm formation | Coulthurst et al. , (2006) | |
| Serratia plymuthica | IC1270 | 3-hydroxy-C6-HSL , 3-hydroxy-C8-HSL | SplI/SplR | Ovadis et al. , (2004) | |
| RVH1 | C4-HSL , C6-HSL , 3-oxo-C6-HSL | SplI/SplR | Production of nuclease, chitinase, protease and antibacterial compound; butanediol fermentation | Van Houdt et al. , (2007) | |
| Serratia proteamaculans | B5a | C6-HSL , 3-oxo-C6-HSL | SprI/SprR | Production of lipase, protease and chitinase | Christensen et al. , (2003) |
AHLs were identified by specific bio-assays ( * ) or by MS ( † ).
Serratia liquefaciens MG1 was recently identified as Serratia marcesens MG1 ( Rice et al. , 2005 ).
The substrate specificity of the AHL synthase (I protein) and the availability of the cellular acyl-ACP pool determine the AHL production profile ( More et al. , 1996 ; Parsek et al. , 1999 ). Structural studies of the I protein from the Pantoea stewartii enzyme EsaI and the Pseudomonas aeruginosa enzyme LasI, which primarily synthesize respectively 3-oxo-C6-HSL and 3-oxo-C12-HSL ( Pearson et al. , 1994 ; Beck von Bodman & Farrand, 1995 ), revealed a common binding site for the acyl-ACP phosphopantetheine prosthetic group. When the acyl group of an acyl-ACP was modelled into the hydrophobic pocket seen in the AHL synthase EsaI, it was observed that this pocket was long enough for a six-carbon acyl chain, but not for a longer AHL, whereas the tunnel structure observed for LasI places no apparent restriction on acyl chain length ( Watson et al. , 2002 ; Gould et al. , 2004 ). Modelling studies implicated a particular threonine in the acyl-chain binding site as being important for hydrogen bonding to the 3-oxo position of acyl-ACP ( Watson et al. , 2002 ; Gould et al. , 2004 ). Furthermore, comparison of AHL synthase sequences and their concurrent AHL production profile suggested that a threonine at that position (quivalent to threonine-140 in EsaI) correlates with the production of 3-oxo-substituted AHLs, whereas alanine and glycine correlate with unsubstituted AHLs and serine correlates with the production of 3-hydroxy-substituted AHLs ( Watson et al. , 2002 ). Replacement of this threonine-140 residue in EsaI by alanine caused the enzyme to drive the synthesis of the unsubstituted C6-HSL ( Watson et al. , 2002 ; Gould et al. , 2006 ). Multiple-sequence alignment of the known I proteins in Serratia revealed that the AHL-synthases SplI from S. plymuthica RVH1 and IC1270, SprI from S. proteomaculans B5a and SpnI from S. marcescens , which are more closely related to EsaI from Pantoea stewartii than to SmaI from S. marcescens strain 12 and Serratia sp. ATCC 39006 and SwrI from S. marcescens MG1, possess a threonine residue at position 140 (relative to EsaI) and direct the synthesis of primarily 3-oxo-substituted AHLs. In contrast, SmaI and SwrI possess an alanine residue at position 140 (relative to EsaI) and produce AHLs without 3-oxo substituent (see also Table 1 ).
LuxR-type AHL receptors
The crystal structures of a dimeric TraR protein ( Agrobacterium tumefaciens ) in complex with its cognate AHL (3-oxo-C8-HSL) and a DNA-promoter fragment containing the TraR-binding site have contributed significantly to the understanding of the function of R proteins ( Vannini et al. , 2002 ; Zhang et al. , 2002 ). The TraR protein (or R protein in general) is composed of two domains that are structurally and functionally different and separated by a linker domain. The N-terminal domain is a helix–sheet–helix sandwich involved in AHL recognition and binding. The AHL produced by A. tumefaciens ( N -3-oxo-octanoyl- l -homoserine lactone) is completely buried in the hydrophobic interior of the domain, indicating that AHL binding and release is associated with large conformational changes of the R protein structure. The C-terminal domain contains a helix–turn–helix motif involved in DNA-binding, particularly adapted for recognition of a DNA sequence with a dyad symmetry, called the lux box. It should be noted that lux boxes are not apparent in all promoter regions of quorum-regulated genes ( Parsek & Greenberg, 2000 ), which in addition can be controlled by other regulatory elements ( Dunlap & Greenberg, 1985 ; Thomson et al. , 1997 ; Ulitzur et al. , 1997 ; Rashid et al. , 2000 ; Winzer et al. , 2000 ; Ovadis et al. , 2004 ). For Serratia species a lux box sequence has thus far only been identified in S. marcescens SS-1 and S. plymuthica RVH1, respectively upstream of the spnR and the splR gene ( Horng et al. , 2002 ; Van Houdt et al. , 2007 ).
Many R proteins act as a transcriptional activator ( Fuqua & Greenberg, 2002 ) for which DNA binding is induced by AHL-dependent conformational changes exposing the DNA-binding site. On the other hand, some R proteins function as repressors. Some of these bind DNA in the absence of AHLs, whereas others require the presence of AHLs to enable initiation of their DNA binding activity ( Qin et al. , 2000 ). For example, the EsaR protein from Pantoea stewartii ssp. stewartii , one of the best characterized repressors, binds to target DNA independently of its cognate AHL ( N -3-oxo-hexanoyl- l -homoserine lactone), sterically blocking transcriptional activation by RNA polymerase. Interaction with the AHL derepresses expression of the quorum sensing-regulated capsular polysaccharide synthesis ( von Bodman et al. , 1998 ; Minogue et al. , 2002 ). In the genus Serratia a negative control of AHL-regulated phenotypes is apparently a general trait given that the characterized R proteins all act as repressor, including SpnR of S. marcescens SS-1 ( Horng et al. , 2002 ), SmaR of Serratia sp. ATCC 30096 ( Slater et al. , 2003 ), SplR of S. plymuthica RVH1 ( Van Houdt et al. , 2007 ) and SprR of S. proteamaculans B5a ( Christensen et al. , 2003 ). Cloning and overexpression of the swrR gene from S. marcescens MG1 indicates a similar function of SwrR (unpublished observations). In contrast to SpnR, which autoactivates its own transcription through interaction with a lux -box sequence, SplR autorepresses its own transcription, whereas SmaR does not appear to regulate its own promoter ( Horng et al. , 2002 ; Slater et al. , 2003 ; Van Houdt et al. , 2007 ).
AHL-regulated phenotypes in Serratia
Swarming and sliding motility
Serratia marcescens MG1 (previously S. liquefaciens ) was isolated from liquefied plant tissue ( Givskov et al. , 1988 ) and is capable of two forms of flagellum-driven motility, swimming and swarming, depending on the viscosity of the growth medium. Swarming is accompanied by differentiation resulting in long, multinucleated, aseptate, hyperflagellated cells, which have the unique ability to move on top of the agar surface ( Eberl et al. , 1996a ). This swarming motility is controlled by two key regulators as depicted in Fig. 1 , which control respectively a biosynthetic pathway and a developmental pathway ( Givskov et al. , 1998 ; Eberl et al. , 1999 ). The latter is controlled by the transcriptional regulators FlhD and FlhC, which control expression of the entire flagellar hierarchy for enteric bacteria ( McNab et al. , 1996 ) and a substantial degree of homology with the Escherichia coli flagellar hierarchy was observed for S. marcescens MG1 (Christensen et al. , unpublished data). An flhDC null mutant of S. marcescens MG1 is incapable of producing flagella and thus completely nonmotile and unable to swim or swarm ( Eberl et al. , 1996a ), whereas controlled expression of the flhDC operon results in flagellar synthesis and restores both swimming and swarming. Moreover, overexpression of flhDC has been demonstrated to induce swarm cell differentiation in liquid medium ( Eberl et al. , 1996a ). Therefore, artificial stimulation of flhDC expression can overcome the otherwise required surface contact and shows that sensing of the surface, a major stimulus for swarm cell differentiation, is channelled through the flhDC operon.
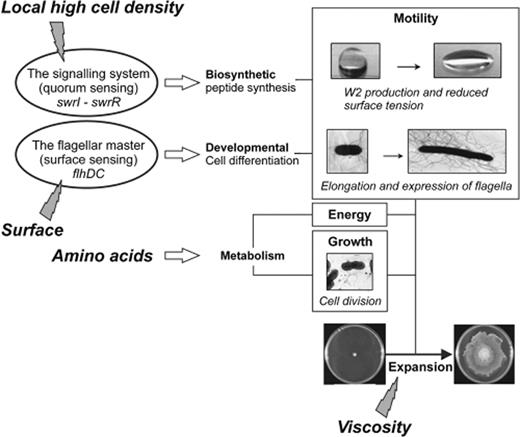
Swarming motility in Serratia marcescens MG1 is controlled by two key regulatory systems (ovals). Inducing stimuli (lightning) point to their respective system. The wide horizontal arrows indicate the pathways targeted by the regulatory systems. The rectangles summarize the biological processes the combined action of which leads to expansion of the colony (adapted with permission from Eberl et al. , 1999 ).
The biosynthetic pathway is controlled by the S. marcescens MG1 SwrI/SwrR quorum sensing system and its cognate AHLs, C4-HSL and C6-HSL synthesized in a molar ratio of 10 to 1 ( Eberl et al. , 1996b ). A knockout mutation of the swrI gene strongly reduced the swarming capability of the strain, which could be restored to the parental level by supplementing the medium with 150 nM C4-HSL ( Eberl et al. , 1996b ). A nonswarming mutant, isolated by transposon mutagenesis, led to the identification of the quorum sensing-controlled gene denoted swrA ( Lindum et al. , 1998 ). The predicted gene product showed homology to a large family of giant, multidomain enzyme complexes responsible for nonribosomal peptide synthesis ( Turgay et al. , 1992 ; Stachelhaus & Marahiel, 1995 ) and has been implicated in the synthesis of serrawettin W2, a cyclical lipodepsipentapeptide carrying a 3-hydroxy-C 10 fatty acid side chain ( Fig. 2a ) ( Matsuyama et al. , 1992 ; Lindum et al. , 1998 ). This secreted extracellular lipopeptide has surface tension-reducing properties that cause water droplets to collapse ( Fig. 2b and c ). In an swrI mutant, serrawettin W2 production and thus surface conditioning is restored when C4-HSL is added exogenously to the medium. In addition, supplementation of media with pure serrawettin W2 also restores the swarming phenotype of surfactant-defective S. marcescens MG1 ( swrI mutant and the swrI swrA double mutant) ( Fig. 2c ), demonstrating that the production of molecules lowering the surface tension of the medium is crucial for swarming motility of S. marcescens MG1. Recently, Coulthurst et al. , (2006) showed that also in S. marcescens strain 12 swarming motility was dependent on smaI and supplementary studies could clarify if this strain uses the same and/or additional regulators.
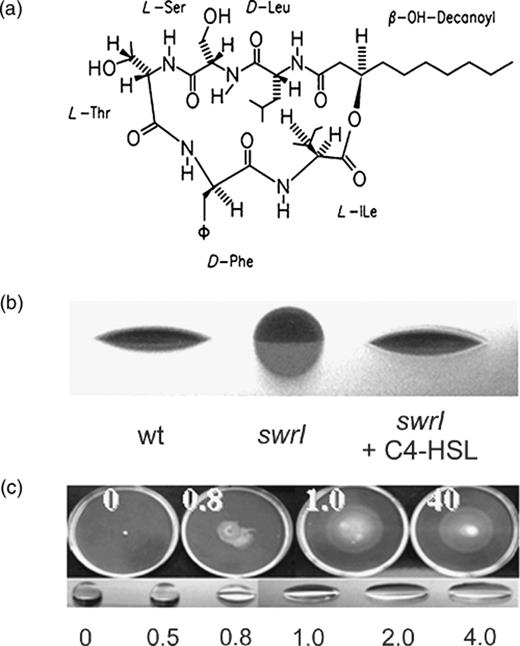
(a) Molecular structure of serrawettin W2. (b) Side views of cultures by the drop-collapsing test, indicating the effect on the surface tension of water ( Lindum et al. , 1998 ). Small volumes of bacterial cultures were placed on the lid of a Petri dish. wt, wild type; swrI , the swrI mutant; +C4-HSL, the strain was grown in the presence of 200 nM N -butanoyl- l -homoserine lactone. (c) Swarming motility of the Serratia marcescens MG1 swrI swrA double mutant, deficient in serrawettin W2, on medium supplemented with serrawettin W2 (0, 0.8, 1 and 40 μg mL −1 ) and drop-collapsing test of water supplemented with 0, 0.5, 0.8, 1, 2 and 40 μg mL −1 serrawettin W2 (adapted with permission from Eberl et al. , 1999 ).
The inability of S. marcescens SS-1 to produce flagella does not permit swimming or swarming motility, but the bacterium is still able to spread rapidly on a 0.35% agar Luria–Bertani plate. This flagellar-independent motility is distinct from swarming motility and is described as ‘sliding motility’ (see also Henrichsen, 1972; Martinez et al. , 1999 ). Deletion of spnI , which encodes the SS-1 LuxI homolog that directs the synthesis of two major (3-oxo-C6-HSL and C6-HSL) and two minor (C7-HSL and C8-HSLs) AHLs, resulted in a substantially reduced sliding motility correlated with the loss of biosurfactant production ( Horng et al. , 2002 ). Although the compound was not chemically characterized the authors suggest a serrawettin due to its surface tension-reducing properties. As described above, the identified LuxR homologue of SS-1, SpnR, acts as a repressor and increasing the SpnR levels has a negative effect on sliding motility, whereas deletion resulted in upregulation. The only cognate AHL able to overcome the SpnR-dependent negative regulation was 3-oxo-C6-HSL, which highlights the AHL-specificity ( Horng et al. , 2002 ). In addition, Horng and colleagues showed that 3-oxo-C8-HSL, although not produced by S. marcescens SS-1, was as effective as 3-oxo-C6-HSL in restoring sliding motility. This ability of bacteria to sense foreign AHLs may reflect their ability to detect and respond to neighbouring microbial species.
The LipB translocation system and exo-enzymes
In S. marcescens a functional lipBCD operon is required for the secretion of several unrelated proteins such as the lipase LipA, the metalloprotease PrtA and the surface-layer protein (S-layer) SlaA ( Nakahama et al. , 1986 ; Akatsuka et al. , 1995 ; Kawai et al. , 1998 ). This LipB protein translocation system is a type I secretion apparatus belonging to the well-characterized ATP-binding cassette (ABC) protein superfamily of transporters, which are involved in the import and export of a wide variety of substrates ( Higgins et al. , 1992 ; Binet et al. , 1997 ), and consists of LipB, the ABC protein (an inner-membrane ATPase), LipC, the membrane fusion protein (MFP) and lipD, the outer-membrane polypeptide ( Akatsuka et al. , 1995 , 1997 ).
In S. marcescens MG1 and S. proteamaculans B5a, transcription of the lipB gene has been demonstrated to be regulated by respectively the SwrI/SwrR and SprI/SprR quorum sensing system and their cognate AHLs, respectively C4-HSL and 3-oxo-C6-HSL ( Riedel et al. , 2001 ; Christensen et al. , 2003 ). This regulation renders production of extracellular PrtA metalloprotease activity in S. marcescens MG1 and of extracellular protease and lipase activity in S. proteamaculans B5a indirectly under the control of quorum sensing ( Riedel et al. , 2001 ; Christensen et al. , 2003 ). For S. marcescens MG1, inactivation of the swr regulatory system also had an effect on the production of S-layer protein but appeared to have no effect on the lipase, both secreted by the LipB apparatus ( Riedel et al. , 2001 ). In addition, Christensen et al. , (2003) demonstrated that for S. proteamaculans B5a these quorum sensing-controlled exo-enzymes secreted by the LipB apparatus contribute to the deterioration of milk ( Fig. 3 ) and that next to LipB-secreted extracellular enzymes, the production of chitinase activity is controlled by quorum sensing.
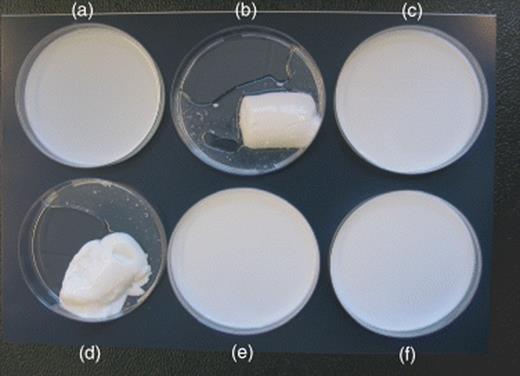
Quorum sensing-controlled secretion of exo-enzymes in Serratia proteamaculans B5a contributes to the deterioration of milk. Milk samples were inoculated with 1 × 10 6 CFU mL −1 of the wild-type or mutants and incubated at room temperature for 18 h. (a) Milk (negative control); (b) milk inoculated with the wild-type B5aN; (c) milk inoculated with the communication-deficient mutant AC1; (d) milk containing 200 nM 3-oxo-C6-HSL inoculated with AC1; (e) milk inoculated with the communication-deficient and secretion-deficient double mutant AC2; (f) milk containing 200 nM of 3-oxo-C6-HSL inoculated with AC2 (reprinted with permission from Christensen et al. , 2003 ).
Quorum sensing-directed production of extracellular enzymes has in addition been reported for other strains of the genus Serratia , for example the production of protease, chitinase and haemolytic activity in S. marcescens strain 12 ( Coulthurst et al. , 2006 ), the production of nuclease in S. marcescens SS-1 ( Horng et al. , 2002 ), the production of nuclease, chitinase and protease in S. plymuthica RVH1 ( Van Houdt et al. , 2007 ), and the production of pectate lyase and cellulase in Serratia sp. ATCC 39006 ( Thomson et al. , 2000 ; Slater et al. , 2003 ).
The quorum sensing-directed control of extracellular enzyme activity, which may contribute to food spoilage and to pathogenesis, indicates that novel preservation techniques or antivirulence therapies employing quorum sensing inhibitors have potential as substitutes or complements to traditional treatments ( Persson et al. , 2005 ).
Prodigiosin production
Three species of the genus Serratia , S. marcescens , S. rubideae and S. plymuthica , produce a reddish pigment called prodigiosin under specific growth conditions, the colour of which ranges from dark red to pale pink ( Grimont & Grimont, 1991 ). Prodigiosin (2-methyl-3-pentyl-6-methoxyprodiginine) has an interesting history (reviewed by Bennett & Bentley, 2000 ; Williamson et al. , 2006 ) and has an unusual structure with three pyrrole rings, two of them directly linked to each other, while the third is attached by way of a methene bridge forming a pyrryldipyrrylmethene ( Gerber et al. , 1975 ; Williams & Qadri, 1980 ). Prodigiosin has no clearly defined physiological functions in the producing organisms and its exact role remains debated, but as a typical secondary metabolite it might play a role in metabolic ‘overflow’ for products from primary metabolism ( Bu'Lock, 1961 ; Hood et al. , 1992 ). However, a possible role in dispersion as well as an active role in competitive survival has been reported ( Williams & Qadri, 1980 ; Burger & Bennett, 1985 ; Matsuyama et al. , 1986 ; Demain et al. , 1995 ). In addition, prodigiosin and family members have been identified as having extremely broad antibacterial, antimalarial, antifungal and antiprotozoal activities ( Castro et al. , 1967 ; Williams & Qadri, 1980 ; Demain et al. , 1995 ). Prodigiosins also have been found to have potent immunosuppressive, proapoptic and anticancer properties (reviewed in Bennett & Bentley, 2000 ; Manderville et al. , 2001 ; Perez-Tomas et al. , 2003 ). Perez-Tomas et al. , (2003) conclude that the current result status suggests that prodigiosin or prodigiosin analogues have the potential to be a new class of anticancer drugs, which hold out considerable promise for the pharmaceutical industry.
The production of prodigiosin in Serratia is dependent on the pig cluster, comprising 15 genes ( pigA – O ) in Serratia sp. ATCC 39006 and of 14 genes ( pigA – N ) in S. marcescens ATCC 274, which is transcribed polycistronically from a promoter upstream of pigA ( Slater et al. , 2003 ; Harris et al. , 2004 ). The additional gene pigO in the Serratia sp. ATCC 39006 pig gene cluster is not involved in prodigiosin biosynthesis, but probably has a regulatory role ( Harris et al. , 2004 ). The most recent model for the biosynthesis of prodigiosin has been proposed by Williamson et al. , (2005) who studied the pathway in Serratia sp. ATCC 39006 and in addition compared and reviewed the biosynthesis and regulation with other bacterial prodiginines ( Williamson et al. , 2006 ). Briefly, MAP (2-methyl-3-amylpyrrole) and MBC (4-methoxy-2,2′bipyrrole-5-carboxyaldehyde), formed from the precursors acetate, serine, alanine, methionine and proline, are believed to be joined in a condensation reaction to prodigiosin ( Morrison et al. , 1966 ; Wasserman et al. , 1973 ; Harris et al. , 2004 ; Williamson et al. , 2005 ).
In Serratia sp. ATCC 39006 production of prodigiosin is regulated by the SmaI/SmaR quorum sensing system and its cognate AHLs, C4-HSL and C6-HSL, the former being the more abundant molecule ( Thomson et al. , 2000 ). At low cell density, the transcription of the pig cluster is repressed by SmaR, while at high cell density binding of C4-HSL/C6-HSL to SmaR derepresses transcription ( Slater et al. , 2003 ; Fineran et al. , 2005b ).
Also in S. marcescens SS-1, prodigiosin production is quorum sensing dependent, as is reflected in the designation of SpnI and SpnR ( s liding, p rodigiosin and n uclease) ( Horng et al. , 2002 ).
Antibiotic production
Although this section is largely dedicated to 1-carbapen-2-em-3-carboxylic acid, the secondary metabolite prodigiosin also has antibacterial activity (as mentioned above). Carbapenem antibiotics, such as 1-carbapen-2-em-3-carboxylic acid (Car), are broad-spectrum β-lactam antibiotics, which function by inhibiting bacterial cell wall peptidoglycan biosynthesis and are characterized by an unsaturated five-membered carbon ring that is fused to the nitrogen and one carbon atom on the β-lactam ring (for review see Coulthurst et al. , 2005 ). Production of carbapenems has been demonstrated in Streptomyces cattleya ( Kahan et al. , 1979 ) and in a small subset of gram-negative species, including Erwinia carotovora ssp. carotovora ( Parker et al. , 1982 ), Photorhabdus luminescens TT01 ( Derzelle et al. , 2002 ) and Serratia sp. ATCC 39006 ( Parker et al. , 1982 ).
The production of carbapenem in Serratia sp. ATCC 39006 is dependent on the car cluster, which consists of nine genes ( carR and carA – H ) and is highly conserved compared with the cluster found in Erwinia carotovora ssp. carotovora ( Cox et al. , 1998 ), the first to be both functionally and transcriptionally defined ( McGowan et al. , 1996 ). In the latter organism, the carA – E genes encode biosynthetic enzymes, which synthesize Car from precursors derived from acetate and glutamate ( Bycroft et al. , 1988 ; McGowan et al. , 1996 ; Li et al. , 2000 ; Stapon et al. , 2003 ), and this pathway is distinct from the biosynthesis of penicillins and cephalosporins ( Demain & Elander, 1999 ). Both CarF and CarG are necessary for the intrinsic resistance to Car by an uncharacterized mechanism, whereas the function of CarH is still unknown ( McGowan et al. , 1997 , 1998 ). The carR gene encodes a LuxR homologous transcriptional activator and is located immediately upstream of the carA – H operon but forms a separate transcriptional unit ( McGowan et al. , 2005 ). In E. carotovora ssp. carotovora , CarR activates carA – H expression depending on the cell density-dependent production of 3-oxo-C6-HSL, which is synthesized by the LuxI homologue CarI ( Welch et al. , 2000 ; Whitehead et al. , 2002 ; McGowan et al. , 2005 ). By contrast, CarR in Serratia sp. ATCC 39006 appears to function independent of AHL signals ( Cox et al. , 1998 ), and carA – H transcription is under control of the repressor SmaR, which binds upstream of the carA transcription start in the absence of AHLs and is released in the presence of C4-HSL/C6-HSL ( Thomson et al. , 2000 ; Slater et al. , 2003 ; Fineran et al. , 2005b ).
Quorum sensing-directed production of antibiotics has in addition been reported for S. plymuthica RVH1 ( Van Houdt et al. , 2007 ) and for S. marcescens strain 12 ( Coulthurst et al. , 2006 ). Coulthurst and colleagues suggested that the compound produced by S. marcescens strain 12 may be a bacteriocin, a compound produced by many S. marcescens strains ( Guasch et al. , 1995 ). Despite the fact that the structure of the compound produced by RVH1 is still unknown, preliminary characteristics of the compound do not reflect the properties of the small carbapenem produced by Serratia sp. ATCC 30096 or of other antibacterial factors identified in Serratia species ( Van Houdt et al. , 2005 , 2007 ).
Biofilm formation
Besides regulation of swarming motility, prodigiosin production and LipB-secreted extracellular enzyme quorum sensing is crucial for normal biofilm development and for elaborate differentiation in S. marcescens MG1 ( Labbate et al. , 2004 ). Biofilms are a structured community of surface-adherent bacterial microcolonies embedded in self-produced exopolymeric substances ( Costerton et al. , 1999 ). Bacterial biofilms in industrial settings may develop into biofouling that can cause problems such as corrosion and reduced mass and heat transfer. For the food industry, formation of biofilms on food and food contact surfaces, and in water distribution systems, constitutes an increased risk for product contamination with spoilage or pathogenic microbial communities ( Carpentier & Cerf, 1993 ). From a medical perspective, biofilm-associated bacteria on implants or catheters are of great concern because they are a source of persistent infections ( Donlan et al. , 2002 ). Furthermore, differentiation of planktonic cells into a complex mature biofilm results in a phenotypic shift with major implications, such as an increased resistance towards antimicrobial agents and towards clearance by host defences (reviewed in Donlan & Costerton, 2002 ).
Biofilms of S. marcescens MG1 are distinct from those of model biofilm-forming bacteria such as P. aeruginosa and E. coli , which consist of undifferentiated cells packed together in microcolonies ( O'Toole & Kolter, 1998 ; Nivens et al. , 2001 ; Reisner et al. , 2003 ; Van Houdt & Michiels, 2005 ). Labbate et al. , (2004) proposed that S. marcescens MG1 biofilm formation followed a genetically encoded programme in which elaborate cellular differentiation and structural differentiation are observed with successive formation of long filamentous cells, aggregation of vegetative bacteria with the filamentous cells and intertwining cell chains where there was biofilm maturation. An swrI mutant of S. marcescens MG1, incapable of synthesizing C4-HSL and C6-HSL, formed a thin and nonmature biofilm lacking cell aggregates and differentiated cell chains. Complementation with C4-HSL resulted in a biofilm with the wild-type architecture. In the same study two additional quorum sensing-regulated genes that are involved in biofilm development were identified. The product of the bsmA gene ( b iofilm s tructure m utant) was proposed to be an adhesin, controlling the size of cell aggregates whereas the product of the bsmB gene was proposed to encode a positive effector of bacterial aggregation necessary for activating aggregation. Both genes were proposed to be engaged in fine-tuning the formation of cell aggregates at a specific point in the proposed genetically encoded programme of biofilm development. Furthermore, quorum sensing is involved in cell detachment or sloughing from the surface, which appears to be an active process in the biofilm life cycle ( Rice et al. , 2005 ). Consequently, quorum sensing in S. marcescens MG1 plays key roles in at least four stages of biofilm development, from attachment to swarming motility, biofilm maturation and detachment ( Eberl et al. , 1999 ; Labbate et al. , 2004 ; Rice et al. , 2005 ).
Recently, Coulthurst et al. , (2006) showed that in S. marcescens strain 12 biofilm formation was dependent on smaI , measured by an attachment assay to a plastic microtitre plate. It would be interesting to explore the developmental pathway of biofilm formation and the role of quorum sensing herein for S. marcescens strain 12, as similar to S. marcescens strain MG1, swarming motility is also under AHL-dependent quorum sensing control in this strain.
Butanediol fermentation
Recently, 2,3-butanediol fermentation was shown to be quorum sensing-regulated in at least two Serratia spp. ( Van Houdt et al. , 2006a ). Members of Klebsiella , Enterobacter , Serratia and a number of other genera in the family Enterobacteriaceae ferment glucose predominantly to 2,3-butanediol ( White et al. , 2000 ). In contrast, members of genera such as Escherichia , Salmonella and Shigella use the mixed acid pathway, producing large amounts of acidic end-products including acetate, lactate, succinate and formate. Mixed acid fermentation causes a strong acidification of their environment, whereas production of acidic end-products is limited when a significant amount of pyruvate from glycolysis is channelled into the butanediol pathway ( Johansen et al. , 1975 ; Magee & Kosaric, 1987 ). The production of neutral compounds is ecologically relevant as it allows butanediol fermenters to prevent lethal acidification as cells approach stationary phase. In both S. plymuthica RVH1 and S. marcescens MG1 inactivation of the AHL synthase encoding gene leads to a reduced production of 2,3-butanediol and to a continued production of acidic end-products at the end of the exponential and throughout the stationary growth phase, which in turn leads to early growth arrest in the presence of fermentable sugars. These findings may also be useful for improving the yield of 2,3-butanediol fermentation via metabolic engineering. The demand and manufacture of 2,3-butanediol is still increasing worldwide (annual rate of 4–7%) due to a variety of applications such as a liquid fuel additive and due to the increased demand for polybutylene terephthalate resin, γ-butyrolactone, spandex, and its precursors ( Syu et al. , 2001 ). Furthermore, rising petroleum prices have revived significant interest in producing feedstock chemicals, including 2,3-butanediol, from biomass.
Integrating quorum sensing in complex networks
The quorum sensing-dependent regulation of particular phenotypes is not absolute; often phenotypes are under multifactor control integrating information from different physiological cues. As described in Fig. 1 swarming motility in S. marcescens MG1 is controlled by two key regulators, respectively the flhDC operon and the swr quorum sensing system. In addition, the above described quorum sensing-dependent S. marcescens MG1 biofilm formation can be circumvented by nutrient cues. In glucose-supplemented minimal medium MG1 was unable to swarm even in the presence of exogenously added AHL signals ( Eberl et al. , 1996b ). In minimal medium supplemented with 0.05% glucose and 0.05% casamino acids, an swrI mutant formed thin, undifferentiated biofilms compared with a filamentous biofilm composed of cell chains and clusters formed by its parent strain, whereas in 0.1 × LB medium both formed biofilms that resembled wild-type architecture. Furthermore, medium composition was demonstrated to shift biofilm morphology. A filamentous biofilm type could reversibly shift to a microcolony biofilm under nutrient-limiting conditions, demonstrating the dynamics of a biofilm structure that responds to the prevailing conditions ( Rice et al. , 2005 ).
In addition, the constitutively expressed estA gene, located upstream of swrR , encodes for the esterase EstA belonging to the class II of lipolytic enzymes (characterized by the active-site consensus motif G-D-S-L, reviewed by Arpigny & Jaeger, 1999 ) and supplies S. marcescens MG1 with precursors required for AHL biosynthesis when cells are grown on certain lipidic substrates such as Tween ( Riedel et al. , 2003 ).
In S. marcescens SS-1, the luxI and luxR homologues ( spnI and spnR ) are organized convergently as in other Serratia species and in most Gammaproteobacteria ( Gray & Garey, 2001 ). However, the spnI gene is present in an operon with spnT , which is as yet only partially characterized. Overexpression of spnT inhibited the production of the secondary metabolites prodigiosin and biosurfactant, the latter exerting a negative effect on sliding motility. In addition, overexpresion of spnT caused inhibition of cell division and chromosomal DNA segregation ( Horng et al. , 2002 ; Wei et al. , 2006a ).
In Serratia sp. ATCC 39006 the biosynthesis of the secondary metabolites prodigiosin and carbapenem is controlled by multiple environmental inputs. The SmaIR quorum sensing system regulates transcription, probably by the same derepressive mechanism, of at least three regulators involved in prodigiosin production: pigR , rap and pigQ . PigQ, a putative response regulator of a GacAS family two-component system PigQW, and Rap, a SlyA/MarR family transcriptional activator, affect prodigiosin production by regulating transcription of pigA – O ( Slater et al. , 2003 ; Fineran et al. , 2005b ). The GacA/GacS cascade is a global regulator of gene expression in gram-negative bacteria ( Heeb & Haas, 2001 ) and production of AHLs is under the control of a GacA/GacS-type two-component signal transduction system in many bacteria, including Pseudomonas species and S. plymuthica IC1270 ( Reimmann et al. , 1997 ; Chancey et al. , 1999 ; Ovadis et al. , 2004 ). In contrast, the PigQW system does not control AHL production and is itself regulated by quorum sensing ( Fineran et al. , 2005b ). The SlyA/MarR family of transcriptional regulators is a growing family of novel bacterial regulatory proteins that play key roles in the global regulation of diverse aspects of bacterial physiology. In addition, Rap controls the production of carbapenem by activating expression of carR ( Thomson et al. , 1997 ; Slater et al. , 2003 ). The exact mechanism of PigR-mediated prodigiosin production is not clear; however, this predicted adenylate cyclase presumably activates production through the synthesis of the common signalling molecule cAMP ( Fineran et al. , 2005a , b ).
Fineran et al. , (2005a) also identified a novel pleiotropic master regulator PigP, which is responsible for modulating prodigiosin and carbapenem production. The latter is affected by activating transcription of carA-H , carR and rap . PigP-mediated prodigiosin production involves at least six regulators: pigQ , pigR , pigS , pigV , pigX and rap . Transcription of pigA – O is activated by the gene products of rap , pigQ and pigS and of pigR and pigV , whose transcription respectively is activated and repressed by PigP. PigS is similar to proteins of the ArsR/SmtB family, the DNA binding activities of which respond to specific divalent metal ions ( Busenlehner et al. , 2003 ). PigV is similar to YgfX, a conserved hypothetical protein in E. coli . In addition, transcription of pigA – O is repressed by the gene product of pigX , a predicted homologue of YhdA from E. coli , whose transcription in turn is repressed by PigP. Besides PigP, the study revealed in addition the presence of PigU, a predicted transcriptional repressor of prodigiosin and carbapenem production.
The availability of inorganic phosphate (P i ) additionally regulates the production of carbapenem and prodigiosin in Serratia sp. ATCC 39006 ( Slater et al. , 2003 ). Under limiting P i conditions, the Pst (phosphate-specific transport) system is the major route for P i uptake (reviewed by Wanner et al. , 1993 ) and negatively regulates the Pho regulon, involving signal transduction to the master regulatory pair PhoB and PhoR ( Steed & Wanner, 1993 ; Wanner et al. , 1995 , 1996 ). When P i is exhausted, the PhoR sensor kinase phosphorylates the response regulator PhoB and phosphorylated PhoB activates genes from the Pho regulon, which contain an upstream activation site with a Pho box consensus sequence. Both pigA and smaI contain a Pho box sequence, which results in increased transcription of smaI and the pig gene cluster, under P i -limiting conditions, leading to increased production of prodigiosin and carbapenem.
It is clear that a complex regulatory network integrates at least cell density (quorum sensing), phosphate limitation and other environmental cues sensed by the master regulator PigP and the histidine kinase PigW of the two-component system in order to control secondary metabolite production in Serratia sp. ATCC 39006 ( Coulthurst et al. , 2005 ; Williamson et al. , 2006 ).
Quorum sensing and horizontal gene transfer
As already described, the ability of Serratia strains to produce AHLs and their AHL production profiles are species- and strain-dependent ( Harris et al. , 2004 ; Van Houdt et al. , 2004 , 2005 ). Furthermore, phenotypes that are regulated by quorum sensing in the diverse Serratia strains are also observed in strains that do not produce AHLs ( Van Houdt et al. , 2005 ). Intriguingly, Wei et al. , (2006b) discovered that in S. marcescens SS-1, the spnIR quorum sensing genes are located within a novel Tn 3 family transposon termed Tn TIR . The location of spnIR on a mobile genetic element can boost its dissemination through horizontal gene transfer, which appears to play an important role in generating the current distribution of the quorum sensing genes across bacterial species ( Lerat & Moran, 2004 ). Coulthurst et al. , (2006) demonstrated that phage-mediated horizontal gene transfer of a complete prodigiosin production cluster to the nonpigmented S. marcescens strain 12 brought prodigiosin production under control of the native quorum sensing system. In addition, phage-mediated transfer of the smaIR locus of S. marcescens strain 12 to S. marcescens strain 274, which lacks SmaIR homologues, brought the production of prodigiosin under control of the foreign quorum sensing locus.
Horizontal gene transfer, due to transposition events or is phage-mediated, can therefore facilitate the incorporation of a quorum sensing module into a microbial genome, which thereupon can directly plug into an existing regulatory circuit. Moreover, the negative control of quorum sensing-dependent phenotypes by R protein-dependent repression of the target genes and AHL-dependent derepression, which apparently is a general trait in the genus Serratia , would facilitate the application possibility of the quorum sensing module in the novel host without the necessity of other adaptations (excellently described by Coulthurst et al. , 2006 ).
The AI-2 molecule
The AI-2 molecule was first described as part of the quorum sensing circuit of the marine bacterium Vibrio harveyi , in which bioluminescence is regulated by at least three parallel quorum sensing systems, each of which synthesizes, detects and responds to different signalling molecules ( Henke & Bassler, 2004 ). HAI-1 is an AHL, N -3-hydroxybutanoyl- l -homoserine lactone, synthesized by the gene products of luxL and luxM , which show no homology to the LuxI family of AHL synthases described above ( Cao & Meighen, 1989 ; Bassler et al. , 1993 ). AI-2 is identified as a furanosyl borate diester ( Chen et al. , 2002 ), and its synthesis depends on luxS ( Surette et al. , 1999 ), whereas system 3 is homologous to system 1 of Vibrio cholerae and uses the CAI-1 (for cholerae autoinducer 1) autoinducer of unknown structure ( Henke & Bassler, 2004 ). The signalling molecules are detected by cognate sensor histidine kinases that all relay phosphate to the shared response regulator LuxO, by switching from kinase mode to phosphatase mode at a particular cell density ( Henke & Bassler, 2004 ; Waters & Bassler, 2006 ). The phosphorylated LuxO activates, in conjunction with σ 54 , the expression of genes encoding small regulatory RNAs (sRNAs), which together with the RNA chaperone Hfq, destabilize the mRNA encoding LuxR ( Lilley & Bassler, 2000 ; Lenz et al. , 2004 ). This LuxR is the master regulator of quorum sensing; however, it is not a homologue of LuxR from V. fischeri . This signalling arrangement provides V. harveryi with a mechanism to integrate the different AI signals ( Waters & Bassler, 2006 ).
Today, there is an extensive and still growing list of bacteria of different genera in which AI-2 have been suggested to play a regulatory role. The putative targets include an assortment of niche-specific genes with diverse functions, such as (but not limited to) genes encoding virulence factors in Actinobacillus actinomycetemcomitans ( Fong et al. , 2001 ), E. coli O157:H7 ( Sperandio et al. , 1999 ), Porphyromonas gingivalis ( Chung et al. , 2001 ; Burgess et al. , 2002 ), Streptococcus pyogenes ( Lyon et al. , 2001 ), V. cholerae ( Miller et al. , 2002 ; Zhu et al. , 2002 ; Lenz et al. , 2004 ) and Vibrio vulnificus ( Kim et al. , 2003 ); antibiotic production in Photorhabdus luminescens ( Derzelle et al. , 2002 ); biofilm formation and carbohydrate metabolism in Streptococcus gordonii ( McNab et al. , 2003 ); and an AI-2 ABC-type transporter in Salmonella enterica serovar Typhimurium ( Taga et al. , 2001 ) and E. coli MG1655 ( Xavier & Bassler, 2005 ). This growing number of both gram-positive and gram-negative bacteria which produce AI-2 or contain a luxS homologue leads to the suggestion that AI-2 is a universal language for interspecies communication ( Xavier & Bassler, 2003 ). However, LuxS can also fulfil a metabolic function as an integral component of the activated methyl cycle, which provides an alternative explanation for its widespread conservation ( Winzer et al. , 2003 ). This metabolic cycle provides activated methyl groups in the form of SAM generating S -adenosylhomocysteine (SAH), a toxic metabolite. SAH can be removed by one of two routes depending on the organism ( Winzer et al. , 2002 ): either in a one-step conversion to homocysteine by SAH hydrolase, or by the production of S -ribosylhomocysteine (SRH) by Pfs nucleosidase (also known as methylthioadenosine/SAH nucleosidase). This SRH is subsequently cleaved to homocysteine and 4,5-dihydroxy-2,3-pentanedione (DPD) by LuxS ( Fig. 4 ). This biosynthetic pathway leading to DPD has been shown to be identical in numerous microorganisms including E. coli , Salmonella enterica serovar Typhimurium, V. harveyi , V. cholerae , Enterococcus faecalis , Neisseria meningitides , Porphyromonas gingivalis and Staphylococcus aureus ( Schauder et al. , 2001 ; Winzer et al. , 2002 ). Next, spontaneous cyclization of DPD results in two epimeric furanones, (2 R ,4 S )- and (2 S ,4 S )-2,4-dihydroxy-2-methyldihydrofuran-3-one (respectively R - and S -DHMF; Fig. 4 ), and hydration of R - and S -DHMF would give rise to (2 R ,4 S )- and (2 S ,4 S )-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran (respectively R - and S -THMF; Fig. 4 ). For V. harveyi , X-ray crystallography of AI-2 bound to LuxP allowed identification of the signal molecule as S -THMF-borate ( Chen et al. , 2002 ), which can be formed by the reaction of the adjacent hydroxyl groups on the furanosyl ring of S -THMF with borate ( Loomis & Durst, 1992 ), abundant in marine environments. For Salmonella enterica serovar Typhimurium, an unborated DPD derivative, R -THMF, is bound by LsrB and transduces the AI-2 signal ( Miller et al. , 2004 ). These results indicate that multiple derivatives of DPD are biologically active. Phenotypes linked to LuxS dependent AI-2 production can therefore be considered either as true behavioural responses of a bacterial population by cell-to-cell signalling or as the result of pleiotropic effects of a disturbed activated methyl cycle on cellular metabolism. The effects observed by disrupting luxS in a bacterium should be verified as unambiguous by establishing the signal transduction/uptake pathways to prove a signalling role of AI-2 in that particular bacterium ( Vendeville et al. , 2005 ).
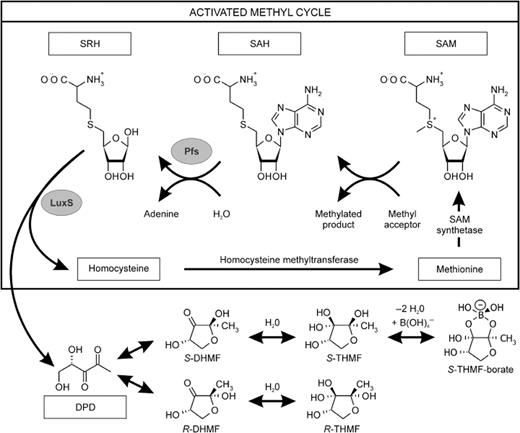
Biosynthetic pathway leading to DPD and AI-2. The activated methyl group of S -adenosylmethionine (SAM) is used for methylation leading to S -adenosylhomocysteine (SAH), which is removed by one of two routes depending on the organism. One route converts SAH first in S -ribosylhomocysteine (SRH) and adenine by Pfs nucleosidase (also known as MTA/SAHase) and SRH is subsequently converted by LuxS to homocysteine and 4,5-dihydroxy-2,3-pentanedione (DPD). Spontaneously cyclization forms either the R or the S form of 2,4-dihydroxy-2-methyldihydrofuran-3-one (DHMF). Hydration of R - DHMF forms R -THMF, which cocrystallized with LsrB, whereas S -THMF can react with borate to generate the diester bound to LuxP.
Functions of AI-2 in Serratia
Coulthurst (2004 , 2006) reported that luxS is present and affects distinct phenotypes in Serratia sp. strain ATCC 39006, S. marcescens ATCC 274 and S. marcescens strain 12, confirming and extending the preceding demonstration of extracellular AI-2 activity in Serratia strains ( Van Houdt et al. , 2004 ). The affected phenotypes in Serratia sp. ATCC 39006 and S. marcescens ATCC 274 were respectively carbapenem production, and prodigiosin and haemolysin production. The luxS mutant of S. marcescens ATCC 274 was also found to exhibit modestly reduced virulence in a Caenorhabditis elegans model. In S. marcescens strain 12, loss of AI-2 production resulted in a slight increase in biofilm formation compared with its parent strain ( Coulthurst et al. , 2006 ). For S. plymuthica RVH1 knock-out of luxS had no detectable effect on the AHL-regulated phenotypes, nor on biofilm formation. However, overproduction of AI-2 in RVH1 strains carrying luxS on a multicopy plasmid caused attenuated growth ( Van Houdt et al. , 2006b ). As the signal transduction pathway has not been identified, these results do not support a role in cell–cell communication for LuxS and AI-2 in S. plymuthica RVH1, but rather suggest that the phenotypes associated with luxS knockout reported earlier in other Serratia spp. may be pleiotropic effects of a disturbed metabolic function.
Concluding remarks
Cell–cell communication is nowadays recognized as a significant aspect of the bacterial world and detailed knowledge of the communication pathways and their regulation will be imperative to improve our comprehension of the physiology of (mixed) bacterial populations, which is of utmost importance from an industrial and medical perspective. AHL-dependent quorum sensing and to a lesser extent (or at least less studied) AI-2-mediated communication is of profound biological and ecological significance for Serratia species, as they adopt such communication systems in multifaceted neural networks that funnel exoenzymes, antimicrobial compounds and other virulence-associated phenotypes. In addition, cell–cell communication systems, especially AI-2 and AHL-mediated systems, are continuously being discovered and characterized in Serratia spp. and other organisms, many having fascinating twists on the central theme. On the other hand, the increasing number of documented infections caused by Serratia spp. other than S. marcescens urges for a more detailed investigation of the physiology, virulence and taxonomy of this genus, which inherently includes communication systems. Moreover, testing of advanced strategies to disrupt cell–cell communication should be included in such studies, with the ultimate goal of preventing or curing diseases.
References
Editor: Miguel Camara



