-
PDF
- Split View
-
Views
-
Cite
Cite
Shruti Menon, Kimberly Alexander, Peter Timms, John A. Allan, Wilhelmina M. Huston, CXCL10, CXCL11, HLA-A and IL-1β are induced in peripheral blood mononuclear cells from women with Chlamydia trachomatis related infertility, Pathogens and Disease, Volume 74, Issue 1, February 2016, ftv099, https://doi.org/10.1093/femspd/ftv099
Close - Share Icon Share
Chlamydia trachomatis infections can result in the development of serious sequelae such as pelvic inflammatory disease and tubal infertility. In this study, peripheral blood mononuclear cells from women who were undergoing or had recently undergone IVF treatment were cultured ex vivo with C. trachomatis to identify the immune responses associated with women who had serological evidence of a history of Chlamydia infection. Cytokines secreted into the supernatant from the cultures were measured using ELISA, and the level of IL-1β was found to be significantly higher in Chlamydia positive women than Chlamydia negative women. qRT-PCR analysis of the expression of 88 immune-related genes showed trends towards an upregulation of CXCL10, CXCL11 and HLA-A in Chlamydia positive women compared with Chlamydia negative women. These findings support that some women launch a more marked proinflammatory response upon infection with C. trachomatis and this may be associated with why C. trachomatis induces infertility in some infected women.
INTRODUCTION
Chlamydia (C.) trachomatis is one of the most common sexually transmitted bacteria in the world (reviewed in Paavonen and Eggert-Kruse 1999). The infection can result in serious complications in women including pelvic inflammatory disease (PID), ectopic pregnancy and tubal infertility (Agrawal et al.2009). Chlamydia trachomatis pathogenesis and the mechanism resulting in female infertility have been investigated using various in vitro techniques, including infection of HeLA cells with C. trachomatis where the expression of genes that regulate innate immunity (Hess et al.2003) and IL-8 (Buchholz and Stephens 2006) has been detected. Specific chlamydial antigens have also been proposed to mediate much of the inflammation; in one example, Zhou et al. (2013) showed that expression of the C. trachomatis pORF5 protein in HeLa cells induced the expression of TNF-α, IL-1β and IL-8. Additionally, ex vivo studies using infection with C. trachomatis serovar D on primary cells isolated from human fallopian tube showed that IFN-γ effector functions in hypoxic conditions facilitated the development of chronic infections (Roth et al.2010). Ex vivo stimulation with C. trachomatis on peripheral blood mononuclear cells (PBMC) and endometrial tissue of women showed an accumulation of IL-4 (Vicetti Miguel, Maryak and Cherpes 2012) and a polarization towards type 2 immunity (Vicetti Miguel et al.2013). Murine models have shown that infection with C. trachomatis alters CD8+ T-cell responses in eliciting protection against the pathogen (Loomis 2006), and ex vivo studies on mouse macrophages and fibroblasts showed an MyD88-dependent induction of IFN-γ and IP-10 (Nagarajan et al.2005). Additionally, when challenged with C. trachomatis MoPn biovar in the ovarian bursa of C3H and C57BL/6 mice, they developed hydrosalpinx that subsequently resulted in infertility (Khamesipour et al.1994). These studies indicate that a proinflammatory response from the local tissue is likely a major contributor to the pathological outcome; however, the paradox is that a proinflammatory or cellular response is essential to clear the intracellular chlamydial infection.
Several studies have identified immune factors expressed from PBMC from Chlamydia-infected or infertile participants. The stimulation of PBMC from infertile women with chlamydia 60 kDa heat shock protein (HSP60) yielded higher production of IFN-γ, IL-10 and IL-12 cytokines (Kinnunen et al.2003). In vitro lymphocyte proliferation of PBMC from women with salpingitis with C. trachomatis 57-kDa Hsp showed a production of cell-mediated responses to the infection, indicating its possible role in tubal pathology (Witkin et al.1993). Proinflammatory or regulatory cytokines such as IL-17 (Jha et al.2011), IFN-γ (Debattista et al.2002), IL-6 (Cunningham et al.2013) and IL-22 (Jha et al.2011) have been previously shown to be secreted from C. trachomatis-stimulated PBMC of patients with acute infections, or chlamydial infertility.
Diagnosis of women with chlamydial infertility is typically conducted using surgical or sonographic investigation for fallopian tubal blockage (Paavonen et al.1987). However, even in the absence of apparent tubal occlusion women who are sero-positive for CT are 50% less likely to conceive other than by IVF treatment (Keltz et al.2013). Approximately 45% of tubal infertility is due to C. trachomatis (Price et al.2012). However, in spite of the prevalence of this condition we still only have limited understanding of the disease mechanism that results in infertility in some women.
Here PBMC from infertile female participants who are undergoing or had recently undergone IVF treatment were isolated and cultured ex vivo in the presence of C. trachomatis. The gene expression of 88 innate and adaptive genes and 10 secreted cytokines were measured from the cultures to detect differences in the responses from women who had serological evidence of chlamydial infertility compared to those with other causes of infertility.
MATERIALS AND METHODS
Whole blood and sera was collected from fully consented voluntary participants. A total of 31 women with infertility (greater than 1 year of attempting to conceive and requiring IVF treatment) who were attending an IVF clinic or who were pregnant having recently conceived from treatment at the IVF clinic participated in the study. The study was reviewed and approved by the Queensland University of Technology and UC Health Human Research Ethics Committees: UC Health Ethics approval 1314, QUT Human Research Ethics approval 1300000505. Chlamydia microimmunofluorescence (MIF) IgG (Focus Diagnostics, USA) assay was conducted on the participant sera to determine the C. trachomatis infection history status.
Chlamydia trachomatis D (ATCC VR-885) and F strain (ATCC VR-346) were cultured in McCoy cells. Confluent cells were infected with the strains and incubated at 37°C for 44 h. Following infection, the strains were semipurified. To prepare a mixture of C. trachomatis D and F strains, the cultures were mixed together (equal ratio of EBs) and purified using density gradient centrifugation (29% v/v urografin Ultravist® (Sigma-Aldrich)), and stored in sucrose phosphate buffer at –80°C.
PBMC were isolated from participant whole blood. PBMC were isolated using Ficoll-Plaque Premium reagent (GE Healthcare) as previously described in Cunningham et al. (2013). PBMC (1×106cells/well) and they were stimulated at an MOI of 5 with purified C. trachomatis serovar D (ATCC: VR-885), purified C. trachomatis serovar F (ATCC VR-346), purified C. trachomatis D and F mix (50:50 elementary bodies (EBs)), phorbol myristate acetate (positive control) and media (negative control). The cells were incubated for 15 h at 37°C. After incubation, the cells were centrifuged at 800 × g, and the supernatant was collected. The cells were resuspended in RNA cell protect® (Qiagen, Victoria, Australia) for gene expression analysis. The levels of IFN-γ, IL-12, IL-1B, IL-2, IL-4, IL-6, IL-8, IL-10, IL-17 and TNF-α were measured from the supernatants using ELISA kits (Elisakit.com, Melbourne, Australia) as per manufacturer's instructions.
Total RNA was extracted and purified using Qiagen RNeasy Micro Kit (Victoria, Australia), and reverse transcribed into cDNA using Qiagen RT2 First strand kit and expression of innate and adaptive immune genes was determined using Qiagen RT2 Profiler PCR arrays. The expression levels of all genes were normalized to five housekeeping genes (ACTB, B2M, GAPDH, RPLP0 and HPRT1) for each participant sample as per manufacturer's instructions. The fold change between CT positive infertile women and CT negative infertile women was determined using the 2−ΔΔCt method (Livak 2001).
RESULTS
Participants were classified as serologically positive for C. trachomatis using C. trachomatis MIF IgG assay (4 of 31 were MIF positive and considered Chlamydia infertile) (Summary Table 1). All participants were women undergoing or recently had undergone IVF treatment. The participants who were seronegative to C. trachomatis infection were characterized as C. trachomatis negative (n = 27). The immune responses were then compared to media as a control C. trachomatis D and F strain treatments in the same groups using cytokine ELISAs and qRT-PCR analysis. Figure 1 shows the level of secreted cytokines from the supernatants of both Chlamydia positive and Chlamydia negative infertile women after culture in the presence of C. trachomatis D and F strains. In response to the culture in the presence of the C. trachomatis D and F EBs, IL-8 (617.5 pg/mL vs 602.5 pg/mL) and TNF-α (111 pg/mL vs 71.73 pg/mL) were produced in both cohorts; however, only IL-1β (96.42 pg/mL vs 41.42 pg/mL) production in Chlamydia positive women was significantly higher than Chlamydia negative women (P < 0.05). Compared with the negative control (unstimulated, media only control) for Chlamydia positive infertile women, the levels of IL-8 (617.5 pg/mL and 515 pg/mL), TNF-α (111 pg/mL and 7.557 pg/mL) and IL-1β (96.42 pg/mL vs 7.76 pg/mL) were significantly higher in the PBMC stimulated with C. trachomatis D and F strains (Fig. 2). Therefore, this is a reflection of the Chlamydia response of the PBMCs from these participants rather than a general response of the PBMCs to culture.
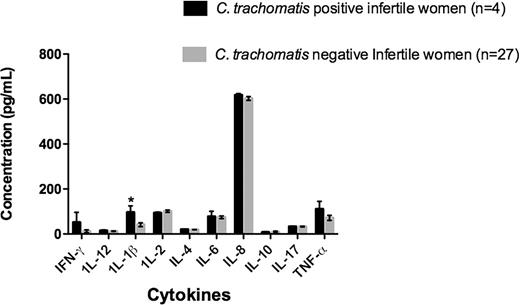
The graph shows the concentration of cytokines detected in the supernatants of the ex vivo PBMC cultures in the presence of C. trachomatis D and F. Chlamydia positive infertile women (n = 4) and Chlamydia negative infertile women (n = 27) (Asterisk indicates P < 0.05).
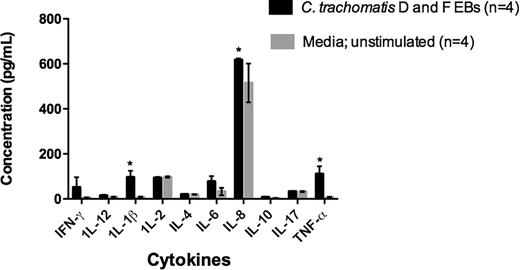
The graph shows the concentration of cytokines detected in the supernatants of the ex vivo PBMC cultures of Chlamydia positive infertile women in the presence of C. trachomatis D and F and control cultures (no Chlamydia added) (Asterisk indicates P < 0.05).
| C. trachomatis serology . | Mean Age . | Type of infertility *ectopic . | . | . | Gravidas at the time . |
|---|---|---|---|---|---|
| status by MIF . | (years) . | (n=) *tubal (n=) . | IVF outcome *success (n=) *failure (n=) . | Gravidas past *nulliparous (n=) *multiparous (n=) . | of collection (n=) . |
| C. trachomatis positive infertile women (n = 4) | 39.5 | *Tubal (n = 3); PID (n = 1); salpingitis (n = 1); tubal adhesion (n = 2)]*Unknown etiology (n = 1) | Success (n = 2) Failure (n = 2) | Nulliparous (n = 3) Multiparous (n = 1) | n = 0 |
| C. trachomatis negative infertile women (n = 19) | 37.4 | *Tubal (n = 4); ovary removal (n = 2); PID (n = 1); salpingitis (n = 1); tubal obstruction (n = 1); ovarian cystecty (n = 1); sterile (n = 1);*Ectopic pregnancy (n = 1)*Polycystic ovarian syndrome (PCOS) (n = 4)*Endometritis (n = 4) + [PID=(n = 2)]*Unknown etiology (n = 6) | Success (n = 11) Failure (n = 8) | Nulliparous (n = 13) Multiparous (n = 6) | n = 1 |
| C. trachomatis serology . | Mean Age . | Type of infertility *ectopic . | . | . | Gravidas at the time . |
|---|---|---|---|---|---|
| status by MIF . | (years) . | (n=) *tubal (n=) . | IVF outcome *success (n=) *failure (n=) . | Gravidas past *nulliparous (n=) *multiparous (n=) . | of collection (n=) . |
| C. trachomatis positive infertile women (n = 4) | 39.5 | *Tubal (n = 3); PID (n = 1); salpingitis (n = 1); tubal adhesion (n = 2)]*Unknown etiology (n = 1) | Success (n = 2) Failure (n = 2) | Nulliparous (n = 3) Multiparous (n = 1) | n = 0 |
| C. trachomatis negative infertile women (n = 19) | 37.4 | *Tubal (n = 4); ovary removal (n = 2); PID (n = 1); salpingitis (n = 1); tubal obstruction (n = 1); ovarian cystecty (n = 1); sterile (n = 1);*Ectopic pregnancy (n = 1)*Polycystic ovarian syndrome (PCOS) (n = 4)*Endometritis (n = 4) + [PID=(n = 2)]*Unknown etiology (n = 6) | Success (n = 11) Failure (n = 8) | Nulliparous (n = 13) Multiparous (n = 6) | n = 1 |
| C. trachomatis serology . | Mean Age . | Type of infertility *ectopic . | . | . | Gravidas at the time . |
|---|---|---|---|---|---|
| status by MIF . | (years) . | (n=) *tubal (n=) . | IVF outcome *success (n=) *failure (n=) . | Gravidas past *nulliparous (n=) *multiparous (n=) . | of collection (n=) . |
| C. trachomatis positive infertile women (n = 4) | 39.5 | *Tubal (n = 3); PID (n = 1); salpingitis (n = 1); tubal adhesion (n = 2)]*Unknown etiology (n = 1) | Success (n = 2) Failure (n = 2) | Nulliparous (n = 3) Multiparous (n = 1) | n = 0 |
| C. trachomatis negative infertile women (n = 19) | 37.4 | *Tubal (n = 4); ovary removal (n = 2); PID (n = 1); salpingitis (n = 1); tubal obstruction (n = 1); ovarian cystecty (n = 1); sterile (n = 1);*Ectopic pregnancy (n = 1)*Polycystic ovarian syndrome (PCOS) (n = 4)*Endometritis (n = 4) + [PID=(n = 2)]*Unknown etiology (n = 6) | Success (n = 11) Failure (n = 8) | Nulliparous (n = 13) Multiparous (n = 6) | n = 1 |
| C. trachomatis serology . | Mean Age . | Type of infertility *ectopic . | . | . | Gravidas at the time . |
|---|---|---|---|---|---|
| status by MIF . | (years) . | (n=) *tubal (n=) . | IVF outcome *success (n=) *failure (n=) . | Gravidas past *nulliparous (n=) *multiparous (n=) . | of collection (n=) . |
| C. trachomatis positive infertile women (n = 4) | 39.5 | *Tubal (n = 3); PID (n = 1); salpingitis (n = 1); tubal adhesion (n = 2)]*Unknown etiology (n = 1) | Success (n = 2) Failure (n = 2) | Nulliparous (n = 3) Multiparous (n = 1) | n = 0 |
| C. trachomatis negative infertile women (n = 19) | 37.4 | *Tubal (n = 4); ovary removal (n = 2); PID (n = 1); salpingitis (n = 1); tubal obstruction (n = 1); ovarian cystecty (n = 1); sterile (n = 1);*Ectopic pregnancy (n = 1)*Polycystic ovarian syndrome (PCOS) (n = 4)*Endometritis (n = 4) + [PID=(n = 2)]*Unknown etiology (n = 6) | Success (n = 11) Failure (n = 8) | Nulliparous (n = 13) Multiparous (n = 6) | n = 1 |
The expression of a selection of 88 innate and adaptive immune genes from PBMC cultured with C. trachomatis D and F was analysed using a RT-PCR array. The gene expression was normalized to housekeeping genes for each participants’ culture and the fold change in gene expression between C. trachomatis infertile women and women with infertility for other reasons was tested (ΔΔct). Three genes showed a trend towards higher expression levels in C. trachomatis positive infertile women (n = 4) compared with C. trachomatis negative infertile women (n = 27) ( Table 2). The chemokines CXCL10 (5.48-fold) and CXCL11 (2.31-fold), and human leukocyte antigen HLA-A (2.22-fold) showed the most notable differences between the two participant groups (although these were not significantly different, possibly due to the low sample size of C. trachomatis positive infertile women).
Gene expression of various immune-related genes from PBMC cultures with C. trachomatis from Chlamydia positive infertile women.
| . | . | Fold change (C. trachomatis positive infertile vs . |
|---|---|---|
| Gene . | Gene function . | C. trachomatis negative infertile)* (2−ΔΔCT) . |
| CXCL10 | CXC motif ligand 10 | 5.480 |
| CXCL11 | Chemotactic for T cells | 2.319 |
| HLA-A | Human leukocyte antigen | 2.224 |
| MX1 | Guanosine triphosphate metabolizing protein | 2.044 |
| TNF | Tumour necrosis factor | 1.689 |
| HLA-E | Human leukocyte antigen | 1.317 |
| C3 | Complement component 3 | 1.190 |
| NFKBIA | Nuclear factor of Kappa light polypeptide gene | 1.105 |
| IRF3 | Interferon regulatory transcription factor | 0.185 |
| IL1B | Interleukin 1 cytokine | 1.08 |
| CCL5 | Chemokine, chemoattractant | 1.055 |
| STAT3 | Transcription factor | 1.010 |
| IL8 | Chemoattractant | 0.995 |
| FASLG | Tumour necrosis factor superfamily FAS/FASLG signalling pathway | 0.988 |
| DDX58 | DEAD box proteins, putative RNA helicases | 0.962 |
| STAT1 | Signal transducer and activator of transcription | 0.926 |
| MYD88 | Cytosolic adapter protein | 0.913 |
| IFNAR1 | Receptor for interferons alpha and beta | 0.912 |
| CD14 | Surface antigen | 0.875 |
| IL1A | Interleukin 1 cytokine family | 0.854 |
| STAT4 | Signal transducer and activator of transcription | 0.831 |
| CSF2 | Colony stimulating factor 2 | 0.818 |
| IL23A | Subunit of cytokine interleukin 23 | 0.782 |
| NFKB1 | Transcription regulator | 0.744 |
| ICAM1 | Intercellular adhesion molecule 1 cell surface glycoprotein | 0.710 |
| CD40 | TNF-receptor superfamily; receptor on antigen-presenting cells | 0.689 |
| CXCR3 | G protein-coupled receptor | 0.629 |
| IL6 | Interleukin 6 | 0.622 |
| TBX21 | Transcription factor | 0.591 |
| CASP1 | Caspase | 0.580 |
| CD80 | Membrane receptor | 0.578 |
| IRF7 | Interferon regulatory factor 7 | 0.546 |
| STAT6 | Signal transduction and transcription | 0.532 |
| IFNG | Type II interferon family | 0.519 |
| IFNGR1 | Gamma interferon receptor | 0.515 |
| MAPK1 | MAP kinases | 0.448 |
| ITGAM | Integrin alpha M chain | 0.441 |
| IFNA1 | Interferon alpha | 0.407 |
| CD8A | Cell surface glycoprotein | 0.403 |
| IFNB1 | Interferon beta | 0.394 |
| TRAF6 | TNF receptor associated factor | 0.392 |
| NOD1 | Nucleotide-binding oligomerization domain | 0.374 |
| CXCR5 | CXC chemokine receptor family | 0.359 |
| CD4 | Membrane glycoprotein | 0.340 |
| CD86 | Cell surface ligand | 0.323 |
| MAPK8 | MAP kinases | 0.310 |
| CCL2 | Chemokine | 0.294 |
| IL10 | Cytokine | 0.292 |
| TICAM1 | Adaptor protein containing (TIR) | 0.271 |
| JAK2 | Janus kinase 2 | 0.257 |
| LYZ | Lysozyme | 0.257 |
| TLR3 | Toll-like receptor | 0.254 |
| IL2 | Interleukin 2 | 0.225 |
| CCR6 | Beta chemokine receptor | 0.211 |
| TLR7 | Toll-like receptor | 0.204 |
| IRAK1 | Interleukin-1 receptor-associated kinase | 0.198 |
| CD40LG | Surface of T cells | 0.191 |
| SLC11A1 | Solute carrier family 11 | 0.183 |
| FOXP3 | Transcriptional regulator | 0.162 |
| NLRP3 | Upstream activator of NF-kappaB | 0.159 |
| TLR2 | Toll-like receptor | 0.143 |
| CCR5 | Beta chemokine receptor family, | 0.142 |
| IL18 | Proinflammatory cytokine | 0.138 |
| IL1R1 | Cytokine receptor interleukin-1 alpha, | 0.117 |
| TLR1 | Toll-like receptor | 0.098 |
| TLR4 | Toll-like receptor | 0.098 |
| LY96 | Associates with Toll-like receptor 4 | 0.089 |
| NOD2 | Nod1/Apaf-1 family | 0.088 |
| TLR8 | Toll-like receptor | 0.086 |
| TLR6 | Toll-like receptor | 0.081 |
| RAG1 | Involved in activation of immunoglobulin V-D-J recombination | 0.081 |
| IL5 | Interleukin 5 cytokine | 0.080 |
| GATA3 | Transcription factors | 0.080 |
| IL17A | Proinflammatory cytokine | 0.078 |
| TLR5 | Receptor mobilizes the nuclear factor NF-kappaB | 0.076 |
| CXCL13 | Chemokine | 0.075 |
| IL4 | Interleukin 4 | 0.073 |
| MBL2 | Soluble mannose-binding lectin | 0.073 |
| APCS | Glycoprotein | 0.072 |
| TLR9 | Toll-like receptor | 0.072 |
| CRP | Host defence-related functions | 0.071 |
| IL22 | Interleukin-22 | 0.045 |
| IL13 | Immunoregulatory cytokine; B-cell maturation | 0.041 |
| MPO | Myeloperoxidase | 0.037 |
| CCR4 | G-protein-coupled receptor family; receptor for the CC chemokine | 0.036 |
| TYK2 | Tyrosine kinase Janus kinases (JAKs) | 0.034 |
| CCR8 | Beta chemokine receptor family | 0.015 |
| RORC | DNA-binding transcription factor | 0.081 |
| . | . | Fold change (C. trachomatis positive infertile vs . |
|---|---|---|
| Gene . | Gene function . | C. trachomatis negative infertile)* (2−ΔΔCT) . |
| CXCL10 | CXC motif ligand 10 | 5.480 |
| CXCL11 | Chemotactic for T cells | 2.319 |
| HLA-A | Human leukocyte antigen | 2.224 |
| MX1 | Guanosine triphosphate metabolizing protein | 2.044 |
| TNF | Tumour necrosis factor | 1.689 |
| HLA-E | Human leukocyte antigen | 1.317 |
| C3 | Complement component 3 | 1.190 |
| NFKBIA | Nuclear factor of Kappa light polypeptide gene | 1.105 |
| IRF3 | Interferon regulatory transcription factor | 0.185 |
| IL1B | Interleukin 1 cytokine | 1.08 |
| CCL5 | Chemokine, chemoattractant | 1.055 |
| STAT3 | Transcription factor | 1.010 |
| IL8 | Chemoattractant | 0.995 |
| FASLG | Tumour necrosis factor superfamily FAS/FASLG signalling pathway | 0.988 |
| DDX58 | DEAD box proteins, putative RNA helicases | 0.962 |
| STAT1 | Signal transducer and activator of transcription | 0.926 |
| MYD88 | Cytosolic adapter protein | 0.913 |
| IFNAR1 | Receptor for interferons alpha and beta | 0.912 |
| CD14 | Surface antigen | 0.875 |
| IL1A | Interleukin 1 cytokine family | 0.854 |
| STAT4 | Signal transducer and activator of transcription | 0.831 |
| CSF2 | Colony stimulating factor 2 | 0.818 |
| IL23A | Subunit of cytokine interleukin 23 | 0.782 |
| NFKB1 | Transcription regulator | 0.744 |
| ICAM1 | Intercellular adhesion molecule 1 cell surface glycoprotein | 0.710 |
| CD40 | TNF-receptor superfamily; receptor on antigen-presenting cells | 0.689 |
| CXCR3 | G protein-coupled receptor | 0.629 |
| IL6 | Interleukin 6 | 0.622 |
| TBX21 | Transcription factor | 0.591 |
| CASP1 | Caspase | 0.580 |
| CD80 | Membrane receptor | 0.578 |
| IRF7 | Interferon regulatory factor 7 | 0.546 |
| STAT6 | Signal transduction and transcription | 0.532 |
| IFNG | Type II interferon family | 0.519 |
| IFNGR1 | Gamma interferon receptor | 0.515 |
| MAPK1 | MAP kinases | 0.448 |
| ITGAM | Integrin alpha M chain | 0.441 |
| IFNA1 | Interferon alpha | 0.407 |
| CD8A | Cell surface glycoprotein | 0.403 |
| IFNB1 | Interferon beta | 0.394 |
| TRAF6 | TNF receptor associated factor | 0.392 |
| NOD1 | Nucleotide-binding oligomerization domain | 0.374 |
| CXCR5 | CXC chemokine receptor family | 0.359 |
| CD4 | Membrane glycoprotein | 0.340 |
| CD86 | Cell surface ligand | 0.323 |
| MAPK8 | MAP kinases | 0.310 |
| CCL2 | Chemokine | 0.294 |
| IL10 | Cytokine | 0.292 |
| TICAM1 | Adaptor protein containing (TIR) | 0.271 |
| JAK2 | Janus kinase 2 | 0.257 |
| LYZ | Lysozyme | 0.257 |
| TLR3 | Toll-like receptor | 0.254 |
| IL2 | Interleukin 2 | 0.225 |
| CCR6 | Beta chemokine receptor | 0.211 |
| TLR7 | Toll-like receptor | 0.204 |
| IRAK1 | Interleukin-1 receptor-associated kinase | 0.198 |
| CD40LG | Surface of T cells | 0.191 |
| SLC11A1 | Solute carrier family 11 | 0.183 |
| FOXP3 | Transcriptional regulator | 0.162 |
| NLRP3 | Upstream activator of NF-kappaB | 0.159 |
| TLR2 | Toll-like receptor | 0.143 |
| CCR5 | Beta chemokine receptor family, | 0.142 |
| IL18 | Proinflammatory cytokine | 0.138 |
| IL1R1 | Cytokine receptor interleukin-1 alpha, | 0.117 |
| TLR1 | Toll-like receptor | 0.098 |
| TLR4 | Toll-like receptor | 0.098 |
| LY96 | Associates with Toll-like receptor 4 | 0.089 |
| NOD2 | Nod1/Apaf-1 family | 0.088 |
| TLR8 | Toll-like receptor | 0.086 |
| TLR6 | Toll-like receptor | 0.081 |
| RAG1 | Involved in activation of immunoglobulin V-D-J recombination | 0.081 |
| IL5 | Interleukin 5 cytokine | 0.080 |
| GATA3 | Transcription factors | 0.080 |
| IL17A | Proinflammatory cytokine | 0.078 |
| TLR5 | Receptor mobilizes the nuclear factor NF-kappaB | 0.076 |
| CXCL13 | Chemokine | 0.075 |
| IL4 | Interleukin 4 | 0.073 |
| MBL2 | Soluble mannose-binding lectin | 0.073 |
| APCS | Glycoprotein | 0.072 |
| TLR9 | Toll-like receptor | 0.072 |
| CRP | Host defence-related functions | 0.071 |
| IL22 | Interleukin-22 | 0.045 |
| IL13 | Immunoregulatory cytokine; B-cell maturation | 0.041 |
| MPO | Myeloperoxidase | 0.037 |
| CCR4 | G-protein-coupled receptor family; receptor for the CC chemokine | 0.036 |
| TYK2 | Tyrosine kinase Janus kinases (JAKs) | 0.034 |
| CCR8 | Beta chemokine receptor family | 0.015 |
| RORC | DNA-binding transcription factor | 0.081 |
*ΔCT was obtained by normalizing the level of expression of gene of interest to the expression level of housekeeping genes (HKG) (ΔCT = CTGOI - CTAVG HKG). Fold change in gene expression (2−ΔΔCT) was determined by dividing the normalized expression of gene of interest of the experimental sample by the normalized expression of the same gene of interest in the control sample; where ΔΔCT = ΔCT (C. trachomatis positive infertile sample)- ΔCT (C. trachomatis negative infertile sample).
Gene expression of various immune-related genes from PBMC cultures with C. trachomatis from Chlamydia positive infertile women.
| . | . | Fold change (C. trachomatis positive infertile vs . |
|---|---|---|
| Gene . | Gene function . | C. trachomatis negative infertile)* (2−ΔΔCT) . |
| CXCL10 | CXC motif ligand 10 | 5.480 |
| CXCL11 | Chemotactic for T cells | 2.319 |
| HLA-A | Human leukocyte antigen | 2.224 |
| MX1 | Guanosine triphosphate metabolizing protein | 2.044 |
| TNF | Tumour necrosis factor | 1.689 |
| HLA-E | Human leukocyte antigen | 1.317 |
| C3 | Complement component 3 | 1.190 |
| NFKBIA | Nuclear factor of Kappa light polypeptide gene | 1.105 |
| IRF3 | Interferon regulatory transcription factor | 0.185 |
| IL1B | Interleukin 1 cytokine | 1.08 |
| CCL5 | Chemokine, chemoattractant | 1.055 |
| STAT3 | Transcription factor | 1.010 |
| IL8 | Chemoattractant | 0.995 |
| FASLG | Tumour necrosis factor superfamily FAS/FASLG signalling pathway | 0.988 |
| DDX58 | DEAD box proteins, putative RNA helicases | 0.962 |
| STAT1 | Signal transducer and activator of transcription | 0.926 |
| MYD88 | Cytosolic adapter protein | 0.913 |
| IFNAR1 | Receptor for interferons alpha and beta | 0.912 |
| CD14 | Surface antigen | 0.875 |
| IL1A | Interleukin 1 cytokine family | 0.854 |
| STAT4 | Signal transducer and activator of transcription | 0.831 |
| CSF2 | Colony stimulating factor 2 | 0.818 |
| IL23A | Subunit of cytokine interleukin 23 | 0.782 |
| NFKB1 | Transcription regulator | 0.744 |
| ICAM1 | Intercellular adhesion molecule 1 cell surface glycoprotein | 0.710 |
| CD40 | TNF-receptor superfamily; receptor on antigen-presenting cells | 0.689 |
| CXCR3 | G protein-coupled receptor | 0.629 |
| IL6 | Interleukin 6 | 0.622 |
| TBX21 | Transcription factor | 0.591 |
| CASP1 | Caspase | 0.580 |
| CD80 | Membrane receptor | 0.578 |
| IRF7 | Interferon regulatory factor 7 | 0.546 |
| STAT6 | Signal transduction and transcription | 0.532 |
| IFNG | Type II interferon family | 0.519 |
| IFNGR1 | Gamma interferon receptor | 0.515 |
| MAPK1 | MAP kinases | 0.448 |
| ITGAM | Integrin alpha M chain | 0.441 |
| IFNA1 | Interferon alpha | 0.407 |
| CD8A | Cell surface glycoprotein | 0.403 |
| IFNB1 | Interferon beta | 0.394 |
| TRAF6 | TNF receptor associated factor | 0.392 |
| NOD1 | Nucleotide-binding oligomerization domain | 0.374 |
| CXCR5 | CXC chemokine receptor family | 0.359 |
| CD4 | Membrane glycoprotein | 0.340 |
| CD86 | Cell surface ligand | 0.323 |
| MAPK8 | MAP kinases | 0.310 |
| CCL2 | Chemokine | 0.294 |
| IL10 | Cytokine | 0.292 |
| TICAM1 | Adaptor protein containing (TIR) | 0.271 |
| JAK2 | Janus kinase 2 | 0.257 |
| LYZ | Lysozyme | 0.257 |
| TLR3 | Toll-like receptor | 0.254 |
| IL2 | Interleukin 2 | 0.225 |
| CCR6 | Beta chemokine receptor | 0.211 |
| TLR7 | Toll-like receptor | 0.204 |
| IRAK1 | Interleukin-1 receptor-associated kinase | 0.198 |
| CD40LG | Surface of T cells | 0.191 |
| SLC11A1 | Solute carrier family 11 | 0.183 |
| FOXP3 | Transcriptional regulator | 0.162 |
| NLRP3 | Upstream activator of NF-kappaB | 0.159 |
| TLR2 | Toll-like receptor | 0.143 |
| CCR5 | Beta chemokine receptor family, | 0.142 |
| IL18 | Proinflammatory cytokine | 0.138 |
| IL1R1 | Cytokine receptor interleukin-1 alpha, | 0.117 |
| TLR1 | Toll-like receptor | 0.098 |
| TLR4 | Toll-like receptor | 0.098 |
| LY96 | Associates with Toll-like receptor 4 | 0.089 |
| NOD2 | Nod1/Apaf-1 family | 0.088 |
| TLR8 | Toll-like receptor | 0.086 |
| TLR6 | Toll-like receptor | 0.081 |
| RAG1 | Involved in activation of immunoglobulin V-D-J recombination | 0.081 |
| IL5 | Interleukin 5 cytokine | 0.080 |
| GATA3 | Transcription factors | 0.080 |
| IL17A | Proinflammatory cytokine | 0.078 |
| TLR5 | Receptor mobilizes the nuclear factor NF-kappaB | 0.076 |
| CXCL13 | Chemokine | 0.075 |
| IL4 | Interleukin 4 | 0.073 |
| MBL2 | Soluble mannose-binding lectin | 0.073 |
| APCS | Glycoprotein | 0.072 |
| TLR9 | Toll-like receptor | 0.072 |
| CRP | Host defence-related functions | 0.071 |
| IL22 | Interleukin-22 | 0.045 |
| IL13 | Immunoregulatory cytokine; B-cell maturation | 0.041 |
| MPO | Myeloperoxidase | 0.037 |
| CCR4 | G-protein-coupled receptor family; receptor for the CC chemokine | 0.036 |
| TYK2 | Tyrosine kinase Janus kinases (JAKs) | 0.034 |
| CCR8 | Beta chemokine receptor family | 0.015 |
| RORC | DNA-binding transcription factor | 0.081 |
| . | . | Fold change (C. trachomatis positive infertile vs . |
|---|---|---|
| Gene . | Gene function . | C. trachomatis negative infertile)* (2−ΔΔCT) . |
| CXCL10 | CXC motif ligand 10 | 5.480 |
| CXCL11 | Chemotactic for T cells | 2.319 |
| HLA-A | Human leukocyte antigen | 2.224 |
| MX1 | Guanosine triphosphate metabolizing protein | 2.044 |
| TNF | Tumour necrosis factor | 1.689 |
| HLA-E | Human leukocyte antigen | 1.317 |
| C3 | Complement component 3 | 1.190 |
| NFKBIA | Nuclear factor of Kappa light polypeptide gene | 1.105 |
| IRF3 | Interferon regulatory transcription factor | 0.185 |
| IL1B | Interleukin 1 cytokine | 1.08 |
| CCL5 | Chemokine, chemoattractant | 1.055 |
| STAT3 | Transcription factor | 1.010 |
| IL8 | Chemoattractant | 0.995 |
| FASLG | Tumour necrosis factor superfamily FAS/FASLG signalling pathway | 0.988 |
| DDX58 | DEAD box proteins, putative RNA helicases | 0.962 |
| STAT1 | Signal transducer and activator of transcription | 0.926 |
| MYD88 | Cytosolic adapter protein | 0.913 |
| IFNAR1 | Receptor for interferons alpha and beta | 0.912 |
| CD14 | Surface antigen | 0.875 |
| IL1A | Interleukin 1 cytokine family | 0.854 |
| STAT4 | Signal transducer and activator of transcription | 0.831 |
| CSF2 | Colony stimulating factor 2 | 0.818 |
| IL23A | Subunit of cytokine interleukin 23 | 0.782 |
| NFKB1 | Transcription regulator | 0.744 |
| ICAM1 | Intercellular adhesion molecule 1 cell surface glycoprotein | 0.710 |
| CD40 | TNF-receptor superfamily; receptor on antigen-presenting cells | 0.689 |
| CXCR3 | G protein-coupled receptor | 0.629 |
| IL6 | Interleukin 6 | 0.622 |
| TBX21 | Transcription factor | 0.591 |
| CASP1 | Caspase | 0.580 |
| CD80 | Membrane receptor | 0.578 |
| IRF7 | Interferon regulatory factor 7 | 0.546 |
| STAT6 | Signal transduction and transcription | 0.532 |
| IFNG | Type II interferon family | 0.519 |
| IFNGR1 | Gamma interferon receptor | 0.515 |
| MAPK1 | MAP kinases | 0.448 |
| ITGAM | Integrin alpha M chain | 0.441 |
| IFNA1 | Interferon alpha | 0.407 |
| CD8A | Cell surface glycoprotein | 0.403 |
| IFNB1 | Interferon beta | 0.394 |
| TRAF6 | TNF receptor associated factor | 0.392 |
| NOD1 | Nucleotide-binding oligomerization domain | 0.374 |
| CXCR5 | CXC chemokine receptor family | 0.359 |
| CD4 | Membrane glycoprotein | 0.340 |
| CD86 | Cell surface ligand | 0.323 |
| MAPK8 | MAP kinases | 0.310 |
| CCL2 | Chemokine | 0.294 |
| IL10 | Cytokine | 0.292 |
| TICAM1 | Adaptor protein containing (TIR) | 0.271 |
| JAK2 | Janus kinase 2 | 0.257 |
| LYZ | Lysozyme | 0.257 |
| TLR3 | Toll-like receptor | 0.254 |
| IL2 | Interleukin 2 | 0.225 |
| CCR6 | Beta chemokine receptor | 0.211 |
| TLR7 | Toll-like receptor | 0.204 |
| IRAK1 | Interleukin-1 receptor-associated kinase | 0.198 |
| CD40LG | Surface of T cells | 0.191 |
| SLC11A1 | Solute carrier family 11 | 0.183 |
| FOXP3 | Transcriptional regulator | 0.162 |
| NLRP3 | Upstream activator of NF-kappaB | 0.159 |
| TLR2 | Toll-like receptor | 0.143 |
| CCR5 | Beta chemokine receptor family, | 0.142 |
| IL18 | Proinflammatory cytokine | 0.138 |
| IL1R1 | Cytokine receptor interleukin-1 alpha, | 0.117 |
| TLR1 | Toll-like receptor | 0.098 |
| TLR4 | Toll-like receptor | 0.098 |
| LY96 | Associates with Toll-like receptor 4 | 0.089 |
| NOD2 | Nod1/Apaf-1 family | 0.088 |
| TLR8 | Toll-like receptor | 0.086 |
| TLR6 | Toll-like receptor | 0.081 |
| RAG1 | Involved in activation of immunoglobulin V-D-J recombination | 0.081 |
| IL5 | Interleukin 5 cytokine | 0.080 |
| GATA3 | Transcription factors | 0.080 |
| IL17A | Proinflammatory cytokine | 0.078 |
| TLR5 | Receptor mobilizes the nuclear factor NF-kappaB | 0.076 |
| CXCL13 | Chemokine | 0.075 |
| IL4 | Interleukin 4 | 0.073 |
| MBL2 | Soluble mannose-binding lectin | 0.073 |
| APCS | Glycoprotein | 0.072 |
| TLR9 | Toll-like receptor | 0.072 |
| CRP | Host defence-related functions | 0.071 |
| IL22 | Interleukin-22 | 0.045 |
| IL13 | Immunoregulatory cytokine; B-cell maturation | 0.041 |
| MPO | Myeloperoxidase | 0.037 |
| CCR4 | G-protein-coupled receptor family; receptor for the CC chemokine | 0.036 |
| TYK2 | Tyrosine kinase Janus kinases (JAKs) | 0.034 |
| CCR8 | Beta chemokine receptor family | 0.015 |
| RORC | DNA-binding transcription factor | 0.081 |
*ΔCT was obtained by normalizing the level of expression of gene of interest to the expression level of housekeeping genes (HKG) (ΔCT = CTGOI - CTAVG HKG). Fold change in gene expression (2−ΔΔCT) was determined by dividing the normalized expression of gene of interest of the experimental sample by the normalized expression of the same gene of interest in the control sample; where ΔΔCT = ΔCT (C. trachomatis positive infertile sample)- ΔCT (C. trachomatis negative infertile sample).
DISCUSSION
The cytokines secreted and immune gene expression in PBMC was tested to provide information into the immune pathways that are activated from women who have C. trachomatis infertility. Within 15 h of coculture with C. trachomatis, three immune genes were expressed at greater than 2-fold higher levels in Chlamydia positive infertile women compared with Chlamydia negative infertile women (not significant). A mix of two well characterized and common C. trachomatis strains was used in the coculture model, rather than a single strain, although these may not have been the same as the strain that originally infected the participants to cause the infertility. These two strains will not cover all of the relevant diversity of C. trachomatis, but were used to provide more diversity in the simulating antigen mix than a single strain. Infection by C. trachomatis is likely to trigger a range of immune responses as a result of antigen-binding human leukocyte antigens (HLA) molecules. Previous studies have elucidated the role of HLA class I and class II molecules and their genotypes in increasing the susceptibility to tubal pathology and infertility caused by C. trachomatis in women (Kimani et al.1996; Cohen et al.2000, 2003; Kinnunen et al.2002; Ness et al.2004). In this study, the HLA-class I molecule, HLA-A gene was upregulated in CT positive infertile women (not significant). Kimani et al. (1996) reported that HLA-A31 allele was a significant risk factor for C. trachomatis PID in a longitudinal study of urban female sex worker in Kenya (n = 23, PID cases). Additionally, HLA-A2 was reported to elicit cytotoxic T lymphocyte responses to C. trachomatis MOMP in peripheral blood of Chlamydia-infected patients (Kim et al.2000).
Our study showed an increased expression of two chemokines CXCL10 (IP-10) and CXCL11 in women with Chlamydia infertility compared to the women with infertility for other reasons. Chemokines are small proinflammatory molecules that induce migration of leukocytes (Costantini et al.2013). The role of CXCL10 in protective immunity against chlamydial infections has been implicated in murine model infected with C. muridarum (Belay et al.2002). In polarized polA2EN epithelial cells, a reduction of CXCL10 was observed on infection with C. trachomatis (Buckner et al.2013), whereas in A2497 epithelial cells, an upregulation of CXCL10 was observed (Porcella et al.2015). CXCL10 recruits CXCR3 and CCR5 positive leukocytes such as T cells and natural killer cells to the site of infection (Buckner et al.2013). Previous studies on mouse models have shown that these chemokines are predominantly expressed in upper genital tract infection and mediate protection through Th1 or proinflammatory immunity (Belay et al.2002; Maxion and Kelly 2002; Sakthivel et al.2008). Wan et al. (2014) showed that CXCL11 (IFN-inducible T-cell α-chemoattractant or i-TAC) gene expression, stimulated by the interferons (Sánchez-Martín, Sánchez-Mateos and Cabañas 2013), was higher in secretory phase cells on infection with C. trachomatis.
Whilst a trend towards an increase in mRNA levels was observed for IL-1β and TNF-α (not significant), ELISAs detection of the secreted protein showed that secreted IL-1β was significantly higher in Chlamydia positive infertile women than those in Chlamydia negative infertile women (P < 0.05). Supporting the previous findings of Hvid et al. (2007), this study also shows that IL-1 is an important proinflammatory cytokine induced by C. trachomatis infection, and therefore likely an important contributor to pathology from C. trachomatis infection. Here the IL-1β was detected from primary ex vivo culture of PBMC rather than reproductive tract epithelia (as in Hvid et al.2007), suggesting that both the innate epithelial response and innate mononuclear response from some women in response to C. trachomatis infection are proinflammatory and involve IL-1β. Both IL-1β and TNF-α are potent inducers of IL-8, hence high levels of the cytokine were observed in the supernatants of Chlamydia positive and Chlamydia infertile women (Osawa et al.2002).
The limitations of our study include a small sample size that might account for the lack of significance between the cohorts for many factors. The observed differences in responses here for women with Chlamydia infertility compared to women with other causes of infertility support the model that some women launch a more marked proinflammatory response to the infection and thus develop the serious pathology and disease sequelae and these responses may reflect the original response to the infection in the local tissue.
FUNDING
This work was partially funded by a Queensland Government Smart State NIRAP project.
The authors would like to thank the voluntary participants involved in this study. The authors acknowledge the involvement of Ms Helen Woodhouse in participant recruitment. The Wesley Research Institute Tissue Bank was involved in sample collection.
Conflict of interest. None declared.
REFERENCES



