-
PDF
- Split View
-
Views
-
Cite
Cite
Deisy Carolina Rodríguez, Marisol Ocampo, Yahson Varela, Hernando Curtidor, Manuel Alfonso Patarroyo, Manuel Elkin Patarroyo, Mce4F Mycobacterium tuberculosis protein peptides can inhibit invasion of human cell lines, Pathogens and Disease, Volume 73, Issue 3, April 2015, ftu020, https://doi.org/10.1093/femspd/ftu020
Close - Share Icon Share
This work was aimed at studying the Mycobacterium tuberculosis H37Rv Rv3494c protein, taking into account that it belongs to the mammalian cell entry family (mce) which is thought to have important functions in the disease's pathogenesis. The protein was characterized in silico; its presence on mycobacterial surface was confirmed by immunoelectron microscopy. High-activity binding peptides (HABPs) were identified by binding assays with 125I; their ability to inhibit mycobacterial entry to two cell lines (U937 alveolar macrophages and A549 epithelial cells) was ascertained and their role in bacterial entry was confirmed by fluorescent microsphere internalization assay. This protein's predicted alpha-helix structure was confirmed by circular dichroism of its peptides. All HABPs inhibited mycobacterial entry to cells and that the 38379 peptide (201IDQAGPFLQAQIRAGGDIKSY220) had high binding ability and inhibited the mycobacterial entry to both cell lines assayed here. Rv3494c peptides 38370 (21LSVMAIFYLRLPATFGIGTY40), 38373 (81HMRLNSGTAIPSNVTATVRSY100) and 38379 (201IDQAGPFLQAQIRAGGDIKSY220) showed to be HABP and inhibited mycobacterial entry to A549 cells and peptide 38382 (261RPSFPALAASLANLGRVGVIY280) bind to U937 and inhibited the mycobacterial entry to this cell line; all of these sequences play an important role in cell line recognition and invasion, and may thus be considered in the search for prophylactic candidates against tuberculosis.
INTRODUCTION
Tuberculosis (TB) is considered to be one of the primary causes of disease and death worldwide; an estimated 8.6 million new cases occurred in 2012, according to the World Health Organisation; 13% of such cases involved co-infection with HIV and 1.3 million people died from TB, including almost 1 million deaths amongst HIV-negative individuals and 320 000 among HIV-positive people (WHO 2013).
TB is an infectious disease caused by species from the Mycobacterium genus belonging to the M. tuberculosis complex (MTC). Antibiotic therapy can effectively control TB; however, such therapy's effectiveness in controlling the spread of TB has become limited owing to treatment often having to be suspended due to secondary effects and because some geographical locations cannot be reached.
Mycobacterium tuberculosis (Mtb), the bacteria occurring most frequently in tuberculous infection, can infect and survive in macrophages and epithelial and dendritic cells by developing defence mechanisms which affect their cellular functioning. It modulates phagosome maturation in macrophages avoiding the lysosomal mechanism and thus mycobacterial degradation and death (Ernst 2012). It has been described in detail regarding dendritic cells where it infects and affects their function as antigen-presenting cell (Wolf et al., 2007). Mtb can also promote macrophage necrosis and recruitment followed by granuloma formation providing additional niches for bacterial population expansion and thus bacterial dissemination in pulmonary tissue (Davis and Ramakrishnan 2009). It is also a bacterium which can remain dormant (maintaining immunological equilibrium) in the human body for decades and then the bacteria becomes reactivated and progresses [when an individual has some alteration in their immune system, such as a reduction in CD4 cells or blocking of TNF (Ernst 2012)], thereby producing active disease.
Its complexity concerning modulating the immune response, the appearance of multiresistant strains and the unfortunate fact that the only vaccine available today (Mycobacterium bovis bacillus Calmette-Guèrin—BCG) has variable effectiveness makes it urgent and necessary to develop new diagnostic methods, medicaments and a vaccine which is truly effective in totally reducing TB cases around the world (Cosma, Sherman and Ramakrishnan 2003; Garcia-Perez, Castrejon-Jimenez and Luna-Herrera 2012; WHO 2013).
Following the methodology for developing multiantigen, subunit-based synthetic vaccines by identifying high-activity binding peptides (HABPs) derived from conserved regions of proteins involved in the pathogen–host interaction (Patarroyo and Patarroyo 2008), our group has studied the surface proteins which have been suggested as being important in Mtb H37Rv pathogenicity and which have peptide sequences which could be candidates for being included in designing such vaccine (Forero et al., 2005; Garcia et al., 2005; Vera-Bravo et al., 2005; Plaza et al., 2007; Chapeton-Montes et al., 2008b; Patarroyo et al., 2008a,b; Cifuentes et al., 2010; Caceres et al., 2011; Rodriguez et al., 2011, 2012; Ocampo et al., 2012a,b).
On assessment of the genome sequence, it was apparent that four copies of mammalian cell entry family (mce) were present and that these were all situated in operons, comprising eight genes, organized in exactly the same manner. In each case, the genes preceding mce code for integral membrane proteins, whereas mce and the following five genes are all predicted to encode proteins with signal sequences or hydrophobic stretches at the N-terminus. These sets of proteins may well be secreted or surface exposed; this is consistent with the proposed role of mce in invasion of host cells (Cole et al., 1998). The mce operon has been implicated in virulence of pathogenic mycobacteria and mce genes' orthologues are present in all bacteria belonging to these species (Joshi et al., 2006).
Rv3494c (Mce4F) is one of the proteins which have been annotated and identified in the genome as possible external membrane proteins which are secreted outside the mycobacteria and have important functions related to mycobacterial pathogenesis (Cole et al., 1998; Song et al., 2008). It has been detected and identified by mass spectrometry in protein concentrate obtained from guinea pig lungs 30 days after being infected with Mtb H37Rv (during the early but not chronic stage of the disease) (Kruh et al., 2010). It is thought that Mce4F expression is controlled by the KstR|Rv3574 transcriptional regulon playing an important role in lipid catabolism which is necessary for bacterial survival (Kendall et al., 2007; Van der Geize et al., 2007). The above suggests that Mce4F could play an important role in mycobacterial virulence and adaptation regarding infection and could be related to host cell invasion, which is why it has been proposed as being a promising protein for the present study.
MATERIALS AND METHODS
Bioinformatics analysis
The Mtb Mce4F protein sequence was obtained from the NCBI database (http://www.ncbi.nlm.nih.gov/protein/NP_218011.1); bearing previous studies in mind (Restrepo-Montoya et al., 2009; Vizcaino et al., 2010), the following validated bioinformatics tools were used for determining its subcellular localization: feature-based approaches were used SignaIP V3.0 (http://www.cbs.dtu.dk/services/SignalP-3.0/), TatP 1.0 (http://www.cbs.dtu.dk/services/TatP/), LipoP 1.0 (http://www.cbs.dtu.dk/services/LipoP/) and SecretomeP 2.0 (http://www.cbs.dtu.dk/services/SecretomeP/), and general localization approaches were used Gpos-PLoc (http://www.csbio.sjtu.edu.cn/bioinf/Gpos/), PSORTb v 2.0.4 (http://www.psort.org/psortb2/) and TBpred (http://www.imtech.res.in/raghava/tbpred/), TMHMM 2.0 (http://www.cbs.dtu.dk/services/TMHMM/) and Phobious (http://phobius.sbc.su.se/index.html) were used to predict transmembrane helices.
Presence and transcription of mce4f gen in MTC
It has been observed that growth conditions influence the expression of different genes (Kim et al., 2008; Gupta, Srivastava and Srivastava 2010); PCR assays were used for confirming the presence and transcription of the mce4f gene in Mycobacterium spp. strains in normal culture conditions.
All mycobacterial strains were cultured for 5 to 20 days in Middlebrook 7H9 Medium (Difco, New Jersey, USA) supplemented with oleic acid-albumin-dextrose-catalase (Becton Dickinson, BBL; Sparks, MD). Genomic DNA was isolated from mycobacterial species and strains using an Ultra Clean Microbial DNA Isolation Kit (MoBio Laboratories, Inc., Carlsbad, CA) following the manufacturer's instructions. The PCR assay was carried out in a LABNET MultiGen Thermal Cycler (Woodbridge, NJ, USA) by incubating 2-μL genomic DNA with a PCR mixture containing 1unit of BioTaq DNA Polymerase (Bioline, London, UK), 1X Taq Polymerase Reaction Buffer, 1.5-mM MgCl2, 1-mM dNTPs Mix and 1 μM of each primer (direct: 5′-CCGGTTGCTGGTGGATG-3′ and reverse: 5′-TTGTTGGGCGGCAGAATG -3′) in a final 25-μL reaction volume.
The reaction was carried out in the following conditions: an initial denaturing step at 95ºC for 5 min followed by 35 cycles consisting of 1 min at 58ºC, 1 min at 72ºC and 1 min at 95ºC. A final extension step was performed at 72ºC for 5 min. Sense and antisense oligonucleotides amplified a 714 base-pair (bp) fragment which was visualized on a 1% agarose gel stained with SYBR Safe (Invitrogen, Carlsbad, CA). Extracted DNA quality was assessed by amplifying a 360-bp fragment from rpoB (encoding RNA polymerase B subunit). DNase- and RNase-free water was used as negative PCR control.
All amplifications were visualized on 1.5% agarose gel stained with SYBR Safe (Invitrogen, Carlsbad, CA).
Total RNA was isolated from the bacterial pellet by homogenization in 1-mL TRIzol Reagent (Invitrogen), following the manufacturer's recommendations, and then 7 μL were treated with amplification grade deoxyribonuclease I, (Invitrogen). Complementary DNA (cDNA) synthesis was carried out using SuperScript III Reverse Transcriptase (Invitrogen) and random hexamers following the manufacturer's recommendations. A negative synthesis control, replacing the SuperScript III enzyme with DEPC-treated water was included for each sample. 2-μL cDNA were used as template for PCR amplification following the same conditions previously described for DNA. The rpoB gene was used as positive transcription control (Lee et al., 2000).
Expression of the Mce4F protein
Once the presence and transcription of the gene encoding the Mce4F protein had been verified, expression and protein localization in the cell were identified by Western blot analysis of M. tuberculosis H37Rv lysate and subcellular fractions (provided by The Biodefense and Emerging Infections Research Resources Repository—BEI Resources) immunoelectron microscopy (IEM), using sera obtained from four non-reactive rabbits which had been inoculated with 5 mg of either polymerized peptide mixture selected according the tool for predicting linear B epitopes.
BepiPred 1.0 Tool (http://www.cbs.dtu.dk/services/BepiPred/) was used to predict B epitopes and then was confirmed using ANTHEPROT Software (http://antheprot-pbil.ibcp.fr/) and modelling with the PyMOL tool.
Two were inoculated with peptides 37910 (107CGQYID-LVPPENPSSTKLRNGFGC126) and 37913 (414CGRNILPPNK-FPYIPPGADPDPGC433) and two with peptides 37911 (494CGPPPPPPEGTGPPPGPAPGPQGC513) and 37912 (452CGPAPHQ-PAQPAPPPNDNGPPPGC471); they were emulsified with Freund's incomplete adjuvant. These sequences were derived from the results obtained using the BepiPred 1.0 tool.
Western blot involved separating the proteins from total sonicate and each mycobacteria subcellular fraction using 12% SDS-PAGE and then transferring them to a nitrocellulose membrane. Sera obtained post-third inoculation was used in 1:100 dilution and pre-immune sera was used as control.
The protein's subcellular localization was determined by IEM using a CM 10 Transmission Electron Microscope (Hitachi HU-12A operated at 100 kV) with thin sections (400 nm) of mycobacteria embedded in LR white resin, rabbit anti-peptide sera as primary antibody and anti-rabbit IgG coupled to 10-nm colloidal gold particles as secondary antibody. Sections were stained with 6% uranyl acetate to enhance image contrast (Stirling and Graff 1995). Pre-immune sera were used as control.
Determining HABPs
Twenty-nine, non-overlapping, 20-mer-long peptides, spanning the entire Rv3494c sequence, were synthesized by solid-phase peptide synthesis (Houghten 1985), purified by RP-HPLC and analysed by MALDI-TOF MS using α-cyano-4-hydroxycinnamic acid matrix and then 125I-radiolabelled, according to previously described techniques (Yamamura, Enna and Kuhar 1978; Ocampo et al., 2012a). A tyrosine residue was added to the carboxyl-terminus of those peptides not containing this residue in their sequence to enable radiolabelling with Na125I.
Peptides were tested for their ability to bind to macrophages derived from U937 monocytes (ATCC CRL-2367) and the A549 alveolar cell line (ATCC CLL-185). U937 cells were differentiated using 200-nM phorbol 12-myristate 13-acetate for 3 days to become adherent. Both adherent cell lines were grown in RPMI 1640 medium supplemented with 10% heat-inactivated foetal bovine serum and kept at 37°C in 5% CO2.
1.5 × 106 cells cultured in Roux flasks were dislodged using 0.3% trypsin, 0.017-mM EDTA and then incubated with increasing 125I-radiolabelled peptide concentrations (0–950 nM) in the absence (total binding) or presence (non-specific binding) of 40-μM unlabelled peptide. Unbound peptide was removed using a dioctylphthalate–dibutylphthalate cushion (d = 1 015 g ml−1), before using a gamma counter (Gamma Counter Cobra II, Packard Instrument Co., Meriden, CT, USA) for measuring cell-associated radioactivity.
Total binding minus non-specific binding yielded the specific binding curve, whose slope corresponded to peptide-specific binding activity. Any peptide with specific curve slope over 1% was considered to be HABP (Forero et al., 2005; Garcia et al., 2005; Vera-Bravo et al., 2005; Plaza et al., 2007; Chapeton-Montes et al., 2008b; Patarroyo et al., 2008a,b; Cifuentes et al., 2010; Caceres et al., 2011; Rodriguez et al., 2011, 2012; Ocampo et al., 2012a,b).
Structural characterization of Mce4F peptides
The protein's tertiary structure was predicted using Robetta software (http://robetta.bakerlab.org/) via searching the PSIpred, SAM and Jufo servers and validated using the Swiss Model protein homology-modelling server (http://swissmodel.expasy.org/).
On the other hand, secondary structure elements of peptides derived from Mce4F protein were studied by circular dichroism (CD). Peptides were dissolved in 30% trifluoroethanol in water and the spectrum was acquired at 20°C by averaging three scans taken on a Jasco J-810 spectropolarimeter, using a 1.00-cm pathway cuvette (Jasco Inc, Easton, MD); this was corrected for baseline subtraction (260–190-nm wavelength range, 20 nm min−1 scan rate, 1-nm bandwidth) (Sreerama, Venyaminov and Woody 1999).
The results were expressed as mean residue ellipticity [Θ], units being degrees × cm2 × dmol, according to [Θ] = Θλ/(100lcn), where Θλ was measured ellipticity, l was the optical path length, c the peptide concentration and n the number of amino acid residues in a particular peptide sequence.
Invasion inhibition assays
Rv3494c HABPs were assessed for their ability to inhibit mycobacterial invasion using a flow cytometry-based assay (Bermudez and Goodman 1996; Chapeton-Montes et al., 2008a). Briefly, A549 and U937 cells (2.5 × 105 per well) adhered to 24-well plates overnight were then incubated with different peptide concentrations (2, 20 and 200 μM) for two h. SYBR-safe stained mycobacteria (2.5 × 107) suspended in RPMI medium were added to each well (1:10 MOI) and incubated overnight at 37°C. Inhibition control consisted of 100 μM cytochalasin B. Extracellular bacilli were removed by washing with Hank's balanced salt solution (HBSS) pH: 7.2 and cells were dislodged from monolayers and fixed with 4% p-formaldehyde. The samples were stained with methylene blue for FAC Scan flow cytometry analysis (Becton Dickinson); 5000 events were collected for determining the percentage of SYBR-safe positive corresponding to infected cells detected on the FL1 channel. Data were statistically analysed using a Student's t-test (P), correlating the distribution of data regarding each treatment with invasion control.
The viability assay involved using an in vitro Toxicology Assay Kit (TOX-8, Sigma) after the cells had been incubated with differing peptide concentrations for 2 h.
Internalization of fluoresbrite carboxylate (GY) microspheres
An internalization assay concerning A549 cell uptake of GY fluorescent microspheres (1.0 Micron Microspheres from Polysciences, Inc) was based on the procedure described by El-Shazly (El-Shazly et al., 2007); it was standardized by our group, following the manufacturer's instructions. Briefly, GY microspheres (2.5 × 106) were coated with the chosen Rv3494c peptides (at 2, 20 and 200 μM) by incubation in PBS1X at 37°C for 1 h and then suspended in incomplete RPMI medium. Peptide-coated microspheres and 2.5 × 105 A549 cells (previously adhered to 24-well plates overnight) were incubated for 1 h at 37°C, MOI 1:10 cell microspheres. The supernatant was removed and cells were washed thrice with HBSS to eliminate extracellular microspheres and then detached using 0.3% trypsin and 0.017-mM EDTA solution. Cells were spun at 1000 × g for 5 min and suspended in 200-μL PBS for reading by flow cytometry. Five thousand events were collected for determining the FITC positive percentage of internalized microspheres detected on the FL1 channel.
As control, 2.5 × 105 A549 adhered cells were incubated with peptides (2, 20 and 200 μM); GY microspheres (2.5 × 106) were then added without peptide to differentiate the peptide's effect on these cells, i.e. to determine whether microsphere internalization was induced by peptide coating the latex GY microspheres or by the peptide alone. GY microspheres without peptide were also incubated with A549 adhered cells as negative control.
Counting in a Neubauer chamber in trypan blue-stained 1:1 dilution using fluorescence microscopy was used for confirming that flow cytometry count really corresponded to internalized microspheres.
RESULTS
Bioinformatics analysis
The 59 646.8 Da molecular weight, 564 amino-acid-long Mce4F protein was predicted as being an extracellular or cytoplasmic membrane protein (Table 1), according to the results obtained using bioinformatics tools to predict bacterial secreted proteins based on a general localization approach (TBpred, Gpos-Ploc and PSORTb software), since the scores obtained for the membrane were 90% higher than those obtained for the cytoplasm. Higher than 0.5 values were obtained in signal peptide presence and secretion type analysis as acceptation threshold for all the tools used. Regarding the presence of a signal sequence, SignalP 3.0 software predicted that the protein had a signal sequence for the classical route, having cleavage sites between residues 25 and 26 and other between 44 and 45 with alanine in position 1 following cleavage, even though not presenting another alanine in position −3 of the cleavage sequence, as would have been expected for type 1 signal peptides for the classical route. SignalP 4.0 analysis confirmed the signal sequence having cleavage sites in amino acids 25 and 26 and predicted that this was not a transmembrane sequence. However, TatP1.0 analysis determined that there was no double-arginine signal sequence, thereby confirming that predicted by SignalP 3.0, whereas LipoP 1.0 predicted a transmembrane helix and thus rejecting a characteristic lipoprotein signal sequence. Despite containing a signal sequence, it was also found that SecretomeP 2.0 predicted that the protein would be secreted also by non-classical route. The TMHMM 2.0 server predicted transmembrane helices, meaning that the protein's amino acids 1 to 11 would be cytoplasmatic, 12 to 34 would form a transmembrane helix and residues 35 to 564 would be exposed to the cell's exterior. A similar result was obtained when using the Phobious tool which indicated a transmembrane helix from residue 12 in cytoplasm to 31 in the non-cytoplasmatic region.
Features of Rv3494c protein related to subcellular localization prediction and major characteristics.
 |
 |
(1) SecP score, a value above ≥0.5 indicates possible non-classical secretion.
(2) In Phobius prediction: TM: the number of predicted transmembrane segments. SP: indicator if a signal peptide was predicted or not. PREDICTION: predicted topology of the protein.
(3) In TMHMM prediction: ExpAA THMs: the expected number of amino acids intransmembrane helices. First 60: the expected number of amino acids in transmembrane helices in the first 60 amino acids of the protein. PredHel: the number of predicted transmembrane helices by N-best. Topology: the topology predicted by N-best.
Features of Rv3494c protein related to subcellular localization prediction and major characteristics.
 |
 |
(1) SecP score, a value above ≥0.5 indicates possible non-classical secretion.
(2) In Phobius prediction: TM: the number of predicted transmembrane segments. SP: indicator if a signal peptide was predicted or not. PREDICTION: predicted topology of the protein.
(3) In TMHMM prediction: ExpAA THMs: the expected number of amino acids intransmembrane helices. First 60: the expected number of amino acids in transmembrane helices in the first 60 amino acids of the protein. PredHel: the number of predicted transmembrane helices by N-best. Topology: the topology predicted by N-best.
mcef gene is present and transcribed in MTC
A 360-bp band was observed in all strains by PCR, corresponding to the rpoB gene and thereby validating PCR experiments’ DNA integrity (Fig. 1A); a 714-bp band was amplified by PCR from Mtb complex (MTC) species’ gDNA using rv3494-specific primers (Fig. 1B). Amplification was observed in Mtb H37Rv (ATCC 27294) and Mtb H37Ra (ATCC 25177) strains and in M. bovis (ATCC 19210), M. bovis BCG (ATCC 27291) and M. africanum (ATCC 25420).
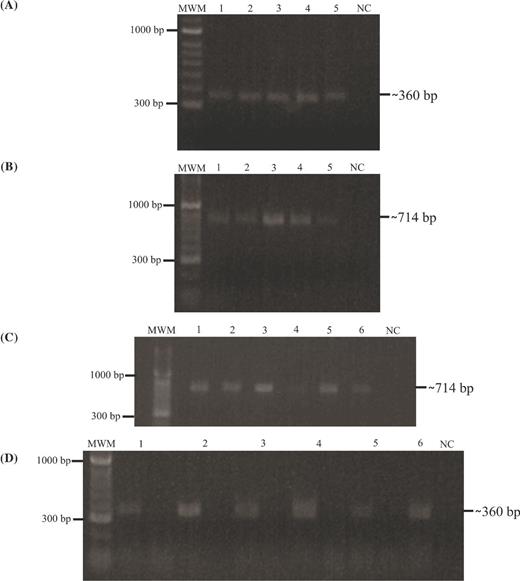
rv3494c gene presence and transcription. MWM: 100-bp molecular weight marker; 1. M. tuberculosis H37Rv; 2. M. tuberculosis H37Ra; 3. M. bovis; 4. M. bovis BCG; 5. M. africanum; 6. Positive PCR control (M. tuberculosis H37Rv gDNA). NC: negative PCR control. (A)rpoB gene amplification from gDNA; (B)rv3494 PCR product amplified from genomic DNA isolated from different mycobacterial species and strain; (C)rpoB gene amplification from cDNA. Left line: plus synthesis and right line: minus synthesis; NC: negative PCR control. (D)rv3494 gene amplification from cDNA of the samples included in this study.
The amplification of the rpoB constitutive gene from plus synthesis cDNA was used as transcription control for each mycobacterial strain. The 714-bp amplification fragment observed in plus synthesis for cDNA indicated that the rv3494 gene was being transcribed in all strains under 7H9 culture medium conditions (Fig. 1C) and the absence of product in the minus synthesis confirmed that there was no gDNA contamination (Fig. 1D)
Detecting Mce4F protein expression
ANTHEPROT software was used for corroborating the selection of linear B epitopes, taking into account that the sequence had to have at least eight favourable contiguous amino acids and surpass hydrophilicity (50), flexibility (−0.1) and solvent accessibility (0.62) validity thresholds. The selected peptides had to fulfil prediction by both tools (Bepipred 1.0 and ANTHEPROT); this was then corroborated using PyMOL software to see whether they were protein surface-exposed peptides in a tertiary structure model obtained in silico. The sequences selected as being better B-epitopes were 107QYIDLVPPENPSSTKLRNGF126, 414RNILPPNKFPYIPPGADPDP433, 494PPPPPPEGTGPPPGPAPGPQ513 and 452PAPHQPAQPAPPPNDNGPPP471.
The sera obtained post-third inoculation from mixtures of polymer sequences from the protein were assayed by Western blot; a characteristic band of around 57 kDa was found close to that expected (i.e. 59.6 kDa) for Rv3494c in the membrane and in cell wall fractions, thereby confirming that predicted by bioinformatics analysis regarding its localization on mycobacterial membrane (Fig. 2A). The decreased molecular weight could have been due to cleavage between amino acids 25 and 26, corresponding to signal sequence, as predicted bioinformatically, since such region's molecular weight is 2.75 kDa (1MIDRLAKIQLSIFAVITVITLSVMA25). Two additional weak bands were observed at 20 kDa (Fig. 2A, lanes 4 and 5) in cytosolic and supernatant fractions, possibly due to proteolytic processing of this protein.
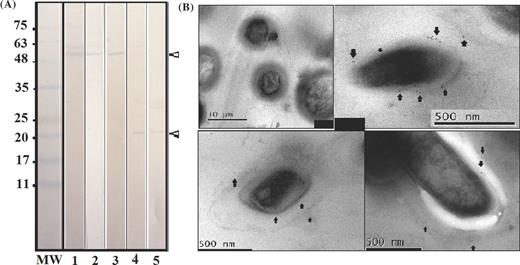
Rv3494c protein recognition. (A) Western blot with rabbit post-third immune serum against M. tuberculosis H37Rv total sonicate (lane 1) and subcellular fractions (lanes 2–5); lane 2 shows M. tuberculosis H37Rv membrane, lane 3 M. tuberculosis H37Rv cell wall, lane 4 the M. tuberculosis H37Rv cytosolic fraction and lane 5 M. tuberculosis H37Rv culture supernatant. The molecular weight marker is shown on the left-hand side of the figure. (B) IEM, upper-left panel: IEM of M. tuberculosis using pre-immune sera. IEM of M. tuberculosis using post-third inoculation sera; the arrows show the presence and location of colloidal gold-labelled antibodies recognizing the Rv3494c protein.
IEM confirmed the presence of Mce4F on mycobacterial surface. Fig. 2B shows pre-immune control and post-third inoculation sera, arrows indicate 10-nm gold particles corresponding to secondary antibody recognition. Consistently in different sections, labelled particles on bacterial surface confirmed this protein's existence on cell envelope; they were seen on the most external part possibly wall and bacterial membrane.
Determining HABPs
An assay involving radiolabelled peptide binding in the absence (total binding) and presence (non-specific binding) of non-radiolabelled peptide facilitated selecting peptides having HABPs. To be selected as such, they had to obtain a score greater than 1% on the specific binding graph slope regarding added peptide (according to previously established criteria). Peptides 38370, 38371, 38373 and 38378 had high specific binding to the A549 cell line and peptide 38377, as well as a region including peptides 38381 to 38384, had high U937 binding activity. Peptide 38379 was of special interest as it had high binding to both cell lines, possibly recognizing a common receptor on cell surface; however, further studies are needed for establishing these HABPs’ receptors on target cells. Fig. 3 shows the sequences for the 20-aminoacid-long peptides forming the protein, their code and position in the protein and binding results.
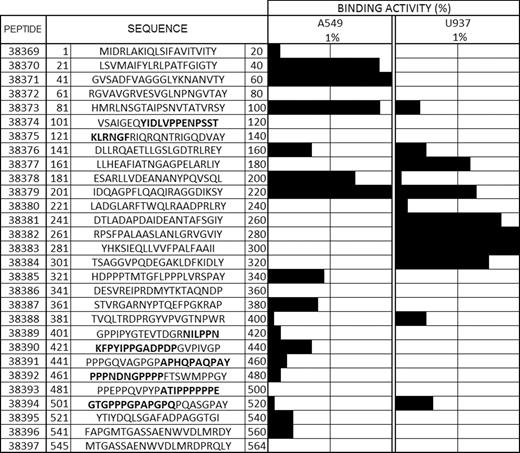
HABPs. The first column refers to the consecutive peptide synthesis number assigned by our institute. The second column shows the amino acid sequence corresponding to each 20-amino-acid-long peptide forming the Rv3494c protein, indicating its position in the sequence. Tyrosine (Y) was added to those peptides not containing it in their sequences. Binding activity is shown by horizontal black bars and the sequences shown in bold are those predicted as being B-epitopes.
A549 type II pneumocytes and U937 monocyte-derived macrophages were used in this study as an in vitro model of invasion, due to M. tuberculosis H37Rv ability to invade both types of cell. U937 cells were used to simulate the mycobacterial colonization of the alveolar space by means of internalization and replication inside macrophages. A549 cells allowed studying a more advanced stage of infection in vitro, which involved not only alveolar monocytes and macrophages but also epithelial and endothelial cells, this being a crucial step for mycobacterial dispersion to the bloodstream and other organs (Mehta et al., 1996).
The M. tuberculosis H37Rv reference strain was also used in mycobacterial invasion assays regarding all experimental procedures (i.e. immunodetection and invasion assays), given that this strain produces full virulence in animal models of TB (Kubica, Kim and Dunbar 1972) and its use ensures that results are obtained which will be comparable and consistent with future trials in animal models.
Structural features of Mce4F protein and peptides
Regarding structural characteristics, Fig. 4A shows the Ramachandran plot for verifying amino acid distribution throughout the model obtained in silico for Rv3494c. It shows that the 3D structure consisted of α-helices in residues 1 to 30 and 140 to 300. Folded β-sheets were also predicted between amino acids 40 and 130, and 314 and 320. Low-complexity, undefined structures predominated from 321 onwards (Fig. 4C) and Fig. 4B shows the final 3D model.
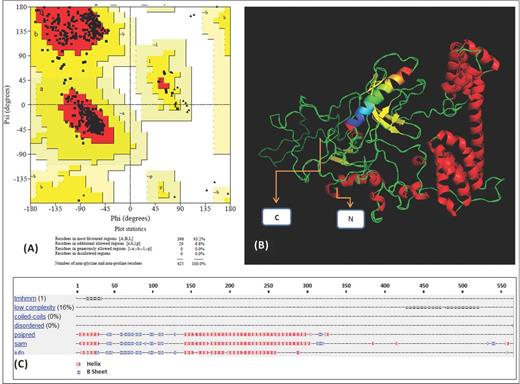
Rv3494c protein structure prediction: (A) Ramachandran plot obtained using Swiss Model software. (B) 3D model of the protein: the β-sheets are drawn as yellow arrows and the helixes are shown in red. The coloured helix (amino acids 12–34) is the transmembrane helix predicted signal peptide. (C) Structural prediction using the Robetta server; a red X indicates the presence of helixes and a blue X the β-sheets.
Bearing in mind the relationship between peptide structure and function as a ligand, Rv3494c structure was analysed by CD from the peptides forming it; peptides were selected and grouped according to their spectrum shape and having a greater than 0.5 deconvolution analysis result given by at least two of the program used (SELCON, CDSSTR and CONTINLL) (Sreerama and Woody 2000). The α-helix regions covered amino acids 1 to 20 and 40 to 60 (peptide 38369 and HABP 38371), 141 to 220 (peptides 38376 and HABPs 38377 to 38379), 261 to 300 (HABPs 38382 and 38383) and 521 to 564 (peptides 38395 to 38397). A β-sheet was defined from amino acid 20 to 40 (HABP 38370), even though part of it was helical. The other residues’ structure could not be determined or was a turn (Fig. 5 shows the spectra). The α-helical and undefined structure region predicted by bioinformatics analysis (Fig 4) was thus confirmed (this did not happen with the β-sheets). A conformational–functional correlation between HABPs could not be determined.
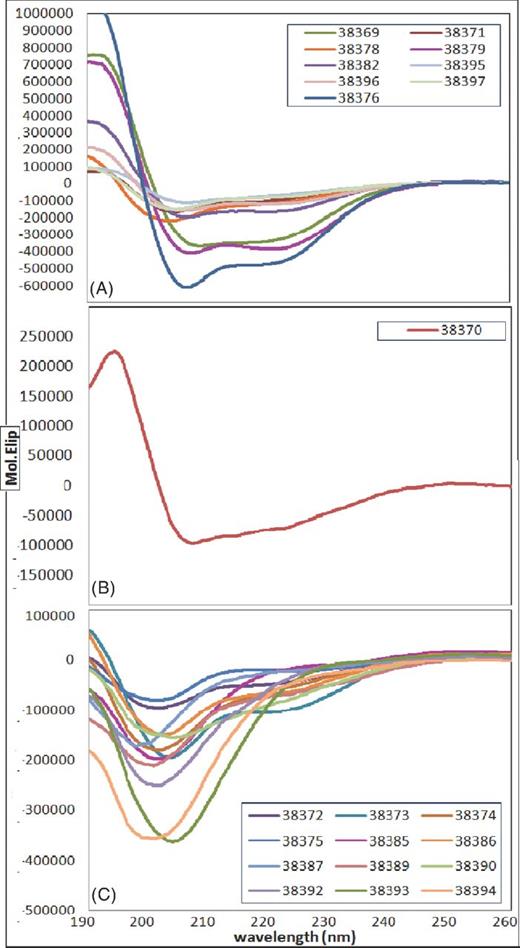
CD of Rv3494c protein peptides: (A) regions having a helix-type structure, (B) β-sheet regions and (C) turn and undetermined structure regions.
Invasion inhibition assays
Peptides identified as being HABPs for each cell line (HABP 38370, 38371, 38373, 38378 and 38379 for A549 and 38377, 38379, 38381, 38182, 38383 and 38384 for U937) as well as two peptides which did not have specific binding (38383 for A549 and 38385 for U937 cells) as negative control were assayed in three logarithmically ascending concentrations for determining their ability to inhibit mycobacterial entry to A549 and U937 cell lines. It had already been verified that no peptide was cytotoxic regarding the target cells, following incubation for 2 h at different peptide concentrations.
Fig. 6 shows that all the HABPs inhibited mycobacterial entry to the respective cell lines; peptides 38379 and 38382 had 5–15% and 35–60% concentration-dependent inhibition in the U937 cell line (at 2, 20 and 200 μM, respectively).
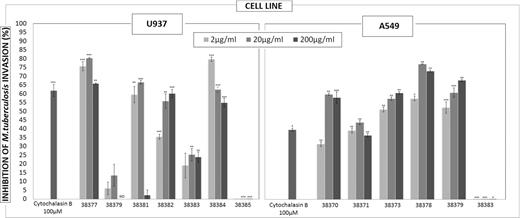
Invasion inhibition assay: inhibition percentages using different peptide concentrations; cytochalasin B was used as control. The results are given as the average inhibition percentages calculated for each treatment. Student's t-test is represented by asterisks *≤ 0.05 P-value, **≤ 0.01 P-value and ***≤ 0.001 P-value.
Peptides 38373 and 38379 inhibited concentration-dependent invasion of the A549 cell line, reaching 60 to 70% at 200 μM and thereby surpassing the result obtained by controlling inhibition with cytochalasin B. Peptide 38385 having low specific binding to U937 cells and 38383 to A549 cells were used as negative controls; they did not inhibit mycobacterial entry.
Peptides having an increasing concentration-dependent inhibition tendency would thus seem to play an important role in both recognition and inhibiting mycobacterial entry. Peptide 38379 which is a HBAP for U937 and A549 cells seemed to have an important role in invading both cell lines since inhibition was concentration-dependent in both cases.
YG microsphere internalization assay
HABPs for A549 cells (38370, 38371, 38373, 38378 and 38379) and peptide 38384 as negative control (and not binding to this cell line) were used in internalization assays involving microspheres for determining peptides ability to mediate microsphere internalization in A549 cells and identify a possible correlation with their ability to inhibit mycobacterial entry to host cells. As control, the assay involved binding peptides to cells and then adding untreated microspheres to confirm that such internalization really resulted from peptides binding to particles and not from peptide action on the cells. Fig. 7A shows the results; peptide 38378 and 38371 led to concentration-dependent internalization, even though there was a really significant difference for 38371 and 38370 regarding control, suggesting that it plays a specific role during cell invasion. Fluorescence microscopy led to establishing that more cells internalize microsphere-coated peptide and there are more beads per cell as this increases peptide coupled concentration. Fig. 7B gives an example of microsphere internalization seen by fluorescence microscopy for counting in a Neubauer chamber, as well as flow cytometry plots.
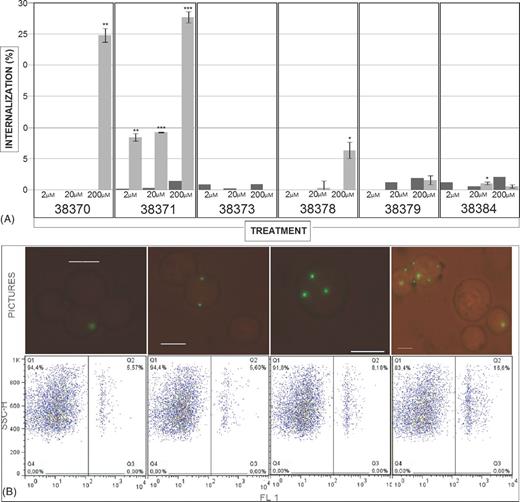
Microsphere internalization assay: (A) percentage of peptide-coated microspheres internalized by A549 cells (clear bars). A549 cells were independently incubated with peptides and then with uncoated microspheres as control treatment (dark bars). The results are given as average internalization/uptake calculated for each treatment ± SD. *≤ 0.05 P-value, **≤ 0.01 P-value and ***≤ 0.001 P-value, according to a two-tailed Student t-test. (B) The upper panel shows Neubauer chamber count using a fluorescence microscope at 1:1 dilution with trypan blue staining. Fluorescent microspheres are well defined within cells and can be seen as fluorescent green dots. The lower panels show density plots for internalization/uptake assay using 38378 HABP-coupled microspheres (2, 20 and 200 μM). The first panel shows non-treated microspheres.
DISCUSSION
The study of microbial Mtb surface molecules is important because they could be involved in specific pathogen–host interactions and could also regulate an immune response. Our group has identified short mycobacterial protein fragments using synthetic peptides having high-affinity interaction with alveolar epithelial cells (A549) and monocyte-derived macrophages (U937) which have blocked the microorganism's entry to target cells in in vitro assays. Such approach has led to about 18 proteins being characterized to date (Ocampo et al., 2013).
The presence of four mce operons in Mtb suggests the essentiality of the functions of the genes of these operons. The differential expression of mce operons reported suggests a probable reason for the apparent redundancy of this operon, mce4 is expressed in both the lung tissues of rabbits and the spleens of guinea pigs following infection, as well as in culture at day 20 (Kumar, Bose and Brahmachari 2003). It has been shown the potential role of mce4 operon in the long-term survival of Mtb and maintenance of the disease TB in infected animals.
This article has thus studied the mce4 (Rv3494c) protein which has been predicted to play an important role in pathogen entry to and survival inside a host cell. The 714-bp band amplified by PCR from mycobacterial complex gDNA and amplification fragment observed in plus synthesis for cDNA showed presence and transcription in all of species belong to MTC tested in this study.
Once the presence and transcription of Rv3494c in the Mtb H37Rv strain from our laboratory cultured under normal conditions has been confirmed, it is necessary to determine its expression. Western blot and electron microscopy results confirmed the presence of the protein on the cell envelop; this position allows it to come into contact with cells during infection so as to fulfil its function. Binding assays involving radiolabelled peptides led to recognizing which protein fragments specifically bound to both cell lines.
It was found that all HABPs inhibited mycobacterial invasion and that for some of them such inhibition was concentration dependent, our non-HABPs controls did not inhibit it. HABPs which were able to inhibit mycobacterial entry in a concentration-dependent manner could then be used for determining which amino acids were critical for binding to target cells and which could then be modified in an attempt to design good candidates for developing a synthetic vaccine (according to our research group's experience).
Regarding the U937 cell line, peptides 38377, 38381, 38382 and 38384 inhibited invasion, by a similar percentage to that for the cytochalasin control reached its maximum inhibition (∼80%) at 20 μM, whereas only 38382 peptide having a concentration-dependent tendency.
HAPBs 38373 and 38379 inhibited mycobacterial entry to the A549 cell line by more than 50% (having a concentration-dependent tendency); peptides 38370, 38371 and 38378 reached maximum inhibition at 20 μM. The inhibition and microsphere internalization results led to deducing which of these peptides played an important role in internalization. As in the inhibition test, the internalization assay showed that A549 cells internalized a greater percentage of microspheres coupled to peptides 38370, 38371 and 38378 compared to controls; the negative result regarding cytotoxicity and control cells pre-treated with peptides confirmed that internalization was not caused by damage within a cell and, being concentration dependent, meant specific peptide-receptor binding. Peptide 38370 corresponded to the signal peptide sequence and transmembrane region which could have involved difficulties in interacting with target cells during infection. Peptides 38373 and 38379, which had an inhibitory role in mycobacteria entry, were not shown to have any effect on the internalization of microspheres coated with these peptides, thereby ruling out any type of role regarding infection.
A common structural characteristic could not be established for Rv3494c peptides having high specific binding and mycobacterial entry inhibition ability. However, structural characterization of the peptides of interest led to modifying them, bearing in mind the results of previous studies (Patarroyo et al., 2010; Patarroyo, Bermudez and Patarroyo 2011)
In conclusion, bearing pathogen–host cell interaction in mind, it was found that the Rv3494c protein was exposed on bacterial surface; it is thus feasible that it could be in contact with human host cells during infection. It was found that only one alpha helix structured 38379 (201IDQAGPFLQAQIRAGGDIKSY220) Rv3494c-derived HAPB played an important role inhibiting the mycobacterial entry, as it bound specifically to prototype cell lines (alveolar epithelial cells A549 and monocyte-derived macrophages U937).
Further studies are thus needed for determining the sequences’ antigenic or immunogenic properties, and according to what has been proposed by other models, modifications to sequences may be required concerning such sequences’ structure to allow them to become included in designing a minimal subunit-based, multiepitope, chemically-synthesized anti-TB vaccine.
We would like to thank Jason Garry for translating and thoroughly revising the manuscript. Mycobacterium tuberculosis H37Rv strain subcellular protein fractions were obtained through the National Institute of Health (NIH) Biodefense and Emerging Infection Research Resources Repository, National Institute of Allergy and Infectious Diseases (NIAID).
FUNDING
This research was supported by the ‘Instituto Colombiano para el Desarrollo de la Ciencia “Francisco José de Caldas”’ (COLCIENCIAS) through contract 709-2013.
AUTHORS’ CONTRIBUTIONS
DCR and MO wrote the manuscript and carried out the data analysis and interpretation. MO, YV, HC, MAP and MEP contributed to the methodological design, supervised its development and critically revised the manuscript's content. MO supervised the research group.
Conflict of interest statement. None declared.
REFERENCES



