-
PDF
- Split View
-
Views
-
Cite
Cite
Mignane B. Ka, Françoise Gondois-Rey, Eric Ghigo, Didier Raoult, Daniel Olive, Jean-Louis Mege, Imbalance of circulating lymphoid cells in Q fever endocarditis, Pathogens and Disease, Volume 73, Issue 2, March 2015, Pages 1–3, https://doi.org/10.1093/femspd/ftu010
Close - Share Icon Share
Q fever endocarditis is characterized by a defective cell-mediated immune response, which may be associated with the dysregulation of circulating subsets of immune cells. In this study, we found that naïve CD8+ T lymphocytes and CD56dim natural killer cells were decreased patients whereas central memory CD8+ T lymphocytes were increased. It is likely that these different subsets of immune cells play a role in the immunosuppression accompanying Q fever endocarditis.
Q fever is an infectious disease caused by Coxiella burnetii. Endocarditis is the major clinical manifestation of chronic Q fever. Endocarditis Q fever was characterized by a defective cell-mediated immune response. Q fever endocarditis is characterized by a defective cell-mediated immune response, and tissue granulomas are replaced by lymphocyte infiltration (Capo and Mege 2012). This defective immune response is often associated with an exacerbated inflammatory response and the increased production of IFN-γ in response to bacterial antigens (Penttila et al., 1998; Schoffelen et al., 2013).
T cells are essential for the elimination of C. burnetii in acute Q fever (Andoh et al., 2003, 2007; Kishimoto et al., 1978). Surprisingly, CD8+ T cells appear to play a significant role in controlling splenomegaly, a marker of a host response to infection (Read et al., 2010). Natural killer (NK) cells may also play an important role in C. burnetii infection (Andoh et al., 2007). In contrast, γδ T cells are likely not required for controlling C. burnetii infection (Read et al., 2010). In humans, the role of lymphoid compartments in the chronic evolution of C. burnetii infection is not well understood. In this study, we investigated CD8+ T lymphocyte subsets: NK cells subsets and γδ T cells in patients with Q fever endocarditis by flow cytometry. We found that central memory (CM) CD8+ T cells were increased, whereas naïve CD8+ T cells and CD56dim NK cells were decreased. Such an imbalance of circulating lymphoid cells may play a major role in the chronic evolution of Q fever.
The study was conducted with the approval of the Ethics Committee of Aix-Marseille University after obtaining informed and written consent from each participant. The diagnosis of Q fever endocarditis was based on standardized questionnaires that included pathological evidence of endocarditis, positive echocardiogram, positive blood culture and high titers of IgG specific for phase I C. burnetii (Raoult 2012). The patients with Q fever endocarditis consisted of 12 men (ranging from 48 to 82 yr old) and 5 women (49 and 54 yr old). Ten age- and sex-matched individuals were included as healthy controls. Peripheral blood mononuclear cells (PBMCs) were obtained after centrifugation on Ficoll cushions. PBMCs were cryopreserved using 10% DMSO and frozen in liquid nitrogen. PBMC samples were incubated with fluorochrome-conjugated monoclonal Abs. Flow cytometry was performed using an LSRII-SORP cytometer (Becton Dickinson). The data were analyzed using FlowJo software (version 9.2, Tree Star Ashland, OR, USA). The gating strategy to explore circulating lymphoid cells is presented in Fig. 1A. The results, expressed as the percentage of living cells and the total number of analyzed cells, were compared with the Mann–Whitney U test. Statistical analyses were performed using GraphPad-Prism software (version 5.0) and differences were considered significant when P value < 0.05.
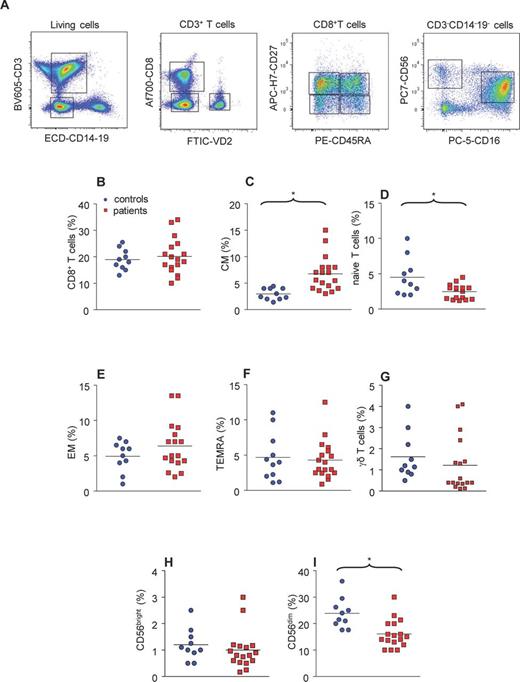
CD8+ T cells in patients with Q fever endocarditis. (A) PBMCs were gated according to their size and after exclusion of dead cells; CD3+ T cells were analyzed according the expression of CD8 and VD2. The CD3+ T cells that expressed VD2 were considered as γδ T cells. CD8+ T cells were analyzed according to the expression of CD27 and CD45RA, resulting in four subsets: naïve, CM, EM and TEMRA T cells. The CD3−CD14−CD19− population was analyzed according the expression of CD56 and CD16. CD56+ cells are considered as NK cells, and two different subsets of NK cells were evidenced according to the expression of CD56 and CD16. (B–I) The CD8+ T cell, NK cells and γδ T cells from 10 healthy controls and 17 patients with Q fever endocarditis were analyzed with flow cytometry and the relative percentage of each population was calculated. The non-parametric Mann–Whitney U test was used to compare patient and control groups. (B) Total CD8+ T cells; (C) CM CD8+ T cells; (D) naïve CD8+ T cells; (E) EM CD8+ T cells; (F) TEMRA CD8+ T cells; (G) γδ T cells; (H) NK cells that highly expressed CD56; (I) NK cells that poorly expressed CD56. *P < 0.05 was considered significative.
As murine models of C. burnetii infection have suggested that CD8+ T cells play a critical role in the control of infection, we determined the total number of circulating CD8+ T cells in patients with Q fever endocarditis. Approximately 20% of PBMCs were CD8+ T cells in the Patients and controls (Fig. 1B). The analysis of different subsets of CD8+ T cells according to the expression of CD27 and CD45RA showed that the proportion of these subsets was dramatically altered in Q fever endocarditis. Indeed, the percentages of CM CD8+ T cells (Fig. 1C) and naïve CD8+ T cells (Fig. 1D) were significantly (P = 0.0004 and 0.0284, respectively) increased and decreased, respectively, in patients with Q fever endocarditis. In contrast, the percentages of effector memory (EM) (Fig. 1E) and terminally differentiated effector (TEMRA) (Fig. 1F) CD8+ T cells were not affected. These results demonstrate that the composition of CD8+ T cell subsets is altered in Q fever endocarditis despite an apparent maintenance of the total CD8+ T cell population.
The modulation of unique types of lymphocytes, such as γδ T cells and NK cells, in C. burnetii infection is poorly understood, and we took the advantage of our gating strategy to explore this with flow cytometry. First, we studied the circulating population of γδ T cells. We found that this population was not affected in patients with Q fever endocarditis compared to healthy controls (Fig. 1G). Second, we evaluated the impact of Q fever endocarditis on NK cell populations and found that the minor subset of NK cells (CD56bright NK cells) was not affected in Q fever endocarditis (Fig. 1H). In contrast, the major subset of NK cells (CD56dim NK cells) was significantly (P = 0.002) decreased in Q fever endocarditis (Fig. 1I).
In summary, we found that the total number of CD8+ T cells was similar in patients with Q fever endocarditis and healthy controls. However, when we examined the relative proportion of the different subsets of CD8+ T cells, we found changes in these subsets. Indeed, the number of EM and TEMRA CD8+ T cells was similar in patients with Q fever endocarditis and healthy controls. In contrast, naïve CD8+ T cells were decreased and CM CD8+ T lymphocytes were increased in Q fever endocarditis. It has been shown that the number of naïve CD8+ T cells is decreased and the number of TEMRA CD8+ T cells was increased in patients with HIV infection (Rosignoli et al., 2009). Similarly, patients with hepatitis C virus infection compared with healthy controls are characterized by a decreased number of naïve CD8+ T cells and an increased number of TEMRA CD8+ T cells (Shen et al., 2010). This suggests that Q fever endocarditis shares a decreased naïve CD8+ T cell distribution with other chronic infectious diseases, though the increase in CM CD8+ T cells appears to be specific.
To our knowledge, the populations of circulating γδ T cells and NK cells in Q fever endocarditis have not been investigated to date. We found that the number of circulating γδ T cells was not modified in Q fever endocarditis compared with healthy controls. In addition, it is generally accepted that γδ T cells do not play a critical role in the control of C. burnetii infection, as shown in murine models of Q fever (Read et al., 2010). NK cells may be involved in the severity of the lesions induced by C. burnetii infection, as found in mice (Andoh et al., 2007). We showed that CD56dim NK cells represented more than 20% of the total number of circulating immune cells. We found that the number of CD56dim NK cells was significantly decreased in Q fever endocarditis whereas the number of CD56bright NK cells, which represents a small proportion of NK cells, was not affected. It has been recently shown that the number of circulating CD56dim and CD56bright NK cells is decreased in sepsis patients compared with healthy controls (Souza-Fonseca-Guimaraes et al., 2012). The decrease in NK cells may have an impact on the efficiency of IFN-γ-mediated immunity against C. burnetii infection.
Our results showed that only the determination of specific subsets of circulating lymphoid cells may be pertinent for analyzing the immune response in Q fever endocarditis. This response was characterized by a subtle imbalance that included a decreased number of naïve CD8+ T cells and CD56dim NK cells, whereas CM CD8+ T cells were increased. It is likely that this imbalance of circulating lymphoid cell subsets plays a major role in the chronic evolution of Q fever.
Conflict of interest statement. None declared.
REFERENCES



