-
PDF
- Split View
-
Views
-
Cite
Cite
Agatha E. Wieczorek, Jolynn L. Troudt, Phillip Knabenbauer, Jennifer Taylor, Rebecca L. Pavlicek, Russell Karls, Anne Hess, Rebecca M. Davidson, Michael Strong, Helle Bielefeldt Ohmann, Angelo A. Izzo, Karen M. Dobos, HspX vaccination and role in virulence in the guinea pig model of tuberculosis, Pathogens and Disease, Volume 71, Issue 3, August 2014, Pages 315–325, https://doi.org/10.1111/2049-632X.12147
Close - Share Icon Share
Abstract
Mycobacterium tuberculosis (Mtb) currently infects billions of people; many of whom are latently infection and at risk for reactivation. Mycobacterium bovis Bacille Calmette Guerin (BCG) while approved as a vaccine, is unable to prevent reactivation of latent tuberculosis infection (LTBI). Subunit vaccines boosting BCG or given alone are being tested for efficacy in LTBI models. Alpha crystallin (Acr, HspX), is a latency associated protein and subunit vaccine candidate. In this report, three HspX formulas (native and two recombinant variants) were used as vaccines in the guinea pig model of tuberculosis; none were protective during challenge with WT Mtb. However, recombinant HspX was protective in animals challenged with a strain of Mtb lacking hspX (X4 19), indicating protection was driven by molecules co purifying with HspX or an adjuvant effect of recombinant HspX in this system. Mtb X4 19 was significantly less virulent than WT Mtb. Quantitative PCR and whole genome sequencing identified several genes (Rv2030cRv2032, Rv1062, Rv1771, Rv1907, and Rv3479) with altered expression that may contribute to loss of virulence. Physiological differences required for the establishment of Mtb infection in different hosts may affect the potential of subunit vaccines to elicit protection, supporting the need for rigorous biochemical and modeling analyses when developing tuberculosis vaccines.
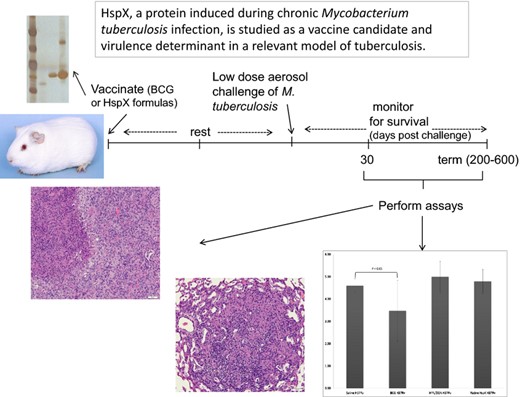
HspX, a protein induced during chronic Mycobacterium tuberculosis infection, is studied as a vaccine candidate and virulence determinant in a relevant model of tuberculosis.
Introduction
Latent tuberculosis infection (LTBI) remains a challenge to prevent, diagnose and treat properly; with upwards of 2 billion people presenting with LTBI. This population needs to be considered when developing new tuberculosis vaccines, as is evidenced by the fact that the current vaccine, Bacille Calmette Guerin (BCG) does not protect adults from pulmonary TB, nor does it prevent reactivation disease as a result of LTBI (Andersen, 2007). BCG has been in use since its development the 1920s, with many countries still vaccinating at birth, with the exception of the US and most of Western Europe. It is one of the most widely given vaccine in the world and for these reasons has an excellent track record for safety; this is particularly important in TB vaccines because much of the target population lives in a resource poor setting and suffer from HIV co infection (Andersen, 2007). Therefore TB vaccine development incorporating BCG uses two general approaches: developing recombinant BCG formulations that either over express known immunogenic antigens or non tuberculosis immune modulators or down regulate virulence factors, and developing new generation subunit vaccines to be given as boosters to the already administered BCG vaccine at birth (reviewed in Aagaard et al., 2009; Cayabyab et al., 2012; Kaufmann & Gengenbacher, 2012); primary articles include (Rao et al., 2013; Shaban et al., 2013; Singh et al., 2014). The latter method will take advantage of the fact that BCG is already given at birth and does offer protection against severe childhood forms of TB (Aagaard et al., 2009), with the hopes that a new subunit will boost immunity to be life long and specific for TB. One such subunit vaccine candidate includes the latency associated antigen and molecular chaperone from Mycobacterium tuberculosis (Mtb), HspX (16kDa, Acr).
Our previous work demonstrated that native HspX purified from Mtb was able to protect mice against pulmonary tuberculosis, whereas the recombinant protein expressed and purified from Escherichia coli (E. coli), failed to protect animals (Taylor et al., 2012). Thus, we hypothesized that HspX mediated protection was based on its ability to chaperone another molecule in vivo, and that this molecule is unique to mycobacteria. HspX is highly up regulated during times of hypoxia and stress in vitro and is also preferentially recognized by LTBI patients resulting in its classification as a latency associated antigen (Geluk et al., 2007). The granuloma in the lung is thought to be a harsh environment of hypoxia, nutrient starvation, and stress (Cunningham & Spreadbury, 1998). For these reasons Acr serves as a potential vaccine candidate to target LTBI and prevent reactivation disease.
HspX contains an α crystallin domain and like all α crystallins, functions as an ATP independent molecular chaperone by preventing improper folding and unfolding of other proteins within the cell (Yuan, 1998). During Mtb infection, HspX can be found in aggregates on the inner side of the cell wall, has been linked to cell wall thickening (Cunningham & Spreadbury, 1998), and its chaperoning activity has been experimentally demonstrated (Yuan, 1998). We hypothesize that because HspX functions as a molecular chaperone, the protein requires binding partners (protein or otherwise) to remain biologically active in vivo. Thus, HspX purified from Mtb in its native form is bound to and co purifies with these binding partners. In contrast, recombinant HspX expressed and purified from E. coli would not contain these mycobacterial binding partners, and thus may not retain all its biological attributes.
Our previous studies in the mouse model of tuberculosis supported this hypothesis since native HspX was protective and the recombinant protein was not. We additionally found that incubation of recombinant HspX with whole cell lysate (WCL) from M. tuberculosis (strain X4 19) restored the protective effect (Taylor et al., 2012). These results suggested that recombinant HspX was able to bind and co purify with binding partners that were present in mycobacterial lysates, and that these co factors could be processed and presented efficiently thus bolstering a protective immune response. The promising results in the mouse model led to this study to further evaluate these same three HspX vaccine formulations in the guinea pig model of tuberculosis.
In contrast to the C57BL/6 mouse model (Orme, 2005a, b), guinea pigs develop an immune response that is not protective and animals succumb to chronic infection and disease. The disease is accompanied by severe pathology mimicking human pathology during active disease (Ordway et al., 2008). The guinea pig is frequently used as a stringent model to assess the potential efficacy of new tuberculosis vaccines via survival studies, as the absence of protective immunity results in death within 20 weeks post infection and BCG vaccination confers long term protection (> 1 year; Orme, 2005a, b; Grover et al., 2009). In the present study, guinea pigs were vaccinated with three preparations of HspX and subsequently challenged with Mtb H37Rv and an Mtb HspX knock out strain (X4 19; ΔHspX) to evaluate the efficacy of HspX as a vaccine. These studies demonstrate that none of the HspX formulations were protective in the guinea pig model of pulmonary tuberculosis when animals were challenged with WT Mtb. Further, the ΔHspX strain displayed a significantly lower virulence phenotype in the guinea pig. Histology of the lung and other affected organs suggests that the physiological establishment of ΔHspX Mtb in the lungs of guinea pigs vs. mice differ, and therefore the protection afforded by the HspX formulations of Mtb differs in the two animal models.
Materials and methods
Preparation of seed stocks
Mycobacterium tuberculosis (Mtb) HspX knock out (ΔHspX, strain X4 19) was made by allelic exchange using a modified protocol of Pelicic et al. (1997), as reported previously (Taylor et al., 2012).One milliliter each of WT and ΔHspX glycerol stocks was transferred into a glass tube containing 9 mL of Proskauer Beck (PB) medium (Kruh et al., 2010) and static cultures incubated at 37 °C for 3 weeks, or until pellicle formation was visible on top of the media. Pellicles were harvested and used for sequential inoculation into 25 and 100 mL of PB media and incubated as before. Pellicles of Mtb from the final passage were transferred to 20 mL of PB media containing 20% glycerol (v/v); cells were mixed by agitation, and suspensions bath sonicated at 4 °C for 10 min. One millilitre seed stock vials were prepared and stored at −80 °C.
Preparation of infectivity (working) stocks
One vial of each strain of seed stock was added to 9 mL of 7H9+OADC medium containing 0.1% Tween 80 and incubated at 37 °C with shaking for 14 days. Cultures were inoculated into 45 mL of 7H9+OADC+0.1% Tween 80 and incubated at 37 °C with shaking, harvested, and 1 mL working stock vials were prepared and stored at −80 °C. Three vials of each strain were randomly recovered, thawed, serially diluted in 7H9+OADC medium and plated in triplicate for determination of colony forming units (CFU).
Preparation of vaccines
Native HspX (nHspX) protein, recombinant HspX (rHspX), and recombinant HspX pull down (rHspX PD) protein were purified as described previously (Taylor et al., 2012). Endotoxin levels were assessed with a Limulus Amebocyte Lysate (LAL) assay as described previously (Yang et al., 2011) and samples were found to contain < 10 ng EU mg−1 protein.
Vaccination and challenge with M. tuberculosis
Pathogen free, female outbred Hartley guinea pigs (c. 500 g) were purchased from Charles River Laboratories (North Wilmington, MA) and held under bio safety level 3 barrier conditions. The animals had unlimited access to guinea pig chow and clean water. Upon arrival, guinea pigs were rested for 14 days, chipped for identification purposes, and rested for another 14 days before vaccination.
Three groups of 10 guinea pigs were vaccinated with nHspX, rHspX or rHspX PD, with three 20 μg doses 3 weeks apart by intramuscular (IM) injection in the hind legs; the vaccines were emulsified with an adjuvant formulation of 250 μg dioctadecylammonium bromide (DDA) and 25 μg monophosphoryl lipid A (MPL) just prior to vaccination. Three additional groups of 10 guinea pigs were vaccinated withsaline, or adjuvant only using the same method as the subunit vaccines, or BCG [1 × 104 CFU intradermally (ID) on the underside of the belly].Guinea pigs were challenged with Mtb strain H37Rv using a Madison chamber aerosol generation device (Madison, WI) to deliver a low dose of c. 20 bacilli per animal following a 10 week resting period after the final vaccination. The vaccination and challenge protocol was repeated in its entirety to monitor protection following infection with Mtb strain ΔHspX.
Virulence assays
Two groups of 13 (n = 26) guinea pigs were infected as above to deliver a low dose of c. 20 bacilli of either Mtb strain H37Rv or ΔHspX per animal. Five guinea pigs per infected group were sacrificed at 30 days post infection to enumerate CFU by plating lung and spleen homogenates on nutrient 7H11 agar with OADC enrichment and incubating at 37 °C for 3 weeks. In addition, samples of tissues (lung, liver, spleen) were fixed in 10% neutral buffered formaldehyde or 4% paraformaldehyde and routine processed through paraffin embedding. Hematoxylin and eosin (H&E) stained sections were assessed by a pathologist (HBO) blinded to the treatments and groupings. Lung lesions were scored based on criteria previously published (Drumm et al., 2009), including an assessment of extent of overall lung involvement, extent of primary and secondary lesions, necrosis, mineralization and fibrosis. Maximum possible score for lung pathology would be 24.
Liver and spleen lesions were scored based on two criteria: (1) lesion type and (2) extent of any change(s) seen. For liver the lesions types were: 0 = no apparent lesion in section; 1 = mild sinusoidal infiltration of macrophages and granulocytes accompanying degeneration and necrosis of few individual hepatocytes; 2 = focal/multifocal coagulation necrosis with or without mild infiltration of macrophages and neutrophils; 3 = granulomatous inflammation with or without bile duct proliferation and minimal fibrosis; 4 = granulomatous inflammation with moderate to severe fibrosis and/or mineralization and/or necrosis. For spleen the lesions types were: 0 = no apparent lesion in section; 1 = small clusters of epitheloid macrophages with or without the presence of a few multinucleated giant cells; 2 = granulomas without necrosis or mineralization; 3 = granulomas with necrosis but without mineralization; 4 = granulomas with necrosis/mineralization and with or without fibrosis. For both liver and spleen the extent scores were as follows: 1 = < 10% involvement of parenchyma; 2 = 11–30% involvement of parenchyma; 3 = 31–60% parenchyma involved; 4 = > 60% of parenchyma involved. For both organs the maximum possible score would be 8.
The remaining eight animals were monitored weekly and subjected to CFU counts upon death or termination of the experiment.
Quantitative real time PCR and whole genome sequencing
Mycobacterium tuberculosis working stocks were plated onto 7H11+OADC media and following incubation for 14 days at 37 °C; one colony from each strain was selected and inoculated into 100 mL of 7H9+OADC+0.1% Tween 80 and allowed to grow for 14 days at 37 °C with shaking; from which three new 100 mL cultures for each strain were inoculated at an OD600 of 0.05 into 7H9+OADC+0.1% Tween 80. Cultures were allowed to grow at 37 °C with shaking and harvested by centrifugation when the OD read between 0.4 and 0.6 (c. 48 h). Cell pellets were washed three times with PBS and resuspended in 10 mL of TRIzol® reagent (Invitrogen, Carlsbad, CA), or processed to isolate genomic DNA (Belisle & Sonnenberg, 1998); all samples were stored at −80 °C or kept on ice.
Cells were broken by probe sonication and chloroform was added to the broken cells at 0.2× volume and centrifuged at 27 000 g for 30 min. The upper organic layer was recovered and precipitated in 0.5× volume isopropyl alcohol. Following 16 h incubation at −20 °C, precipitates were recovered by centrifugation and pellets washed in 80% ethanol; RNA was extracted as per manufacturer's instructions (RNeasy© spin kit; Qiagen, Valencia, CA). To confirm RNA integrity, 5 μg was run on a 1% agarose gel and visualized with ethidium bromide for the presence of 16s and 23s RNA bands. Concentration and purity was determined by measuring A260:A280 ratio on a Nanodrop® spectrophotometer (Thermo, Waltham, MA). 100 μg RNA was reverse transcribed to cDNA using Invitrogen Superscript III First Strand Synthesis SuperMix, as per manufacturers' instructions. Specifically, RNA samples from Mtb cultures were transcribed using following primers for 5 Mtb genes:
Rv2030c: forward 5′TTATCTACCGGCAACGGAAC 3′ reverse 5′CTGATCGATGTGGATCATGG 3′;
Rv2031c (HspX): forward 5′TAAGGCCACCTACGACAAGG 3′; reverse 5′CCGGATCTGAATGTGCTTTT 3′;
Rv2032 (acg): forward 5′CTGACCCACATCACCGAACT 3′ reverse 5′TAGCCCGAACGTGAAACACT 3′;
Rv0251c (acr2): forward 5′CCATCGCGGCTTCCTATG 3′ reverse 5′CTACTTCGTGATGGCGATGC 3′;
Rv2703 (sigA, housekeeping gene): forward 5′CGACGACGAAGACCACGAAG 3′ reverse 5′TGTCCTTTTCGGTGGGTTCA 3′.
The RNA polymerase sigma factor A gene (sigA) was used as a housekeeping gene and genomic DNA from Mtb was used to set a standard curve. Reactions were prepared in 96 well plates in triplicate; three biological replicates for each strain and three technical replicates for each gene/primer pair: 10 μL SYBR green (Quanta Biosciences, cat no: 84067), 2 μL forward primer at 10 mM, 2 μL reverse primer at 10 mM, 2 μL DNA sample and QS to 20 μL with water. The program was run on a BioRad® iQ5 (Hercules, CA) instrument: 2 min at 55 °C; 2 min at 95 °C; 40 cycles of 95 °C for 15 s; 60 °C for 30 s; 72 °C for 45 s; and finally 5 min at 72 °C then 10 min at 55 °C; gene expression profiles were analyzed with the accompanying program software.
Whole genome sequencing was conducted as described previously (Ioerger et al., 2009). Briefly, genomic DNA was processed and multiplexed using a SOLiD array run (75 bp fragment reads) and analyzed with a high confidence threshold to obtain 11 315 504 filtered reads, which after mapping yielded an average genome coverage of 118×. Single nucleotide polymorphisms were identified with the samtools pileup algorithm (Li et al., 2009) and were filtered based on SNP quality (≥ 20), coverage (> 10×) and proportion of reads supporting the SNP (> 50%) with a custom perl script. SNPs were annotated relative to the H37Rv gene models using the annovar software program (Wang et al., 2010).
Statistical analysis
All survival data was analyzed using graphpad prism® software (La Jolla, CA); survival data was analyzed with the log rank test and CFU were analyzed using the student's t test. In vitro qPCR data (used to verify gene expression in the mutant strain) was normalized to a housekeeping gene to determine relative gene expression. In vivo qPCR data (used to determine hspX gene expression in the lungs of vaccinated guinea pigs) was analyzed using sas® statistical software, running a sas proc mixed program. The analysis included random effects from animal and gene interactions and biological effects. A P value of < 0.05 was considered statistically significant for all assays performed.
Results
HspX mediated protection in the guinea pig model of tuberculosis
Previously, mice vaccinated with some, but not all, HspX vaccine formulations were found to be protected when challenged with Mtb strain H37Rv (Taylor et al., 2012). These studies also demonstrated that while native HspX purified from Mtb culture lysates (nHspX) conferred protection, the recombinant protein expressed in and purified from E. coli (rHspX) did not, unless the purified recombinant protein was incubated with Mtb lysate and re purified from this lysate (rHspX PD) prior to vaccination, suggesting that HspX mediated protection was driven in part, by other products co chaperoned with HspX. This finding was further supported by evidence that nHspX and rHspX PD could protect mice against challenge with an HspX deletion strain of Mtb (X4 19; ΔHspX; Taylor et al., 2012).
To build upon these studies, groups of 10 guinea pigs each were vaccinated with either nHspX, rHspX, or rHspX PD in adjuvant, or with BCG, adjuvant alone, or saline, and after a 10 week interval were challenged with a low dose aerosol of Mtb H37Rv or ΔHspX and monitored for survival. The efficacy of each of these sub unit vaccines was compared against infection in saline controls; BCG vaccination is used as a positive control in this model. Guinea pigs succumb to tuberculosis within about 25 weeks post infection with no prophylactic intervention. BCG vaccinated guinea pigs can live up to 70 weeks (Orme, 2005a, b); survival rates are used to access vaccine protection. Kaplan Meier survival plots of the wild type H37Rv infected guinea pigs demonstrated that none of the HspX sub unit vaccine formulations were able to significantly prolong their survival after Mtb challenge when compared to the Saline treated group (Fig. 1a; P > 0.05, log rank test).
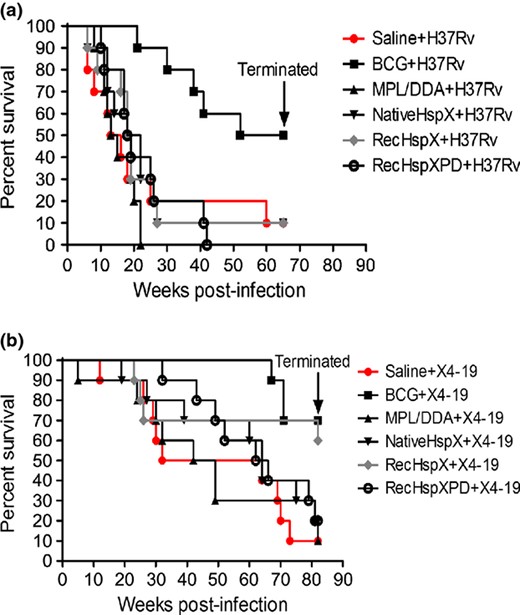
Vaccination and Challenge with WT (a) or ΔHspX (strain X4 19; b) Mtb. (a) Survival of WT Mtb infected guinea pigs through 64 weeks post infection (PI); all surviving animals were euthanized at 65 weeks PI. BCG alone was protective (P < 0.01). (b) Survival of ΔHspX Mtb infected guinea pigs through 84 weeks PI; all surviving animals were euthanized at 84 weeks PI. BCG (P < 0.01) and recHspX (P < 0.05) were protective in this experiment.
The survival rates of the ΔHspX Mtb infected group differ from those challenged with WT Mtb, in which rHspX significantly prolonged survival, similar to BCG (Fig. 1b; P < 0.05 for BCG and rHspX vaccinated animals). In addition, the number of animals surviving challenge at the termination of each study was significantly different, with over 35% of saline or adjuvant control animals surviving more than 50 weeks post ΔHspX Mtb infection, compared to 12% and 0% of animals surviving in the respective saline and adjuvant groups infected with WT Mtb (Fig. 1). A comparison of CFU in animal groups at necropsy demonstrated differences within and between challenge groups as well (Supporting Information, Table S1). Specifically, significant reductions in CFU were observed in the lungs (Fig. 2) and spleens (Table S1) of animals vaccinated with BCG when challenged with WT Mtb. In contrast, a significant reduction in CFU was observed in the lungs of both BCG and rHspX PD vaccinated animals when challenged with ΔHspX Mtb (Fig. 2); no significant reduction in CFU was observed in the spleens of any of the vaccinated animals challenged with ΔHspX Mtb (Table S1). These data suggest that ΔHspX Mtb is less virulent in the guinea pig model of TB; and that the general antigenic properties of HspX confer a protective benefit to the host during infection with ΔHspX Mtb.
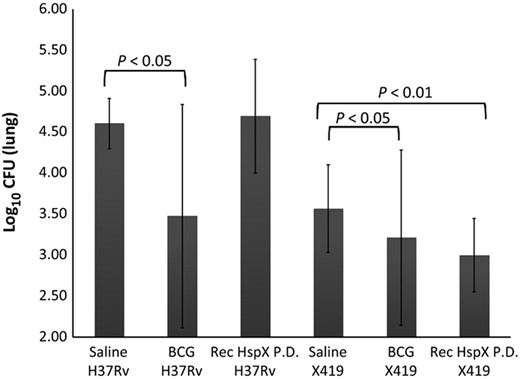
CFU in the lungs of infected, vaccinated animals. Lungs from animals succumbing to disease or euthanized at termination of the vaccine study were harvested, homogenized, and lysates plated for enumeration of bacilli by CFU. Only BCG vaccinated animals demonstrated significantly less CFU when WT Mtb was used as the challenge strain (P < 0.05); however both BCG (P < 0.05) and rHspX PD (P < 0.01) demonstrated significantly lower CFU levels when ΔHspX Mtb was used as the challenge strain.
ΔHspX Mtb virulence in the guinea pig model of tuberculosis
The results in the survival rates of ΔHspX infected guinea pigs were somewhat unexpected, thus a comparison of the virulence of H37Rv and ΔHspX in guinea pigs was performed. Groups of 13 guinea pigs each were infected with a low dose aerosol of either Mtb H37Rv or ΔHspX Mtb. Five of these animals were sacrificed at day 30 post infection to determine the bacterial burden in affected organs and assess pathology; the remaining eight animals were monitored for survival. Bacterial burden was measured by plating the right cranial lung lobe and a portion of spleen homogenates and enumerating CFU. At 30 days post infection, the CFU data (Fig. 3) was consistent with the reduction in virulence that was observed in the vaccine study (Fig. 1), with a significantly higher CFU burden (P < 0.01) in the lungs and spleens of H37Rv vs. ΔHspX Mtb infected animals. Survival data for the remaining eight animals corroborated the CFU data, demonstrating a significant increase in survival for animals infected with ΔHspX Mtb vs. WT Mtb (Fig. 4; P < 0.001).
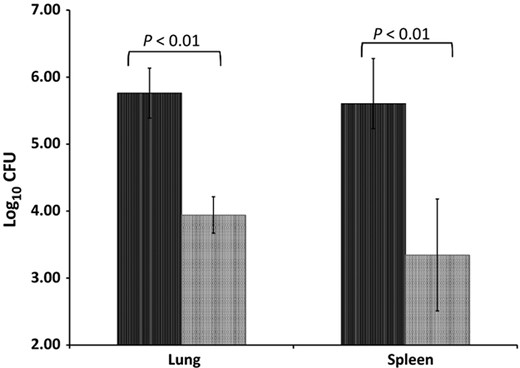
CFU in the lungs and spleens of infected animals 30 days post infection. Five animals were infected with either WT (H37Rv; black bars) or ΔHspX (X4 19; grey bars) Mtb and sacrificed 30 days PI. Lungs and spleens were harvested, homogenized, and plated to enumerate bacilli by CFU. Significant differences in bacillary burden (P < 0.01) were observed in both organs.
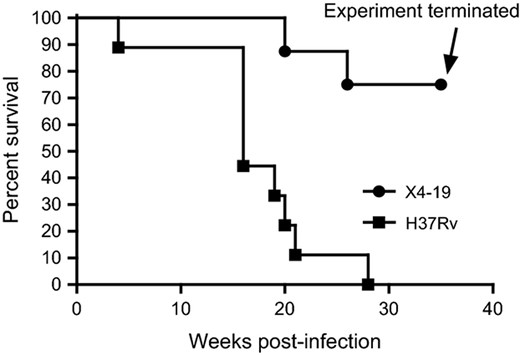
Survival of Guinea Pigs experimentally infected with WT vs. ΔHspX Mtb. Eight animals were infected with either WT (H37Rv; squares) or ΔHspX (X4 19; circles) Mtb and monitored for survival through 35 weeks PI. Significant difference in survival rate (P < 0.01) was observed between the two groups.
The lungs, spleens, and livers from five animals infected with each strain were harvested at day 30 post infection and examined by a veterinary pathologist blinded to the groups. Lungs of ΔHspX infected animals demonstrate minimal pathology, limited primary lesions, with rare, small secondary lesions involving infiltration of lymphocytes and fewer macrophages in randomly distributed areas (Fig. 5). In contrast, animals infected with WT Mtb show significant evidence of tissue destruction with scattered primary granulomas with central mineralization and secondary lesions with dense infiltration of lymphocytes and macrophages (Fig. 6). Similarly, spleen lesions were characterized by occasional primary lesions in ΔHspX Mtb infected animals compared to extensive granuloma development in the parenchyma of H37Rv infected animals (Fig. 7). Overall, the ΔHspX Mtb infected animals displayed less organ pathology than WT Mtb with less over all involvement of infected tissues, consistent with a loss of virulence phenotype for ΔHspX Mtb in the guinea pig model of tuberculosis.
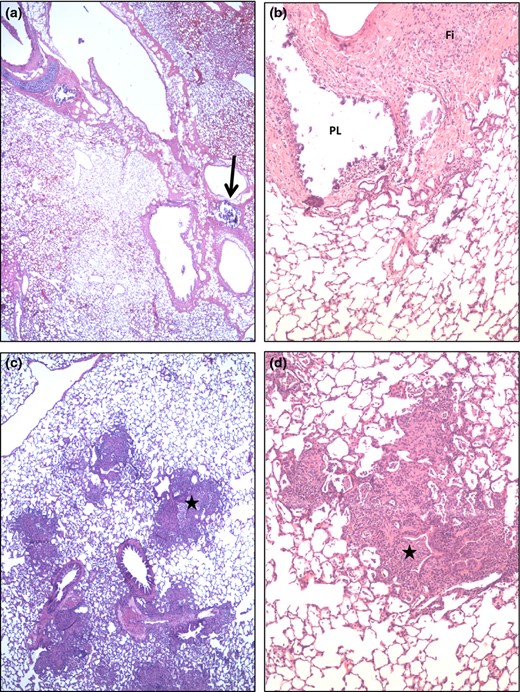
Lung micrographs from ΔHspX (X4 19) infected guinea pigs. (a and b) Occasional remnants of primary lesions (PL, arrow) are apparent in the interstitium supporting bronchioles and larger pulmonary vessels. In b the mineralized material, characteristic of late stage primary lesions, have fallen out during tissue processing. Such lesions are often accompanied by marked fibrosis (Fi). (c and d) Small/restricted areas of secondary lesions are seen in some lungs of this group and characterized by a mild to moderate, mostly compact lympho histiocytic infiltrate in the alveoli and bronchiolitis (stars) also involving hetrophil infiltration.
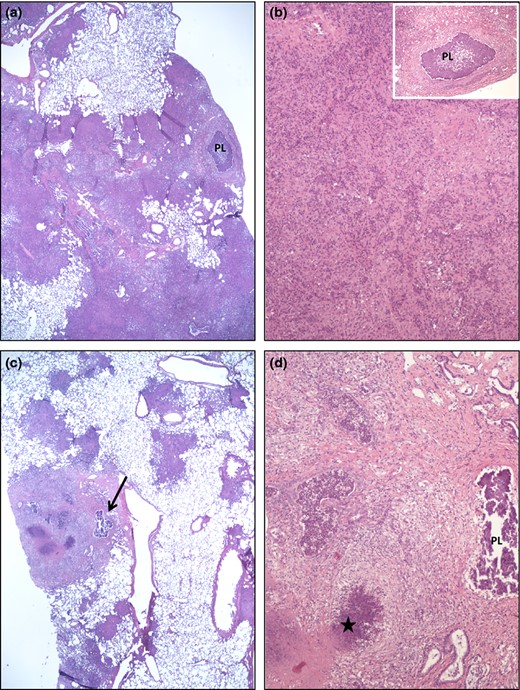
Lung micrographs from WT (H37Rv) infected guinea pigs. The pathological changes are dominated by secondary lesions with dense, coalescing (a) and (c) multifocal infiltrates of lymphocytes and macrophages. Occasional primary granulomas with central mineralization (PL in a, b insert, d; arrow in c) are seen within the extensive secondary changes. Areas of necrosis (star in d) and variable fibrosis characterize the secondary lesions (minimal fibrosis in a and b; moderate fibrosis in d).
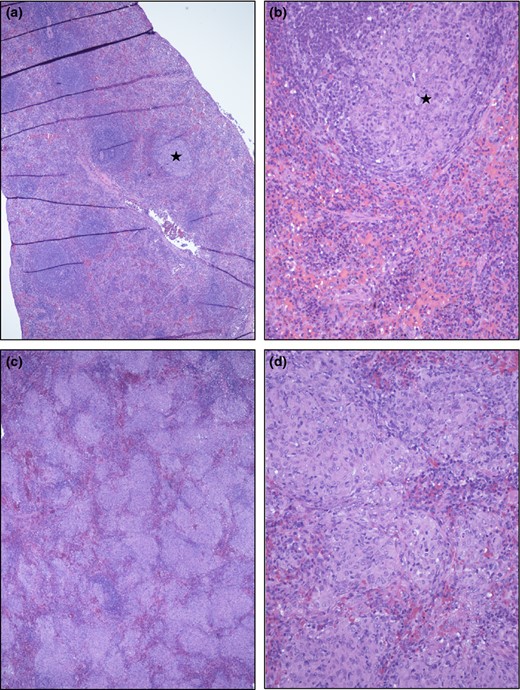
Spleen micrographs from ΔHspX (a and b) and WT (c and d) Mtb infected guinea pigs. (a and b) No or only occasional small granulomatous foci (star in a and b) were seen, involving < 10% of the parenchyma. (c and d). In contrast, more than 30% and often more than 60% of the spleen parenchyma was involved, as illustrated in c and d. The multifocal to coalescing cellular infiltrates are composed mainly of epitheloid macrophages with variable admixtures of lymphocytes and occasional multinucleated giant cells.
Gene expression and whole genome sequence analyses of Mtb X4 19 (ΔHspX)
Studies performed by ourselves (Taylor et al., 2012), and others (Stewart et al., 2006; Rustad et al., 2008), suggest that hspX deletion strains of Mtb display a similar virulence phenotype; in addition, notable work of other researchers suggests a hypervirulent phenotype for hspX deletion strains of Mtb (Parish et al., 2003; Stewart, 2005; Hu et al., 2006). In contrast, the studies performed here suggest that the X4 19 ΔHspX deletion strain is significantly less virulent in the guinea pig model of TB; an observation that is consistent with two other reports (Yuan, 1998; Converse et al., 2009). This may be due to the loss of expression of hspX, neighboring genes (Rv2030c and Rv2032), or other single nucleotide polymorphisms coevolving with the deletion of hspX. The hspX gene lies within close proximity to gene Rv2032, a putative nitrate reductase termed acg (for acr co regulated gene; Purkayastha, 2002), and Rv2030c, a hypothetical protein with unknown function. It has been suggested that reactive nitrogen intermediates (RNI) and nitric oxide (NO) are effective anti mycobacterial agents in vivo and can influence the expression of other latency related genes, including hspX (Garbe et al., 1999; Hu et al., 2006, 2011). A recent report by Hu and Coates suggests that Acg is a key virulence determinant in Mtb (Hu et al., 2011). Thus a lack of acg expression in X4 19, may explain the loss of virulence phenotype observed here. Rv0251c, encoding an acr/hspX homologue in Mtb) was also included in the qPCR study because it is also highly up regulated during heat shock and stress, although its location on the genome is not in the DosR regulon as the other listed genes. Figure 1 represents relative gene expression, normalized to the TB housekeeping gene, sigA, of Rv2030c 2032c in Mtb strain X4 19 (ΔHspX) vs. H37Rv (WT). The results demonstrate that, in addition to the loss of hspX expression, neither Rv2030c nor acg are expressed, suggesting that the loss of virulence described for X4 19 may be attributed to the loss of expression of any one, or a combination of, these genes.
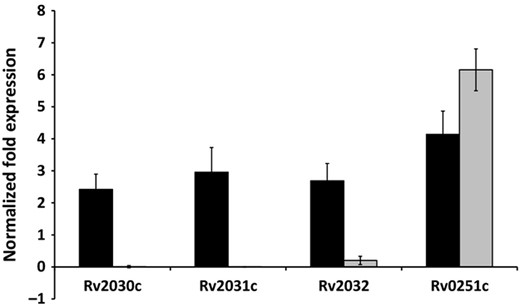
qPCR of Rv2030c, Rv2031c, and Rv2032c in ΔHspX strain X4 19 vs. WT strain H37Rv of Mtb. Gene expression levels were normalized to RNA sigma factor A, sigA. In addition to lack of expression of hspX, the ΔHspX strain X4 19 (grey bars), demonstrated significantly lower to no detectable level of expression of Rv2030c and Rv2032c (acg) in comparison to WT (black bars) Mtb.
Because our in vitro qPCR data revealed that in addition to hspX, Rv2030c and acg expression was lower in strain X4 19 than in WT Mtb, whole genome sequence analysis was used to determine if additional mutations could potentially affect the virulence associated with Mtb strain X4 19. Whole genome sequence (WGS) analysis of X4 19 was performed in reference to the parental H37Rv strain, with the goal of discovering unique single nucleotide polymorphisms (SNPs) present in X4 19 only.
The sequence of X4 19 demonstrated that in addition to ablation of hspX (Rv2031c), 65 SNPs were discovered, including 29 non synonymous mutations, three stopgain mutations, two loss of stop codon mutations, 25 synonymous mutations, and six intergenic mutations. A comparison of these SNPs to all publically available Mtb WGSs (www.tbdb.org) demonstrated 16 SNPs unique to X4 19 (Table 1). Of the mutations that were unique to this strain, three non synonymous mutations were found in genes Rv1062 (hypothetical protein, possible patatin like phospholipase), Rv1771 (oxidoreductase, possible L gulono 1,4 lactone dehydrogenase, involved in vitamin C biosynthesis), and Rv1907c (hypothetical protein, located upstream of katG in a number of mycobacteria, and may be in a common operon based on the distance between genes). In addition, one unique early termination (stopgain) in Rv3479 (hypothetical protein) was found. Unique synonymous mutations in Rv0543c (hypothetical protein), Rv0861c (DNA helicase ErcC3), Rv1087A (hypothetical protein), Rv1308 (atpA, ATP synthase subunit), and Rv2948c (acyl CoA synthase), were also found; a functional implication of these synonymous mutations is unlikely. Unique intergenic mutations include mutations upstream from otsB (trehalose biosynthesis), upstream of nadD (NAD biosynthesis), upstream of Rv0383c, upstream of PPE34, and upstream from the rrs (Table 1). In summary, in addition to reduced expression of acg and Rv2030c unique SNPs in X4 19 that may affect the function of four additional proteins (Rv1062, Rv1771, Rv1907, and Rv3479) are present and may also contribute to the loss of virulence phenotype observed for this ΔHspX strain of Mtb.
| Coding annotation | Reference | Query | NT change | AA change | Locus | Functional implication |
| Nonsynonymous | G | T | c.G173T | p.S58I | Rv1062 | Patatin like phospholipase |
| Nonsynonymous | A | G | c.A872G | p.Q291R | Rv1771 | L gulono 1,4 lactone dehydrogenase, vit C biosynthesis |
| Nonsynonymous | A | G | c.T473C | p.V158A | Rv1907c | Near katG |
| Stopgain | G | A | c.G2922A | p.W974X | Rv3479 | Toward end of protein, 1021aa |
| Synonymous | C | T | c.G243A | p.A81A | Rv0543c | |
| Synonymous | C | A | c.G1230T | p.A410A | Rv0861c | |
| Synonymous | C | T | c.C696T | p.T232T | Rv1308 | |
| Synonymous | C | T | c.C600T | p.Y200Y | Rv1520 | |
| Synonymous | G | A | c.C273T | p.R91R | Rv2948c | |
| Intergenic | A | G | – | – | Rv2005, Rv2006 | Upstream of otsB, trehalose biosynthesis |
| Intergenic | A | G | – | – | Rv2231c, Rv2232 | Possible toxin gene coding region |
| Intergenic | T | G | – | – | Rv2420c,Rv2421c,Rv2422,Rv2423 | Upstream of nadD, NAD biosynthesis |
| Intergenic | A | C | – | – | Rv0383c,Rv0384c | Upstream of Rv0383c |
| Intergenic | T | C | – | – | Rv1917c,Rv1918c | Upstream of PPE34 |
| Intergenic | C | T | – | – | Rv1315 | Probable UDP N acetylglucosamine 1 carboxyvinyltransferase MurA, upstream of rrs |
| Coding annotation | Reference | Query | NT change | AA change | Locus | Functional implication |
| Nonsynonymous | G | T | c.G173T | p.S58I | Rv1062 | Patatin like phospholipase |
| Nonsynonymous | A | G | c.A872G | p.Q291R | Rv1771 | L gulono 1,4 lactone dehydrogenase, vit C biosynthesis |
| Nonsynonymous | A | G | c.T473C | p.V158A | Rv1907c | Near katG |
| Stopgain | G | A | c.G2922A | p.W974X | Rv3479 | Toward end of protein, 1021aa |
| Synonymous | C | T | c.G243A | p.A81A | Rv0543c | |
| Synonymous | C | A | c.G1230T | p.A410A | Rv0861c | |
| Synonymous | C | T | c.C696T | p.T232T | Rv1308 | |
| Synonymous | C | T | c.C600T | p.Y200Y | Rv1520 | |
| Synonymous | G | A | c.C273T | p.R91R | Rv2948c | |
| Intergenic | A | G | – | – | Rv2005, Rv2006 | Upstream of otsB, trehalose biosynthesis |
| Intergenic | A | G | – | – | Rv2231c, Rv2232 | Possible toxin gene coding region |
| Intergenic | T | G | – | – | Rv2420c,Rv2421c,Rv2422,Rv2423 | Upstream of nadD, NAD biosynthesis |
| Intergenic | A | C | – | – | Rv0383c,Rv0384c | Upstream of Rv0383c |
| Intergenic | T | C | – | – | Rv1917c,Rv1918c | Upstream of PPE34 |
| Intergenic | C | T | – | – | Rv1315 | Probable UDP N acetylglucosamine 1 carboxyvinyltransferase MurA, upstream of rrs |
| Coding annotation | Reference | Query | NT change | AA change | Locus | Functional implication |
| Nonsynonymous | G | T | c.G173T | p.S58I | Rv1062 | Patatin like phospholipase |
| Nonsynonymous | A | G | c.A872G | p.Q291R | Rv1771 | L gulono 1,4 lactone dehydrogenase, vit C biosynthesis |
| Nonsynonymous | A | G | c.T473C | p.V158A | Rv1907c | Near katG |
| Stopgain | G | A | c.G2922A | p.W974X | Rv3479 | Toward end of protein, 1021aa |
| Synonymous | C | T | c.G243A | p.A81A | Rv0543c | |
| Synonymous | C | A | c.G1230T | p.A410A | Rv0861c | |
| Synonymous | C | T | c.C696T | p.T232T | Rv1308 | |
| Synonymous | C | T | c.C600T | p.Y200Y | Rv1520 | |
| Synonymous | G | A | c.C273T | p.R91R | Rv2948c | |
| Intergenic | A | G | – | – | Rv2005, Rv2006 | Upstream of otsB, trehalose biosynthesis |
| Intergenic | A | G | – | – | Rv2231c, Rv2232 | Possible toxin gene coding region |
| Intergenic | T | G | – | – | Rv2420c,Rv2421c,Rv2422,Rv2423 | Upstream of nadD, NAD biosynthesis |
| Intergenic | A | C | – | – | Rv0383c,Rv0384c | Upstream of Rv0383c |
| Intergenic | T | C | – | – | Rv1917c,Rv1918c | Upstream of PPE34 |
| Intergenic | C | T | – | – | Rv1315 | Probable UDP N acetylglucosamine 1 carboxyvinyltransferase MurA, upstream of rrs |
| Coding annotation | Reference | Query | NT change | AA change | Locus | Functional implication |
| Nonsynonymous | G | T | c.G173T | p.S58I | Rv1062 | Patatin like phospholipase |
| Nonsynonymous | A | G | c.A872G | p.Q291R | Rv1771 | L gulono 1,4 lactone dehydrogenase, vit C biosynthesis |
| Nonsynonymous | A | G | c.T473C | p.V158A | Rv1907c | Near katG |
| Stopgain | G | A | c.G2922A | p.W974X | Rv3479 | Toward end of protein, 1021aa |
| Synonymous | C | T | c.G243A | p.A81A | Rv0543c | |
| Synonymous | C | A | c.G1230T | p.A410A | Rv0861c | |
| Synonymous | C | T | c.C696T | p.T232T | Rv1308 | |
| Synonymous | C | T | c.C600T | p.Y200Y | Rv1520 | |
| Synonymous | G | A | c.C273T | p.R91R | Rv2948c | |
| Intergenic | A | G | – | – | Rv2005, Rv2006 | Upstream of otsB, trehalose biosynthesis |
| Intergenic | A | G | – | – | Rv2231c, Rv2232 | Possible toxin gene coding region |
| Intergenic | T | G | – | – | Rv2420c,Rv2421c,Rv2422,Rv2423 | Upstream of nadD, NAD biosynthesis |
| Intergenic | A | C | – | – | Rv0383c,Rv0384c | Upstream of Rv0383c |
| Intergenic | T | C | – | – | Rv1917c,Rv1918c | Upstream of PPE34 |
| Intergenic | C | T | – | – | Rv1315 | Probable UDP N acetylglucosamine 1 carboxyvinyltransferase MurA, upstream of rrs |
Discussion
Our initial study of three HspX subunit vaccine formulas suggested that HspX may be a possible vaccine candidate based on the ability of two formulas to confer protection in the mouse model of tuberculosis (Taylor et al., 2012). The data reported here demonstrates that HspX did not induce a protective immune reponse in the guinea pig model of tuberculosis when animals are challenged with a standard WT strain of Mtb. The physiology of tubercle bacillus in the lungs of mice may be different compared to guinea pigs over the course of infection. Chronic infection is difficult to match in animal models; indeed bacteria grown in vitro demonstrates significant differences in protein abundance vs. resident in vivo bacilli (Kruh et al., 2010). Accurate measurement of the expression of Mtb proteins between animal models in vivo may further address these differences; such studies focused on the expression of genes in the DosR regulon would be particularly interesting, since these products have transcriptional influences on each other (Chauhan & Tyagi, 2008a, b; Vasudeva Rao & McDonough, 2008).
Several studies have attempted to decipher the specific role of hspX in virulence and many discrepancies have been described (Yuan, 1998; Parish et al., 2003; Hu et al., 2006; Stewart et al., 2006; Rustad et al., 2008; Converse et al., 2009). In one particular study, an hspX mutant was originally described with attenuated growth in macrophages (Yuan, 1998), however, these studies were conducted before the function of other genes in the proximity of Rv2031c (hspX) were identified, nor were their promoters known. More recently it was shown that an in frame acg internal deletion strain (with hspX and Rv2030c intact) significantly reduced the virulence of Mtb in mice, while a similar Rv2030c mutant did not, thereby suggesting that Acg is needed for mycobacterial virulence and growth in the host (Hu et al., 2011). Acg has been described as putative nitrate reductase, which are known virulence determinants in bacteria (Moreno Vivian et al., 1999). The data presented in this study supports this concept; as acg expression was nearly ablated in X4 19 (Fig. 8), concomitant with a loss in virulence. In addition to the contribution that acg or hspX expression has on virulence, other phenotypic differences may be required for the establishment of a productive Mtb infection in guinea pigs. Indeed, a comparison of the virulence of WT and ΔHspX Mtb in the guinea pig demonstrated marked differences between these two strains, in contrast to our studies in the mouse (Taylor et al., 2012). In this study, 16 SNPs unique to X4 19 were identified by whole genome sequence analysis, including four which affect the amino acid sequence (Rv1062, Rv1771, and Rv1907c) or translation (Rv3479) of the resultant protein, and thus may also contribute to the loss of virulence phenotype that was observed in the present study. Further studies are required to determine the relevance of these SNPs.
The idea for using HspX as a vaccine target is tempting because it is up regulated during hypoxia and stress (Sherman et al., 2001), can be found in the sera of latently infected patients (Yuan, 1998) and is preferentially recognized by persons presenting with LTBI (Geluk et al., 2007). At the present time, the only vaccine available for use in humans is live, attenuated M. bovis BCG, which offers very limited protection against adult pulmonary TB. Since BCG does not protect against reactivation from LTBI, mycobacterial antigens associated with LTBI, including HspX, are being pursued in promising new generation multi protein sub unit vaccine platforms (Chen et al., 2010; Shi et al., 2010; Jeon et al., 2011; Li et al., 2011; Niu et al., 2011; Xin et al., 2013); it is important to note that none of our formulations were tested in a BCG prime boost model and may still be promising vaccine candidates when used as sub unit booster vaccines.
Our studies presented here demonstrated that the 16 kDa heat shock protein from M. tuberculosis did not elicit a protective immune response when used as a single antigen subunit vaccine in the guinea pig model of tuberculosis. The complete elucidation of the binding partners or physiochemical requirements for HspX to retain its biological activity and how this relates to establishment of active disease vs. chronic infection and vaccine protection are ongoing studies in our laboratory. Although our current hypothesis is that native HspX requires its binding partners to retain its biological potency as a vaccine, the physiochemical requirements of HspX folding and properties as a chaperone are unknown. Thus far, proteomic analyses using mass spectrometry have not provided compelling evidence of discreet chaperoned proteins or peptides bound to HspX (our unpublished observations). Other experiments aimed at determining the requisite binding partners required to maintain the biological potency of HspX as a vaccine are in progress.
Acknowledgements
The authors wish to acknowledge Ms. Rachel Flinkstrom, Haley Conde, and Mr. Nick May for technical assistance with the animal experiments. The authors also thank Drs. Nicole Kruh Garcia and Lisa Wolfe for their insightful discussions during the preparation of this draft. This work was funded in part by the NIH NIAID Contract # HHSN266200400091c (KMD), and internal funds granted from Colorado State University (KMD).
References
Supporting Information
Table S1. CFU of Mtb in the lung and spleen (measured at necropsy for all animals).
Alpha crystallin (Acr, HspX), is a latency associated protein of Mycobacterium tuberculosis and a subunit vaccine candidate. Several subunit HspX formulations in a guinea pig model of TB were not protective against challenge with virulent Mtb. However, recombinant HspX was protective in animals challenged with a strain of Mtb lacking hspX (X4 19). Mtb X4 19 was significantly less virulent than wild type Mtb perhaps due to altered expression of several genes that were identified, emphasizing the need for careful biochemical analysis of Mtb challenge strains in vaccine modelling experiments.
Editor: Patrick Brennan



