-
PDF
- Split View
-
Views
-
Cite
Cite
Barry Rockx, Recent developments in experimental animal models of Henipavirus infection, Pathogens and Disease, Volume 71, Issue 2, 1 July 2014, Pages 199–206, https://doi.org/10.1111/2049-632X.12149
Close - Share Icon Share
Abstract
Hendra (HeV) and Nipah (NiV) viruses (genus Henipavirus (HNV; family Paramyxoviridae) are emerging zoonotic agents that can cause severe respiratory distress and acute encephalitis in humans. Given the lack of effective therapeutics and vaccines for human use, these viruses are considered as public health concerns. Several experimental animal models of HNV infection have been developed in recent years. Here, we review the current status of four of the most promising experimental animal models (mice, hamsters, ferrets, and African green monkeys) and their suitability for modeling the clinical disease, transmission, pathogenesis, prevention, and treatment for HNV infection in humans.
Review of current animal models of henipaviruses available for studying disease and testing treatments.
Introduction
Hendra (HeV) and Nipah (NiV) viruses (genus Henipavirus (HNV; family Paramyxoviridae) are emerging zoonotic agents that can cause severe respiratory distress and acute encephalitis in humans and are considered biosafety level 4 (BSL 4) pathogens. Following the initial identification of HeV and NiV in 1994 and 1998, respectively, almost yearly outbreaks of HeV and NiV have occurred in Australia (HeV) and Bangladesh (NiV) over the past decade (Rockx et al., 2012).
Fruit bats (Pteropodidae family) are considered to be the reservoir for HNV (Young et al., 1996; Olson et al., 2002) with a geographic distribution extending beyond South East Asia and Australia to West Africa (Hayman et al., 2008). Bats infected with HNV primarily shed virus in urine and transmit to humans through infection of intermediate hosts such as horses (HeV) and pigs (NiV, Malaysia) (Luby et al., 2009). Outbreaks of NiV in Bangladesh have primarily been associated with the consumption of raw date palm sap, which is believed to be contaminated with NiV from bats urinating in the sap collection jars (Rahman et al., 2012). Importantly, person to person transmission has been observed in several outbreaks of NiV in Bangladesh (Gurley et al., 2007), highlighting the potential of wider spread of this virus (Luby, 2013).
Given the lack of effective therapeutics and vaccines for human use, these viruses are considered as public health concerns and listed as category C priority pathogens for biodefense research by the National Institute of Allergy and Infectious Diseases.
Unlike many other paramyxoviruses, HNV displays a broad species tropism. Here, we review the current status of four of the most promising experimental animal models and their suitability for modeling the clinical disease, transmission, pathogenesis, prevention, and treatment for henipavirus infection in humans.
Pathogenesis in humans
Hendra virus
Hendra virus (HeV) was first isolated in 1994 during an outbreak of severe respiratory disease and febrile illness in horses and humans in Queensland, Australia (Selvey et al., 1995). A total of seven human cases have been reported to date, with a case fatality rate of 57% (Selvey et al., 1995; O’sullivan et al., 1997; Wong et al., 2009; Playford et al., 2010; Rockx et al., 2012). Following an incubation period of 7–16 days, human cases develop an influenza like illness (ILI). Cases can develop acute meningitis and/or encephalitis with tonic–clonic seizures, recurrent focal, and motor seizures, which can rapidly progress to coma (O’sullivan et al., 1997). Of four fatal human cases, three were due to severe neurological disease (encephalitis), while one case exhibited severe respiratory distress, multiorgan failure, and arterial thrombosis with chest radiographs showing bilateral alveolar and interstitial infiltration. One of the cases who recovered from the initial infection died from a fatal relapse of acute encephalitis 13 months later (O’sullivan et al., 1997). In survivors, residual neurological symptoms have been observed in survivors including residual ataxia (Playford et al., 2010).
At autopsy, histopathological changes associated with HeV infection in humans are characterized by vasculitis and syncytial formation in the endothelium of target organs such as brain, liver, spleen, and lungs. In the brain, leptomeningitis with lymphocyte and plasma cell infiltration is observed (O’sullivan et al., 1997). Focal areas of necrosis are present in the neocortex, basal ganglia, brainstem, and cerebellum, whereas in the lungs, gross pathological changes include congestions and hemorrhages (Selvey et al., 1995). Histopathological changes in the lungs include severe parenchymal inflammation and necrosis with focal necrotizing alveolitis and intra alveolar macrophages/inflammatory cells (Selvey et al., 1995; Wong et al., 2009). Type II pneumocytes, alveolar macrophages as well as vascular endothelium are positive for viral antigen (Wong et al., 2009).
Nipah virus
NiV was first isolated during an outbreak of respiratory disease and acute encephalitis in pigs and humans in Malaysia and Singapore (NiV M) in 1998–1999 (Chua et al., 1999, 2000). During that outbreak, a total of 276 human cases were identified with a case fatality rate of 40%. Following an incubation period of 4 days to 2 weeks, cases initially presented with ILI. The majority of cases presented with fever, headache, dizziness, and vomiting. More than half of patients exhibited reduced levels of consciousness, cognitive impairment, and prominent brain stem dysfunction. Distinctive clinical signs included segmental myoclonus and hypotonia, suggesting involvement in the brain stem and the upper cervical spinal cord.
Respiratory symptoms following NiV M infection are observed in c. 20% of cases and characterized by nonproductive cough, sore throat, dyspnea, and chest pain. In these cases, chest radiographs are abnormal with upper/lower lobe alveolar consolidation and reticular changes.
Since the initial isolation of the Malaysia strain of NiV (NiV M) in 1998–1999, a genetically distinct strain of NiV (Bangladesh, NiV B) has been the cause of outbreaks in humans in Bangladesh and India from 2001 to 2013 (Lo & Rota, 2008; Rockx et al., 2012). These genetically distinct NiV strains are distinguished by differences in clinical outcome in human cases. Specifically, the incubation period during the NiV B outbreaks in Bangladesh/India is shorter compared with NiV M outbreak in Malaysia with an onset of clinical symptom ranging from 6 to 11 days (Hossain et al., 2008; Lo & Rota, 2008). In addition, the proportion of cases presenting with severe respiratory symptoms is higher during the outbreaks of NiV B (69%) compared with NiV M (c. 20%). Finally, the case fatality rate of NiV B is higher compared with that of NiV M, 73% vs. 40%, respectively (Luby et al., 2006).
During NiV B infection, 69% experienced respiratory difficulties and chest radiographs, showing diffuse bilateral opacities consistent with acute respiratory distress syndrome (ARDS). Respiratory distress during NiV B infection was significantly associated with death (Hossain et al., 2008).
The majority of patients who survive acute NiV encephalitis make a full recovery. Interestingly, about 22% of survivors are left with residual neurological sequelae such as persistent convulsions and personality changes. Similar to HeV, c. 8% of patients, who recover from infection with NiV, subsequently develop relapse encephalitis as late as 4 years after infection. In addition, 3% of cases with an initial asymptomatic or nonencephalitic infection develop delayed onset encephalitis (Tan et al., 2002). In the long term, persistent neurological dysfunctions are observed in more than 15% of people.
Histopathological changes include vasculitis and syncytial endothelial cells in the central nervous system, lung heart, and kidney and are characterized by endothelial destruction, fibrinoid necrosis, and inflammatory cell infiltration (Wong et al., 2002).
The main histopathological findings in the brain include necrotic plaques, perivascular cuffing, thrombosis, parenchymal inflammation, and meningitis (Wong et al., 2002). Eosinophilic inclusions in the cytoplasm of neurons were highly positive for viral antigen.
In the lung, histopathological changes were mainly characterized by alveolar hemorrhage, pulmonary edema, and aspiration pneumonia (Wong et al., 2002). Fibrinoid necrosis and vasculitis in the lung were observed in a majority of cases. Multinucleated giant cells were occasionally observed in the alveolar spaces.
Additional organs with histopathological changes involved lymph nodes, kidney, and spleen, with white pulp depletion and acute necrotizing inflammation for the latter. In lymph nodes, large reactive mononuclear cells were observed as well as necrosis. Finally, histological changes in the kidney primarily included glomerular inflammation and necrosis.
Henipavirus infection in animals
Unlike other paramyxoviruses, HNV can infect a wide range of animal species. Natural hosts include bats, pigs, horses, cats, and dogs. Experimental challenge studies in larger species such as pigs and horses have been limited to two research facilities studying these zoonotic pathogens in their natural hosts (Weingartl et al., 2005; Marsh et al., 2011).
Experimental infection in cats primarily results in respiratory disease and does not consistently recapitulate neurological disease (Middleton et al., 2002). Conversely, experimental infection in guinea pigs is primarily associated with encephalitis (Torres Velez et al., 2008). More recently, hamster, ferret, and African green monkey (AGM) models were developed and characterized, which more closely mimic the disease progression, both respiratory and neurological, seen in human cases (Wong et al., 2003; Bossart et al., 2009; Geisbert et al., 2010; Rockx et al., 2010, 2011a). In addition, two mouse models have recently been described that primarily develop fatal encephalitis (Dups et al., 2012; Dhondt et al., 2013). Here, the recent development and suitability of the mouse, hamster, ferret, and AGM models for studying the pathogenesis of HNV as well as their use in testing countermeasures are reviewed (Table 1).
Characteristics of Henipavirus animal models and their use in studies of pathogenesis and testing of countermeasures
AGM, African green monkey.
Only recapitulates neurological disease.
Immunosuppressed model (aged or type I interferon signaling knockout).
Limited reagents available.
Characteristics of Henipavirus animal models and their use in studies of pathogenesis and testing of countermeasures
AGM, African green monkey.
Only recapitulates neurological disease.
Immunosuppressed model (aged or type I interferon signaling knockout).
Limited reagents available.
Hamster
Syrian golden hamsters (Mesocricetus auratus) can be lethally challenged with HNV via the intranasal or intraperitoneal routes (Wong et al., 2003; Guillaume et al., 2009; Rockx et al., 2011a; DeBuysscher et al., 2013). Infection with a high dose of HNV results in severe acute respiratory distress characterized by labored breathing, serosanguineous nasal and oral exudates, and radiological changes, including rapidly progressive increases in pulmonary infiltrates occurring as early as day one postchallenge. Death typically occurs within 3–5 days postchallenge. Interestingly, animals that are challenged with a low dose of HNV develop respiratory distress, but in addition, also develop severe neurological disease at the end stage of disease by day 7–10. Neurological disease is characterized by partial or complete paralysis, muscle fasciculations, and seizures.
Gross pathological lesions are primarily seen in lungs and brain. Large hemorrhagic lesions are present on the lungs and can cover as much as 100% of the surface of the lung by the end stage. Congestion of vessels in the brain was also observed occasionally.
Histopathological changes are similar between the HNV strains but can differ in severity and occur at earlier time points in HeV vs. NiV M vs. NiV B infected animals (Rockx et al., 2011a; DeBuysscher et al., 2013). Pulmonary lesions begin as focal to multifocal, predominantly peribronchial, areas of mononuclear inflammation admixed with scattered neutrophils and associated with necrosis of alveolar walls. Over time lesions expand in size, and viral syncytial cells are observed in both bronchial epithelium and the interstitium. At the end stage of disease, multifocal areas of inflammation, necrosis, fibrin exudation, and hemorrhage are observed in the lungs with severe consolidation of entire lung lobes in several cases.
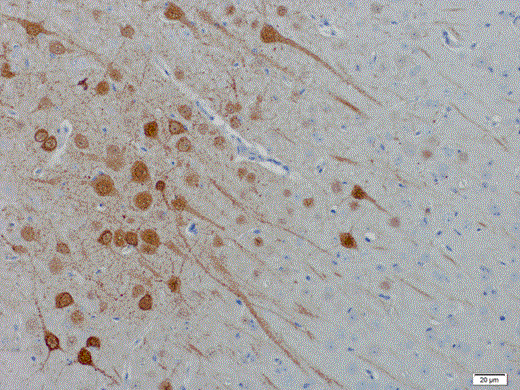
Histopathological lesions in the brain are characterized by multifocal areas of neuropil vacuolation (Fig. 1a), neuronal necrosis, and moderate to severe meningitis (Fig. 1b). Blood vessels, neurons, glial cells, neuropil, ependyma, and meninges stain positive for viral antigen. Lesions are randomly distributed throughout the brain, affecting cerebrum, cerebellum, hippocampus, and olfactory cortex simultaneously. Additional organs that are affected include heart, liver, kidneys, and bladder and are characterized by multifocal neutrophilic, histiocytic, and lymphocytic inflammation with or without necrotizing vasculitis. Infectious HNV can be recovered in a range of tissues, although HeV seems to be more efficient in systemic dissemination.
Tropism of lethal Henipavirus infection in hamsters. Hendra virus antigen is present in (a) focal areas of neuropil vacuolization in the brain, (b) areas of meningitis, and (c) olfactory epithelium in nasal turbinates. Sections were stained for HNV nucleoprotein (brown).
The hamster model has been used to study transmission and pathogenesis as well as testing of countermeasures. Interestingly, a difference in initial sites of replication was observed between HeV and NiV M. NiV M initially replicates in the epithelium of the trachea and bronchi and progressing to the alveoli and lung interstitium, whereas HeV only replicates in the alveolar epithelium and interstitium. This provides a potential mechanism for the differences in the efficiency of zoonotic transmission observed between NiV M and HeV outbreaks (Rockx et al., 2011a). More recently, this model was use to study animal to animal transmission of NiV M (De Wit et al., 2011). Virus transmission primarily occurred through direct contact and not via aerosols (De Wit et al., 2011). Overall, animal to animal transmission was not very efficient which is in line with the limited human to human transmission observed during outbreaks. Transmission studies have not been reported for HeV or the Bangladesh strain of NiV.
Studies into the pathogenesis of these viruses in hamsters have been limited by the lack of hamster specific reagents. The recent development of molecular assays for the characterization of hamster gene expression (Zivcec et al., 2011) has been crucial in characterizing the host response to HNV infection (Rockx et al., 2011a; DeBuysscher et al., 2013). Interestingly, HNV infection of the brain results in increased permeability of the blood–brain barrier (BBB) and expression of TNF α and IL 1β (Rockx et al., 2011a). These pro inflammatory cytokines play a role in BBB permeability and the induction of neuronal injury and death. It is unknown whether disruption of the BBB is a direct cytopathic effect of virus replication in the endothelium or an indirect effect through expression of TNF α and IL 1β by neurons and microglia.
Two routes of HNV entry into the CNS have been proposed. First, following intranasal challenge, HNV initially targets the olfactory epithelium in the nasal turbinates (Munster et al., 2012) (Fig. 1c). It has been shown that NiV M infected neurons can extend through the cribriform plate and into the olfactory bulb as a mode of entry into the CNS (Munster et al., 2012). Second, while lymphocytes are not permissive to infection with NiV, it has been shown that the virus can efficiently bind to leukocytes and transfer infection to endothelial and Vero cells (Mathieu et al., 2011). In hamsters, NiV bound leukocytes can transfer lethal NiV infection into naïve animals, demonstrating efficient virus transinfection in vivo as a method of viral dissemination.
Finally, the hamster model has been used to test the efficacy of a variety of antiviral and vaccine candidates such as chloroquine, ribavirin, poly I:C, neutralizing antibodies, and adeno associated virus vectored vaccines (Guillaume et al., 2004, 2006, 2009; Georges Courbot et al., 2006; Freiberg et al., 2010; Porotto et al., 2010; Ploquin et al., 2013).
Ferrets
In ferrets (Mustela putorius furo), HNV infection via the oro nasal route results in the development of fever at days 4–7 postchallenge and rapidly progresses to severe respiratory distress and neurological signs between days 6 and 10 (depending on challenge dose). Clinical signs include cough, nasal discharge, shortness of breath, edema of the head, tremors, and partial or complete limb paralysis (Bossart et al., 2009; Pallister et al., 2011). Unlike hamsters (Rockx et al., 2011a), and no correlation between challenge dose and clinical outcome is observed in these animals (Bossart et al., 2009).
Gross pathological changes are primarily limited to the respiratory tract and include scattered small pinpoint hemorrhagic lesions on the surface of the lungs. These lesions are notably distinct from the large hemorrhagic lesions observed in hamsters and AGM (Geisbert et al., 2010; Rockx et al., 2010, 2011a). In addition to lesions in the lungs, hemorrhages in lymph nodes can be observed in some animals.
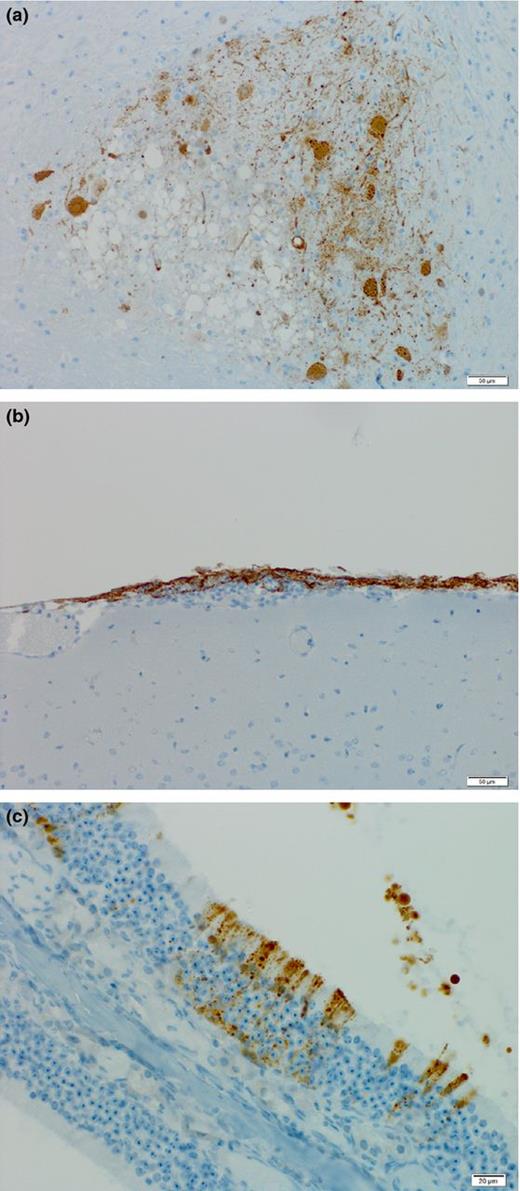
Histopathological changes primarily involve the lungs, spleen, and kidneys (Bossart et al., 2009). They are characterized by acute focal necrotizing alveolitis and pulmonary vasculitis in the lungs (Fig. 2a) and by necrosis in spleen and kidney. While typically no evidence of vasculitis or encephalitis is observed in these animals, nonsuppurative meningitis can be observed occasionally.
Tropism of lethal Henipavirus infection in ferrets. Hendra virus antigen is present in (a) focal areas of necrotizing alveolitis and (b) neurons in the brain. Sections were stained for HNV nucleoprotein (brown).
Viral antigen is detected in syncytial cells of small blood vessels and the alveolar walls in lungs as well as in the necrotic glomerular and tubular epithelium in the kidneys. Despite the near absence of histopathological changes in the brain, HNV antigen can be detected in meningeal blood vessels, choroid plexus endothelium and in neurons (Fig. 2b). HNV genome can be detected in a variety of tissues including the respiratory tract, brain, liver, spleen, and kidneys. In addition, low level viremia can be detected in most animals. Finally, low level virus shedding is observed in both pharyngeal and rectal swabs.
This model has been used to compare the possible differences in transmission and pathogenesis between the Malaysia and Bangladesh strain of NiV (Clayton et al., 2012). Over the course of infection in ferrets, significantly higher levels of virus are recovered from oral secretions of animals infected with the Bangladesh strain compared with NiV M. However, no attempt at studying animal to animal transmission has been made in this model.
While reagents for ferrets are limited, several immunological and molecular assays are now available to study the host responses following viral infection (Rowe et al., 2010; Ljungberg et al., 2012; Leon et al., 2013). In addition to transmission and pathogenesis studies, these models have also been successfully used to test both passive immunization and vaccine candidates, as well as studying long term immunity of a vaccine candidate (Bossart et al., 2009; Pallister et al., 2011, 2013).
Nonhuman primates
To date, experimental challenge studies with HNV have only been reported for squirrel monkeys (Saimiri sciureus) and African green monkeys (AGM; Chlorocebus aethiops) (Geisbert et al., 2010; Marianneau et al., 2010; Rockx et al., 2010). Experimental infection of squirrel monkeys requires an intravenous challenge and does not result in a uniformly lethal model (Marianneau et al., 2010).
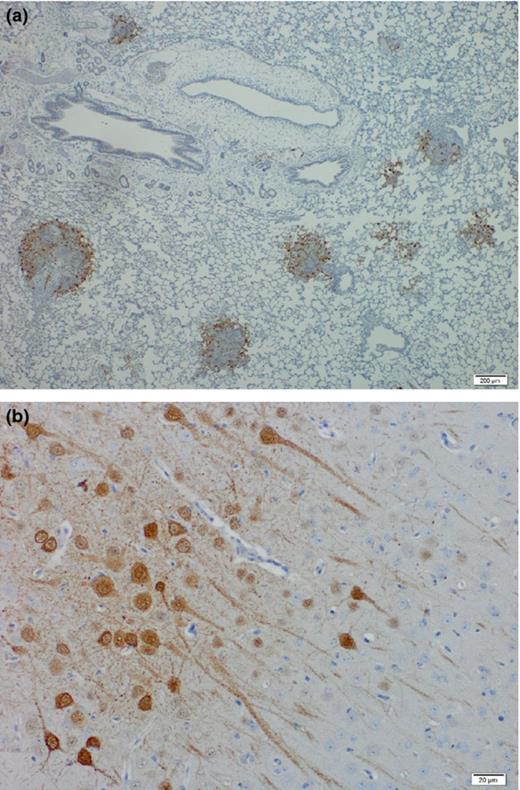
Intratracheal inoculation of AGM with HeV or NiV (Malaysia strain) results in a uniformly lethal infection with animals dying of acute respiratory distress within 7–9 or 9–12 days postchallenge, respectively (Geisbert et al., 2010; Rockx et al., 2010). Clinical signs include nasal discharge and labored breathing. Chest x rays show an acute onset of extensive diffuse interstitial infiltrates 1–2 days prior to death. Gross pathological changes are primarily limited to the respiratory tract and include sanguineous discharge from the nares, edema, hemorrhagic lesions of the lungs covering over 90% of the surface and the presence of serous fluid in the thoracic cavity (Rockx et al., 2010). In addition to the lesions in the lung, congestion of vessels in the brain is also observed as well as focal hemorrhages in the bladder in some animals.
Interestingly, while neurological signs are typically not observed in HNV infected animals that succumb, animals that either show a delay in time to death (Rockx et al., 2010) or survived due to treatment strategies (Bossart et al., 2011) exhibit neurological signs. These include behavioral changes, muscle fasciculations of the arms and face and seizures (Rockx et al., 2010; Bossart et al., 2011).
Histopathology in the lungs is characterized by acute respiratory distress syndrome (ARDS) such as changes including alveolar hemorrhaging, pulmonary edema, and inflammation. Syncytia formation in lung endothelial cells is prominent. HNV antigen in the lungs is primarily detected in endothelial cells of small blood vessels and the alveolar walls. In the brain, focal areas of HNV antigen are observed in neuron cell bodies and axons as well as surrounding cells in animals with and without neurological signs.
Similar to hamsters and ferrets, infectious HNV can be recovered in a wide range of tissues, including respiratory tract, brain, heart, liver, spleen, and kidney suggesting efficient dissemination of the virus and a broad tissue tropism (Geisbert et al., 2010; Rockx et al., 2010).
Information on the pathogenesis of HNV in the AGM model has been limited to data from nonlethal procedures (x ray, blood sampling, virus shedding) and the end stage of disease for HeV and the Malaysia strain of NiV. To date, no studies have been published using the Bangladesh strain. A wide variety of reagents are available to study the pathogenesis of viruses in NHP (Rockx et al., 2011b; Safronetz et al., 2011; Richt et al., 2012).
Despite the recent development and limited characterization of the AGM model, it is considered the ‘gold standard’ for testing the efficacy of countermeasures against HNV infection. These models have already been instrumental in testing the efficacy of antivirals (Rockx et al., 2010), passive immunization (Bossart et al., 2011), and vaccination (Bossart et al., 2012; Yoneda et al., 2013).
Mice
During initial studies, mice were found to be resistant to infection with HeV and NiV, with exception of intracranial injection in juvenile mice (Westbury et al., 1995; Wong et al., 2003). More recently, two mouse models have been described (Dups et al., 2012; Dhondt et al., 2013). First, a new model for HeV encephalitis in mice shows that aged animals (Balb/c and C57BL/6 strains) are susceptible to HeV infection via the intranasal route (Dups et al., 2012). Infected animals exhibit signs of ataxia, muscle tremors, and hypersensitivity. Histopathological changes include neuronal degeneration, microglial activation, glial reaction, perivascular cuffing, and nonsuppurative meningitis with a lack of vasculitis. Viral antigen is present in the olfactory tract, the cortex by the olfactory tract and the piriform lobe, suggesting that HeV potentially enters the CNS via an anterograde route along the olfactory sensory neurons (Dups et al., 2012), similar to what has been shown in hamsters (Munster et al., 2012).
In addition to aged animals, it has also been shown that mice lacking the receptor for type I interferon are susceptible to both HeV and NiV (Dhondt et al., 2013). In this model, animals challenged via the intraperitoneal route develop acute and fatal encephalitis within 8 days postchallenge. Histopathological changes in the brain include parenchymal and meningeal nonsuppurative inflammation, vasculitis, and perivascular cuffing. Ependymal cells and neurons are positive for viral antigen.
While these models do not recapitulate the respiratory disease seen in human cases, they can be useful in studying the pathogenesis of HNV in the CNS. In addition, the availability of an extensive range of reagents for mice as well as transgenic mice on these genetic backgrounds will greatly improve the understanding of HNV neuropathogenesis. Finally, these models will be useful for early testing for efficacy of novel antiviral drugs.
Conclusion
Experimental models of HNV infection that mimic the disease progression seen in humans are crucial in furthering our understanding of the pathogenesis of these viruses. The availability of these well characterized animal models and the recent development of reverse genetics systems for HNV will allow for in depth studies into the role of host and virus genes in HNV pathogenesis (Yoneda et al., 2006, 2010; Marsh et al., 2013). In addition, these recombinant HNV can now be used to express a reporter gene (Marsh et al., 2013) for detection by in vivo small animal molecular optical imaging.
While these animal models are very promising, caveats exist in the translation of findings from animal models to human cases of HNV infection. In particular, it has been shown that entry into the CNS can be achieved via the olfactory bulb; however, it is unknown whether this route is also biologically relevant in human infections, as the olfactory epithelial surface is relatively large in these species compared with man. No evidence of olfactory bulb involvement was reported in a limited number of human cases (Wong et al., 2002). It also remains unclear whether the genetic differences between the Bangladesh and Malaysia strains of NiV are responsible for the observed differences in transmission and pathogenesis. In human cases, the case fatality rate is higher in outbreaks of NiV B compared with those with NiV M; however, in hamsters and ferrets, there is only a limited difference in virulence (Clayton et al., 2012; DeBuysscher et al., 2013). And in fact, in these models, NiV M appears to be more virulent. The availability of a reverse genetics system will now allow us to study the role of this genetic variability in the differences in disease outcome between the distinct HNV strains by creating chimeric viruses and testing them in these animal models. Finally, while late onset encephalitis and recrudescence of HNV infection have been observed in human cases, these have not been studied in any of the animal models.
With regard to the development of countermeasures, the availability of a lethal mouse model will be invaluable for initial screening of antiviral candidates in the mouse model. As HNV is highly virulent, human efficacy studies are neither ethical nor feasible during the development of countermeasures. Therefore, the Food and Drug Administration has published the ‘Animal Rule’, which requires that experimental animal models of HNV are available which recapitulate the disease progression seen in humans and are expected to react to infection and treatment in these models with a response predictive for humans (Snoy, 2010). For these purposes, evaluation of these countermeasures, using the hamster or ferret model, is recommended with the ultimate goal of efficacy testing in the AGM model.
Acknowledgements
This work was funded by start up funds of Department of Pathology and the Institute of Human Infections & Immunity, University of Texas Medical Branch, Galveston, TX. Thanks to Drs. Olivier Escaffre and Viktoriya Borisevich at the UTMB for critically reading this manuscript.
References
A review of these BSL 4 level select agents is a timely addition to the repertoire of papers that address animal models that may be used to study these highly dangerous agents.
Editor: Gerald Byrne



