-
PDF
- Split View
-
Views
-
Cite
Cite
Geetha Subramanian, Abdoulaye O. Barry, Eric Ghigo, Didier Raoult, Oleg Mediannikov, Antibiotic susceptibility and intracellular localization of Diplorickettsia massiliensis, FEMS Immunology & Medical Microbiology, Volume 64, Issue 1, February 2012, Pages 48–56, https://doi.org/10.1111/j.1574-695X.2011.00885.x
Close - Share Icon Share
Abstract
Diplorickettsia massiliensis is an obligate intracellular bacterium from the Coxiellaceae family recently isolated from Ixodes ricinus ticks. The inhibitory effects of antimicrobial agents were assessed by two different methods, immunofluorescence and Gimenez staining assay. Different markers (EEA1, Lamp-1, Cathepsin D, and LysoTracker Red DND99) were used to reveal the nature of the vacuole containing the bacterium. Ciprofloxacin, levofloxacin, and rifampin had MIC values of 2 µg mL−1. We found that 4 µg mL−1 of Doxycycline inhibited the growth of D. massiliensis strain. Surprisingly,D. massiliensis was resistant to chloramphenicol up to the concentration of 64 µg mL−1. We found that penicillin G, ammonium chloride, gentamycin, omeprazole, bafilomycin A1, and chloroquine were not active against D. massiliensis. Studies performed with markers EEA1, Lamp-1, Cathepsin D, and LysoTracker Red DND99 showed that D. massiliensis is localized within an acidic compartment that is not an early phagosome, but a late phagosome or a phagolysosome. Gimenez staining stays a good method that will work with a very low number of bacteria and can be used to determine the MICs of new therapeutic antibiotics precisely. The resistance profile of D. massiliensis was found to be quite unusual for intracellular Gram-negative bacterium with marked resistance to chloramphenicol. Despite of localization in acidic compartment, pH-neutralizing agents do not significantly inhibit intracellular growth of bacterium. The results of these studies prove that antibiotic resistance does not depend on pH of vacuole. This pH-related mechanism seems not to play a contributing role in the overall resistance of D. massiliensis.
Introduction
The recently described bacterium Diplorickettsia massiliensis is an obligate intracellular Gram-negative bacterium (Mediannikov et al., 2010). The only available strain of D. massiliensis was isolated from Ixodes ricinus tick collected from southeastern part of the Slovak republic forest Rovinka in 2006. Comparative sequence analysis of the 16S rRNA gene showed that it is phylogenetically related to the genus Rickettsiella; further, it can be grouped into the family Coxiellaceae and the order Legionellales of γ-proteobacteria. The order Legionellales is comprised of two families, Legionellaceae and Coxiellaceae. Many species of Legionella in this monotypic family cause legionellosis, most notably Legionella pneumophila. The Coxiellaceae family comprises two genera, Coxiella and Rickettsiella (La Scola et al., 2001); Coxiella burnetii, a Coxiella family member and intracellular bacterium, is the causative agent of Q fever (Maurin & Raoult, 1999). The genus Rickettsiella currently includes four officially recognized species: Rickettsiella popilliae, Rickettsiella grylli, Rickettsiella chironomi, and Rickettsiella stethorae. These intracellular pathogens infect a wide range of arthropods including insects, crustaceans, and arachnids (Fournier & Raoult, 2005). The tick Ixodes ricinius from which D. massiliensis was isolated harbors a wide spectrum of microorganisms that are pathogenic to both humans and animals including: Borrelia burgdorferi sensu lato, Rickettsia spp., Babesia spp., Ehrlichia spp., Anaplasma phagocytophilum, Bartonella henselae, and Bartonella quintana (Reye et al., 2010). Recently, we observed three cases from Marseille, France (one has just returned from Slovenia). Patients symptoms were fever, skin eschar, arthralgia, and myalgia (Subramanian et al., 2011).
As D. massiliensis is an obligate intracellular bacterium (Mediannikov et al., 2010) localized within a compartment, in vitro studies to assess antibiotic susceptibility necessitate the use of cell culture systems. The intracellular environment is likely to influence both the susceptibility of the intracellular pathogen and drug activity. It has long been assumed that the intracellular accumulation of an antibiotic is indicative of efficient activity against intracellular bacteria. Antimicrobial therapy targeting an intracellular pathogen is more complex than an extracellular target; intracellular antimicrobial activity additionally depends on drug penetration into and accumulation within the cell, cellular metabolism, sub-cellular disposition, and the bioavailability of the drug (Van et al., 2006). Further, the efficiency of an antibiotic is related to the intracellular localization of the targeted bacteria. Pathogenic bacteria, including Mycobacterium spp., Ehrlichia spp., Salmonella spp., Francisella tularensis, Legionella spp., Brucella spp., and Yersinia enterocolitica (Maurin & Raoult, 1997), reside in atypical phagosomes; in general, these atypical phagosomes are more or less permissive for antibiotics or contain (i.e. pH) affecting the chemical properties of the antibiotic. Thus, the relative inefficiency of doxycycline treatment in Q fever (Maurin & Raoult, 1999) is closely related to the fact that C. burnetii is localized in an acidic compartment of the host cell (Beron et al., 2002). In this study, we have determined the kinetics of activity on growth and the minimum inhibitory concentration (MIC) of 12 antibiotics against D. massiliensis; results were obtained through the observation of bacterial growth in shell vials using Gimenez staining for visualization. We also studied the intracellular localization of D. massiliensis to examine different patterns of antibiotic resistance.
Materials and methods
Bacterial strain and MIC determination
Diplorickettsia massiliensis was cultured as described previously (Mediannikov et al., 2010). Briefly, D. massiliensis was cultured in XTC-2 cells at 28 °C with Leibovitz-15 media (Invitrogen) supplemented with 2% fetal bovine serum (FBS) and 2% tryptose-phosphate broth solution (Sigma-Aldrich, Ayrshire, UK). Diplorickettsia massiliensis was also grown in the human MRC-5 and human erythroleukemia (HEL) cell lines (Mediannikov et al., 2010) at 32 °C using minimum essential media containing 5% fetal calf serum and supplemented with 2 mM l-glutamine (Gibco).
Cells became heavily infected usually after 5 days of incubation for XTC-2 cells and 7 days of incubation for MRC-5 cells. Cell supernatants were then discarded from the flasks, and the infected cells were detached using sterile glass beads. The supernatants were diluted 1 : 200 in the culture media. This dilution was used to infect XTC-2 and MRC-5 monolayers in shell vials. The XTC-2 vials were incubated at 28 °C, and the MRC-5 vials were incubated at 37 °C in 5% CO2.
Antibiotics and other tested substances used in this study were tested in serial twofold dilutions as follows: doxycycline, 0.25–8 µg mL−1 (Pfizer, Neuilly, France); chloramphenicol, 0.25–64 µg mL−1 (Roussel, Paris, France); erythromycin, 0.25–132 µg mL−1 (Roussel); rifampin, 0.25–8 µg mL−1 (Merrel Dow, Neuilly/Seine, France); ciprofloxacin, 0.25–8 µg mL−1 (Bayer Pharma, Sebs, France); levofloxacin, 0.25–8 µg mL−1 (Hoechst Marion, Roussel); penicillin G, 0.25–132 µg mL−1 (Diamant); gentamicin, 0.25–132 µg mL−1 (Dakota Pharm, Creteil, France); ammonium chloride, 1 to 100 mM (Coger, Paris, France); bafilomycin A1, 2.5–80 nM, (Sigma, Germany); omeprazole (Sigma, France); and chloroquine, 0.25–8 µg mL−1 (France). Stock solutions were prepared according to the manufacturer's instructions and stored at −80 °C until used. Working solutions were prepared just prior to antibiotic treatment by diluting stock solutions in the respective media required. Dilutions of each antibiotic were added to rows of infected cells; positive controls consisted of infected cells in the absence of antibiotics, and negative controls consisted of uninfected cells in the presence of antibiotics. We harvested cells in 15-day period to be sure that the effect of antibiotic or other substances developed fully. After incubation, shell vials were stained with the Gimenez stain. Smears were prepared on glass slides with a centrifugal slide maker (Cytospin II; Shandon, Cheshire, UK), and the number of intracellular D. massiliensis was counted at 100× magnification. The MIC was defined as the lowest antibiotic concentration that inhibited growth of the D. massiliensis. Experiments were performed three times with an interval time to confirm the results.
Determination of degree of infection
The percentage of infected cells was determined by the direct microscopic examination of cells stained utilizing the Gimenez technique (Gimenez, 1964). A minimum of 200 cells were examined in each prepared slide to determine the proportion of infected cells. The percentage of infected cells was assessed at a magnification of 100×.
A minimum of 20–40 cells were observed for D. massiliensis counting of per host cell under the microscope. So we checked and calculated the number of bacteria per cells.
Indirect immunofluorescence assay (IFA)
IFA was used to confirm the bacteriostatic activity of antibiotics by the inhibition of growth in shell vials (Raoult et al., 1991). After 15 days of growth, infected cells were fixed in the shell vials for 10 min with absolute ethanol, rehydrated for 5 min in phosphate-buffered saline (PBS), and incubated for 30 min at 37 °C with 0.3 mL of anti-D. massiliensis mouse serum. Polyclonal antibodies directed against D. massiliensis were generated in our laboratory and suspended in PBS (titer, 1 : 400). Next, cover slips were rinsed, washed three times with PBS (10 min each), and then incubated for 30 min at 37 °C with 0.3 mL of fluorescein-conjugated goat anti-mouse immunoglobulin (1 : 100) (Bio-Merieux, Charbonnieres-les-Bains, France). After incubation, the cover slips were washed three times for 5 min each in PBS, mounted on slides, and examined by fluorescence microscope at a magnification of 40×. The MIC was defined as the lowest antibiotic concentration that caused growth inhibition of bacteria compared with the control sample of day 0. Several visible intracellular or extracellular bacteria in the field of light microscope were not considered as bacterial growth.
Intracellular localization of D. massiliensis
Infected cells were fixed in 3% paraformaldehyde and permeabilized with 0.1% Triton X-100, and then immunofluorescence labeling was performed. Rat antibodies specific for Lamp-1 (clone 1D4B) (DSHB, IA, USA) and rabbit antibodies specific for EEA-1 (M. Zerial, MPI-CBG, Dresden, Germany) and Cathepsin D (M. Zerial, MPI-CBG) were purchased. Secondary Alexa antibodies were purchased from Invitrogen. To study the acidity of the compartments, infected cells were incubated with LysoTracker Red DND99 (Molecular Probes) at 100 nM for 2 h and then washed and fixed with 3% paraformaldehyde. After fluorescent labeling, these cells were mounted with Mowiol and examined by laser scanning microscopy using a confocal microscope (Leica TCSSP5) with a 63×/1.32-0.6 oil CS lens and an electronic Zoom 4×. Optical sections of fluorescent images were collected at 0.25-µm intervals using Leica confocal software and processed using adobe photoshop V5.5 software. At least 60 cells were examined for each experimental condition; results are expressed as the percentage of D. massiliensis that colocalized with each of the fluorescent markers.
Results
Antibiotic susceptibility using immunofluorescence staining and Gimenez staining
The degree of infection, defined by the proportion of cells infected by D. massiliensis over the total number of morphologically identifiable cells, corresponded to the mean number of bacteria per cell. These numbers were used in the evaluation of the bacteriostatic effects of the antibiotics and other chemical substances tested (Fig. 3). As expected, IFA was found to be more sensitive than Gimenez staining in the visualization of infected cells (Fig. 1).
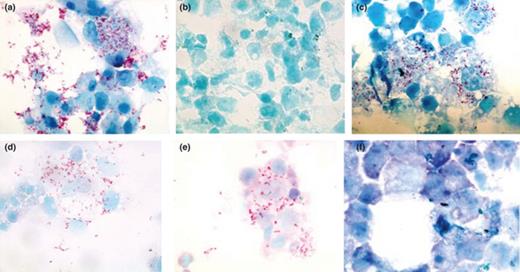
Photomicrographs illustrating the effect of antibiotic treatment on Diplorickettsia massiliensis-infected XTC cells by Gimenez staining (100× magnification). Control, 15 days postinfection (a); negative control (b); rifampin (0.25 µg mL−1) (c); rifampin (0.5 µg mL−1) (d); rifampin (1 µg mL−1) showed little infection compare with control (e); rifampin (2 µg mL−1) showed no sign of infection after 15 days of treatment (f).
Although the Gimenez staining protocol was easy to perform, the counting of the organisms was laborious and time-consuming, as was the subsequent calculation of the percent reduction in infection. The MIC results for the 12 antibiotics tested against D. massiliensis obtained using Gimenez staining showed no significant differences to the MIC values obtained utilizing IFA (Table 1, and Figs 1 and 2).

Photomicrographs illustrating the effect of antibiotic treatment on Diplorickettsia massiliensis-infected XTC cells by IFA, 100×. Control, 15 days postinfection (a); negative control (b); rifampin (0.25 µg mL−1) (c); rifampin (0.5 µg mL−1) (d); rifampin (1 µg mL−1) (e); rifampin (2 µg mL−1) showed little infection compare with control (f); rifampin (4 µg mL−1) showed no sign of infection after 15 days of treatment (g).
Comparison of antibiotic MICs for Diplorickettsia massiliensis using Gimenez and immunofluorescence staining
| MIC of antibiotics (XTC-2 and MRC-5) | ||
| Antibiotics | Gimenez staining | IFA |
| Penicillin G | > 64 µg mL−1 | > 64 µg mL−1 |
| Chloramphenicol | > 64 µg mL−1 | > 64 µg mL−1 |
| Erythromycin | > 64 µg mL−1 | > 64 µg mL−1 |
| Bafilomycin | > 80 nM mL−1 | > 80 nM mL−1 |
| Ammonium chloride | > 100 mM mL−1 | > 100 mM mL−1 |
| Omeprazole | > 8 µg mL−1 | > 8 µg mL−1 |
| Chloroquine | > 8 µg mL−1 | > 8 µg mL−1 |
| Gentamicin | 16 µg mL−1 | 32 µg mL−1 |
| Doxycycline | 4 µg mL−1 | 8 µg mL−1 |
| Ciprofloxacin | 2 µg mL−1 | 8 µg mL−1 |
| Levofloxacin | 2 µg mL−1 | 4 µg mL−1 |
| Rifampin | 2 µg mL−1 | 4 µg mL−1 |
| MIC of antibiotics (XTC-2 and MRC-5) | ||
| Antibiotics | Gimenez staining | IFA |
| Penicillin G | > 64 µg mL−1 | > 64 µg mL−1 |
| Chloramphenicol | > 64 µg mL−1 | > 64 µg mL−1 |
| Erythromycin | > 64 µg mL−1 | > 64 µg mL−1 |
| Bafilomycin | > 80 nM mL−1 | > 80 nM mL−1 |
| Ammonium chloride | > 100 mM mL−1 | > 100 mM mL−1 |
| Omeprazole | > 8 µg mL−1 | > 8 µg mL−1 |
| Chloroquine | > 8 µg mL−1 | > 8 µg mL−1 |
| Gentamicin | 16 µg mL−1 | 32 µg mL−1 |
| Doxycycline | 4 µg mL−1 | 8 µg mL−1 |
| Ciprofloxacin | 2 µg mL−1 | 8 µg mL−1 |
| Levofloxacin | 2 µg mL−1 | 4 µg mL−1 |
| Rifampin | 2 µg mL−1 | 4 µg mL−1 |
Comparison of antibiotic MICs for Diplorickettsia massiliensis using Gimenez and immunofluorescence staining
| MIC of antibiotics (XTC-2 and MRC-5) | ||
| Antibiotics | Gimenez staining | IFA |
| Penicillin G | > 64 µg mL−1 | > 64 µg mL−1 |
| Chloramphenicol | > 64 µg mL−1 | > 64 µg mL−1 |
| Erythromycin | > 64 µg mL−1 | > 64 µg mL−1 |
| Bafilomycin | > 80 nM mL−1 | > 80 nM mL−1 |
| Ammonium chloride | > 100 mM mL−1 | > 100 mM mL−1 |
| Omeprazole | > 8 µg mL−1 | > 8 µg mL−1 |
| Chloroquine | > 8 µg mL−1 | > 8 µg mL−1 |
| Gentamicin | 16 µg mL−1 | 32 µg mL−1 |
| Doxycycline | 4 µg mL−1 | 8 µg mL−1 |
| Ciprofloxacin | 2 µg mL−1 | 8 µg mL−1 |
| Levofloxacin | 2 µg mL−1 | 4 µg mL−1 |
| Rifampin | 2 µg mL−1 | 4 µg mL−1 |
| MIC of antibiotics (XTC-2 and MRC-5) | ||
| Antibiotics | Gimenez staining | IFA |
| Penicillin G | > 64 µg mL−1 | > 64 µg mL−1 |
| Chloramphenicol | > 64 µg mL−1 | > 64 µg mL−1 |
| Erythromycin | > 64 µg mL−1 | > 64 µg mL−1 |
| Bafilomycin | > 80 nM mL−1 | > 80 nM mL−1 |
| Ammonium chloride | > 100 mM mL−1 | > 100 mM mL−1 |
| Omeprazole | > 8 µg mL−1 | > 8 µg mL−1 |
| Chloroquine | > 8 µg mL−1 | > 8 µg mL−1 |
| Gentamicin | 16 µg mL−1 | 32 µg mL−1 |
| Doxycycline | 4 µg mL−1 | 8 µg mL−1 |
| Ciprofloxacin | 2 µg mL−1 | 8 µg mL−1 |
| Levofloxacin | 2 µg mL−1 | 4 µg mL−1 |
| Rifampin | 2 µg mL−1 | 4 µg mL−1 |
All experiments were run concurrently and in triplicate. While doxycycline was able to inhibit D. massiliensis growth at a concentration of 4 µg mL−1, the MIC of gentamicin was 16 µg mL−1. Surprisingly, D. massiliensis was resistant to chloramphenicol up to 64 µg mL−1. Bacteria were also resistant to erythromycin, with a MIC value of 64 µg mL−1. In contrast, ciprofloxacin, levofloxacin, and rifampin had MIC values of 2 µg mL−1 (Table 1).
Figures 3 and 4 illustrate the percentage of infection for each concentration. Doxycycline, ciprofloxacin, levofloxacin, and rifampin each showed a reduction in the percentage of infected cells, with no infection detected at concentrations as low as 4 and 2 µg mL−1. However, in the cases of omeprazole (proton pompe inhibitor), penicillin G, and chloramphenicol, D. massiliensis bacteria were observed at all concentrations. Additionally, no bacteriostatic activity was detected for ammonium chloride (nonspecific inhibitor of phagocytosis), bafilomycin (inhibitor of vacuolar-type H+-ATPase), and chloroquine (lysosomotropic at low pH), substances that are all considered to be vacuole directed.
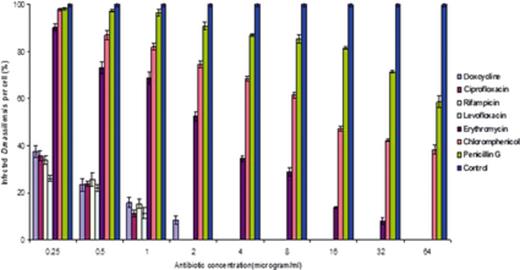
Effect of different antibiotic treatments on Diplorickettsia massiliensis-infected XTC cells counting bacteria per cell. Effects of chloramphenicol, penicillin G, erythromycin, gentamycin, rifampin, ciprofloxacin, levofloxacin, and doxycycline on D. massiliensis in XTC-2 cell lines. A minimum of 20–40 cells were examined for each concentration. Results were compared with a positive control as the standard (minimum 100 bacteria per cell). The results are reported as the means and standard errors for the three replicates. The anova, four drugs (doxycycline, ciprofloxacin, rifampin, and levofloxacin) states that the variation among the treatments (drugs) and the effect of dosage (4 µg mL−1) are highly significant (P < 0.01).
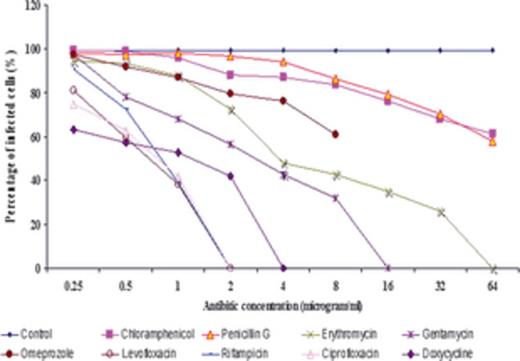
Determination of degree of infection. A minimum 200 cells were counted for each concentration. We found that 90–99% of cells were infected in the control samples. Ciprofloxacin, levofloxacin, rifampin, and doxycycline showed that at higher concentrations, there are no bacteria. However, chloramphenicol and penicillin G showed an even higher concentration infection that was more than 50% when compared with the control. Also the interaction effects between the drugs and its dosage is also highly significant (P < 0.01). Among lowest drug, dosage of 0.5 ug could show significant difference (P < 0.01) from the control. The significant highest static effect was shown by at 0.5 ug compared with other drugs. Also the Diplorickettsia effect of rifampin at 0.5 (µg mL−1) and 4 µg mL−1) was not significantly different even at P value 5%. So rifampin can be considered at best and only Diploricketistatic candidate at 0.5 ug. A significant (P < 0.01) effect was observed in three drugs at a dosage of 2 (µg mL−1) in ciprofloxacin, rifampin, and levofloxacin.
Combinations of chemicals
The experiments were also repeated with various combinations of drugs and antibacterial substances, including those that influence on phagocytosis and intravacuolar environment. The effect of chloramphenicol (from 0.25 to 64 µg mL−1) was assessed in the presence of 20 nM of bafilomycin. The results of this experiment are shown in Table 1. The combination of bafilomycin and chloramphenicol was observed to be more effective than chloramphenicol only; no growth was observed by Gimenez staining at concentrations of 4 µg mL−1 of chloramphenicol and 20 nM mL−1 of bafilomycin. Interestingly, when used individually, bafilomycin and chloramphenicol failed to exhibit an inhibitory effect on D. massiliensis growth.
Intracellular localization of D. massiliensis
Electron microscopy has shown that D. massiliensis is localized within vacuoles in the cells, indicating that the bacteria does not replicate in the cytosol. (Mediannikov et al., 2010) However, the nature of the vacuole containing the bacterium is unknown. Hypothesizing that the cellular localization of D. massiliensis might play a role in antibiotic resistance and susceptibility, we investigated the vacuoles containing D. massiliensis. To examine the nature of the vacuoles, we utilized markers of the early phagosome (EEA1, early endosome-1), late phagosome and phagolysosome (Lamp-1, lysosomal-associated membrane protein-1), and phagolysosomes (active Cathepsin D).
First, we investigated EEA1, a marker of the early phagosome; D. massiliensis does not colocalize either with EEA1 in either HEL cells (Fig. 5a and Table 2) or in MRC-5 cells (Fig. 5b and Table 2). We also investigated markers for late phagosomes and phagolysosomes (Lamp-1) and phagolysosomes (Cathepsin D). Diplorickettsia massiliensis was not observed to colocalize with Cathepsin (Fig. 5c and Table 2) or Lamp-1 (Fig. 5d and Table 2) in HEL cells. Similar results were obtained with MRC-5 cells (Fig. 5e and f, and Table 2). These data suggest that D. massiliensis is not localized in early phagosomes, late phagosomes, or phagolysosomes. Next, the acidic nature of the compartments containing D. massiliensis was investigated using LysoTracker Red DND99, a weakly basic amine that selectively accumulates in compartments with low pH. Confocal microscopy revealed that more than 90% (Table 2) of D. massiliensis bacteria colocalized with DND99 in HEL (Fig. 5g) and MRC-5 cells (Fig. 5h). These data suggest that D. massiliensis is localized inside an acidic compartment within cells.
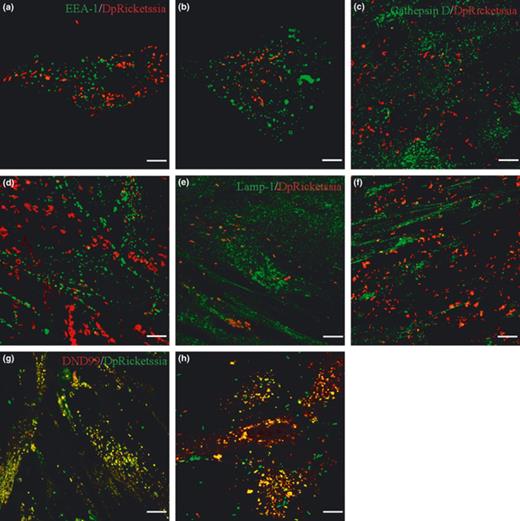
Intracellular localization of Diplorickettsia massiliensis with HEL and MRC-5 cells. Intracellular localization D. massiliensis was studies in HEL cells (a, c, e, g) and in MRC-5 cells (b, c, f, h) by immunofluorescence and confocal microscopy. (a, b) Colocalization of D. massiliensis (red) with EEA-1 (green). (c, d) Colocalization of D. massiliensis with Cathepsin D (green). (e, f) colocalization of D. massiliensis (red) with Lamp-1 (green). (g, f) colocalization of D. massiliensis (green) with Lysotracker DND99 (red).
| Percentage of colocalization with | ||||
| Cell lines | EEA-1 | Cathepsin D | Lamp-1 | DND99 |
| HEL | 0 ± 0 | 0 | 2 ± 1 | 97 ± 2 |
| MRC-5 | 1 ± 0.5 | 0 | 3 ± 2 | 91 ± 7 |
| Percentage of colocalization with | ||||
| Cell lines | EEA-1 | Cathepsin D | Lamp-1 | DND99 |
| HEL | 0 ± 0 | 0 | 2 ± 1 | 97 ± 2 |
| MRC-5 | 1 ± 0.5 | 0 | 3 ± 2 | 91 ± 7 |
Cells were incubated with D. massiliensis, after 7 days the number of bacteria that colocalized with EEA-1, Cathepsin D, Lamp-1, and Lysotracker red DND99 was scored. The results are expressed as the percentage of D. massiliensis that colocalized with EEA-1, Cathepsin D, Lamp-1, and Lysotracker red DND99 are the mean ± SD of three experiments.
| Percentage of colocalization with | ||||
| Cell lines | EEA-1 | Cathepsin D | Lamp-1 | DND99 |
| HEL | 0 ± 0 | 0 | 2 ± 1 | 97 ± 2 |
| MRC-5 | 1 ± 0.5 | 0 | 3 ± 2 | 91 ± 7 |
| Percentage of colocalization with | ||||
| Cell lines | EEA-1 | Cathepsin D | Lamp-1 | DND99 |
| HEL | 0 ± 0 | 0 | 2 ± 1 | 97 ± 2 |
| MRC-5 | 1 ± 0.5 | 0 | 3 ± 2 | 91 ± 7 |
Cells were incubated with D. massiliensis, after 7 days the number of bacteria that colocalized with EEA-1, Cathepsin D, Lamp-1, and Lysotracker red DND99 was scored. The results are expressed as the percentage of D. massiliensis that colocalized with EEA-1, Cathepsin D, Lamp-1, and Lysotracker red DND99 are the mean ± SD of three experiments.
Discussion
The recently described bacterium D. massiliensis is phylogenetically related to Rickettsiella spp., Legionella spp., and C. burnetii, the etiologic agent of Q fever. This bacterium has been shown to grow successfully in humans cells (Mediannikov et al., 2010). Epidemiologically, the bacterium is associated with the tick I. ricinus, a species known to bite humans. Despite of a number of infectious diseases transmitted by Ixodes ticks in Europe (Socolovschi et al., 2009), there is still many cases without a proven etiological diagnosis (Hofmann, 1996; Sharma et al., 2010). Some of these cases may be due to D. massiliensis infection.
In our study, the inhibitory effects of antimicrobial agents were assessed by two different methods, immunofluorescence and Gimenez staining. Intracellular bacteria in shell vial cultures were clearly visualized when infected cells were stained by immunofluorescence using mouse anti-D. massiliensis polyclonal antibody. We did not use real-time PCR for the evaluation because of its disadvantages: genomic DNA evaluation does dot correspond to live bacteria and does not take into account the degradation of bacterial DNA; cDNA reflect the number of metabolically active bacteria but is too sensitive and dependent of a gene selection.
In summary, our report describes for the first time the susceptibilities of D. massiliensis to a wide range of antibiotics as determined by Gimenez staining and immunofluorescence staining. The efficacies of all antibiotics tested in the control of D. massiliensis are summarized in Table 1. Our results show that doxycycline, ciprofloxacin, levofloxacin, and rifampin are effective in inhibiting D. massiliensis growth; however, we found that penicillin G, chloramphenicol, erythromycin, ammonium chloride, gentamycin, omeprazole, bafilomycin A1, and chloroquine were not.
The efficacies of antibiotics tested in the control of D. massiliensis were compared with Coxiella, F. tularensis, and L. pneumophila (Table 3). Consistent with our findings, the two quinolone antibiotics, ciprofloxacin, and levofloxacin as well as the drugs doxycycline and rifampin were more effective against Coxeilla (Raoult et al., 1991), F. tularensis (Maurin & Raoult, 2001), and L. pneumophila (Edelstein & Meyer, 1980; Roch & Maurin, 2005).
Antibiotic MICs of Diplorickettsia massiliensis, Coxiella burnetii, Legionella pneumophila, and Francisella tularensis using Gimenez staining
| MIC (µg mL−1) | ||||||
| Coxiella burnetii | ||||||
| S. no | Antibiotic | Nine mile | Q212 | Legionella pneumophila | Francisella tularensis | Diploricketssia massiliensis |
| 1 | Penicillin G | NA | NA | 4–16 | 256 | > 64 |
| 2 | Gentamycin | > 10 | > 10 | 0.25–2 | 4 | > 16 |
| 3 | Erythromycin | 4 | 2 | 1 | 4 | 64 |
| 4 | Doxycycline | 4 | 2 | 1 | 8 | 4 |
| 5 | Rifampin | 4 | 4 | ≤ 0.001 | 0.5 | 2 |
| 6 | Ciprofloxacin | 4 | 2 | 0.06 | 0.25 | 2 |
| 7 | Levofloxacin | 2 | 2 | 0.03 | 0.12 | 2 |
| 8 | Chloramphenicol | > 8 | > 8 | < 1 | 4 | > 64 |
| MIC (µg mL−1) | ||||||
| Coxiella burnetii | ||||||
| S. no | Antibiotic | Nine mile | Q212 | Legionella pneumophila | Francisella tularensis | Diploricketssia massiliensis |
| 1 | Penicillin G | NA | NA | 4–16 | 256 | > 64 |
| 2 | Gentamycin | > 10 | > 10 | 0.25–2 | 4 | > 16 |
| 3 | Erythromycin | 4 | 2 | 1 | 4 | 64 |
| 4 | Doxycycline | 4 | 2 | 1 | 8 | 4 |
| 5 | Rifampin | 4 | 4 | ≤ 0.001 | 0.5 | 2 |
| 6 | Ciprofloxacin | 4 | 2 | 0.06 | 0.25 | 2 |
| 7 | Levofloxacin | 2 | 2 | 0.03 | 0.12 | 2 |
| 8 | Chloramphenicol | > 8 | > 8 | < 1 | 4 | > 64 |
NA, data not available.
Antibiotic MICs of Diplorickettsia massiliensis, Coxiella burnetii, Legionella pneumophila, and Francisella tularensis using Gimenez staining
| MIC (µg mL−1) | ||||||
| Coxiella burnetii | ||||||
| S. no | Antibiotic | Nine mile | Q212 | Legionella pneumophila | Francisella tularensis | Diploricketssia massiliensis |
| 1 | Penicillin G | NA | NA | 4–16 | 256 | > 64 |
| 2 | Gentamycin | > 10 | > 10 | 0.25–2 | 4 | > 16 |
| 3 | Erythromycin | 4 | 2 | 1 | 4 | 64 |
| 4 | Doxycycline | 4 | 2 | 1 | 8 | 4 |
| 5 | Rifampin | 4 | 4 | ≤ 0.001 | 0.5 | 2 |
| 6 | Ciprofloxacin | 4 | 2 | 0.06 | 0.25 | 2 |
| 7 | Levofloxacin | 2 | 2 | 0.03 | 0.12 | 2 |
| 8 | Chloramphenicol | > 8 | > 8 | < 1 | 4 | > 64 |
| MIC (µg mL−1) | ||||||
| Coxiella burnetii | ||||||
| S. no | Antibiotic | Nine mile | Q212 | Legionella pneumophila | Francisella tularensis | Diploricketssia massiliensis |
| 1 | Penicillin G | NA | NA | 4–16 | 256 | > 64 |
| 2 | Gentamycin | > 10 | > 10 | 0.25–2 | 4 | > 16 |
| 3 | Erythromycin | 4 | 2 | 1 | 4 | 64 |
| 4 | Doxycycline | 4 | 2 | 1 | 8 | 4 |
| 5 | Rifampin | 4 | 4 | ≤ 0.001 | 0.5 | 2 |
| 6 | Ciprofloxacin | 4 | 2 | 0.06 | 0.25 | 2 |
| 7 | Levofloxacin | 2 | 2 | 0.03 | 0.12 | 2 |
| 8 | Chloramphenicol | > 8 | > 8 | < 1 | 4 | > 64 |
NA, data not available.
The resistance documented to chloramphenicol is quite unusual for most gamma-proteobacteria (Vester & Douthwaite, 2001; Maurin et al., 2003). However, resistance has been reported in strict intracellular bacteria, such as Ehrlichia chaffeensis and A. phagocytophilum (Maurin et al., 2003). The mechanisms whereby D. massiliensis gains resistance to chloramphenicol are yet unknown, but it could be explained by the activity of chloramphenicol acetyltransferase, which is encoded by the cat gene in Escherichia coli (Potrykus & Wegrzyn, 2001).
Doxycycline, rifampin, and macrolides have been shown to be highly active against strict intracellular bacteria such as Rickettsia spp., C. burnetii, and Ehrlichia spp.(Spicer et al., 1981; Maurin & Raoult, 1996, 2001) In contrast, resistance to macrolides was identified in the case of D. massiliensis. In contrast, resistance to macrolides was identified in the case of D. massiliensis. One possible explanation is the alteration of specific nucleotides in the 23S rRNA gene, contained within the large ribosomal subunit (Vester & Douthwaite, 2001). We analyzed the 23S gene of D. massiliensis derived from the genome-sequencing project (data not shown). The sequence of the gene coding for 23S rRNA of D. massiliensis was compared with sequences from bacteria resistant to erythromycin (Branger et al., 2004). However, no mutation corresponding for erythromycin resistance was found. Other ribosomal genes may also be involved (protein L4, L22) as well as erm genes and/or efflux pumps; however, these remain to be investigated.
We investigated the effect of pH-neutralizing agents on D. massiliensis survival by Gimenez staining. The agents were used at the lowest concentrations that neutralized intracellular pH without affecting the viability of XTC-2 and MRC-5 cells. We found that D. massiliensis growth is not inhibited at a neutral pH with high concentrations of bacteria being detected.
Bafilomycin A1 is toxic macrolide antibiotic derived from Streptomyces griseus (Werner et al., 1984). It is a strong inhibitor of the vacuolar-type H+-ATPase in vitro at nanomolar concentrations (Bowman et al., 1988; Hanada et al., 1990). In this study, we showed that when used alone, bafilomycin A1 is not effective against D. massiliensis infection. Nevertheless, in combination with chloramphenicol, it was active at a lower concentration. Decreased susceptibility of D. massiliensis to the antibiotics may be due to an alteration in the target of the drugs. Alkalinization of vacuoles has been shown to result in bactericidal activity of doxycycline against C. burnetii (Maurin et al., 1992) omeprazole and bafilomycin and chloroquine when used individually have been found to be ineffective. A combination of doxycycline and chloroquine also displays bactericidal activity against C. burnetii (Raoult et al., 1990).
Our study shows that D. massiliensis does not require an acidic pH for growth and that it can grow even under neutral pH conditions. The results of these studies indicate that antibiotic resistance does not depend on vacuolar pH; thus, a pH-related mechanism does not appear to play a contributing role in the overall resistance profile of D. massiliensis. Diplorickettsia massiliensis is localized within an acidic compartment that is not an early phagosome, late phagosome, or a phagolysosome. Therefore, although D. massiliensis survival does not require an acidic pH, it localizes within an acidic compartment inside infected cells.
In conclusion, our results suggest that the Gimenez staining method will work with a very low number of bacteria and can be used to determine the MICs of new therapeutic antibiotics more precisely. Additionally, this tool can be used in the future to help better define mechanisms of antibiotic resistance and to aid in the screening of new therapeutic drugs. According to possible acute infection because of D. massiliensis after tick bite, Doxycycline should be used as a reference treatment.
Acknowledgements
Authors thank Guy Vestris and Lionel Pretat for their helpful technical assistance. Geetha Subramanian is a receiver of a stipend from APHM (Assistance Publique-Hôpitaux de Marseille). Abdoulaye O. Barry is a fellow at the Scientific Cooperation Foundation ‘Infectiopole Sud’. No funding of any kind has been received, and all data have been generated as part of the routine work (URMITE UMR IRD 198-CNRS 6236.). We declare that we have no competing interests.
References
Author notes
Editor: Achilles Gikas



