-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Østrup Jensen, Michael Givskov, Thomas Bjarnsholt, Claus Moser, The immune system vs. Pseudomonas aeruginosa biofilms, FEMS Immunology & Medical Microbiology, Volume 59, Issue 3, August 2010, Pages 292–305, https://doi.org/10.1111/j.1574-695X.2010.00706.x
Close - Share Icon Share
Abstract
Ilya Metchnikoff and Paul Ehrlich were awarded the Nobel price in 1908. Since then, numerous studies have unraveled a multitude of mechanistically different immune responses to intruding microorganisms. However, in the vast majority of these studies, the underlying infectious agents have appeared in the planktonic state. Accordingly, much less is known about the immune responses to the presence of biofilm-based infections (which is probably also due to the relatively short period of time in which the immune response to biofilms has been studied). Nevertheless, more recent in vivo and in vitro studies have revealed both innate as well as adaptive immune responses to biofilms. On the other hand, measures launched by biofilm bacteria to achieve protection against the various immune responses have also been demonstrated. Whether particular immune responses to biofilm infections exist remains to be firmly established. However, because biofilm infections are often persistent (or chronic), an odd situation appears with the simultaneous activation of both arms of the host immune response, neither of which can eliminate the biofilm pathogen, but instead, in synergy, causes collateral tissue damage. Although the present review on the immune system vs. biofilm bacteria is focused on Pseudomonas aeruginosa (mainly because this is the most thoroughly studied), many of the same mechanisms are also seen with biofilm infections generated by other microorganisms.
Introduction
The study of the immune system was introduced as an academic discipline when Ilya Metchnikoff and Paul Ehrlich were awarded the Nobel price in 1908 (Kaufmann, 2008; Nathan, 2008). Since then, numerous studies have unraveled a multitude of mechanistically different immune responses to intruding microorganisms. However, in the vast majority of these studies, the underlying infectious agents have appeared in the planktonic state. Accordingly, much less is known about the immune responses to the presence of biofilm-based infections (which is probably also due to the relatively short period of time in which the immune response to biofilms has been studied). Nevertheless, more recent in vivo and in vitro studies have revealed both innate as well as adaptive immune responses to biofilms. On the other hand, measures launched by biofilm bacteria to achieve protection against the various immune responses have also been demonstrated. Whether particular immune responses to biofilm infections exist remains to be firmly established. However, because biofilm infections are often persistent (or chronic), an odd situation appears with the simultaneous activation of both arms of the host immune response, neither of which can eliminate the biofilm pathogen, but instead, in synergy, causes collateral tissue damages. Although the present review on the immune vs. biofilm bacteria is focused on Pseudomonas aeruginosa (mainly because this is the most thoroughly studied), many of the same mechanisms are also seen with biofilm infections generated by other microorganisms.
Innate immune response to biofilms
Innate immunity involves germline-encoded, nonclonal mechanisms that provide nonspecific protection against pathogens by mechanisms that are not influenced inherently by repeated encounters with infectious intruders (Kimbrell & Beutler, 2001). The most solid evidence for an innate immune response to biofilm bacteria was obtained by exposing P. aeruginosa biofilms that in principle had been depleted for planktonic cells to freshly isolated human neutrophils and macrophages (Jesaitis et al., 2003; Bjarnsholt et al., 2005; Leid et al., 2005; Jensen et al., 2007). The responses observed included respiratory burst, as well as accumulation, penetration, phagocytosis and killing of the biofilm bacteria. Early sampling before establishment of the acquired immune response during experimental biofilm infections in mouse lungs has also demonstrated that accumulation of activated neutrophils in the airways is a part of the innate immune response to lung infections with P. aeruginosa biofilms (Jensen et al., 2004, 2007; Bjarnsholt et al., 2005; Alhede et al., 2009; van Gennip et al., 2009). These studies aimed at understanding the interactions between P. aeruginosa biofilms and the neutrophils and macrophages during a chronic lung infection in patients with cystic fibrosis (CF), which is probably the most intensively studied biofilm infection. In this condition, the response by the neutrophils has gained particular attention due to the suspicion of collateral, the detrimental effects of the numerous neutrophils and their failure to eradicate the biofilm in the airways. The chronic lung infection in CF patients is believed to result from deficient, apical ion transport caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) (Knowles & Boucher, 2002). Infected CF airways are dominated by endobronchial P. aeruginosa growing as biofilms in the shape of dense, aggregated bacteria surrounded by numerous neutrophils (Bjarnsholt et al., 2009) and few planktonic bacteria, which are readily phagocytosed by the neutrophils (Bjarnsholt et al., 2009; Kolpen et al., 2010). The response by the neutrophils in infected endobronchial secretions in CF resembles the reaction of neutrophils responding to experimental in vitro and in vivo biofilms with regard to an intense accumulation of neutrophils close to the biofilm (Bjarnsholt et al., 2009) including accelerated oxygen depletion (Kolpen et al., 2010), which is caused by an active respiratory burst where molecular oxygen is reduced to superoxide (Babior et al., 1973). Therefore, the active participation of neutrophils during the immune response to biofilms of the chronic lung infection in CF may account for the anaerobic conditions in infected endobronchial mucus (Wörlitzsch et al., 2002). In addition, because active neutrophils are mainly fueled by anaerobic glycolysis (Borregaard & Herlin, 1982), the increased glucose uptake in neutrophils in CF lungs (Chen et al., 2006) and the high concentration of l-lactate in sputum from CF patients with chronic P. aeruginosa lung infection (Wörlitzsch et al., 2007) further support the presence of active neutrophils during biofilm infection in CF lungs. It may be argued that the neutrophil response to planktonic P. aeruginosa also includes activation of the respiratory burst (Kolpen et al., 2010) and thus makes it difficult to attribute the activation of the neutrophils in the infected CF lungs as a specific response to the presence of a biofilm infection. The neutrophils in the infected CF airways may be activated by direct contact with the bacteria, by lipopolysaccharide-immune complexes (Kronborg et al., 1993), or by alginate (Pedersen, 1992), and the neutrophils may be primed by bacterial endotoxins (Kharazmi et al., 1987), and by soluble components of the innate immune response such as tumor necrosis factor-α (TNF-α), platelet-activating factor, leukotriene B4, and interleukin-8 (IL-8) (Kharazmi et al., 1988; Yuo et al., 1991; Jones et al., 2003; Jensen et al., 2006; Downey et al., 2009). In addition, activation of the neutrophils may occur already during the migration in the inflamed tissue by the binary signaling from the engagement of the integrins and binding to inflammatory cytokines (Nathan et al., 1989). The functionality of the neutrophils in CF patients is apparently not affected by the mutations in the gene encoding CFTR (McKeon et al., 2010). Therefore, the response of the neutrophils to P. aeruginosa biofilms observed in the study of CF patients may also apply to other patients with P. aeruginosa biofilm infections. In fact, intense accumulation of neutrophils at the site of biofilms has been demonstrated recently in biopsies from chronic wounds (Bjarnsholt et al., 2008; Kirketerp-Møller et al., 2008). In addition, the induction of biofilm formation observed during the interaction between normal, human neutrophils and P. aeruginosa (Mathee et al., 1999; Walker et al., 2005; Parks et al., 2009) may also apply for the chronic lung infections in CF.
As illustrated in the previous section, cellular components of the innate immune system, such as the neutrophils and macrophages, are able to respond to biofilms. The ability to detect intruding microorganisms is aided by pattern recognition receptors (PRRs) that recognize conserved microbial pathogen-associated molecular patterns and signal their presence, resulting in the activation of host response. Even though several types of PRRs and their corresponding ligands are known, PRRs specific for biofilm-growing microorganisms have not yet been identified. Instead, the resilience of biofilms may at least in part result in continuous stimulation and activation of PRRs belonging to the innate immune system. Both secreted and membrane-bound PRRs are known. Even though the soluble receptors of the complement system are among the most studied secreted PRRs, a role of the complement system during biofilm infections is far from firmly established. Apparently, no cases of biofilm infections have been reported for patients with complement deficiency so far. This may in part be due to the ability of biofilm-growing microorganisms to develop resistance against the complement system and thus to establish biofilm infections in spite of the activation of the complement systems. In this respect, the secretion of alkaline protease and elastase by P. aeruginosa has long been known to inactivate complement (Kharazmi, 1991).
In CF, activated complement (C3c) was more frequent in the sputum from patients with chronic P. aeruginosa lung infection (Schiøtz et al., 1979). However, it could not be determined whether complement activation was only due to biofilm formation because planktonic bacteria activated the complement system more than biofilm bacteria (Jensen et al., 1993) and both planktonic and biofilm-growing P. aeruginosa are frequently found in the same samples from CF patients. Nevertheless, evasion from binding to complement receptors and from the subsequent complement activation has been demonstrated in P. aeruginosa isolated from CF sputum samples (Davies et al., 2000). This protection against complement opsonization may be provided by the high content of alginate with O-acetylation in mucoid P. aeruginosa biofilms (Pier et al., 2001). Biofilm formation has also been suggested to prevent activation of the complement system by Mycoplasma pulmonis (Simmons & Dybvig, 2007) and Mycobacterium abscessus (Rhoades et al., 2009).
No membrane-bound PRRs have so far been demonstrated to mount a response specific for biofilm infections. However, recent studies have demonstrated that toll-like receptors (TLRs) may mediate responses to matrix components of biofilms and to bacterial products of both biofilm and planktonic infections. Evidence for the involvement of TLRs in clinical biofilm infections has mainly accumulated from chronically infected CF patients and from dental plaques. Because the pathogen recognition capacity of neutrophils mostly relies on TLRs (Parker et al., 2005), investigations of the expression of TLRs on the neutrophils from the airways of chronically infected CF patients have been performed recently. TLR5 was the only MyD88-dependent TLR that was increased on neutrophils from the lungs of chronically infected CF patients (Koller et al., 2008). This elevated TLR5 expression on the neutrophils was probably mediated by IL-8, TNF-α, and granulocyte-colony-stimulating factor (G-CSF), and by the interaction of TLR1 and TLR2 resulting from the binding to the bacterial lipoprotein (Koller et al., 2008). Whether the expression of the flagellin receptor, TLR5, on the neutrophils results in the expected innate response against the biofilms in the CF lungs is difficult to establish because flagella were absent in biofilms of mucoid P. aeruginosa that are frequently isolated from CF airways (Garrett et al., 1999). However, when neutrophils encounter nonmucoid biofilms in vitro, the absence of functional flagella induces killing of the bacteria due to the secretion of bactericidal concentrations of lactoferrin (Leid et al., 2009), which has the ability to prevent biofilm formation (Singh et al., 2002). In addition, TLR5-mediated enhanced phagocytosis may reinforce the host defense against the planktonic, flagellin-intact P. aeruginosa subpopulations in the CF airways. In fact, apparently only planktonic bacteria were engulfed by neutrophils in explanted lungs and sputum from chronically infected CF patients (Bjarnsholt et al., 2009; Kolpen et al., 2010) and acute lung infections with flagellin-defective planktonic P. aeruginosa were cleared later in mice (Balloy et al., 2007).
In the pursuit of a biofilm-specific innate response, the ability of bacterial DNA, a matrix component of biofilms (Whitchurch et al., 2002), to activate neutrophils has been examined. Bacterial DNA may activate neutrophils through TLR9-independent mechanisms resulting in the upregulation of intracellular signaling pathways and IL-8 production (Alvarez et al., 2006; Fuxman Bass et al., 2008). Increased alginate content is another hallmark of the matrix in the mucoid P. aeruginosa biofilm and is considered the strongest virulence factor in chronically infected CF patients (Koch & Høiby, 1993). Although neutrophils are able to respond to alginate by an increased respiratory burst (Pedersen et al., 1990) and alginate may stimulate monocytes to produce cytokines (Otterlei et al., 1993) in vitro, the involved receptors have not been identified. It has, however, been demonstrated that both TLR2 and TLR4 participate in the activation of monocytes by the mannuronic acid polymeric components of alginate produced by P. aeruginosa (Flo et al., 2002). In addition to alginate, other polysaccharide components of the extracellular polymeric substance of P. aeruginosa biofilms, such as Psl and Pel (Ryder et al., 2007), may potentially stimulate an immune response that is unique for biofilm. In this respect, a unique immune response to biofilm is probably less likely to be mounted against proteins, because the existence of biofilm-specific proteomes has been doubted (Vilain et al., 2004).
Pseudomonas aeruginosa biofilms are now also believed to play a key role in nonhealing leg ulcers (chronic wounds) (Bjarnsholt et al., 2008; Kirketerp-Møller et al., 2008). Chronic wounds consist primarily of granulation tissue composed of a network of collagen fibers, new capillaries, and extracellular matrix, together with neutrophils, macrophages, and fibroblasts. Embedded in this, we have found the aggregated microcolonies of bacteria. This is in accordance with our observations from the chronically infected CF lung; here, within the lumen of the bronchial airways, P. aeruginosa is also detected in aggregated microcolonies. Because the patients in both diseases do not suffer from defects in the cellular defense, the neutrophils would be expected to eradicate the bacteria. The key to this persistence is caused by particular components of the biofilm matrix. Pseudomonas aeruginosa produces rhamnolipids (Jensen et al., 2007), powerful detergents that cause cellular necrosis and the concomitant elimination of the neutrophils (Jensen et al., 2007; Alhede et al., 2009; van Gennip et al., 2009). Accordingly, the rhamnolipids function as a neutrophil shield, and we hypothesize that the capability of mounting this shield significantly contributes to the ability of P. aeruginosa to persist in the CF lung as well as in the chronic wound. The implications of sustained neutrophil lysis are that antimicrobials as well as tissue-devastating compounds, such as myeloperoxidase, elastase, and matrix metallopeptidase 9, spill out, examples in which chronic wound fluids are particularly rich, in contrast to fluid from acute wounds in humans (Wysocki et al., 1993). This also applies for CF patients, in contrast to patients suffering from acute respiratory failure (Gaggar et al., 2007). The regulation of rhamnolipid synthesis is governed by quorum sensing (QS) (Alhede et al., 2009). This suggests that quora (which are likely to be represented by the observed bacterial biofilm aggregates) have amassed at certain locations in chronic wounds (Kirketerp-Møller et al., 2008) and in the CF lung (Bjarnsholt et al., 2009). Such quora are then capable of eliminating neutrophils by the production of rhamnolipid, which, in turn, would reduce the number of functional neutrophils at their location (Jensen et al., 2007; Alhede et al., 2009). Furthermore, the QS signal molecule OdDHL functions as a neutrophil chemoattractant (Zimmermann et al., 2006) and may therefore help attract neutrophils to the site of infection, where they burst and are eliminated by the rhamnolipid shield. Interestingly, AlgR was recently shown to reduce the expression of RhlR-controlled gene expression in the biofilm mode (Morici et al., 2007). Concurrently, we found that the contents of rhamnolipids were below the detection limit in our in vitro biofilms (Alhede et al., 2009). However, exposure to freshly isolated neutrophils superseded AlgR repression and significantly increased the expression of rhamnolipids up to a level of 50 µg g−1 biofilm, which, in a pure form, would cause lysis of neutrophils within 30 min of exposure (Jensen et al., 2007; Alhede et al., 2009). The inducing effect was found to require a functional QS system to induce transcription initiation of the RhlR and PQS-regulated genes and it indicates that P. aeruginosa, in order to upregulate its neutrophil shield, receives and responds to signal molecules originating from the host defense cells. Zaborina (2007) have reported that the endogenous opioid, dynorphin, can induce QS in P. aeruginosa. We cannot reproduce this effect on biofilm cells (analyzed by transcriptomics, reporter genes or reverse transcriptase-PCR), but because freshly isolated neutrophils (Alhede et al., 2009) as well extracts of neutrophils (unpublished data) selectively induce QS in biofilm cells and not in planktonic cells, we expect that there is another signal that specifically overrules the AlgR repression of QS genes in the biofilm mode of growth. Specific effects of cytokines such as interferon gamma (IFN-γ) has recently been shown to be transmitted through IFN-γ binding to OprF, resulting in the expression of the QS-dependent virulence determinants PA-I lectin and pyocyanin (Wu et al., 2005). The observation that lungs of mice infected with wild-type P. aeruginosa contain significantly less intact neutrophils compared with lungs of mice infected with an rhlA mutant supports our current ‘shield model’ (Alhede et al., 2009); P. aeruginosa biofilms resist phagocytosis by eradicating the neutrophils on contact.
Adaptive immune response to biofilms
The adaptive immune response has developed to distinguish between self and nonself just as the innate immune response. However, in comparison with the innate immune response, the adaptive immune response is characterized by a higher degree of specificity and so-called memory and the adaptive immune response recognizes species- or even strain-specific antigens. The memory is characterized by a clonal expansion of specialized subtypes of lymphocytes (effector and central memory cells) during the first exposure, resulting in a significantly faster, stronger, and higher affinity response as compared with the first response. In contrast, the innate response by itself cannot distinguish between a primary or a subsequent exposure (Janeway & Travers, 1997; Roitt et al., 2006).
Activation of the adaptive immune response is initiated simultaneously or shortly after activation of the innate immune response, although with some inertia. In accordance to what is published, activation of the adaptive immune response during biofilm infections follows the same mechanisms as during infection with the same microorganism during a non-biofilm-forming infection. Therefore, the difference between the adaptive immune response to a biofilm and a nonbiofilm infection lies in the impaired clearance of the microorganism and the contribution of the adaptive immune responses to the pathology (Høiby et al., 2001; Brady et al., 2008; Schaudinn et al., 2009). The effector mechanisms of the adaptive immune response often act in synergy with the innate immune response, and the type of the innate immune response influences the type of adaptive immune response generated. In biofilm infections, however, the persistent infection can resist the released antibodies, chemoattracted, activated, and opsonized phagocytes, as well as other components of the host response. Instead, the surrounding tissue is subject to deleterious oxidative radicals and enzymes released from the host itself. In addition to various pathogen-specific virulence factors, the release of proteases and other exoenzymes from the host cells can result in the degradation of important surface molecules on the immune cells and thereby contribute to the impaired antibiofilm effect of the host (Kharazmi et al., 1984, Horvat & Parmely, 1988; Theander et al., 1988; Kharazmi & Nielsen, 1991; McCormick et al., 1997). Also, in this case, the host response itself may be the major cause of tissue damage, because neutralizing antibodies directed against a number of bacterial virulence factors during biofilm infections have been reported (Döring & Høiby, 1983; Döring et al., 1985; Petersen et al., 1996). CF patients have been reported to develop specific antibodies against elastase, lipopolysaccharide, flagella, etc., indicating that these antigenic determinants are likely to be neutralized during the chronic infection (Table 1). These virulence factors may therefore exert their actions mainly during the early stages of colonization and infection, but they are not directly harmful to the tissue. Instead, the antibodies have been shown to result in immune complexes precipitating in the parenchyma, leading to the activation of complement and opsonization of neutropils, and thereby indirectly inducing tissue damage (Høiby et al., 1977; Koch & Høiby, 1993).
Chronic infections where visualization of biofilm on human material has been reported
Based on originality.
TEM, transmission electron microscopy; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; SEM, scanning electron microscopy; CLSM, confocal laser scanning microscopy; HE: hematoxylin–eosin stain; EC, echocardiography.
Chronic infections where visualization of biofilm on human material has been reported
Based on originality.
TEM, transmission electron microscopy; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; SEM, scanning electron microscopy; CLSM, confocal laser scanning microscopy; HE: hematoxylin–eosin stain; EC, echocardiography.
Activation of the adaptive immune response of the dendritic cells (DC)
Macrophages (Mφ) and, in particular, DC are links to the adaptive immune response, and are specialized in antigen uptake and presentation and function as activator cells for the adaptive immune response. The adaptive host response will not be sufficiently activated without the action of the DCs (Banchereau & Steinman, 1998).
Based on the observation that human DCs can be divided into myeloid (mDCs) or lymphoid (later plasmacytoid DCs, pDCs) depending on their surface markers and cytokine response, and that these DC subtypes depended on different cytokines in their surroundings, and that they induced distinct T-helper cell responses initiated a study on inflammatory cytokines and T-helper cell responses (Moser et al., 2005). mDCs were characterized as IL-12 producers and dependent on granulocyte-macrophage-colony-stimulating factor (GM-CSF) and therefore designated DC1 cells due to their Th1-inducing ability. Lymphoid DCs (later pDCs) are weak IL-12 producers and dependent on IL-3. Furthermore, they were shown to be induced by G-CSF and were designated as DC2 cells due to their ability to induce Th2 responses. It was speculated that G-CSF in CF patients not only recruited neutrophils from the bone marrow, but in addition, also induced DC2 cells and promoted a Th2 response. Indeed, a positive correlation was observed between the GM-CSF/G-CSF ratio and the IFN-γ response as well as the lung function in CF patients with chronic P. aeruginosa lung infection. In addition, an inverse correlation between IL-3 and the IFN-γ response was also observed (Moser et al., 2005). Basically, DCs have not been studied in biofilm infections and the nomenclature of DCs is currently undergoing adjustments and DCs are only present in very limited numbers, which, together with their plasticity, makes them difficult to investigate. However, they are a potential treatment target and research in this area is ongoing (Jarrossay et al., 2001; Kadowaki et al., 2001; Penna et al., 2002; Demedts et al., 2006; Ito et al., 2006; Piccioli et al., 2007).
Cross-kingdom signaling is likely to play a significant role in P. aeruginosa–host interactions. Over the last decade, several researchers have demonstrated that QS signals, when administered in in vitro experimental scenarios, can modulate the production of proinflammatory cytokines (in particular inhibit Il-12 and TNF-α) in lymphocytes and induce apoptosis in neutrophils and macrophages (Telford et al., 1998; Chhabra et al., 2003; Tateda et al., 2003; Horikawa et al., 2006). Recent evidence by (Kristiansen et al., 2008) also supports the view of Jahoor (2008) and Williams (2004) that QS signal molecules interact with distinct eukaryotic target proteins and alter gene expression in mammalian cells. (Skindersoe et al., 2009) reported that P. aeruginosa QS signals also decrease the production of IL-12 by murine DCs without altering the IL-10 release. DCs exposed to QS signals during antigen stimulation exhibit a decreased ability to induce specific T-cell proliferation. These in vitro results suggest that the P. aeruginosa QS signal molecules impede DCs in exerting their T-cell-stimulatory effects and function as immunomodulators, which, in the host, may change the milieu away from the host-protecting proinflammatory Th1, thereby possibly enabling the establishment of P. aeruginosa infections within the host. Vikstrom (2005) have shown that high concentrations (100 µM) of OdDHL specifically stimulate the phagocytic activity of macrophages by selectively engaging the p38, MAPK signaling pathway. However, counteracting forces exist; the QS signals are prone to degradation by host tissues in a particular airway epithelium that produces a lactonase that degrades OdDHL (but not BHL) (Chun et al., 2004). Because the QS-controlled rhamnolipid shield is not launched until a significant number of cells have been amassed, it may be speculated that such a mechanism, in addition to antimicrobial peptides and mucociliary clearance, plays a role in the innate defense against bacterial biofilm infections, where it may aid innate immunity, counteracting young and undeveloped biofilms as suggested by Gunther (2009), but not mature, well-established biofilms that will be protected by the rhamnolipid shield.
Activation of the adaptive immune response — a diagnostic tool
The use of serological tests in diagnosing chronic (e.g. syphilis, borreliosis, Q-fever) and sometimes even acute infections (e.g. legionnaires' disease or mycoplasma) as well as during disentangling of hyper-reactive phenomena initiated by infectious diseases (e.g. reactive arthritis, Reiter's syndrome) is well established. However, serological tests can also be involved in biofilm infections. The majority of patients with CF acquire chronic P. aeruginosa lung infections with time. The infection cannot be completely eradicated from the lungs due to the biofilm mode of growth. A significant characteristic of the disease is the development of a pronounced humoral response against P. aeruginosa when the patients become chronically infected, and several CF centers use the detection of a specific antibody response as a marker of chronic infection per se in contrast to harmless colonization (Høiby et al., 1977; Pressler et al., 2009). Similarly, antibody responses in chronic or lente endocarditis can be used as a diagnostic tool, either by the ELISA technique or as precipitating antibodies, a useful diagnostic tool because the diagnosis of infective endocarditis is often delayed (Kjerulf et al., 1998). This is in contrast to acute endocarditis, where an adaptive immune response is of limited value due to the inertia of the adaptive immune response, combined with the aggressive infection.
Patients suffering from spinal cord lesions are at a high risk of acquiring recurrent or chronic urinary tract infections due to their impaired ability to empty the urinary bladder. Many of those patients empty their bladder using catheters either by intermittent catheterization or through permanent catheters. To distinguish whether those patients have developed a chronic infection, antibody response to the most prevalent pathogens of the urinary tract can be estimated (Moser et al., 1998). Actually, the finding of precipitating antibodies to cultured pathogens of the urinary tract in a subgroup of those patients (patients with myelomeningocele) correlates to levels of serum creatinine indicating impaired renal function, probably due to immune complex disease (Moser et al., 1998).
T-cell responses have, to our knowledge, not been used as a diagnostic tool in biofilm infections, although specific changes may occur when the infection changes from intermittent colonization to chronic infection. T-cell responses as a diagnostic tool are best known in tuberculosis as a delayed-type hypersensitivity response in the skin after injection of a purified TB antigen (the Mantoux test) or the so-called quantiferon test, where peripheral blood cells are exposed to a specific TB antigen and the release of IFN-γ is measured.
Antibody responses and biofilm infections
Antibody responses and biofilm infections are best studied in CF patients with chronic P. aeruginosa lung infections, and humoral responses in CF were investigated to reveal whether P. aeruginosa could be considered as a pathogen in CF or whether it was a harmless colonizer. Because classes and subclasses of antibodies have distinct functions, their levels have been correlated to the course of the chronic P. aeruginosa lung infections in CF patients. Interestingly, both specific subclass IgG2 and IgG3 correlated to a poor lung function and poor clinical condition in CF. The mechanism behind this correlation was believed to be the ability of IgG3 antibodies to activate, complement and thereby contribute to inflammation (Pressler et al., 1988, 1990). Although not to the benefit of the patient, this is an example of how the two immune responses can act in synergy.
Interestingly, and in contrast to what is usually reported during the course of an infection, there was no maturation in avidity (binding strength between antibody and antigen) of antibodies directed against chromosomal β-lactamase of P. aeruginosa or the P. aeruginosa heat shock protein Gro-EL during an 11-year follow-up period of the chronic P. aeruginosa lung infection in CF (Ciofu et al., 1999). In accordance, other investigators have reported the development of reduced opsonic killing by antibody responses directed against P. aeruginosa exopolysaccharide (Meluleni et al., 1995). Such a failure in maturation probably results in a reduced ability of the humoral response to control the infection and increase the tendency to develop immune complex disease (Devey et al., 1984). Furthermore, T cell responses have shown a reduced mitogenic response during the chronic lung infection. The significance of this observation still needs to be clarified.
In contrast, examples of protective antibody responses in biofilm infections have been shown. In infected CF patients, specific antibodies directed against protease and elastase were able to neutralize the enzymatic activity of those virulence factors and measurements of these exoenzymes (including exotoxin A) were negative in the sputum from the CF patients (Döring et al., 1985). Another interesting example emerges from the observation that β-lactamases was secreted outside P. aeruginosa in small blebs (vesicles). Furthermore, a high-avidity anti-β-lactamase antibody response correlated to a better lung function and this initiated a study to investigate whether the CF patients generated a humoral response directed against β-lactamases. Not only was this the case, but the production of high-avidity anti-β-lactamase antibodies correlated to a better lung function (Ciofu et al., 2002). An animal study where β-lactamase vaccination was performed in rats, later infected with a P. aeruginosa strain producing high levels of β-lactamase, showed that vaccinated rats that responded to the vaccine had a more beneficial lung inflammation and reduced quantitative lung bacteriology as nonvaccinated and nonresponding vaccinated rats when the vaccination was combined with ceftazidim treatment (Ciofu et al., 2002).
Finally, autoantibodies to parts of the immune system have also been reported. This is probably best described as antineutrophil cytoplasmic autoantibodies (ANCA) directed against bacterial/permeability increasing protein (BPI) in patients suffering from chronic biofilm infections with P. aeruginosa, for example in patients with diffuse panbronchiolitis (Ohtami et al., 2001). The authors reported that serum BPI-ANCA correlated to the severity of clinical symptoms and that the titer was reduced with improvements in clinical status (Ohtami et al., 2001). Later on, this has also been shown in CF patients, because BPI-ANCA was inversely correlated to the lung function in CF patients chronically infected with P. aeruginosa (Carlsson et al., 2003). The mechanism was suggested to be inhibition of the phagocytic activity because BPI-ANCA reduced this activity in a dose-dependent manner (Ohtami et al., 2001).
Adaptive immune response and CF
In the spontaneous course of the chronic P. aeruginosa lung infection before modern aggressive antibiotic treatments were implemented, there seemed to be a dichotomized course of infection. Either a deteriorating course with a poor prognosis in the majority of the patients where the antibody response was pronounced or rapidly increasing or a more beneficial course of the chronic infection in a minority of the patients where the antibody response remained low was observed (Høiby et al., 1977). The suggested decisive role of the adaptive immune response during the chronic P. aeruginosa lung infection initiated a number of studies in an attempt to reveal the mechanisms behind this observation.
Mosmann and Coffman first reported the background for the division of the T-helper cell response into two subtypes based on their cytokine profile (Mosmann et al., 1986; Locksley et al., 1987). The two subsets were designated Th1 and Th2 cells, and cytokines from one subset could downregulate the other subset. In addition to IFN-γ (Th1) and IL-4 and IL-5 (Th2), IL-9 and IL-13 are also considered Th2 cytokines. The two Th-cell subsets were also shown to influence major parts of the immune system differently; Th1 responses are thus related to the activation of Mφ and the cellular immune response, whereas Th2 responses stimulate the humoral immune response and mast cells (Fig. 1).
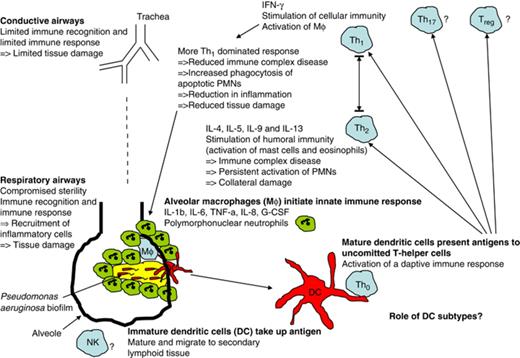
The Th1/Th2 dichotomy in chronic pulmonary Pseudomonas aeruginosa biofilm infection.
A specific significantly reduced release of IFN-γ (Th1 marker) from peripheral blood mononuclear cells (PBMCs) was observed from chronically infected CF patients as compared with CF patients who were not yet chronically infected (Moser et al., 2000). IL-4 release (Th2 marker) was almost exclusively seen in PBMCs from the chronically infected CF patients, indicating a skewing of the Th1/Th2 balance to a Th2-dominated response in CF patients with chronic P. aeruginosa lung infection. Moreover, IFN-γ release from PBMCs correlated to the lung function of the chronically infected CF patients, indicating a possible beneficial effect if the Th1/Th2 balance could be tipped in favor of a more Th1-dominated response (Moser et al., 2000). A skewing of the Th1/Th2 balance in CF has been confirmed by other groups (Moss et al., 2000; Brazova et al., 2005; Hartl et al., 2006). IFN-γ treatment of chronically infected rats was shown to render the lung inflammation from an acute type dominated by neutrophils to an inflammation dominated by MN cells (Johansen et al., 1996). Infecting two different inbred mouse strains with alginate embedded P. aeruginosa in the lungs revealed that the C3H/HeN mouse strain had a Th1-dominated response and a beneficial course of the infection, in contrast to the BALB/c mouse strain, which had a Th2-dominated response and a more serious course of the infection (Moser et al., 1997, 1999). However, in case of reinfection of the susceptible BALB/c mice, the mice became resistant and the immune response changed to a Th1-dominated response resembling the course of a primary infection in the resistant C3H/HeN mice (Moser et al., 2002).
An improved course of the chronic P. aeruginosa lung infection through a Th1-dominated response is primarily believed to be mediated through increased stimulation of the alveolar Mφ. An increase in the number and activation of the alveolar Mφ would presumably improve the resolution of the pulmonary inflammation by phagocytosis of apoptotic neutrophils and cell debris from necrotic neutrophils (Ware & Matthay, 2000). Especially, the removal of apoptotic neutrophils before they proceed to necrosis (which may strongly be promoted by contact with the rhamnolipid shield), and thereby further increase the inflammation, is thought to be important. In addition, a more Th1-dominated response may also result in the reduced production of IL-8 (Cassatella et al., 1993; Schnyder-Candrian et al., 1995) and thereby reduced chemoattraction of neutrophils.
In addition, a downregulated Th2 response and thereby reduced B-cell stimulation would result in reduced antibody response and reduced formation of immune complexes and therefore reduced tissue damage. Furthermore, reduced IL-13 production could result in diminished mucus production, reducing the tendency for aspirated pathogens to be captured by the copious mucus in the CF lung. However, any relationship between such mechanisms and the Th1/Th2 balance in CF remains to be investigated.
In contrast, a direct antimicrobial effect on the P. aeruginosa biofilms by Mφ does not seem to be the mechanism; for example J. Leid and colleagues have observed an increased phagocytosis of young P. aeruginosa biofilm after activation with IFN-γ. However, when exogenous alginate was added to the biofilms, the increased killing of IFN-γ-activated Mφ was impaired (Leid et al., 2005). This observation further supports that the beneficial effect of a more Th1-dominated response in CF patients with chronic biofilm infections is probably mediated through modulation of the host responses and not by a direct antibiofilm mode of action per se.
In the case of S. aureus biofilm infection, there also seems to be a skewing of the T-helper cell response. Release of the Th1-inducing cytokines IL-12 and IFN-γ by leukocytes exposed to an S. aureus biofilm may promote a Th1-dominated response to the early biofilm where a Th2-dominated response may be more appropriate (Leid et al., 2002; Shkreta et al., 2004; Sun et al., 2005). Although a Th2-dominated humoral response develops at later stages of the biofilm infection, such a response is ineffective in clearing the infection, like the chronic P. aeruginosa lung infection in CF. A Th17 subtype (producing IL-17 and IL-22) and a regulatory T-cell subset (Treg1) subset producing IL-10 and TGF-β (as well as IFN-γ and IL-5) seems to be readily accepted. Generally, it is acknowledged that the responses are characterized by a balance of all subsets, which, however, can be an inappropriate balance.
To the best of our knowledge, the possible role of a Th17 response or Treg cells in biofilms has not been published, and similarly with respect to T suppressor cells.
In conclusion, detailed knowledge of the immune responses, cross-kingdom communication and bacterial defense mechanisms under conditions of biofilm infections is important — not only because the response is part of the immunopathology in biofilm infections, but because it is likely to provide important treatment tools during the otherwise immunotolerant biofilm infections.
References
Author notes
Editor: Gianfranco Donelli



