-
PDF
- Split View
-
Views
-
Cite
Cite
Motomichi Takahashi, Haruhiko Taguchi, Hiroyuki Yamaguchi, Takako Osaki, Akio Komatsu, Shigeru Kamiya, The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice, FEMS Immunology & Medical Microbiology, Volume 41, Issue 3, July 2004, Pages 219–226, https://doi.org/10.1016/j.femsim.2004.03.010
Close - Share Icon Share
Abstract
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 has been considered as an agent responsible for outbreak of hemorrhagic colitis and the hemolytic uremic syndrome. We examined the effect of the probiotic agent Clostridium butyricum MIYAIRI strain 588 on EHEC O157:H7 infections in vitro and in vivo using gnotobiotic mice. The growth of EHEC O157:H7 and the production of Shiga-like toxins in broth cultures were inhibited by co-incubation with C. butyricum. The antibacterial effects of butyric and lactic acid were demonstrated in a dose-dependent manner. In addition, the inhibitory effect of butyric acid on the viability of EHEC was demonstrated not only at low pH, but also at neutral pH adjusted to 7.0. Flowcytometric analysis showed that pre-incubation of Caco-2 cells with C. butyricum and E. coli K12 inhibited the adhesion of EHEC O157:H7. However, the effect of C. butyricum on adhesion of EHEC to Caco-2 cells was more inhibitory than that of E. coli K12. Gnotobiotic mice mono-associated with EHEC O157:H7 died within 4–7 days after the infection. On the other hand, all gnotobiotic mice prophylactically pre-treated with C. butyricum survived exposure to EHEC O157:H7 and of the gnotobiotic mice therapeutically post-treated with C. butyricum, 50% survived. Both counts of EHEC O157:H7 and the amounts of shiga-like toxins (Stx1 and Stx2) in fecal contents of gnotobiotic mice di-associated with EHEC O157:H7 and C. butyricum were less than those of gnotobiotic mice mono-associated with EHEC O157:H7. These results indicated that the probiotic bacterium C. butyricum MIYAIRI strain 588 has preventive and therapeutic effects on EHEC O157:H7 infection in gnotobiotic mice.
1 Introduction
The Escherichia coli strain of the serotype O157:H7 has been considered as an agent responsible for outbreak of hemorrhagic colitis and the hemolytic uremic syndrome (HUS) [1–3]. This strain belongs to the family of enterohemorrhagic E. coli (EHEC). The EHEC O157:H7 produces intimin [4], Shiga toxins (Stx1, Stx2 or both) [3,5,6], lipopolysaccharide (LPS) and hemolysins [6]. Stx and LPS have been suggested to be involved in the pathogenesis of HUS [7]. These Stx inhibit protein synthesis by cleaving a specific adenine residue in the 28S subunit of eukaryotic RNA. They are multi-subunit toxins, consisting of one enzymatically active A subunit and five receptor-binding B subunits and are cytotoxic to vero tissue culture cells [8].
A variety of animal models has been employed for studying the pathogenesis in EHEC O157:H7 infections associated with HUS. Stx-producing strains cause gastrointestinal, neurologic or systemic symptoms, resulting in death in gnotobiotic piglets [9], rabbits [10] and mice [11]. Histopathologic lesions such as inflammatory colitis [10], endothelial cell necrosis in the brain [7,11] and acute tubular necrosis of the kidneys [11,12] were found in the inoculated animals, but glomerular pathology was not observed [3]. Strains differing in Stx production have been studied in the piglet and rabbit colitis model [9,10], but HUS-like symptoms and their pathology were not compared.
Probiotics are viable cell preparations that have beneficial effects on the health of the host [13]. Clostridium butyricum is a butyric-acid producing gram-positive anaerobe, found in soil and intestines of healthy animals and humans. The MIYAIRI 588 strain of C. butyricum has been used as a probiotic for the treatment and prevention of non-antimicrobial induced diarrhea as well as antimicrobial-associated diarrhea in human and animal [14,15]. This strain has been approved for human clinical use since 1968 in Japan. The mechanism, by which C. butyricum controls diarrhea, is considered to be complex. For example, C. butyricum has been shown to have antagonistic interaction against Candida albicans, Clostridium difficile, enterotoxigenic E. coli, Klebsiella spp., Salmonella spp., Vibrio spp. and Helicobacter pylori[16–21]. In addition, butyric acid produced by C. butyricum has a proliferative effect on mucosal cells in the intestine [22] and it was reported that butyric acid produced by C. butyricum has therapeutic efficiency against the inflammatory bowel disease [23].
The aim of this study was to examine the possible antagonistic effect of C. butyricum MIYAIRI strain 588 on EHEC O157:H7 infections in vitro and in vivo using germ-free mice.
2 Materials and methods
2.1 Bacterial strains
C. butyricum MIYAIRI strain 588 used in this study was obtained from Miyarisan Pharmaceutical Co., Ltd., Tokyo, Japan. The biochemical characteristics of this strain have been described elsewhere [24–26]. Clinically isolated EHEC O157:H7 strain 6 (Stx1+, Stx2+, eaeA+), strain 20 (Stx1+, Stx2+, eaeA+) and strain 120 (Stx2+, eaeA+) were kindly provided by Dr. K. Tamura, National Institute of Infectious Diseases, Tokyo, Japan. The strain 6 used in the animal experiments has an enhanced ability to produce both Stx1 and Stx2 in vitro compared with other strains [27]. Laboratory-stocked E. coli K12 (non-pathogenic E. coli) was also used.
2.2 Media and culture conditions
Brain heart infusion (BHI) (Difco Laboratories, Detroit, MI) broth and sorbitol MacConkey agar no. 3 (Oxoid Ltd., Basing stoke, UK) were used for routine culture of EHEC under aerobic conditions. EHEC O157:H7 strain 6 could not utilize sorbitol. Thus, this strain forms white colonies on sorbitol MacConkey agar no. 3. GAM agar (Nissui Pharmaceutical Co., Ltd.) was used for routine culture of C. butyricum as described previously. Cultures on GAM agar were incubated at 37 °C in an anaerobic chamber (H2 10%, CO2 10%, N2 80%).
2.3 Cell culture
Enterocyte-like Caco-2 cells [28] were used for the adherence assay by flowcytometry. Cells were routinely grown in Dulbecco modified Eagle's minimal essential medium (DMEM; Gibco, BRL), supplemented with 10% inactivated fetal calf serum in a humidified atmosphere of 5% CO2 at 37 °C for 48 h.
2.4 Animals
Germ-free mice (IQI/jic, female, 8 weeks old) were obtained from Japan Clea Inc., Tokyo, Japan. Germ-free mice were maintained in a sterilized vinyl isolator and fed a sterilized diet and water ad libitum.
2.5 Inhibitory effect of C. butyricum on growth of EHEC O157:H7 in vitro
EHEC O157:H7 and C. butyricum, 18 h old, were separately cultured on GAM agar and resuspended in phosphate buffer saline (PBS; pH 7.2), followed by centrifugation at 10,000g for 5 min. The supernatant was discarded, and the bacteria were washed three times with sterile PBS and resuspended in BHI broth. The EHEC O157:H7 suspension was diluted to approximately 104 cfu ml−1 and the C. butyricum suspension was diluted to 106 cfu ml−1. Growth inhibition assays were performed by incubating 1 ml of EHEC O157:H7 and 1 ml of C. butyricum in 8 ml BHI broth (103 cfu ml−1 of EHEC O157:H7, 105 cfu ml−1 of C. butyricum) at 37 °C under anaerobic conditions. Initially, and further at predetermined intervals, aliquots were removed, serially diluted, plated on BHI agar and incubated at 37 °C for 24 h under aerobic conditions to determine colony counts of EHEC O157:H7.
2.6 Effect of butyric and lactic acid on the viability of EHEC O157:H7
Various concentrations (6, 3 and 0 mM) of butyric (Wako Junyaku Co. Ltd., Tokyo, Japan) and lactic (Wako Junyaku Co. Ltd., Tokyo, Japan) acid were used to examine their effect on the viability of EHEC O157:H7. The acids were diluted by sterilized distilled water and then 1 ml of EHEC O157:H7 culture (106 cfu ml−1) were inoculated into 9 ml of the acid solution. The pH of 6, 3 and 0 mM solutions of butyric acid was 3.7, 4.3 and 7.0, respectively and the pH of the lactic acid solutions was 3.4, 4.1 and 7.0, respectively. One ml of bacterial suspension was sampled at 3 h after anaerobic incubation at 37 °C. These bacterial suspensions were centrifuged for 5 min and washed three times by PBS. After centrifugation, the pellets were resuspended in 1 ml of PBS and then 100 µl of suspension were plated on BHI agar and anaerobically incubated at 37 °C under for 24 h for quantification of EHEC O157:H7.
2.7 Effect of C. butyricum and E. coli on the adhesion of EHEC O157:H7 to Caco-2 cells
An adhesion assay was performed using flowcytometric analysis as described previously [21,29,30]. Six hours old EHEC O157:H7 strain 6, E. coli K12 and C. butyricum were cultured in 10 ml of BHI broth and then 1 ml of the culture were centrifuged at 10,000g for 5 min and washed three times by PBS. After centrifugation, the pellets were resuspended in 1 ml of PBS. Of the bacterial suspension, 50 µl was mixed with 500 µl of labeling buffer (Zynaxis Cell Sciences, Phoenixville, USA), and then 8 µl of PKH-2 lipophilic dye (Zynaxis Cell Sciences, Phoenixville, USA) was added. After incubation at room temperature for 10 min, the bacterial suspension was centrifuged at 10,000g for 5 min, and resuspended in 5 ml Hanks' balanced salt solution (HBSS) containing gelatin 0.1% (HGS). Caco-2 cells (2×105 cells) were pre-incubated with C. butyricum (2.1×107 cfu) or E. coli K12 (1.6×107 cfu) in 5 ml of HGS at room temperature for 1 h with gentle shaking. Then the cells were centrifuged at 1000 rpm for 10 min and further incubated with the PKH-2 labeled EHEC O157:H7 (107 cfu) at room temperature for 1 h with gentle shaking. Non-adherent bacteria were removed by centrifugation at 1000 rpm for 10 min and then resuspended in sucrose 15% solution. The cells were washed three times with HGS and then resuspended in 1 ml of HGS for flowcytometric analysis. A flowcytometer (FACS Vantage, Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) was used for the measurement of fluorescence intensity. Fluorescence data were obtained in a logarithmic mode scale.
2.8 Experimental infection of germ-free mice with EHEC O157:H7
We used the infection model of EHEC O157:H7 as reported by Taguchi et al. [27]. EHEC O157:H7 was grown in 50 ml of BHI broth at 37 °C for 6 h during continuous shaking, and the cell count was approximately 108 cfu ml−1 at exponential to logarithmic phase. Cells were sedimented by centrifugation (3000g, 20 min) and then washed three times with phosphate-buffered saline (PBS) and resuspended in 10 ml PBS (108 cfu ml−1). 0.5 ml of the bacterial suspension was inoculated intragastrically. The control mice received 0.5 ml of PBS. After the inoculation, the population level of EHEC O157:H7 in fecal contents and the cytotoxic activity of the fecal fluid were examined. This experimental infection was performed under the guidelines on animal experiments in Kyorin University and Japanese Government Animal Protection and Management Law (No. 105).
2.9 Probiotic treatment
C. butyricum was grown on GAM agar at 37 °C for 48 h under anaerobic conditions and 10 colonies were picked up and suspended into 10 ml of PBS. C. butyricum cells were suspended in 10 ml phosphate-buffered saline (PBS; pH 7.2), washed three times by centrifugation (3000g, 20 min) and resuspended in 10 ml PBS (2×108 cfu ml−1). Germ-free mice were orally inoculated with C. butyricum suspension (108 cfu mouse−1) 4 days before the challenge with EHEC O157:H7 (prophylactic treatment), or 2 days after the infection with EHEC O157:H7 (therapeutic treatment).
2.10 Quantitative assay of bacteria and detection of Stx1 and Stx2
At various times after the infection with EHEC, 1 g of feces was collected and homogenized in 1 ml of PBS. From the homogenate, 10-fold serial dilutions were prepared, plated on sorbitol-MacConkey agar media and then incubated at 37 °C for 24 h under aerobic conditions. Stx1 and Stx2 titers were quantified using a kit for the detection of E. coli verotoxins 1 and 2, based on reversed passive latex agglutination (VTEC-RPLA “SEIKEN”, Denka Seiken Co., Ltd.), as described previously [27,31].
2.11 Statistical analysis
Fluorescence intensity, cell counts of EHEC O157:H7 and Stx levels in the fecal contents were determined as described above, and the significance of the difference between means of the groups was determined by Student's t-test. Probability values of <0.01 were considered significant.
3 Results
3.1 Inhibitory effect of C. butyricum on growth of EHEC O157:H7 in vitro
The inhibitory effect of C. butyricum on the growth of EHEC O157:H7 was examined in vitro (Fig. 1). The growth of three strains (strains 6, 20 and 120) of EHEC O157:H7 in BHI broth under anaerobic conditions was strongly inhibited by co-incubation with C. butyricum. These inhibitory effects were not dependent on the strain used. In addition, the ability to produce Stx1 and Stx2 was inhibited by co-incubation with C. butyricum (Fig. 2).
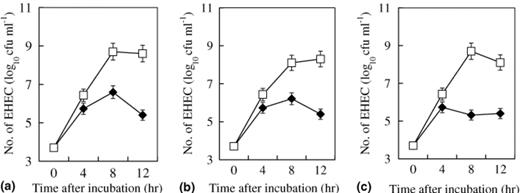
Inhibitory effect of C. butyricum on the growth of (a) EHEC O157:H7 strain 6 (Stx1+, Stx2+, eaeA+), (b) EHEC O157:H7 strain 20 (Stx1+, Stx2+, eaeA+), (c) EHEC O157:H7 strain 120 (Stx2+, eaeA+). (–□–) Growth curve of EHEC strain in BHI broth; (–♦–) Growth curve of EHEC strain co-inoculated with C. butyricum in BHI broth.
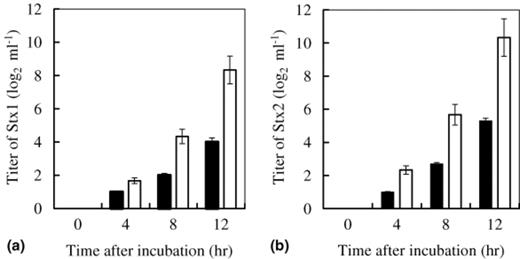
Inhibitory effect of C. butyricum on the production of (a) Stx1 and (b) Stx2 from EHEC O157:H7 strain 6 in BHI broth: (□) The titer of Stx produced by EHEC; (▄) The titer of Stx produced by EHEC co-inoculated with C. butyricum.
3.2 Antibacterial effect of butyric and lactic acid on EHEC O157:H7 in vitro
The antibacterial effect of butyric and lactic acid on the viability of EHEC O157:H7 was determined in vitro (Fig. 3) and was demonstrated in a dose-dependent manner without adjusting the pH. In addition, antibacterial effects of butyric acid were demonstrated not only at low pH but also at pH 7.0. However, antibacterial effects of lactic acid were only demonstrated at low pH.
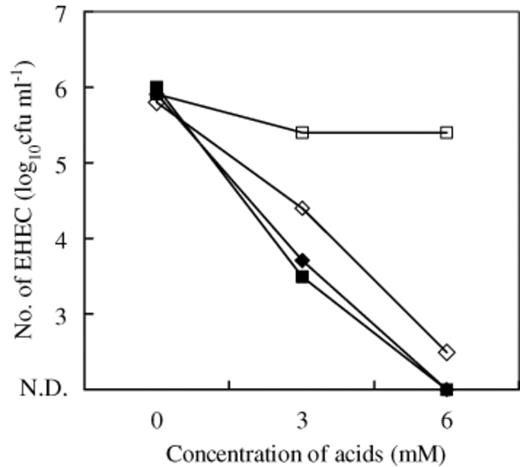
Inhibitory effect of butyric and lactic acid on EHEC O157:H7 strain 6: (–♦–) butyric acid (pH non-adjusted); (–◊–) butyric acid (pH adjusted to 7.0); (–▄–) lactic acid (pH non-adjusted); (–□–) lactic acid (pH adjusted to 7.0); N.D., not detectable. Results were averages of duplicate studies.
3.3 Inhibitory effect of C. butyricum on adhesion of EHEC O157:H7 to Caco-2 cells
The effect of C. butyricum on the adhesion of EHEC O157:H7 to human intestinal epithelial Caco-2 cells was examined by flowcytometric analysis. The mean fluorescence intensity (mean±SD) of control Caco-2 cells was 3.27±2.35. The mean fluorescence intensity of Caco-2 cells with adherent PKH-2 labeled EHEC O157:H7 was 9.44±8.26. After pre-incubation of the Caco-2 cells with 107 cfu of E. coli K12 or C. butyricum, the mean fluorescence intensity of the Caco-2 cells incubated with EHEC O157:H7 was 8.37±8.08 or 4.91±2.69, respectively. The adherence activity of EHEC O157:H7 was significantly inhibited by pre-incubation with E. coli K12 or C. butyricum (p<0.01), and the inhibitory activity of C. butyricum was stronger than that of E. coli (p<0.01).
3.4 Preventive and therapeutic effects of C. butyricum on lethal infection with EHEC in gnotobiotic mice
The effect of C. butyricum on lethal infection with EHEC O157:H7 was examined using germ-free mice (Fig. 4). All of the gnotobiotic mice mono-associated with EHEC O157:H7 died within 7 days post infection. The infected mice did not develop any gastrointestinal symptoms such as loose, watery or bloody stools during the infection. However, neurological symptoms such as hindleg weakness or paralysis, intention tremor and spastic paralysis developed within 3–6 days after the inoculation. In contrast, the survival rate of the therapeutically treated mice was 50% at 14 days after the infection with EHEC. Interestingly, the survival rate of the mice prophylactically treated with C. butyricum was 100% at 14 days after the infection with EHEC.
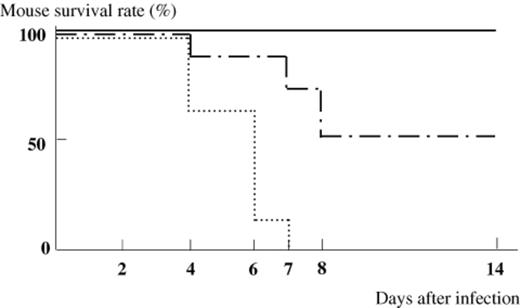
Survival rate of gnotobiotic mice infected with EHEC O157:H7. (▀) gnotobiotic mice mono-associated with EHEC O157:H7 (n=8). (-⋄-) EHEC-associated gnotobiotic mice prophylactically treated with C. butyricum (n=8). (-·-·-·-) EHEC-associated gnotobiotic mice therapeutically treated with C. butyricum (n=8).
3.5 Bacterial counts and Stx1 and Stx2 production
After infection of germ-free mice with EHEC O157:H7, 108–109 cfu g−1 of EHEC O157:H7 was continuously detected in the feces during 6 days (Table 1). In the gnotobiotic mice prophylactically treated with C. butyricum, the number of EHEC O157:H7 in the feces was detected at the level of 107 cfu g−1, which was nearly 100 times less compared to that in the feces of the EHEC mono-associated mice. The level of 108 cfu g−1 of EHEC O157:H7 was detected in the gnotobiotic mice therapeutically treated with C. butyricum. A statistically significant difference was observed in the counts of EHEC O157:H7 between non-treated mice and therapeutically treated mice (p<0.01).
Quantification of EHEC O157:H7 in the feces of the EHEC-associated gnotobiotic mice with or without treatment with C. butyricum
Days after infection with EHEC O157:H7.
Statistically significant (p<0.01) compared with the gnotobiotic mice mono-associated with EHEC O157:H7.
Quantification of EHEC O157:H7 in the feces of the EHEC-associated gnotobiotic mice with or without treatment with C. butyricum
Days after infection with EHEC O157:H7.
Statistically significant (p<0.01) compared with the gnotobiotic mice mono-associated with EHEC O157:H7.
The titer of Stx1 and Stx2 in the feces of the infected gnotobiotic mice was determined (Fig. 5). The titers of Stx1 and Stx2 in the feces of the mice mono-associated with EHEC O157:H7 were 24.5–27.1 and 27–29.5 g−1, respectively. In contrast, the production of both Stx1 and Stx2 significantly decreased in the feces of the EHEC-associated gnotobiotic mice prophylactically treated with C. butyricum, with titers of 22.8–23.5 and 23.9–26.5 g−1 for Stx1 and Stx2, respectively. The titers of Stx1 and Stx2 at 2 days after the infection in the feces of the mice therapeutically treated with C. butyricum was significantly lower than that in the EHEC mono-associated mice (p<0.01).
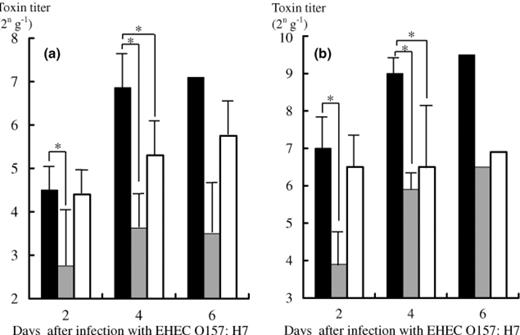
Detection of Stx1 (a) and Stx2 (b) in the feces of the EHEC-associated gnotobiotic mice with or without treatment with C. butyricum. (▄) toxin titer (2n g−1) in the gnotobiotic mice mono-associated with EHEC O157:H7. (

Persistent colonisation with C. butyricum in the intestine was observed in the gnotobiotic mice. The level of C. butyricum in mouse feces was 109 cfu g−1 and no significant change was observed between with or without EHEC O157:H7 infection (data not shown).
4 Discussion
In 1996, serious outbreaks of diarrheal diseases caused by EHEC strains of the serotype O157:H7, with sporadic cases of hemorrhagic colitis and HUS, were reported in Japan [32]. The mainstay of the therapy for hemorrhagic colitis is the management of dehydration, electrolyte abnormalities and gastrointestinal blood loss. The antibiotic susceptibilities of EHEC have been investigated [33]. However, no controlled trials of antibiotic treatment of EHEC infection have been reported. Some investigators suggested that antibiotic therapy increased Stx production by EHEC, with enhanced risk of the illness [34,35]. Thus, the probiotic treatment of these infectious diseases is an important strategy for clinical therapies. The inhibitory effect of probiotics on the infection with enteropathogens was reported to be based on several properties such as production of substances that inhibit or kill the pathogen [36], competition with the pathogen for adhesion to enterocytes [37], nutritional antagonism [38] and inhibition of toxin production [39].
Several investigators suggested that probiotic bacteria have inhibitory effects on the growth of EHECs [40–42]. Our data indicated that C. butyricum has an inhibitory effect on growth of EHECs as described in other reports. This inhibition may be due to the production of short chain fatty acids, such as butyric acid, by C. butyricum. The possible mechanisms responsible for the antagonism between two bacteria in the intestinal ecosystem include competition of either nutrients or adhesion sites and production of either bacteriocins or volatile fatty acids. C. butyricum produces butyric acid, and in the present study butyric acid inhibited the growth of EHEC even when the pH was adjusted to 7.0, suggesting that butyric acid has as antibacterial activity in addition to its acidity.
Bernet et al. [37] reported that Lactobacillus acidophilus LA 1 with binding activity to Caco-2 cells inhibited adhesion and invasion by enteropathogenic bacteria. We demonstrated the inhibitory effect of C. butyricm on adhesion of EHEC to Caco-2 cells using flowcytometric analysis. Interestingly, the inhibitory effect of C. butyricum on adhesion of EHEC O157:H7 was much stronger than that of E. coli.
It has been considered that the LEE type III secretion system of EHEC is important for bacterial adhesion to host's intestinal cells during the early steps of infection [43]. It is possible that binding of C. butyricum to Caco-2 cells might interfere with the adhesion of EHEC. However, the mutual interaction between C. butyricum and the LEE type III secretion system remains to be determined.
In the present investigation, we defined three groups of gnotobiotic mice, which were associated with C. butyricum before or after challenge with EHEC O157:H7. In the group non-treated with C. butyricum, we noted the persistent colonization of EHEC O157:H7 at the levels of 108–109 cfu g−1 and all of the infected mice died within 7 days. On the other hand, in the experimental group of the gnotobiotic mice pre-treated with C. butyricum all these mice survived. The preventive effect of C. butyricum on mouse lethality was induced by inhibition of bacterial growth and toxin production of EHEC O157:H7 (Table 1 and Fig. 5). Similar results were obtained in an independent experiment using four prophylactically treated mice with C. butyricum and five untreated gnotobiotic mice mono-associated with EHEC O157:H7 (data not shown). The level of growth inhibition by C. butyricum in mouse intestine was similar to the in vitro inhibitory effects. The concentration of butyric acid in the fecal specimen was not as high as expected (data not shown), and it is likely that 95% of short chain fatty acids, including butyric acid produced by intestinal microflora, could be absorbed quickly from intestinal epithelial cells as described before [22]. However, it is also possible that butyric acid produced might induce bactericidal effects on EHEC O157:H7 during a very short period. Isogai et al. [7] stated that the kidneys of gnotobiotic mice with EHEC infection were pale and swollen. In addition, Taguchi et al. [27] reported that gnotobiotic mice mono-infected with EHEC O157:H7 strain 6 showed severe damages in kidney, brain and colon. It is possible that C. butyricum directly inhibited the growth and toxin production of EHEC O157:H7, resulting in the suppression of the induction of histopathological changes.
The results obtained in this study suggest that the probiotic agent C. butyricum MIYAIRI strain 588 has prophylactic and therapeutic effects on EHEC O157:H7 infection in gnotobiotic mice.
References



