-
PDF
- Split View
-
Views
-
Cite
Cite
Hyesuk Seo, Ti Lu, Rahul M Nandre, Qiangde Duan, Weiping Zhang, Immunogenicity characterization of genetically fused or chemically conjugated heat-stable toxin toxoids of enterotoxigenic Escherichia coli in mice and pigs, FEMS Microbiology Letters, Volume 366, Issue 4, February 2019, fnz037, https://doi.org/10.1093/femsle/fnz037
Close - Share Icon Share
ABSTRACT
Enterotoxigenic Escherichia coli (ETEC) producing type Ib heat-stable toxin (STa) are a main cause of children's diarrhea and travelers’ diarrhea, thus STa needs to be targeted in ETEC vaccine development. However, because this 19-amino acid STa is poorly immunogenic, attempts to genetically fuse or chemically couple it to carrier proteins have been made to enhance STa immunogenicity. In this study, we selected one genetic fusion and one chemical conjugate to comparatively evaluate STa immunogenicity. The genetic fusion is 3xSTaN12S-mnLTR192G/L211A carrying three toxoid (STaN12S) genetically fused to a double mutant LT monomer (mnLTR192G/L211A); the chemical conjugate is BSA-STaA14T, which has toxoid STaA14T chemically coupled to bovine serum albumin (BSA). We immunized mice with the STa toxoid fusion and chemical conjugates, and examined antibody responses. Furthermore, we immunized pigs and evaluated derived antibodies for efficacy to passively provide protection against ETEC diarrhea using a piglet model. Data showed that mice subcutaneously immunized with BSA-STaA14T or 3xSTaN12S-mnLTR192G/L211A developed a strong anti-STa antibody, and the induced antibodies exhibited equivalent toxin-neutralizing activities. Pigs immunized with 3xSTaN12S-mnLTR192G/L211A or BSA-STaA14T developed similar levels of anti-STa antibodies; piglets with passively acquired antibodies induced by the genetic fusion appeared better protected against STa + ETEC. Results from the current study indicate that the fusion and conjugate approaches are viable options for facilitating STa immunogenicity and developing ETEC vaccines.
INTRODUCTION
Escherichia coli strains producing enterotoxins, particularly heat-stable toxin (STa), alone or together with heat-labile toxin (LT), known as enterotoxigenic E. coli (ETEC), are a leading cause of moderate-to-severe diarrhea in children living in low- and- middle income countries (children's diarrhea) and diarrhea in international travelers (travelers’ diarrhea). STa is a key virulence determinant and remains highly prevalent among ETEC strains causing diarrhea. STa recognizes intestinal receptor guanylyl cyclase C (GC-C) and enzymatically disrupts intestinal epithelial cell fluid homeostasis, which leads to water and electrolyte hyper-secretion through the elevation of intracellular guanylate cyclase (cGMP) level, resulting in watery diarrhea (Nataro and Kaper, 1998, Zhang and Sack, 2015).
STa, a 19-amino acids peptide (the porcine-type ETEC STa consists of 18 amino acids) is poorly immunogenic and potently toxic. That becomes a major challenge to identify safe and immunogenic STa antigens for use in ETEC vaccination (Taxt et al., 2010, Zhang et al., 2010, Zegeye et al., 2018). STa toxoids (derived from mutations of certain amino acid residues) showed significant reductions in enterotoxicity (biological activity) but retained STa antigenicity (Zhang et al., 2010, Liu et al., 2011, Taxt et al., 2014), thus increasing the prospects that these toxoids could be safe ETEC vaccine components. In an effort to enhance STa immunogenicity, STa molecules (native STa or STa mutants) were coupled chemically or fused genetically to various carrier proteins to form chemical conjugates or fusion proteins to be valuated immunologically (Frantz and Robertson, 1981, Sanchez, Hirst and Uhlin, 1988, Zhang et al., 2010). Recently, a study revealed 3xSTaN12S-mnLTR192G/L211A, a peptide with three STa toxoid STaN12S genetically fused to a double mutant LT monomer (mnLTR192G/L211A), induced antibodies that neutralized biological activity of both STa and LT toxins when administered either intraperitoneally (IP) or subcutaneously (SC) to mice, or intramuscularly (IM) to pigs (Ruan et al., 2014, Nandre et al., 2016). An alternative approach to improve STa immunogenicity is to chemically conjugate STa to a carrier protein such as bovine serum albumin (BSA) or chicken ovalbumin (Frantz and Robertson, 1981). Chemical conjugation has been successfully applied to facilitate immunogenicity of some poorly immunogenic proteins from other bacterial pathogens including Vibrio cholerae, Streptococcus pneumonia, Haemophilus influenzae Type b and Francisella tularensis (Xu et al., 2011, Black et al., 2000, Conlan et al., 2002, Cutts et al., 2005). However, little is known about the ability of STa toxoid conjugates in inducing anti-STa antibodies and contributing to protective immunity. In addition, no studies have attempted to directly compare the effectiveness of genetic fusion versus chemical conjugation in enhancing STa toxoid immunogenicity or their protective efficacy in any challenge models.
In the current study, we immunized mice with 3xSTaN12S-mnLTR192G/L211A genetic fusion side-by-side with chemical conjugates BSA-STaA14T or BSA-STa and examined mouse anti-STa antibody responses, but also antibody neutralization activity against STa biological activity. Moreover, we IM injected pregnant pigs with 3xSTaN12S-mnLTR192G/L211A or BSA-STaA14T to assess anti-STa antigenicity. Moreover, we challenged newborn piglets after 24 h suckling with an ETEC strain producing STa to evaluate protection of the fusion- and conjugate-induced anti-STa antibodies (passively acquired) over STa + ETEC diarrhea to assess STa toxoid fusion or conjugate antigen for potential application in ETEC vaccine development.
MATERIALS AND METHODS
Antigens, adjuvants and ETEC challenge strains used in this study
Chemical conjugates BSA-STaA14T and BSA-STa and toxoid genetic fusion 3xSTaN12S-mnLTR192G/L211A were used as antigens in mouse immunization, whereas 3xSTaN12S-mnLTR192G/L211A and BSA-STaA14T were used to immunize pregnant gilts. 3xSTaN12S-mnLTR192G/L211A toxoid fusion, previously named 3xSTaN12S-dmLT (Ruan et al., 2014, Duan and Zhang, 2017, Duan et al., 2018a), is a his-tag-less recombinant protein. This toxoid fusion protein is derived by genetically embedding three STa toxoid molecules (STaN12S) at the C- and N-terminals, as well as between the A and B subunits of modified LT mutant mnLTR192G/L211A monomer. This monomeric mnLTR192G/L211A was created by fusing a mutant LTA subunit to a single LTB subunit for a single peptide. 3xSTaN12S-mnLTR192G/L211A was expressed by vector pET28a in E. coli BL21 strain (Ruan et al., 2014, Nandre et al., 2017). Chemical conjugates BSA-STaA14T and BSA-STa were produced by chemically conjugating the purified STa toxoid STaA14T or native STa to BSA (provided by Dr. John Clements at Tulane University, LA). Double mutant LT (dmLT, LTR192G/L211A; AB5 molecule with one mutated LTA subunit noncovalently linked to a pentamer of five LTB subunits) obtained from PATH (produced by Walter Reed Army Institute of Research; Silver Spring, MD) was the adjuvant for mouse and pig immunization.
Recombinant ETEC strain 8823 producing STa and 987P fimbriae was used in ETEC infection study with the pig challenge model as described previously (Francis and Willgohs, 1991, Zhang et al., 2010, Nandre et al., 2017). Strain 8823 was derived from transformation of G58–1, a nonpathogenic porcine E. coli isolate (Francis and Willgohs, 1991), first with pDMS158 plasmid, which carries 987P fimbrial gene cassette to express 987P fimbriae (Schifferli and Alrutz, 1994; gifted by Dr. Richard Isaascon from University of Minnesota College of Veterinary Medicine, MN), and then with p8755 plasmid, which has porcine-type STa gene (estA) cloned to produce STa.
Mouse immunization
Mouse immunization study followed the protocol previously described (Duan et al., 2018a). Briefly, a total of 40 mice were used in this immunization study. Eight-week-old female BALB/c mice purchased from Charles River Laboratories International, Inc. (Wilmington, MA) were randomly divided into four groups (10 mice per group). Three groups were SC immunized with 60 µg of 3xSTaN12S-mnLTR192G/L211A, BSA-STaA14T or BSA-STa, respectively, with 1 µg dmLT adjuvant. The fourth group receiving no immunization served as the control. Immunized mice received two booster injections at an interval of two weeks. Mouse serum samples collected two weeks after the final booster were stored at -80°C.
Mouse (and pig) serum anti-STa antibody titration
Anti-STa-specific IgG antibodies were titrated by enzyme-linked immunosorbent assay (ELISA). As described previously (Zhang et al., 2010, Nandre et al., 2016, Nandre et al., 2017), STa-ovalbumin conjugates (10 ng in 100 µl STa ELISA buffer per well) coated in Costar plates (Corning Inc., Corning, NY) were used to titrate anti-STa IgG and IgA antibodies in mouse or pig serum samples (2-fold serially diluted, from 1:400 to 1:25 600, in triplicate). Anti-STa IgG or IgA titers were determined and presented at log10 scale (Zhang et al., 2010, Nandre et al., 2016, Nandre et al., 2017). ELISA for antibody titration in serum or colostrum samples was repeated three times.
Mouse serum anti-STa antibody neutralization assay
T-84 cells (ATCC, #CCL-248) and a cGMP EIA kit (Enzo Life, Farmingdale, NY) were used to examine mouse serum anti-STa antibodies neutralization activity against STa biological activity or enterotoxicity, as we described previously (Nandre et al., 2017, Duan et al., 2018a). Briefly, serum samples pooled from each group or from each individual mouse (30 µl) premixed with STa (2 ng) were incubated with T-84 cells. After 1 h incubation in a 5% CO2 incubator, cells were washed, lysed and measured for intracellular cGMP levels by following the manufacturer's instructions (Enzo Life). Intracellular cGMP in T-84 cells exposed to STa (2 ng) alone or to culture medium served as the control for STa biological activity or a baseline intracellular cGMP level, respectively. Toxin neutralization assay using mouse serum was repeated three times.
Mouse serum anti-STa antibody guanylin and uroguanylin cross-reactivity
To examine cross-reactivity of toxoid fusion- or conjugate-induced anti-STa antibodies with guanylin or uroguanylin, competitive STa ELISA was performed by following the protocol described previously (Duan et al., 2018a). Briefly, STa-ovalbumin conjugates (50 ng per well) coated in wells of costar plates (Corning Inc.) were used to compete against phosphate buffered saline (PBS), STa (50 ng), guanylin (50 ng) or uroguanylin (50 ng), respectively, for binding to anti-STa antibodies from the immunized mouse serum samples (2-fold serially diluted, from 1:1000 to 128 000). Optical density at 650 nm after incubation with horseradish peroxidase-conjugated goat-anti-mouse IgG and 3,3',5,5'-tetramethylbenzidine peroxidase substrates (KPL, Gaithersburg, MD) were measured for anti-STa antibody cross-reactivity with uroguanylin or guanylin (Duan et al., 2018a). ELISA for guanylin and uroguanylin cross-reactivity with anti-STa antibody was repeated three times.
Pig immunization and STa + ETEC challenge study
A total of six pregnant gilts with no pre-existing anti-LT and anti-STa antibodies were used in this study. Two pigs were IM immunized with 500 µg toxoid fusion 3xSTaN12S-mnLTR192G/L211A or chemical conjugate BSA-STaA14T, respectively, with 5 µg dmLT adjuvant, at eight weeks prior to farrowing, followed with a boost injection four weeks later. The two other pregnant gilts which received no injection served as the control. Serum samples collected prior to each injection and four weeks after final injection, and colostrum samples collected prior to farrowing, were stored at -80°C.
Piglet challenge study followed the protocol previously published (Zhang et al, 2010, Nanre et al. 2016). Fifty-two piglets born to two immunization groups and one control group were orally challenged with STa-producing strain 8823. Each piglet after 24 h suckling was gavage-fed with 1 × 109 CFU 8823 bacteria. Challenged piglets were returned to their mothers and were monitored every 2–4 h during 24 h post-inoculation for clinical diarrhea. Piglets were also weighed prior to challenge and 24 h post-inoculation to assess daily weight gain. Mild (semi-formed stool, yellow-stained butt) or watery diarrhea (entirely unformed liquid stool) in piglets were recorded for evaluating clinical diarrhea.
The mouse immunization, pig immunization and the pig challenge study complied with the Animal Welfare Act (1996 National Research Council Guidelines) and the USDA Animal Welfare Act Regulations, respectively, and were supervised and approved by the Institutional Animal Care and Use Committee of Kansas State University.
Statistical analyses
Data are presented in means ± standard deviations. A standard one-way ANOVA to compare different treatment groups was used in this study. Significance in clinical outcome between two groups was determined with Fisher's exact test. A post hoc Tukey's test was used to assess differences between treatments following ANOVA. Calculating a P value of <0.05 was considered significant difference.
RESULTS
Mice SC immunized with 3xSTaN12S-mnLTR192G/L211A genetic fusion or chemical conjugates developed antibody response specific to STa
Mice SC immunized with 3xSTaN12S-mnLTR192G/L211A, BSA-STaA14T or BSA-STa developed IgG antibody specific to STa (Fig. 1). Anti-STa IgG titers were 4.5 ± 0.3 (log10) from the serum samples of the mice injected with BSA-STa conjugate which carried native STa. These titers were higher than those detected in the group immunized with conjugate BSA-STaA14T (3.9 ± 0.4) or the group immunized with toxoid fusion 3xSTaN12S-mnLTR192G/L211A (3.7 ± 0.8). Statistical analyses indicated that only differences between the group injected with conjugate BSA-STa and the group administered with toxoid fusion were significant (P < 0.01).
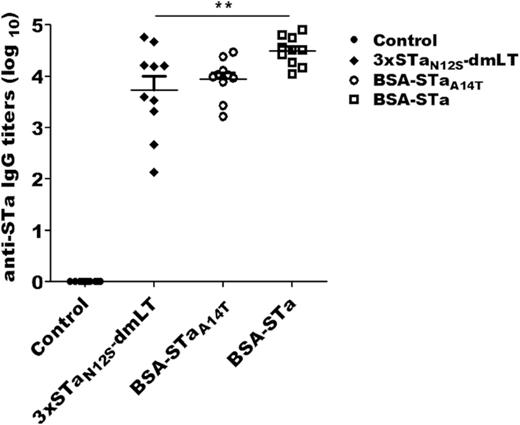
Anti-STa IgG antibody titers in the serum of mice subcutaneously immunized with toxoid fusion 3xSTaN12S-mnLTR192G/L211A (3xSTaN12S-dmLT), conjugate BSA-STaA14T (BSA-STaA14T) or conjugate BSA-STa (BSA-STa), and the control mice (Control). Bars indicate the means and standard deviations of antibody titers. Each dot represents the STa antibody titer of a mouse. ** indicates a P value of <0.01 for the difference between 3xSTaN12S-dmLT and BSA-STa groups.
Toxoid fusion- and chemical conjugate-induced antibodies showed a similar level of activity in neutralizing STa biological activity
In vitro antibody neutralization activity against STa was detected from the serum pooled from each immunized group, but not the control group (Fig. 2A). T-84 cell intracellular cGMP concentrations were 0.1 ± 0.1, 0.2 ± 0.1 and 0.4 ± 0.3 (pmol/ml), after incubation with STa exposed to the mouse serum of the group SC injected with genetic fusion 3xSTaN12S-mnLTR192G/L211A, conjugate BSA-STaA14T or conjugate BSA-STa, respectively. These cGMP levels showed no significant differences, but they significantly differed from the cGMP from the cells exposed to STa alone (9.0 ± 0.3 pmol/ml; P < 0.001) or STa with the control mouse serum (9.5 ± 0.1 pmol/ml; P < 0.001).
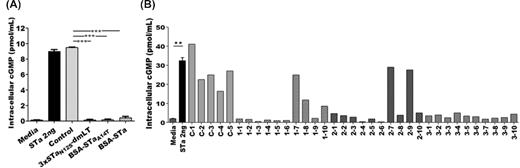
Mouse serum antibody in vitro neutralization activity against STa using T-84 cells and an intracellular cyclic GMP EIA kit. (A) Neutralization activity against STa from pooled serum samples from mice immunized with toxoid fusion 3xSTaN12S-mnLTR192G/L211A (3xSTaN12S-dmLT), conjugate BSA-STaA14T (BSA-STaA14T), conjugate BSA-STa (BSA-STa), or the control group (Control). Each pooled serum sample (30 µl) mixed with 2 ng of STa was added to T-84 cells. After 1 h incubation, intracellular cGMP levels were measured by using the cGMP EIA kit. Cell culture medium alone was included as a control for baseline cGMP in T-84 cells. Two ng of STa alone was used to show stimulation of cGMP by STa. (B) Neutralization activity against STa from each individual mouse serum sample. C indicates the control, and groups 1, 2 and 3 represent the groups immunized with 3xSTaN12S-mnLTR192G/L211A (3xSTaN12S-dmLT), BSA-STaA14T and BSA-STa, respectively. Error bars indicate standard deviations. ** indicates a P value of <0.01 and *** indicates a P value of <0.001.
Of the individual mouse serum samples analyzed, all 10 mice SC administered with conjugate BSA-STa, 8 out of 10 mice with conjugate BSA-STaA14T, and 7 out of 10 mice immunized with the toxoid fusion, gave rise to antibodies that demonstrated STa-neutralizing activity (Fig. 2B). Similar to the neutralizing activity of the pooled serum samples, anti-STa neutralizing activities across the three immunized groups were not significantly different, but they all significantly differed compared with the control group (P < 0.001).
Toxoid fusion- and chemical conjugate-induced anti-STa antibodies showed no or low cross-reactivity with guanylin peptides
The data from competitive ELISA using the mouse serum of the group SC administered with genetic fusion 3xSTaN12S-mnLTR192G/L211A demonstrated little cross-reactivity with either guanylin peptide (Fig. 3). The level of reactivity of anti-STa antibodies induced by 3xSTaN12S-mnLTR192G/L211A to the coated STa-ovalbumin was 46.8 ± 0.7% when 50 ng of STa (as the competitive agent) was added. However, when guanylin or uroguanylin were included, the levels of reactivity were 91.2 ± 1.4% and 90.2 ± 2.5%, respectively, showing that only 8.8% or 9.8% of anti-STa antibodies cross-react with guanylin or uroguanylin, respectively. The background reading was 3.4 ± 0.1% in wells without the STa-ovalbumin-coating antigen. Similarly, the levels of reactivity of anti-STa antibodies derived from conjugate BSA-STaA14T or BSA-STa with the coated STa-ovalbumin were 46.0 ± 1.6% and 43.3 ± 5.1% when combined with STa, respectively; however, the levels of reactivity to guanylin or uroguanylin ranged from 85.4 ± 3.6% to 99.0 ± 2.8%, respectively.
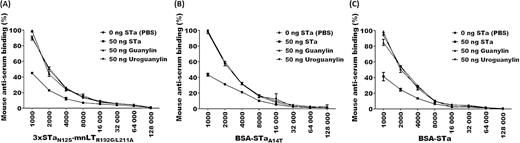
Mouse serum anti-STa antibody cross-reactivity with guanylin and uroguanylin determined by STa competitive ELISA. (A) 3xSTaN12S-mnLTR192G/L211A-induced mouse serum antibody cross-reactivity with STa, guanylin and uroguanylin. (B) BSA-STaA14T-induced mouse serum antibody cross-reactivity with STa, guanylin and uroguanylin. (C) BSA-STa-induced mouse serum antibody cross-reactivity with STa, guanylin and uroguanylin. Competitive ELISA used 50 ng of STa, guanylin or uroguanylin, or PBS as the competitive agent to compete for mouse serum anti-STa antibody dilutions from STa-ovalbumin conjugate (coated at ELISA plate wells). OD values were converted to percentages to present antibody cross-reactivity, with the degree of reactivity of serum antibodies at a 1:1000 serum dilution with PBS being 100%.
IM administered 3xSTaN12S-mnLTR192G/L211A genetic fusion or chemical conjugate BSA-STaA14T induced STa-specific IgG and IgA antibodies in pregnant pigs
IgG or IgA antibody response specific to STa was detected in the serum and colostrum of each pregnant gilt IM injected with the STa toxoid genetic fusion or chemical conjugate (Fig. 4). Anti-pig serum STa IgG titers were 2.65 ± 0.25 and 2.08 ± 0.16 in the pigs administered with 3xSTaN12S-mnLTR192G/L211A or BSA-STaA14T (log10), respectively. Anti-STa IgG and IgA were detected at the titers of 3.24 ± 0.63 and 1.3 ± 0.26 from the colostrum of the pigs injected with genetic fusion 3xSTaN12S-mnLTR192G/L211A, respectively. Anti-STa IgG (2.87 ± 0.16) and IgA (1.38 ± 0.06) were also detected from pigs immunized with conjugate BSA-STaA14T. While the serum IgG titer in the pigs immunized with 3xSTaN12S-mnLTR192G/L211A was significantly higher than those in the pigs immunized with BSA-STaA14T, the colostrum IgG and IgA titers between the two groups were not significantly different. No serum or colostrum anti-STa antibody was detected from the control pigs.
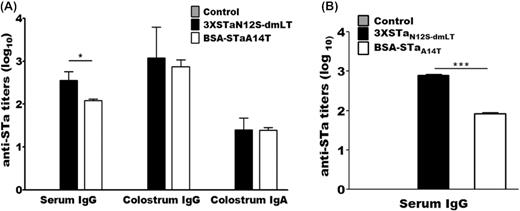
Pig anti-STa IgG or IgA titers (in log10) in the serum and colostrum samples of immunized and control groups and the serum of the suckling piglets born to the immunized or control mothers. (A) Anti-STa antibody IgG and IgA titers in serum and colostrum samples of pregnant pigs immunized with 3xSTaN12S-mnLTR192G/L211A or BSA-STaA14T or the control group. (B) Anti-STa IgG in serum samples of piglets born to control mothers or gilts immunized with toxoid fusion 3xSTaN12S-mnLTR192G/L211A (3xSTaN12S-dmLT) or conjugate BSA-STaA14T. Box and bar indicate the mean and standard deviation of antibody titer in each group. * represents a P value of <0.05 and *** indicates a P value of <0.001.
Suckling piglets passively acquired anti-STa antibodies from the immunized mothers. Anti-STa IgG titers were 2.87 ± 0.04 (log10) in the serum of the piglets delivered by the mothers administered with 3xSTaN12S-mnLTR192G/L211A, which were significantly higher compared with the titers from the serum of the piglets suckling to mothers immunized with conjugate BSA-STaA14T (1.93 ± 0.08; P < 0.001). No serum anti-STa antibodies were detected in the piglets born to the control mothers.
Suckling piglets born to the mothers administered with 3xSTaN12S-mnLTR192G/L211A or BSA-STaA14T were protected against STa + ETEC challenge
Following challenge of passively immunized piglets with STa + ETEC strain 8823, a significant reduction of mild to severe diarrhea was observed in piglets suckling to the mothers injected with the genetic fusion or the chemical conjugate compared with piglets delivered by the control mothers. While 17 out of 19 (89.5%) piglets from the control mothers developed watery diarrhea during 24 h post-inoculation (Table 1), only one (6.3%) out of 16 challenged piglets delivered by the mothers administered with genetic fusion 3xSTaN12S-mnLTR192G/L211A developed watery diarrhea (P < 0.001), and five (31.3%) showed mild diarrhea, significantly different from the outcomes of the control piglets (P < 0.001). For piglets suckling to the mothers injected with conjugate BSA-STaA14T, three (17.6%) out of 17 developed watery diarrhea and two more (11.8%) had mild diarrhea when challenged with the same STa strain. The weight gain rates were 16%, 11% or 7%, respectively, during 24 h post-challenge for the piglets suckling to the mothers administered with genetic fusion 3xSTaN12S-mnLTR192G/L211A, chemical conjugate BSA-STaA14T, or the control.
Clinical outcomes after 24 h challenge with STa + ETEC strain 8823 in suckling piglets born to the control pigs or to the mothers immunized with toxoid fusion 3xSTaN12S-mnLTR192G/L211A or chemical conjugate BSA-STaA14T.
| Outcomes . | Control . | 3xSTaN12S-mnLTR192G/L211A . | BSA-STaA14T . |
|---|---|---|---|
| Normal (%) | 2/19 (10.5) | 10/16 (62.5) | 12/17 (70.6) |
| Mild diarrhea (%) | 0/19 (0) | 5/16 (31.2) | 2/17 (11.8) |
| Watery diarrhea (%) | 17/19 (89.5) | 1/16 (6.3) | 3/17 (17.6) |
| Weight gain rate (%) | 6.9 ± 3.9 | 15.5 ± 6.0a | 10.8 ± 6.1a |
| Outcomes . | Control . | 3xSTaN12S-mnLTR192G/L211A . | BSA-STaA14T . |
|---|---|---|---|
| Normal (%) | 2/19 (10.5) | 10/16 (62.5) | 12/17 (70.6) |
| Mild diarrhea (%) | 0/19 (0) | 5/16 (31.2) | 2/17 (11.8) |
| Watery diarrhea (%) | 17/19 (89.5) | 1/16 (6.3) | 3/17 (17.6) |
| Weight gain rate (%) | 6.9 ± 3.9 | 15.5 ± 6.0a | 10.8 ± 6.1a |
Note: ‘a’ indicates a significant difference from the control group (P < 0.01 and < 0.05).
Clinical outcomes after 24 h challenge with STa + ETEC strain 8823 in suckling piglets born to the control pigs or to the mothers immunized with toxoid fusion 3xSTaN12S-mnLTR192G/L211A or chemical conjugate BSA-STaA14T.
| Outcomes . | Control . | 3xSTaN12S-mnLTR192G/L211A . | BSA-STaA14T . |
|---|---|---|---|
| Normal (%) | 2/19 (10.5) | 10/16 (62.5) | 12/17 (70.6) |
| Mild diarrhea (%) | 0/19 (0) | 5/16 (31.2) | 2/17 (11.8) |
| Watery diarrhea (%) | 17/19 (89.5) | 1/16 (6.3) | 3/17 (17.6) |
| Weight gain rate (%) | 6.9 ± 3.9 | 15.5 ± 6.0a | 10.8 ± 6.1a |
| Outcomes . | Control . | 3xSTaN12S-mnLTR192G/L211A . | BSA-STaA14T . |
|---|---|---|---|
| Normal (%) | 2/19 (10.5) | 10/16 (62.5) | 12/17 (70.6) |
| Mild diarrhea (%) | 0/19 (0) | 5/16 (31.2) | 2/17 (11.8) |
| Watery diarrhea (%) | 17/19 (89.5) | 1/16 (6.3) | 3/17 (17.6) |
| Weight gain rate (%) | 6.9 ± 3.9 | 15.5 ± 6.0a | 10.8 ± 6.1a |
Note: ‘a’ indicates a significant difference from the control group (P < 0.01 and < 0.05).
DISCUSSION
Since STa plays a key role in ETEC-associated moderate-to-severe diarrhea in children who live in developing countries and travelers’ diarrhea, an effective ETEC vaccine would need to incorporate STa antigens to elicit protective neutralizing antibodies against STa enterotoxicity and a possible role in modulating host responses to ETEC antigens (Sack et al., 2007, Kotloff et al., 2012, Zhang and Sack, 2012, Read et al., 2014). In order to be included as a component for ETEC vaccines, STa antigens would need to possess decreased enterotoxicity and maintained antigenicity but more importantly enhanced immunogenicity to induce protective anti-STa antibody responses. In the current study, both approaches to generate STa antigens, i.e. genetically fusing an STa toxoid to double mutant LT monomer mnLTR192G/L211A or chemically conjugating it to BSA, substantially conferred protective immunity against ETEC-producing STa, as demonstrated by high titers of antibody responses to STa in the mice and pigs injected with STa toxoid fusion 3xSTaN12S-mnLTR192G/L211A or chemical conjugate BSA-STaA14T. Anti-STa antibodies raised by both the STaN12S toxoid genetic fusion and the STaA14T chemical conjugate exhibited similar levels of STa-neutralizing activity and protection against diarrhea in piglets challenged with a STa ETEC strain. Taken together, the data strongly support the inclusion of STa toxoids (both fusion 3xSTaN12S-mnLTR192G/L211A and chemical conjugate BSA-STaA14T) as promising antigens in future ETEC vaccine development efforts.
Toxoid genetic fusion 3xSTaN12S-mnLTR192G/L211A (previously referred to as 3xSTaN12S-dmLT), when IP and SC immunized (alone or together with an ETEC colonization factor antigen (CFA) adhesin antigen) to mice or in IM immunized to pigs, induced neutralizing anti-STa (and anti-LT) antibodies (Ruan et al., 2014, Nandre et al., 2016, Nandre et al., 2017, Duan et al., 2018a). Chemical conjugate BSA-STaA14T, on the other hand, has not been investigated for STa immunogenicity, and this is the first study to evaluate its ability to induce anti-STa antibodies in pigs that can passively protect piglets against ETEC challenge.
The current data suggested that mouse anti-STa antibodies raised from genetic fusion 3xSTaN12S-mnLTR192G/L211A and conjugate BSA-STaA14T exhibited a similar level in neutralizing STa biological activity. Moreover, passively acquired antibodies derived from 3xSTaN12S-mnLTR192G/L211A fusion and BSA-STaA14T conjugate were equivalently protective against STa ETEC diarrhea to challenged piglets (P = 0.30). That suggests that both 3xSTaN12S-mnLTR192G/L211A and BSA-STaA14T could be potential STa antigens for inclusion in candidates of ETEC vaccines. In future studies, the adjuvant control group immunized with dmLT alone or the same carrier protein with an irrelevant antigen need to be included to better elucidate that STa toxoid candidates enhance STa immunogenicity.
Looking towards the clinical development of these toxoid candidates, there are manufacturing challenges that will have to be systematically addressed for STa toxoids. The chemical conjugation process may be lengthy involving a complicated purification process, particularly when the STa or STa toxoids have to be purified from wild-type organisms. By cloning native STa gene into high expression vector pUC19 and transforming wild type STa-producing ETEC strain 8633 with STa plasmid p8835, we produced STa recombinant ETEC strain 9115 for STa purification. Subsequently, we mutated the STa gene cloned in pUC19 for toxoids STaN12S (p9270) and STaA14T (p9215) for STa toxoid purification, but only STaA14T toxoid was purified (by Dr. J. Clements at Tulane University, LA). We also cloned native STa gene in pUC19 and STa was able to be purified, and BSA-STa conjugate induced anti-STa antibodies in mice that were significantly greater compared with those induced by the toxoid fusion. However, BSA-STa is not considered safe for ETEC vaccines because a small amount of native STa (at nanogram level) may be disassociated from the conjugate and could cause adverse effects (members of the STa toxoid vaccine consortium group, pers. comm.). Conjugate BSA-STaA14T was thus the main conjugate target in the current study because of its substantially reduced biological activity of STa. In future studies to purify toxoid STaN12S with a modified STa purification protocol, and then to study BSA-STaN12S conjugate comparatively with toxoid fusion 3xSTaN12S-mnLTR192G/L211A, we can directly compare genetic fusion or chemical conjugation for effectiveness in enhancing STa immunogenicity. However, it needs to be pointed out that STa immunogenicity from the same STa toxoid in conjugate BSA-STaN12S and genetic fusion 3xSTaN12S-mnLTR192G/L211A can be different, because the genetic fusion and chemical conjugation process may affect STaN12S antigenic topology differently.
While a recombinant protein of toxoid genetic fusion 3xSTaN12S-mnLTR192G/L211A can be purified at 130–150 mg per liter broth medium (Duan and Zhang, 2017, Duan et al., 2018b), STa toxoid conjugation approach needs to improve STa toxoid purification yield as well as chemical conjugation efficacy. Current STa purification is a lengthy process (three days for completing the purification process) and often yields one to a few mg STa from one liter of medium culture, and the STa-BSA chemical conjugation method shows 30% efficiency (Dr. Don Robertson at Kansas State University, KS, pers. comm.). Additionally, some STa toxoids cannot be purified with the current procedure (communication with Dr. John Clements at Tulane University, LA, pers. comm.). Direct chemical synthesis of STa toxoids may help to overcome this drawback, particularly with recent progress made to synthesize STaA14T toxoid with three disulfide bonds formed (Dr. Pal Puntervoll at University of Bergen, Norway, pers. comm.), making STa toxoid conjugation a feasible approach for developing ETEC vaccines.
In conclusion, current data confirmed STa immunogenicity and protective efficacy for toxoid fusion 3xSTaN12S-mnLTR192G/L211A and its antigen candidacy for ETEC vaccine development. Our data also demonstrated that STa toxoid conjugate BSA-STaA14T could potentially be an additional option for future ETEC vaccines. Future antigen safety and immunogenicity studies of toxoid fusion 3xSTaN12S-mnLTR192G/L211A in preference to chemical conjugate may be needed in human volunteers to better characterize toxoid candidate for its use in improving ETEC vaccines against diarrhea.
ACKNOWLEDGEMENTS
The authors thank Dr. John Clements from Tulane University for supplying chemical conjugates BSA-STa and BSA-STaA14T, and the STa toxoid vaccine consortium group (Drs. James Nataro, Eileen Barry, John Clements, Halvor Sommerfelt, Weiping Zhang and Pal Puntervoll) for providing valuable suggestions of study design, but particularly Drs. A. Louis Bourgeois, Richard Walker and Sachin Mani from PATH for reviewing and commenting in order to improve the quality of this manuscript. Financial support for this work was provided by PATH and NIH grant R01AI121067.
Conflicts of interest. None declared.



