-
PDF
- Split View
-
Views
-
Cite
Cite
Antony T. Vincent, Luca Freschi, Julie Jeukens, Irena Kukavica-Ibrulj, Jean-Guillaume Emond-Rheault, Annie Leduc, Brian Boyle, Fabrice Jean-Pierre, Marie-Christine Groleau, Eric Déziel, Jean Barbeau, Steve J. Charette, Roger C. Levesque, Genomic characterisation of environmental Pseudomonas aeruginosa isolated from dental unit waterlines revealed the insertion sequence ISPa11 as a chaotropic element, FEMS Microbiology Ecology, Volume 93, Issue 9, September 2017, fix106, https://doi.org/10.1093/femsec/fix106
Close - Share Icon Share
Abstract
The bacterium Pseudomonas aeruginosa is well known to have a remarkable adaptive capacity allowing it to colonise many environments. A recent study on environmental isolates from dental unit waterlines (DUWLs) hinted at a genetic clustering into two groups. Isolates from one of these groups, named cluster III, were shown to have unusual phenotypes for environmental isolates, such as an increased biofilm production. To have a better ecological view, more specifically on isolates from cluster III, the complete genomes of 39 isolates including 16 from DUWLs were sequenced. In addition to an investigation of antibiotic resistance and secretion system gene content, a molecular phylogeny allowed confirmation of the split of the 16 environmental isolates in two groups and also sheds light on a correlation between the phylogenetic positions and the serotypes of the isolates. Isolates from cluster III harboured insertion sequences ISPa11 inserted into the O-specific antigen biosynthesis clusters and the gene lasR, encoding for a master regulator of the quorum sensing. Investigation of key regulators revealed another truncated gene, gacS. Alteration in lasR and gacS genes was consistent with phenotypic assays confirming their inactivation. These results bring new perspectives regarding the ecological adaptive potential of P. aeruginosa.
INTRODUCTION
The Gram-negative bacterium Pseudomonas aeruginosa is well known to be ubiquitous and one of the most common pathogens causing lung infection in patients suffering from cystic fibrosis (CF; Winstanley, O’Brien and Brockhurst 2016).
The CF disease is an autosomal recessive disorder caused by an alteration in the gene CFTR, encoding for a chloride channel (Kartner et al.1991). As well reviewed elsewhere (Cutting 2015), several phenotypes are associated with CF disease, including among others, the production of viscous secretions within lungs and in the ducts of the pancreas leading to malfunctions of the affected organs.
In fact, CF lungs contain a complex microbial community changing over the years, beginning with a higher proportion of Staphylococcus aureus during childhood of the patients while P. aeruginosa becomes dominant during adolescence (Harrison 2007). Interestingly, the P. aeruginosa population itself evolves to adapt to the lung environment, transitioning from an acute- to a chronic-infection lifestyle (Smith et al.2006). This lifestyle switch generates a recurring scheme of alterations in genes encoding regulatory proteins implicated in various phenotypes such as quorum sensing (lasR), regulation of virulence factors (retS, gacS and exsD), iron acquisition (phuR), antibiotic resistance (mexZ, mexR, mexT, ampR, pmrA and nalD), mucoidy (mucA, retS) and transport (ompR) in what is called a pathoadaptation (Winstanley, O’Brien and Brockhurst 2016).
It is also known that dental unit waterlines (DUWLs), which are heavily colonised by bacterial species (Barbeau et al.1996; Barbot et al.2012), are favourable environments for P. aeruginosa (Barbeau et al.1996; De Oliveira et al.2008; Abdouchakour et al.2015). Knowing that DUWLs can be a source of infections, especially when patients are immunocompromised (Barbot et al.2012), it is crucial to investigate how P. aeruginosa isolates adapt to this anthropogenic environment to minimise the spread of the bacterium. A recent study investigated phenotypic differences between 16 P. aeruginosa strains from CF patients and 13 strains from DUWLs, the latter being considered as a particular type of environmental strains (Ouellet et al.2015). A random amplified polymorphic DNA (RAPD) analysis revealed that the strains were distributed between three clusters, the first one (cluster I) being exclusively composed of clinical strains, while the second (cluster II) and third (cluster III) included those from DUWLs (Fig. S1, Supporting Information). Interestingly, cluster II, containing DUWLs strains, was considered closer to the clinical strains (cluster I) than cluster III, also composed of DUWLs strains. In fact, isolates from cluster III were shown to have opposite phenotypes compared to those of clusters I and II: flat colonies, high production of biofilm and inability to lyse amoeba. Even if this study allowed new insights into the diversity of P. aeruginosa with an emphasis on strains from DUWLs, many questions remained concerning the genomic particularities of these environmental strains, more especially regarding those from cluster III.
The main objective of the present study was to investigate the genomics of the P. aeruginosa isolates studied by Ouellet et al., more specifically those from cluster III showing distinctive phenotypes when compared to other clusters, to have a better understanding of what are the causes of this divergence. To have a better view of the particularity of cluster III isolates, those from clusters I and II were also analysed at the genomics level as well as new CF and DUWLs isolates.
MATERIALS AND METHODS
Bacterial growth and sequencing
Bacterial colonies were isolated on Difco Pseudomonas Isolation Agar (BD, Sparks, MD, USA). Genomic DNA was extracted from overnight liquid cultures (37°C, LB medium) using the E-Z 96 Tissue DNA Kit (Omega Bio-tek, Norcross, GA, USA). Genomic DNA (250–750 ng) was mechanically fragmented for 40 seconds using a Covaris M220 (Covaris, Woburn, MA, USA) with default settings. Library synthesis was performed on fragmented DNA using NEB Next Ultra II DNA library prep kit for Illumina (New England Biolabs, Ipswich, MA, USA) according to manufacturer's instructions. TruSeq HT adapters (Illumina, SanDiego, CA, USA) were used to barcode the libraries, which were sequenced in 1/48 of an Illumina MiSeq 300 bp paired-end run at the Plateforme d’Analyses Génomiques of the Institut de Biologie Intégrative et des Systèmes (Laval University, Quebec, Canada). Finally, the resulting sequencing reads were de novo assembled using A5-miseq pipeline version 20160825 (Coil, Jospin and Darling 2014).
Pan-genome analysis and molecular phylogenies
A total of 1355 Pseudomonas aeruginosa genomes having fewer than 251 contigs were downloaded from the Pseudomonas Genome Database (Winsor et al.2016) and were combined with the 39 genomes sequenced in the present study, thus composing a dataset of 1394 isolates. The pan-genome was found by Saturn V version 1.0 (https://github.com/ejfresch/saturnV) using the algorithm ‘lazy,’ prodigal version 2.6.2 (Hyatt et al.2010) for the annotation and USEARCH version 8 (Edgar 2010) (at least 50% of identity over 85% of the alignment length) for the homology search.
Sequences of genes in the softcore, defined here as the orthologous genes present in at least 95% of the dataset and without paralogous ambiguity, were extracted and codon aligned by muscle version 3.7 (Edgar 2004) through TranslatorX (Abascal, Zardoya and Telford 2010). The resulting 3354 alignments were filtered by Block Mapping and Gathering with Entropy (BMGE) version 1.2 (Criscuolo and Gribaldo 2010) to remove sites without variation. All the filtrated sequences were concatenated and partitioned into a supermatrix of 624 578 sites by Alignment Manipulation And Summary (AMAS) (Borowiec 2016). Given the intensive computational requirement needed to infer a phylogenetic tree based on such matrices, FastTree version 2.1.9 (Price, Dehal and Arkin 2010) with the model GTR+CAT was used to infer an approximately maximum-likelihood phylogenetic tree. The same bioinformatics procedure was used to build a tree containing only the 39 isolates from the present study, with the exception that the best-fit model was inferred from the matrix (135 212 positions) by IQ-TREE version 1.5.3 (Nguyen et al.2015) and that the tree was computed by maximum likelihood under the model GTR+R2 and 10 000 ultrafast bootstraps (Minh, Nguyen and Von Haeseler 2013), also by using IQ-TREE.
The presence/absence matrix generated by Saturn V was binary encoded and evaluated using Count version 10.04 (Csurös 2010) alongside with the previously generated phylogenetic tree under the Dollo parsimony model. The sequences of genes that were gained at the DUWLs node were analysed by eggNOG-mapper online (Huerta-Cepas et al.2016).
Other bioinformatics analyses
Molecular modelling of LasR was done by using I-TASSER version 5.0 (Yang et al.2015). The best model had a C-score of 1.28 and a TM-score of 0.89 ± 0.07 for the complete LasR from P. aeruginosa PAO1, while the one of the truncated LasR from PPF-1 had a C-score of 1.11 and a TM-score of 0.87 ± 0.07. Various alignments were performed using Easyfig version 2.2.2 (Sullivan, Petty and Beatson 2011). Antibiotic resistance genes and those implicated in secretion systems were predicted using the Resistance Gene Identifier, from the Comprehensive Antibiotic Resistance Database (CARD) (Jia et al.2017) and TXSScan (Abby et al.2016), respectively. The in silico serotyping of P. aeruginosa isolates was done by PAst version 1.0 (Thrane et al.2016).
LC-MS/MS quantification of N-Acyl homoserine lactones and pyocyanin
N-Acyl homoserine lactones (AHL) and pyocyanin quantifications were performed using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) as previously described (Lépine and Déziel 2010). 5,6,7,8-tetradeutero-4-hydroxy-2-heptylquinoline (HHQ-d4) was used as an internal standard. Briefly, 5-mL cultures were grown in Tryptic Soy Broth (TSB; BD, Sparks, MD, USA) until OD600 = 2 and 4. Cultures with added internal standard HHQ-d4 were then extracted twice with ethyl acetate. Extracts were evaporated under a gentle nitrogen stream and suspended in 500 μL acetonitrile containing 1% acetic acid. Analyses were performed using a high-performance liquid chromatograph (Waters 2795, Mississauga, ON, Canada) equipped with a C8 reverse-phase column (Eclipse XDB-C8, Agilent Technologies, Mississauga, ON, Canada), coupled to a mass spectrometer (Quattro Premier XE, Waters). Experiments were performed on two different sets of cultures.
RNA preparation
Total RNA was extracted from liquid bacterial cultures grown in biological triplicate in TSB cultivated to late exponential phase (OD600 = 2.8) at 37°C. The cells were then centrifuged for 5 min at 12 000 × g, and the supernatant was discarded. Cells were resuspended in PureZOL (BioRad), and RNA extraction was performed following the manufacturer's recommendations.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Reverse transcription (RT) was performed using 50 ng of the extracted RNAs with the iScript RT Supermix kit (Bio-Rad, St-Laurent, QC, Canada) following the manufacturer's recommendations. The qPCR step was done with the Sso Advanced Universal SYBR Green Supermix kit (Bio-Rad) and a RotorGene 6000 thermocycler (QIAGEN, Valencia, CA, USA). The rsmY primer set previously published (Jean-Pierre, Tremblay and Déziel 2017) was used for the quantitative real-time polymerase chain reaction (qRT-PCR) analyses. The nadB gene was used as the control. Each qRT-PCR cycle was performed in triplicate. The threshold cycle (Ct) was normalised to nadB Ct amplified in each corresponding samples using the prototypic strain PA14 as a reference (Rahme et al.1995). Variations in expression were calculated using the –2ΔΔCt method (Livak and Schmittgen 2001). The experiments were carried out using biological triplicates.
Swarming assays
Swarming motility assays were performed as previously described with culture medium solidified with 0.5% agar (Tremblay and Déziel 2008). Once poured, the plates were dried in a laminar biological safety cabinet for 75 min. Swarming plates were inoculated with 5 μL of bacterial suspension adjusted to an OD600 of 3.0 and incubated at 34°C for 24–48 h.
RESULTS AND DISCUSSION
The main objective of the present study was to elucidate at the genomic level the diversity of Pseudomonas aeruginosa isolates from DUWLs. Here, we focused efforts on isolates identified as part of cluster III based upon RAPD analysis (Ouellet et al.2015). Strains from this cluster had divergent phenotypes from other DUWLs and CF isolates. The genomes of the 29 isolates (16 from CF patients and 13 from DUWLs) from Ouellet et al., were combined with 10 new isolates (7 from CF patients and 3 from DUWLs) (Tables 1 and 2) and their complete genomes sequenced by next-generation sequencing.
Pseudomonas aeruginosa isolates having a CF origin analysed by the present study.
| Isolate . | Patient's gender . | Patient's birth date . | Patient's city of origin . | Sample date . | Source . | Clustera . | GenBank . | Reference . |
|---|---|---|---|---|---|---|---|---|
| VD171 | Female | 04-11-75 | McMasterville | 27-10-87 | Infected lung | I | MWYO00000000 | (Ouellet et al.2015) |
| VD329 | Female | 04-11-75 | McMasterville | 23-08-88 | Infected lung | I | MWYR00000000 | (Ouellet et al.2015) |
| VD706 | Female | 04-11-75 | McMasterville | 17-10-89 | Infected lung | I | MWYS00000000 | (Ouellet et al.2015) |
| VD564 | Female | 04-11-75 | McMasterville | 15-05-90 | Infected lung | I | MWYP00000000 | (Ouellet et al.2015) |
| VD609 | Female | 04-11-75 | McMasterville | 05-12-90 | Infected lung | I | MWYN00000000 | (Ouellet et al.2015) |
| 297 | Female | 25-08-80 | Saint-Pie-de-Bagot | 01-06-88 | Infected lung | I | MWYQ00000000 | (Ouellet et al.2015) |
| 403 | Female | 19-04-74 | Trois-Rivières | 10-01-89 | Infected lung | I | MWYM00000000 | (Ouellet et al.2015) |
| 585 | Female | 18-07-72 | Saint-Jean sur Richelieu | 21-08-90 | Infected lung | I | MWYL00000000 | (Ouellet et al.2015) |
| 359 | Female | 10-03-75 | Répentigny | 04-10-88 | Infected lung | I | MWYK00000000 | (Ouellet et al.2015) |
| E-500 | Female | 24-02-76 | Mont-Laurier | 26-09-89 | Infected lung | I | MWYJ00000000 | (Ouellet et al.2015) |
| 392 | Male | 16-11-84 | Saint-Roch-de-l’Achigan | 24-11-88 | Infected lung | I | MWYI00000000 | (Ouellet et al.2015) |
| 279 | Female | 09-04-69 | Montréal-Nord | 17-05-88 | Infected lung | I | MWYH00000000 | (Ouellet et al.2015) |
| 358 | Female | 28-11-80 | Laprairie | 04-10-88 | Infected lung | I | MWYG00000000 | (Ouellet et al.2015) |
| 578-A | Male | 25-09-73 | Montréal-Nord | 31-06-90 | Infected lung | I | MWYF00000000 | (Ouellet et al.2015) |
| 578-B | Female | 15-06-75 | Mont-Laurier | 31-06-90 | Infected lung | II | MWXW00000000 | (Ouellet et al.2015) |
| 506 | Female | 26-08-75 | Saint-Blaise | 10-10-89 | Infected lung | II | MWXV00000000 | (Ouellet et al.2015) |
| 173 | Unknown | Unknown | Montréal | 1997 | Sputum | N/Ab | MWXG00000000 | This study |
| 265 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXJ00000000 | This study |
| 462 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXK00000000 | This study |
| 707A | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXM00000000 | This study |
| 712 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXH00000000 | This study |
| 282 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXL00000000 | This study |
| 354 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXI00000000 | This study |
| Isolate . | Patient's gender . | Patient's birth date . | Patient's city of origin . | Sample date . | Source . | Clustera . | GenBank . | Reference . |
|---|---|---|---|---|---|---|---|---|
| VD171 | Female | 04-11-75 | McMasterville | 27-10-87 | Infected lung | I | MWYO00000000 | (Ouellet et al.2015) |
| VD329 | Female | 04-11-75 | McMasterville | 23-08-88 | Infected lung | I | MWYR00000000 | (Ouellet et al.2015) |
| VD706 | Female | 04-11-75 | McMasterville | 17-10-89 | Infected lung | I | MWYS00000000 | (Ouellet et al.2015) |
| VD564 | Female | 04-11-75 | McMasterville | 15-05-90 | Infected lung | I | MWYP00000000 | (Ouellet et al.2015) |
| VD609 | Female | 04-11-75 | McMasterville | 05-12-90 | Infected lung | I | MWYN00000000 | (Ouellet et al.2015) |
| 297 | Female | 25-08-80 | Saint-Pie-de-Bagot | 01-06-88 | Infected lung | I | MWYQ00000000 | (Ouellet et al.2015) |
| 403 | Female | 19-04-74 | Trois-Rivières | 10-01-89 | Infected lung | I | MWYM00000000 | (Ouellet et al.2015) |
| 585 | Female | 18-07-72 | Saint-Jean sur Richelieu | 21-08-90 | Infected lung | I | MWYL00000000 | (Ouellet et al.2015) |
| 359 | Female | 10-03-75 | Répentigny | 04-10-88 | Infected lung | I | MWYK00000000 | (Ouellet et al.2015) |
| E-500 | Female | 24-02-76 | Mont-Laurier | 26-09-89 | Infected lung | I | MWYJ00000000 | (Ouellet et al.2015) |
| 392 | Male | 16-11-84 | Saint-Roch-de-l’Achigan | 24-11-88 | Infected lung | I | MWYI00000000 | (Ouellet et al.2015) |
| 279 | Female | 09-04-69 | Montréal-Nord | 17-05-88 | Infected lung | I | MWYH00000000 | (Ouellet et al.2015) |
| 358 | Female | 28-11-80 | Laprairie | 04-10-88 | Infected lung | I | MWYG00000000 | (Ouellet et al.2015) |
| 578-A | Male | 25-09-73 | Montréal-Nord | 31-06-90 | Infected lung | I | MWYF00000000 | (Ouellet et al.2015) |
| 578-B | Female | 15-06-75 | Mont-Laurier | 31-06-90 | Infected lung | II | MWXW00000000 | (Ouellet et al.2015) |
| 506 | Female | 26-08-75 | Saint-Blaise | 10-10-89 | Infected lung | II | MWXV00000000 | (Ouellet et al.2015) |
| 173 | Unknown | Unknown | Montréal | 1997 | Sputum | N/Ab | MWXG00000000 | This study |
| 265 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXJ00000000 | This study |
| 462 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXK00000000 | This study |
| 707A | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXM00000000 | This study |
| 712 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXH00000000 | This study |
| 282 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXL00000000 | This study |
| 354 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXI00000000 | This study |
Based on the study of Ouellet et al.
N/A: None applicable
Pseudomonas aeruginosa isolates having a CF origin analysed by the present study.
| Isolate . | Patient's gender . | Patient's birth date . | Patient's city of origin . | Sample date . | Source . | Clustera . | GenBank . | Reference . |
|---|---|---|---|---|---|---|---|---|
| VD171 | Female | 04-11-75 | McMasterville | 27-10-87 | Infected lung | I | MWYO00000000 | (Ouellet et al.2015) |
| VD329 | Female | 04-11-75 | McMasterville | 23-08-88 | Infected lung | I | MWYR00000000 | (Ouellet et al.2015) |
| VD706 | Female | 04-11-75 | McMasterville | 17-10-89 | Infected lung | I | MWYS00000000 | (Ouellet et al.2015) |
| VD564 | Female | 04-11-75 | McMasterville | 15-05-90 | Infected lung | I | MWYP00000000 | (Ouellet et al.2015) |
| VD609 | Female | 04-11-75 | McMasterville | 05-12-90 | Infected lung | I | MWYN00000000 | (Ouellet et al.2015) |
| 297 | Female | 25-08-80 | Saint-Pie-de-Bagot | 01-06-88 | Infected lung | I | MWYQ00000000 | (Ouellet et al.2015) |
| 403 | Female | 19-04-74 | Trois-Rivières | 10-01-89 | Infected lung | I | MWYM00000000 | (Ouellet et al.2015) |
| 585 | Female | 18-07-72 | Saint-Jean sur Richelieu | 21-08-90 | Infected lung | I | MWYL00000000 | (Ouellet et al.2015) |
| 359 | Female | 10-03-75 | Répentigny | 04-10-88 | Infected lung | I | MWYK00000000 | (Ouellet et al.2015) |
| E-500 | Female | 24-02-76 | Mont-Laurier | 26-09-89 | Infected lung | I | MWYJ00000000 | (Ouellet et al.2015) |
| 392 | Male | 16-11-84 | Saint-Roch-de-l’Achigan | 24-11-88 | Infected lung | I | MWYI00000000 | (Ouellet et al.2015) |
| 279 | Female | 09-04-69 | Montréal-Nord | 17-05-88 | Infected lung | I | MWYH00000000 | (Ouellet et al.2015) |
| 358 | Female | 28-11-80 | Laprairie | 04-10-88 | Infected lung | I | MWYG00000000 | (Ouellet et al.2015) |
| 578-A | Male | 25-09-73 | Montréal-Nord | 31-06-90 | Infected lung | I | MWYF00000000 | (Ouellet et al.2015) |
| 578-B | Female | 15-06-75 | Mont-Laurier | 31-06-90 | Infected lung | II | MWXW00000000 | (Ouellet et al.2015) |
| 506 | Female | 26-08-75 | Saint-Blaise | 10-10-89 | Infected lung | II | MWXV00000000 | (Ouellet et al.2015) |
| 173 | Unknown | Unknown | Montréal | 1997 | Sputum | N/Ab | MWXG00000000 | This study |
| 265 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXJ00000000 | This study |
| 462 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXK00000000 | This study |
| 707A | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXM00000000 | This study |
| 712 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXH00000000 | This study |
| 282 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXL00000000 | This study |
| 354 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXI00000000 | This study |
| Isolate . | Patient's gender . | Patient's birth date . | Patient's city of origin . | Sample date . | Source . | Clustera . | GenBank . | Reference . |
|---|---|---|---|---|---|---|---|---|
| VD171 | Female | 04-11-75 | McMasterville | 27-10-87 | Infected lung | I | MWYO00000000 | (Ouellet et al.2015) |
| VD329 | Female | 04-11-75 | McMasterville | 23-08-88 | Infected lung | I | MWYR00000000 | (Ouellet et al.2015) |
| VD706 | Female | 04-11-75 | McMasterville | 17-10-89 | Infected lung | I | MWYS00000000 | (Ouellet et al.2015) |
| VD564 | Female | 04-11-75 | McMasterville | 15-05-90 | Infected lung | I | MWYP00000000 | (Ouellet et al.2015) |
| VD609 | Female | 04-11-75 | McMasterville | 05-12-90 | Infected lung | I | MWYN00000000 | (Ouellet et al.2015) |
| 297 | Female | 25-08-80 | Saint-Pie-de-Bagot | 01-06-88 | Infected lung | I | MWYQ00000000 | (Ouellet et al.2015) |
| 403 | Female | 19-04-74 | Trois-Rivières | 10-01-89 | Infected lung | I | MWYM00000000 | (Ouellet et al.2015) |
| 585 | Female | 18-07-72 | Saint-Jean sur Richelieu | 21-08-90 | Infected lung | I | MWYL00000000 | (Ouellet et al.2015) |
| 359 | Female | 10-03-75 | Répentigny | 04-10-88 | Infected lung | I | MWYK00000000 | (Ouellet et al.2015) |
| E-500 | Female | 24-02-76 | Mont-Laurier | 26-09-89 | Infected lung | I | MWYJ00000000 | (Ouellet et al.2015) |
| 392 | Male | 16-11-84 | Saint-Roch-de-l’Achigan | 24-11-88 | Infected lung | I | MWYI00000000 | (Ouellet et al.2015) |
| 279 | Female | 09-04-69 | Montréal-Nord | 17-05-88 | Infected lung | I | MWYH00000000 | (Ouellet et al.2015) |
| 358 | Female | 28-11-80 | Laprairie | 04-10-88 | Infected lung | I | MWYG00000000 | (Ouellet et al.2015) |
| 578-A | Male | 25-09-73 | Montréal-Nord | 31-06-90 | Infected lung | I | MWYF00000000 | (Ouellet et al.2015) |
| 578-B | Female | 15-06-75 | Mont-Laurier | 31-06-90 | Infected lung | II | MWXW00000000 | (Ouellet et al.2015) |
| 506 | Female | 26-08-75 | Saint-Blaise | 10-10-89 | Infected lung | II | MWXV00000000 | (Ouellet et al.2015) |
| 173 | Unknown | Unknown | Montréal | 1997 | Sputum | N/Ab | MWXG00000000 | This study |
| 265 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXJ00000000 | This study |
| 462 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXK00000000 | This study |
| 707A | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXM00000000 | This study |
| 712 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXH00000000 | This study |
| 282 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXL00000000 | This study |
| 354 | Unknown | Unknown | Montréal | 1997 | Sputum | N/A | MWXI00000000 | This study |
Based on the study of Ouellet et al.
N/A: None applicable
| Isolate . | Dental clinic section . | Dental unit number . | Clustera . | GenBank . | Reference . |
|---|---|---|---|---|---|
| Urg-7 | Emergency | 7 | II | MWYB00000000 | (Ouellet et al.2015) |
| Urg-5 | Emergency | 5 | II | MWYA00000000 | (Ouellet et al.2015) |
| Ortho-1 | Orthodontics | 1 | II | MWYD00000000 | (Ouellet et al.2015) |
| Chir_D-144-ass. | Surgery | D-144 assistant | II | MWYC00000000 | (Ouellet et al.2015) |
| PPF-18 | PPFb | 18 | II | MWYE00000000 | (Ouellet et al.2015) |
| PPF-19 | PPF | 19 | II | MWXZ00000000 | (Ouellet et al.2015) |
| PPF-20 | PPF | 20 | II | MWXY00000000 | (Ouellet et al.2015) |
| PPF-13 | PPF | 13 | II | MWXX00000000 | (Ouellet et al.2015) |
| PPF-2 | PPF | 2 | III | MWXU00000000 | (Ouellet et al.2015) |
| PPF-1 | PPF | 1 | III | MWXT00000000 | (Ouellet et al.2015) |
| PPF-21 | PPF | 21 | III | MWXS00000000 | (Ouellet et al.2015) |
| PPF-7 | PPF | 7 | III | MWXR00000000 | (Ouellet et al.2015) |
| Chir_D-144 | Surgery | D-144 | III | MWXQ00000000 | (Ouellet et al.2015) |
| 151 ass. | Surgery | 151 assistant | N/Ac | MWXO00000000 | This study |
| 146 ass. | Surgery | 146 assistant | N/A | MWXP00000000 | This study |
| Site 7 | Dental equipment | N/A | N/A | MWXN00000000 | This study |
| Isolate . | Dental clinic section . | Dental unit number . | Clustera . | GenBank . | Reference . |
|---|---|---|---|---|---|
| Urg-7 | Emergency | 7 | II | MWYB00000000 | (Ouellet et al.2015) |
| Urg-5 | Emergency | 5 | II | MWYA00000000 | (Ouellet et al.2015) |
| Ortho-1 | Orthodontics | 1 | II | MWYD00000000 | (Ouellet et al.2015) |
| Chir_D-144-ass. | Surgery | D-144 assistant | II | MWYC00000000 | (Ouellet et al.2015) |
| PPF-18 | PPFb | 18 | II | MWYE00000000 | (Ouellet et al.2015) |
| PPF-19 | PPF | 19 | II | MWXZ00000000 | (Ouellet et al.2015) |
| PPF-20 | PPF | 20 | II | MWXY00000000 | (Ouellet et al.2015) |
| PPF-13 | PPF | 13 | II | MWXX00000000 | (Ouellet et al.2015) |
| PPF-2 | PPF | 2 | III | MWXU00000000 | (Ouellet et al.2015) |
| PPF-1 | PPF | 1 | III | MWXT00000000 | (Ouellet et al.2015) |
| PPF-21 | PPF | 21 | III | MWXS00000000 | (Ouellet et al.2015) |
| PPF-7 | PPF | 7 | III | MWXR00000000 | (Ouellet et al.2015) |
| Chir_D-144 | Surgery | D-144 | III | MWXQ00000000 | (Ouellet et al.2015) |
| 151 ass. | Surgery | 151 assistant | N/Ac | MWXO00000000 | This study |
| 146 ass. | Surgery | 146 assistant | N/A | MWXP00000000 | This study |
| Site 7 | Dental equipment | N/A | N/A | MWXN00000000 | This study |
Based on the study of Ouellet et al.
Partial/fixed prosthesis
N/A: None applicable
| Isolate . | Dental clinic section . | Dental unit number . | Clustera . | GenBank . | Reference . |
|---|---|---|---|---|---|
| Urg-7 | Emergency | 7 | II | MWYB00000000 | (Ouellet et al.2015) |
| Urg-5 | Emergency | 5 | II | MWYA00000000 | (Ouellet et al.2015) |
| Ortho-1 | Orthodontics | 1 | II | MWYD00000000 | (Ouellet et al.2015) |
| Chir_D-144-ass. | Surgery | D-144 assistant | II | MWYC00000000 | (Ouellet et al.2015) |
| PPF-18 | PPFb | 18 | II | MWYE00000000 | (Ouellet et al.2015) |
| PPF-19 | PPF | 19 | II | MWXZ00000000 | (Ouellet et al.2015) |
| PPF-20 | PPF | 20 | II | MWXY00000000 | (Ouellet et al.2015) |
| PPF-13 | PPF | 13 | II | MWXX00000000 | (Ouellet et al.2015) |
| PPF-2 | PPF | 2 | III | MWXU00000000 | (Ouellet et al.2015) |
| PPF-1 | PPF | 1 | III | MWXT00000000 | (Ouellet et al.2015) |
| PPF-21 | PPF | 21 | III | MWXS00000000 | (Ouellet et al.2015) |
| PPF-7 | PPF | 7 | III | MWXR00000000 | (Ouellet et al.2015) |
| Chir_D-144 | Surgery | D-144 | III | MWXQ00000000 | (Ouellet et al.2015) |
| 151 ass. | Surgery | 151 assistant | N/Ac | MWXO00000000 | This study |
| 146 ass. | Surgery | 146 assistant | N/A | MWXP00000000 | This study |
| Site 7 | Dental equipment | N/A | N/A | MWXN00000000 | This study |
| Isolate . | Dental clinic section . | Dental unit number . | Clustera . | GenBank . | Reference . |
|---|---|---|---|---|---|
| Urg-7 | Emergency | 7 | II | MWYB00000000 | (Ouellet et al.2015) |
| Urg-5 | Emergency | 5 | II | MWYA00000000 | (Ouellet et al.2015) |
| Ortho-1 | Orthodontics | 1 | II | MWYD00000000 | (Ouellet et al.2015) |
| Chir_D-144-ass. | Surgery | D-144 assistant | II | MWYC00000000 | (Ouellet et al.2015) |
| PPF-18 | PPFb | 18 | II | MWYE00000000 | (Ouellet et al.2015) |
| PPF-19 | PPF | 19 | II | MWXZ00000000 | (Ouellet et al.2015) |
| PPF-20 | PPF | 20 | II | MWXY00000000 | (Ouellet et al.2015) |
| PPF-13 | PPF | 13 | II | MWXX00000000 | (Ouellet et al.2015) |
| PPF-2 | PPF | 2 | III | MWXU00000000 | (Ouellet et al.2015) |
| PPF-1 | PPF | 1 | III | MWXT00000000 | (Ouellet et al.2015) |
| PPF-21 | PPF | 21 | III | MWXS00000000 | (Ouellet et al.2015) |
| PPF-7 | PPF | 7 | III | MWXR00000000 | (Ouellet et al.2015) |
| Chir_D-144 | Surgery | D-144 | III | MWXQ00000000 | (Ouellet et al.2015) |
| 151 ass. | Surgery | 151 assistant | N/Ac | MWXO00000000 | This study |
| 146 ass. | Surgery | 146 assistant | N/A | MWXP00000000 | This study |
| Site 7 | Dental equipment | N/A | N/A | MWXN00000000 | This study |
Based on the study of Ouellet et al.
Partial/fixed prosthesis
N/A: None applicable
Phylogenetics
A large-scale molecular phylogeny containing 1394 isolates was performed and defined three large groups (Fig. 1), as it was already suggested by studies having fewer genomes (Stewart et al.2013; Freschi et al.2015). Focusing on the newly sequenced genomes described in the present study, we found that the CF isolates are within Group 1 as reference strains PAO1, LESB58 and PAK; while those from DUWLs are in Group 2 (Fig. 2), which also includes PA14. Cluster I of Ouellet et al. corresponds to Group 1 while clusters II and III are in Group 2. We noted two exceptions: clinical isolates 506 and 578-B are comprised within Group 1 with the CF, whereas they were previously found in cluster II, containing the environmental isolates (Ouellet et al.2015). However, in the RAPD-based tree, these two isolates were part of a clade basal to the core cluster II isolates (Fig. S1, Supporting Information), suggesting an unclear cladistics position. In addition, the positions of these two isolates in the new tree are strongly supported by the bootstrap values, while the RAPD-based tree does not have any statistical weight. Finally, DUWLs isolates originate from at least three populations likely divergent of PA14 as showed by the molecular phylogeny (Figs 1 and 2) and also the presence of three serotypes (O1, O7 and O17-like) (see below).
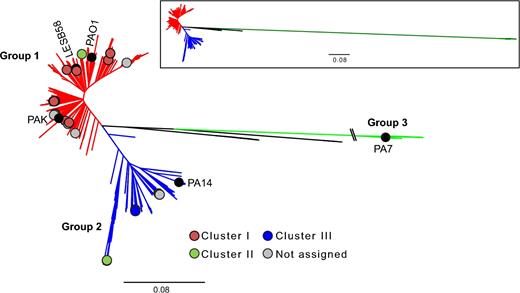
Molecular phylogeny of P. aeruginosa. 1394 P. aeruginosa isolates based on 3354 softcore genes, showing three major groups labelled as Groups 1–3 (Freschi et al.2015). The position of reference strains PAO1, PA14, PAK, LESB58 and PA7 are indicated with black circles. The position of the isolates from the study of Ouellet et al. is shown with coloured circles and the one of newly described isolates in this study with grey circles. Pseudomonas aeruginosa isolated from DUWLs are clustered into Group 2. The scale bar represents the substitutions per site. The branch length for the Group 3 was truncated for esthetical purpose but correctly represented in the tree located in the upper rectangle.
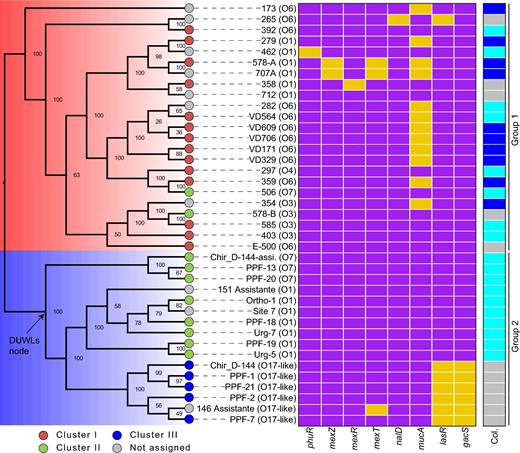
Molecular phylogeny performed by using the softcore of 39 P. aeruginosa isolates. The Groups 1 (blue) and 2 (red) correspond to the large groups as defined elsewhere (Stewart et al.2013; Freschi et al.2015) and derived from Fig. 1. The coloured circles define the isolates and their corresponding clustering as established by Ouellet et al. while the grey circles correspond to the new isolates. Finally, the predicted serotype was indicated for each isolate. The heatmap represents the genes encoding key regulators that are disrupted (yellow rectangles) or not (violet rectangle) in every isolate. The Col. column indicates the appearance of the colonies: mucoid (blue), slightly shiny (light blue) and flat (grey).
Since the molecular phylogeny showed that all P. aeruginosa isolated from DUWLs are monophyletic (Fig. 2), comparatively to the RAPD tree (Fig. S1), it was tempting to find genes that were gained or lost at the common ancestor of all DUWLs isolates (DUWLs node, Fig. 2). These events (gains or losses) could be the results of evolutionary adaptations to the DUWLs. Interestingly, 98 genes were gained at the DUWLs node while no gene was lost. Of the 98 genes, 82 were grouped into functional categories (Table S1 and Fig. S2, Supporting Information). The most represented categories were: function unknown (31%), replication and repair (16%), transcription (11%) and cell wall/membrane/envelope biogenesis (10%). Even if it is perilous to conclude about a possible evolutionary scenario, the fact that 10% of genes are related to membrane lets us believe that these genes may help to enhance an adaptation to the DUWLs given the high pressure within the pipes.
Presence of the ISPa11 in the O17-like O-antigen gene cluster
Cluster III isolates of Ouellet et al. were found to have divergent phenotypes, such as increased biofilm formation, compared to other DUWLs isolates and CF isolates (clinical). A characteristic that was relevant and found by Ouellet et al., was that all the isolates from cluster III harboured the wbjC and wbjD genes compared to other isolates included in this previous study. These two genes are implicated in the O11 O-antigen biosynthesis (Kneidinger et al.2003; Mulrooney et al.2005). This result suggested that isolates from cluster III had a different serotype, probably O11, than other isolates.
Investigating the genome of these isolates allowed not only to find these two genes, but more importantly, to identify an alteration of the wzx gene, encoding a putative O-antigen flippase (Liu, Cole and Reeves 1996) playing a crucial role in the Wzx/Wzy-dependent polysaccharide assembly pathway (Islam and Lam 2013). In fact, the wzx gene is truncated by two insertion sequences (ISs) (Fig. 3). Verification in GenBank revealed only two perfect hits (GenBank: AF498409.1 and AF236052.1) corresponding to ISPa11. This IS has previously been described to have altered wzx, wbjA and wzy genes in the O-specific antigen (OSA) biosynthesis clusters in isolates forming the serotype O17 group (Dean and Goldberg 2000). There is evidence that the genes encoding the O17 antigens could have been acquired by horizontal gene transfer (HGT) and are identical to those from Burkholderia cepacia serogroup O5. In the case of the isolates from the cluster III, the two ISs are not directly repeated, as for the typical O17, but inversely repeated (Fig. 3) and wbjA and wzy are intact in the genome of cluster III isolates. This suggests that ISPa11 may act as a chaotropic element in more than one scenario, leading to innovations in terms of O-antigen. As reviewed elsewhere (Lam et al.2011), HGT is already known to contribute to the diversity of P. aeruginosa lipopolysaccharides (LPS), and the present study shows that by investigating novel genome sequences in details, it could be possible to learn more about how mobile genetic elements can shape LPS structures.
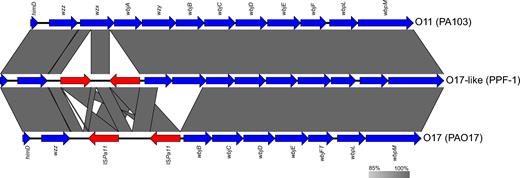
Multiple alignments of OSA biosynthesis clusters O11, O17-like and O17. Genes are represented by blue arrows, while the ISPa11s are represented by the red arrows.
Alteration of lasR and gacS
Given the discovery of ISPa11 in the OSA cluster, it was tempting to investigate if this IS also inserted elsewhere within the genomes of isolates from cluster III. Nine ISPa11 sequences are inserted in known genes (Table 3), of which five were genes encoding hypothetical proteins. In addition to these nine ISs, three others were found to be likely inserted into intergenic regions.
| Target . | Locus tag . | Localisationa . | Presence of the gene in . | |
|---|---|---|---|---|
| . | . | . | PAO1 . | PA14 . |
| wzx | G655_09050b | Cytoplasmic membrane | No | No |
| wzx | G655_09050b | Cytoplasmic membrane | No | No |
| Ankyrin-like | A0K_RS20560 | Periplasmic | Yes | Yes |
| lasR | PA1430 | Cytoplasmic | Yes | Yes |
| Hypothetical protein | T225_RS27665 | Extracellular | Yesc | Yes |
| Hypothetical protein | A4W92_RS10840 | Cytoplasmic | No | No |
| Hypothetical protein | PHAGEPCYII10_RS14555 | Cytoplasmic | No | Yes |
| Hypothetical protein | BG483_RS18925 | Unknown | Yes | Yes |
| Hypothetical protein | PHAGEPCYII10_RS14555 | Cytoplasmic | No | Yes |
| Target . | Locus tag . | Localisationa . | Presence of the gene in . | |
|---|---|---|---|---|
| . | . | . | PAO1 . | PA14 . |
| wzx | G655_09050b | Cytoplasmic membrane | No | No |
| wzx | G655_09050b | Cytoplasmic membrane | No | No |
| Ankyrin-like | A0K_RS20560 | Periplasmic | Yes | Yes |
| lasR | PA1430 | Cytoplasmic | Yes | Yes |
| Hypothetical protein | T225_RS27665 | Extracellular | Yesc | Yes |
| Hypothetical protein | A4W92_RS10840 | Cytoplasmic | No | No |
| Hypothetical protein | PHAGEPCYII10_RS14555 | Cytoplasmic | No | Yes |
| Hypothetical protein | BG483_RS18925 | Unknown | Yes | Yes |
| Hypothetical protein | PHAGEPCYII10_RS14555 | Cytoplasmic | No | Yes |
Based on the prediction of pseudomonas.com
Annotated as an hypothetical on pseudomonas.com
Slightly differs in length
| Target . | Locus tag . | Localisationa . | Presence of the gene in . | |
|---|---|---|---|---|
| . | . | . | PAO1 . | PA14 . |
| wzx | G655_09050b | Cytoplasmic membrane | No | No |
| wzx | G655_09050b | Cytoplasmic membrane | No | No |
| Ankyrin-like | A0K_RS20560 | Periplasmic | Yes | Yes |
| lasR | PA1430 | Cytoplasmic | Yes | Yes |
| Hypothetical protein | T225_RS27665 | Extracellular | Yesc | Yes |
| Hypothetical protein | A4W92_RS10840 | Cytoplasmic | No | No |
| Hypothetical protein | PHAGEPCYII10_RS14555 | Cytoplasmic | No | Yes |
| Hypothetical protein | BG483_RS18925 | Unknown | Yes | Yes |
| Hypothetical protein | PHAGEPCYII10_RS14555 | Cytoplasmic | No | Yes |
| Target . | Locus tag . | Localisationa . | Presence of the gene in . | |
|---|---|---|---|---|
| . | . | . | PAO1 . | PA14 . |
| wzx | G655_09050b | Cytoplasmic membrane | No | No |
| wzx | G655_09050b | Cytoplasmic membrane | No | No |
| Ankyrin-like | A0K_RS20560 | Periplasmic | Yes | Yes |
| lasR | PA1430 | Cytoplasmic | Yes | Yes |
| Hypothetical protein | T225_RS27665 | Extracellular | Yesc | Yes |
| Hypothetical protein | A4W92_RS10840 | Cytoplasmic | No | No |
| Hypothetical protein | PHAGEPCYII10_RS14555 | Cytoplasmic | No | Yes |
| Hypothetical protein | BG483_RS18925 | Unknown | Yes | Yes |
| Hypothetical protein | PHAGEPCYII10_RS14555 | Cytoplasmic | No | Yes |
Based on the prediction of pseudomonas.com
Annotated as an hypothetical on pseudomonas.com
Slightly differs in length
A striking observation was that an ISPa11 was found inserted into lasR (Fig. 4), encoding for LasR, a master regulator of quorum sensing (Papenfort and Bassler 2016). LasR is active in vivo in a dimeric form (Kiratisin, Tucker and Passador 2002; Schuster, Urbanowski and Greenberg 2004), each monomer being composed of two domains: an N-terminal autoinducer-binding domain and a C-terminal DNA-binding domain (Chowdhury and Bagchi 2016) (Fig. 4A). ISPa11 targeted the codon 5'-GCA-3' (glycine at position 213) and thus was inserted into the DNA-binding domain (Fig. 4B), truncating almost half of the domain (Fig. 4C) and introducing five residues from the IS open reading frame (Fig. 4D). As defined in a publication reporting the predicted structure of LasR (Chowdhury and Bagchi 2016) and also within the GenBank accession of the protein (GenBank: BAA06489.1), several important residues involved in DNA binding and dimerisation were located in the truncated portion of the protein, suggesting that LasR is inactive in the cluster III isolates.
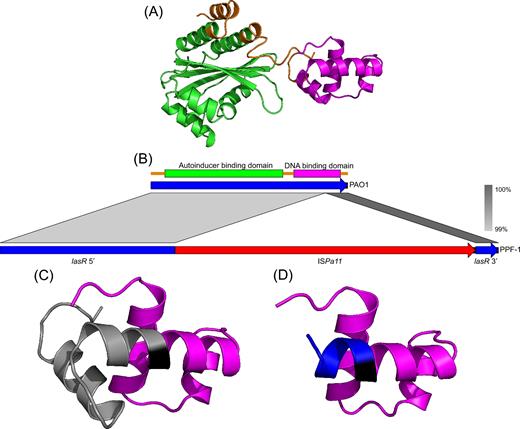
ISPa11 insertion in lasR gene. (A) Predicted structure of LasR from the strain PAO1. The N-terminal autoinducer-binding domain and the C-terminal DNA-binding domain are in green and purple, respectively, while the residues not annotated (GenBank: BAA06489) as being in a domain are in orange. (B) Alignment of the gene sequences of lasR found in PAO1 and PPF-1 (cluster III) showing the insertion of the ISPa11 into the DNA-binding domain coded by lasR from PPF-1. (C) Focus on the DNA-binding domain of PPF-1, showing the intact part in purple, the residue encoded by the codon 5'-GCA-3' that was targeted by the transposase in black and finally the missing residues in grey. (D) Still a focus on the PPF-1 DNA-binding domain, but showing in blue the few new residues produced by the in frame insertion of the ISPa11.
LasR is implicated in biosynthesis of the autoinducer 3-oxo-C12-homoserine lactone (3-oxo-C12-HSL) by controlling transcription of the gene encoding for the AHL synthase LasI (Pearson et al.1994). The production of 3-oxo-C12-HSL was thus verified to confirm that LasR is inactive in the cluster III isolates. Indeed, isolates from cluster III (PPF-1, PPF-2, PPF-7, PPF-21 and Chir_D-144-assistant) produced less than 0.1 μg/L 3-oxo-C12-HSL whereas cluster I isolate 297 produced 6 μg/L. Accordingly, the cluster III isolates did not produce pyocyanin (0.00 mg/L), a phenazine pigment produced under regulation of the quorum sensing machinery in P. aeruginosa while the cluster I isolate 297 produced significant amounts (1.05 ± 0.07 mg/L).
Mutations in lasR are well known to occur in strains of P. aeruginosa found in chronic lung infections (Hoffman et al.2009; Feltner et al.2016; Winstanley, O’Brien and Brockhurst 2016). In addition, cases of lasR mutants were also listed in acute infections, where the LasR-inactive cells gain in fitness by exploiting the public goods, such as catabolic enzymes, produced by the LasR-intact cells (Köhler, Buckling and van Delden 2009). Environmental P. aeruginosa lasR mutants were found to date from swimming pools and rivers (Cabrol et al.2003), and recently, clinical lasR mutants implicated in severe corneal ulcers were inferred to have an environmental origin (Hammond et al.2016). Inactivation of lasR produces a series of pleiotropic phenotypes such as increased β-lactamase activity, growth advantage with particular carbon and nitrogen sources (amino acids) and an overall decrease in virulence (Rumbaugh et al.1999; D’Argenio et al.2007). The elastase pseudolysin (LasB) is an elastolytic zinc metalloproteinase and a major virulence factor that is under the control of LasR (Azghani 1996; Kuang et al.2011). Isolates from cluster III produce significantly less elastase than other isolates studied (Ouellet et al.2015) which is to be expected from our 3-oxo-C12-HSL data, as the gene lasB, encoding for the protein LasB, is up-regulated by LasR when activated by its cognate ligand 3-oxo-C12-HSL (Pearson, Pesci and Iglewski 1997; Nouwens et al.2003). Finally, Ouellet et al. reported colony flattening for cluster III isolates (Ouellet et al.2015). This colonial morphology is due to cell autolysis and is known to be correlated with an inactive lasR gene (D’Argenio et al.2007).
Since inactivation of lasR plays a role in switching P. aeruginosa from an acute to a chronic infection, we investigated the other genes coding for key regulators and known to be altered when P. aeruginosa is found in a chronic lung infection (Winstanley, O’Brien and Brockhurst 2016). As expected, many isolates from the Group 1 (CF isolates) harboured characteristic disruptions into these genes (Fig. 2). The gene mucA, encoding the anti-σ-factor MucA, is the most mutated gene among the studied isolates. Knockout mutations into mucA are well known to occur in CF isolates, leading to increased alginate production, resulting in mucoid isolates (Folkesson et al.2012). Disruptions of mucA are consistent with the mucoid phenotypes already observed for these isolates (Ouellet et al.2015) (Fig. 2).
The most striking observation was that isolates from cluster III have a truncated gacS in addition to the lasR defect. GacS is a key sensor kinase implicated into a complex activation phosphorylation cascade regulating several genes critical in either acute or chronic infections (Jimenez et al.2012; Kumari, Balasubramanian and Mathee 2016). All the six isolates from cluster III harboured a deletion of 1 bp (cytosine at position 1695) in gacS.
The truncation of lasR and gacS are involved in a pathoadaptation when the isolates establish chronic lung infection (Winstanley, O’Brien and Brockhurst 2016). However, cluster III isolates were all from DUWLs, not from chronic lung infection. We cannot exclude the scenario that a pathoadapation-like could enhance the survival of strains under specific environmental conditions, such those provided by DUWLs, that could mimic a lung. For example, DUWLs are environments promoting microbial biofilm (Barbot et al.2012), similar to CF lungs. Moreover, in addition to a contamination from the water network, biological fluid retraction (suck-back) can be a cause of DUWLs contamination and pathogen transmission (Nikaeen et al.2009; Ricci et al.2012; Petti et al.2013). However, this scenario is unlikely in the present study since all isolates were obtained from different dental units (Table 2). Moreover, the phylogenetic positions of the isolates from cluster III are clearly along other DUWLs isolates suggesting a true environmental origin of cluster III isolates. Knowing that quorum sensing in P. aeruginosa is a complex regulatory network (Lee and Zhang 2014; Papenfort and Bassler 2016), it is still unclear whether the truncation of lasR, caused by ISPa11, may have decreased the selective pressure on gacS, leading to the point mutation in the gene sequence of the latter, or the reverse. Also, in a less parsimonious idea, we cannot rule out the scenario of two independent events. To confirm at the phenotypical level the inactivation of gacS, we performed qRT-PCR experiments to verify the expression of the small RNA rsmY. This small RNA is positively controlled by the GacS/A global regulation system in P. aeruginosa (Brencic et al.2009). Accordingly, expression of rsmY was found to be decreased in the isolates from cluster III compared to the PA14 strain (Fig. S3, Supporting Information).
Genes involved in antibiotic resistance and secretion systems
It is well known that P. aeruginosa is a major challenge because of its resistance to most classes of antibiotics, acquired either by HGT or by point mutations (Lister, Wolter and Hanson 2009). It was relevant to verify if differences in the resistome exist between environmental and clinical isolates (Fig. S4, Supporting Information). The genes PDC-3, PDC-5 and PDC-8, providing resistance to β-lactams, are missing in all environmental isolates. The triC gene, encoding a Resistance-Nodulation-Cell Division (RND) transporter implicated in triclosan resistance (Mima et al.2007), is also missing in environmental isolates. Interestingly, the isolates Chir_D-144-assistant, PPF-13 and PPF-20, which form the most basal clade among environmental isolates, exhibit a divergent pattern from the other strains with the presence of golS (efflux pump), mdsA (membrane fusion protein), ceoB (cytoplasmic membrane component) and mdsC (outer membrane channel). It is unclear why these three isolates are distant from other environmental isolates in both their phylogenetic positioning and in antibiotic resistance genes.
Another concern about P. aeruginosa is its high number of secretion systems, which are complex machineries exporting proteins outside the cells and often implicated in virulence (Bleves et al.2010; Filloux 2011). Investigation of the presence/absence of genes found in various secretion systems permitted to find several distinct patterns correlating with the phylogenetic positions of the isolates (Fig. S5, Supporting Information). The presence of several genes implicated in T4SS was surprising since all known secretion systems were already observed in P. aeruginosa, with the exception of the T4SS (Bleves et al.2010; Filloux 2011). However, a more precise investigation (Table S2, Supporting Information) revealed that the mandatory genes to have functional T4SS are missing and the variations were among accessory genes. The environmental isolates are those with the most conserved pool of genes implicated in secretion systems. However, here again, the isolates Chir_D-144-assistant, PPF-13 and PPF-20 exhibit a divergent pattern from other environmental isolates, mainly for the T4SS. This is interesting since Chir_D-144-assistant was isolated from a surgery dental clinic section, while both PPF-13 and PPF-20 were isolated from a prosthodontics clinic (Ouellet et al.2015), thus suggesting that these three isolates may have undergone selective pressure leading to convergent evolution. Other DUWLs isolates could increase the taxon sampling and thus help to better understand why these three isolates show divergence in their phylogenetic positions, their resistome and in their set of genes involved in secretion systems.
Ecological implications
Overall, the present study was able to shed light on an interesting genomic diversity between DUWLs and clinical isolates of P. aeruginosa, allowed to correlate several genotypes with previously reported phenotypes (Ouellet et al.2015) and finally illustrate how the dissemination of an IS into a genome can have multiple effects. However, one question remains unanswered: Why the environmental isolates from cluster III have truncated lasR and gacS, which are known to be involved in the pathoadaptation process? Few environmental isolates have been found with deletions in lasR (Cabrol et al.2003; Hammond et al.2016). Since these isolates are from DUWLs, it would be reasonable to propose the hypothesis that such mutations could help the bacterium to colonise the pipes more efficiently, which is a hostile environment with high-pressure flow. Supporting that hypothesis, inactivation of the gacS gene results in a significant increase in biofilm formation of the PA14 strain (Yeung et al.2009). Interestingly, that sessile phenotype is commonly found to be regulated in an opposite manner to swarming motility, a multicellular bacterial behaviour where cells are highly motile on a semi-solid surface (Kuchma et al.2007; Merritt et al.2007; Kearns 2010). As expected, isolates from the cluster III exhibit a major defect in swarming motility even after a prolonged incubation (Fig. S6, Supporting Information). These findings further supports that GacS is one of the key factors implicated in the adaptation of P. aeruginosa to DUWLs, an environment where biofilm formation occurs (Barbot et al.2012).
The actual knowledge on P. aeruginosa is based on a dichotomy between environmental and clinical isolates. However, environmental P. aeruginosa can be found in atypical niches. This impressive capacity to colonise efficiently such different environments necessarily compels the bacterium to quickly adapt its genome, mainly by acquiring mobile genetic elements to increase its available pool of genes and also by decreasing the conservative pressure on dispensable genes. Consequently, by pushing this idea, it is possible to believe that there are as many P. aeruginosa strains that there are colonisable environments. This study, by showing that isolates from DUWLs, an environment where humans are frequently in contact with, present several genomic and phenotypic characteristics of pathoadaptation urges the investigation of P. aeruginosa from atypical human-made environments, since these isolates could bring important insights to potential new environmental sources of infection.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSEC online.
Acknowledgements
The authors want to thank Arnaud N’guessan for his technical help with the preliminary data and François D. Rouleau for his critical reading of the manuscript.
FUNDING
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC; Discovery grant RGPIN-2014-04595 to SJC) and by Cystic Fibrosis Canada [CF Canada grant ID number 2610 to RCL]. ATV holds an Alexander Graham Bell Canada Graduate Scholarships from the NSERC. SJC is a research scholar from the Fonds de Recherche du Québec en Santé.
Conflicts of interest. None declared.



