-
PDF
- Split View
-
Views
-
Cite
Cite
Gabriele Berg, Martina Köberl, Daria Rybakova, Henry Müller, Rita Grosch, Kornelia Smalla, Plant microbial diversity is suggested as the key to future biocontrol and health trends, FEMS Microbiology Ecology, Volume 93, Issue 5, May 2017, fix050, https://doi.org/10.1093/femsec/fix050
Close - Share Icon Share
Abstract
The microbiome of plants plays a crucial role in both plant and ecosystem health. Rapid advances in multi-omics tools are dramatically increasing access to the plant microbiome and consequently to the identification of its links with diseases and to the control of those diseases. Recent insights reveal a close, often symbiotic relationship between microorganisms and plants. Microorganisms can stimulate germination and plant growth, prevent diseases, and promote stress resistance and general fitness. Plants and their associated microorganisms form a holobiont and have to be considered as co-evolved species assemblages consisting of bacterial, archaeal and diverse eukaryotic species. The beneficial interplay of the host and its microbiome is responsible for maintaining the health of the holobiont, while diseases are often correlated with microbial dysbioses. Microbial diversity was identified as a key factor in preventing diseases and can be implemented as a biomarker in plant protection strategies. Targeted and predictive biocontrol approaches are possible by developing microbiome-based solutions. Moreover, combined breeding and biocontrol strategies maintaining diversity and ecosystem health are required. The analysis of plant microbiome data has brought about a paradigm shift in our understanding of its role in health and disease and has substantial consequences for biocontrol and health issues.
INTRODUCTION
The plant microbiome has been known to be one of the key determinants of plant health and productivity for more than a century. Lorenz Hiltner began intensive research on this topic as early as 1901 (Hartmann, Rothballer and Schmid 2008). Important discoveries relating to plant-associated microorganisms followed, for example, the rhizosphere effect and the importance of rhizosphere microorganisms in the protection of roots against soil-borne pathogens was discovered (Cook et al.1995; Weller et al.2002) as well as the communication of microorganisms with their plant host (Ryu et al.2003; Hartmann and Schikora 2012). Despite a taxonomic and functional overlap within the plant microbiota (Bai et al.2015), distinct microbiomes were identified for each organ (Vorholt 2012; Philippot et al.2013; Hardoim et al.2015) and plant species (Berg and Smalla 2009). The enrichment of microorganisms by the plant root is not a random, but rather a targeted process. The current model shows the involvement of seed- and soil-borne microorganisms (Adam et al.2016a; Johnston-Monje et al.2016) and the attraction of microbes to roots by nutrients such as carbohydrates and amino acids in combination with plant-specific secondary metabolites (Moe 2013; Weston and Mathesius 2013). Differences in plant root exudates play an important role in the functioning of both chemoattractants and repellents (Badri and Vivanco 2009). Plant defense signaling plays an additional role in these processes (Doornbos, Van Loon and Bakker 2012). Although the structure of plant microbiomes is well studied, there are many knowledge gaps because of the plant species-specific component and because most of the studies were performed on crops and model plants like Arabidopsis (Bulgarelli et al.2012). The gaps are especially related to plants in natural ecosystems and their relationship to plant health.
Multi-omics technologies allow much deeper insights into the structure of plant-associated microbial communities, which support and often extend the current body of knowledge (Berg et al.2016; Jansson and Baker 2016). In addition, the tools revealed new functions of the plant microbiome and interactions within the ecosystem. Altogether the plant microbiota has profound effects on soil, plant and agro-ecosystem health (Sessitsch and Mitter 2015). Moreover, the One Health and EcoHealth concepts, which emphasize a holistic and interdisciplinary understanding of health, need plant health involvement and strategies, which are just in the beginning stages of investigation (Zinsstag et al.2011). This overview of the state-of-the-art in current literature in addition to own research insights shows that the analysis of plant microbiome data has brought about a paradigm shift in our understanding of its role in health and disease and has substantial consequences for biocontrol and beyond.
NOVEL INSIGHTS INTO PLANT MICROBIOMES
Plant-associated microbial communities and their functions for plant hosts
Microbial diversity and balance is a key for healthy plants (Yan et al.2017). Traditional knowledge and recent insights form a clearer picture of the functioning of the plant holobiont (Fig. 1). Microbial species have the ability to contribute multiple aspects to the system, including essential functions such as (i) seed germination and growth support through the provision of hormones; (ii) nutrient supply, e.g. by fixation of nitrogen, and the mobilization of phosphorus and minerals such as iron; (iii) resistance against biotic stress factors (pathogen and parasite defense); (iv) resistance against abiotic factors; and (v) physiology and production of bioactive metabolites. In detail, already in the first step of a plant's life cycle, the plant and especially the seed microbiome are crucial for the host (Truyens et al.2015). Seed-associated microorganisms can be essential for the germination procedure in different plant phyla (Hornschuh, Grotha and Kutschera 2006; Alavi et al.2013; Jacquemyn et al.2015; van der Heijden et al.2016). The underlying mechanisms comprise the production of phytohormones, fixation of nitrogen and the mobilization of phosphorus and minerals (Tkacz and Poole 2015). In these cases of germination support, microorganisms are essential, and this may be one reason that these keystone microorganisms are vertically transmitted (Bragina et al.2012; Truyens et al.2015). The plant microbiome is involved in resistance against biotic and abiotic stress factors (Bragina et al.2013). While the mechanisms involved in pathogen defense are well studied (Weller et al.2002; Berg 2009; Lugtenberg and Kamilova 2009), the phenomenon of abiotic stress protection, for example, against high salinities and drought, is only at the beginning of the investigations (Yang, Kloepper and Ryu 2009; Zolla et al.2013; Rolli et al.2015). The plant microbiota also has an influence on the physiology and production of bioactive metabolites (Zabetakis, Moutevelis-Minakakis and Gramshaw 1999; Verginer et al.2010). This was also shown for medicinal plants and their active ingredients (Köberl et al.2013b; Schmidt et al.2014). Interestingly, the rhizosphere microbiome was able to be linked to insect feeding behavior, which was most probably a result of microbiome-driven changes in the leaf metabolome of Arabidopsis (Badri et al.2013). Peñuelas et al. (2014) were able to show that the removal of the floral microbiome of Sambucus nigra resulted in a reduced floral terpene emission which plays a key role in pollination and consequently in fruit and seed production. Recent studies also revealed the direct impact of the root microbiome on plant phenology (Wagner et al.2014; Panke-Buisse et al.2015). Additional essential roles of the plant microbiome for phenotypic and epigenetic plasticity as well as the evolution of plants were suggested by Partida-Martínez and Heil (2011) and recently evidenced for mycorrhizal fungi (van der Heijden et al.2016).
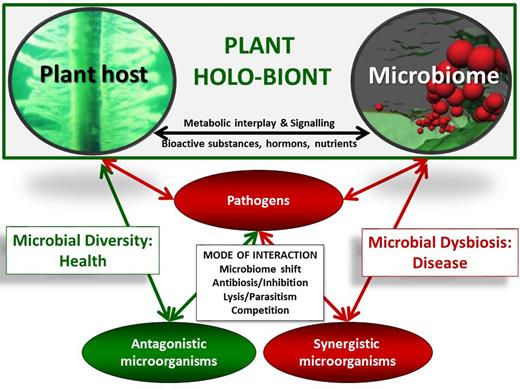
The interplay between the plant and its microbiome in health and disease. Healthy plants are associated with their microorganisms by metabolic co-operation and exchange of signals, hormones and nutrients. Diseases are characterized by a microbial dysbiosis and a response of specific microbes, which can act as antagonists or synergists towards pathogens.
Interkingdom signaling and interconnections of the plant microbiome
The cooperation between plants and millions of microbes as well as between them requires an intense communication (Venturi and Keel 2016). A high number of signatures encoding interaction via quorum sensing and other signaling molecules were found in plant-associated microbial metagenomes (Bragina, Berg and Berg 2015); however, the mechanism by which microorganisms interact as a community to confer beneficial traits to plants is still poorly understood. Volatile organic compounds are responsible for ‘microbial small talk’ but can also act as long-distance messengers for interaction with the plant host (Schmidt et al.2016). A recent review by Rosier et al. (2016) highlights the importance of chemical signaling, and biochemical and genetic events which determine the efficacy of benign microbes in promoting the development of beneficial traits in plants.
Beyond the plant host, the plant microbiome is interconnected with the ecosystem. Although the plant microbiome is divided into specific compartments linked with specific microorganisms, it is also connected with the surrounding environment. The rhizosphere represents the plant–soil interface, from which organic material and signaling compounds migrate into subsoil zones facilitating long-term mineralization processes as part of the biogeochemical and ecological disequilibrium (Berendsen, Pieterse and Bakker 2012; Gocke et al.2017). The phyllosphere and all above-ground organs are connected with the atmosphere and are at the interface for permanent exchange with the air microbiome (Lindow and Brandl 2003). Both ectophytic microhabitats—the phyllosphere and the rhizosphere—present a higher microbial diversity than their endophytic counterparts, the endorhiza and endosphere, because resident and transient microorganisms are co-occurring. Endosphere, carposphere and spermosphere are colonized by specifically adapted microorganisms representing an intimate interaction; they present the internal phytomicrobiome (Fig. 2). The sporosphere included the spores of mosses as well as pollen grains of higher plants, which are morphologically homolog.
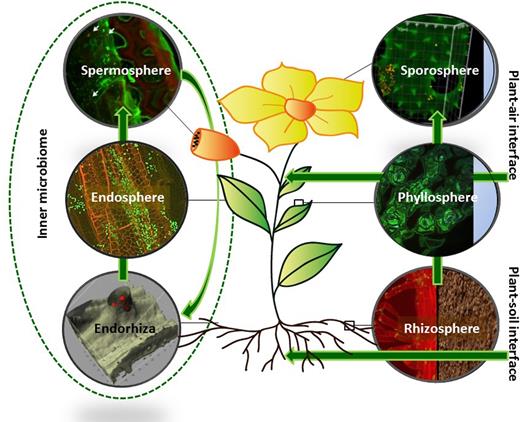
The relationship between plant habitats and their interconnection with the ecosystem.
The seed microbiome plays a crucial role in a plant's development
The seed microbiome is crucial because it ensures the dissemination and preservation of a co-evolved and specific microbiome to the next generation. In seeds, most of the microorganisms are in a dormant stage and are difficult to isolate (Truyens et al.2015). Through the application of DNA-based techniques, highly diverse seed microbiomes were shown to exist in many plant species (Adam et al.2016a; Johnston-Monje et al.2016; Klaedtke et al.2016). There are still only a few plant species being analyzed but it seems that seeds transfer a core microbiome to the new plantlets similar to all other organisms including mosses (Bragina et al.2012) and humans (Blaser 2014). However, in textbooks and international trade rules there is evidence that the use of pathogen-free seeds is very important in the production of healthy crops. A large amount of physical and chemical treatments have been developed to obtain pathogen-free seeds. These measures can also lead to the general reduction of microbial diversity and could potentially cause diseases in seedlings due to the fact that beneficial microorganisms supporting germination and growth were also killed. Therefore, we recommend the evaluation of existing methods by omics technologies. We also believe that a combination with biologicals (biopesticides, biostimulants, biofertilizers) can improve the effects of common seed disinfection techniques as suggested by Adam et al. (2016b).
The plant microbiome and agriculture
Nearly half of the world's land is used for agriculture according to FAO (Food and Agriculture Organization) statistics. All our crops are ‘domesticated’ by breeding and man-made evolution. Co-evolution between plants and associated microbial communities, known for wild-type species, has already been hypothesized based on culture-dependent results obtained for the rhizosphere of ancient and modern wheat cultivars (Germida and Siciliano 2001). Co-evolution was recently shown to be prevalent among other plants, e.g. maize, sugar beet, wheat, pumpkin and lettuce, by application of deep sequencing techniques (Peiffer et al.2013; Bulgarelli et al.2015; Cardinale et al.2015; Adam et al.2016a). Crop breeding was identified as strong driver of natural evolution (Berg et al.2013). In some cases, the breeding strategy was targeted at combating pathogens, increasing yield and various growth characteristics, but historically it was a mainly random selection process for plant phenotypes, and changes in the plant microbiome were neglected.
In general terms, most of the management strategies and treatments in agriculture have an impact on microbial diversity and community composition of soils. The concept of the soil memory effect as a potential mechanism for facilitating sustainable plant growth and productivity was recently suggested by Lapsansky et al. (2016). Evidence was found in field studies that organically managed crops enrich their own biocontrol potential (Cooper et al.2006; Fließbach et al.2007; Crowder et al.2010, Schmid et al.2011). For example, statistically significant differences in the structure and function of above-ground grapevine-associated microorganisms from organically and conventionally managed vineyards were found in a study by Schmid et al. (2011). Aureobasidium pullulans, a copper-detoxifying fungus and biocontrol agent, played a key role in explaining these differences.
PLANT PATHOGENS, DISEASE OUTBREAKS AND SUPPRESSIVENESS
What is a plant pathogen?
The presence of microbes in plants became initially noticeable when diseases appeared. However, the vast majority of microbes in plants are not causal agents of damage in plants (Mendes, Garbeva and Raaijmakers 2013). Microbes that are in general able to incite diseases on plant are collectively called plant pathogens. The most common plant diseases are caused by fungi, oomycetes, bacteria, viruses, viroids, virus-like organisms, phytoplasmas, nematodes and parasitic higher plants. In the context of this review, we discuss fungi and bacteria, the largest groups of plant pathogens, as causal agents of plant diseases. A classical definition of plant pathogens assumes that in order to fulfill Koch's postulates, the pathogenic microorganisms must be characterized by pathogenicity/virulence factors. Pathogenicity is defined as the ability of a pathogen to infect and cause disease on a particular host plant, and virulence as a quantitative property is defined as the degree of damage caused to the host (Sacristán and García-Arenal 2008). The degree of a disease is assumed to be negatively correlated with the host fitness (D’Arcy, Eastburn and Schumann 2001). In addition, plant–fungal pathogen interaction can be race-specific and non-specific. Race-specific interaction is known for a large number of leaf pathogens such as rust or powdery mildew fungi (Hovmøller et al.2008), while race-non-specific interaction is described for various soil-borne pathogens. Fungi enter plants through natural openings (e.g. stomata), wounds or by direct penetration of plant tissue. In contrast, bacteria can enter plants only through wounds or natural openings. Diverse pathogens thrive on plant surfaces, while other pathogens inhabit internal plant tissues. Our present knowledge of the factors driving the ecology of plant pathogens is still rather limited as plant pathogens are typically studied mainly on diseased plants. However, there is increasing evidence that members of the microbiome have an important role in the establishment and development of plant diseases (Trivedi et al.2012; Erlacher et al.2014).
Many plant pathogens are either cultivable or diagnostic tools exist for known, not cultivable obligate pathogens. However, despite the substantial progress made in cultivation-dependent methodology (Bai et al.2015), not all microbes retrieved from natural environments are cultivable. Hence, aside from obligate parasites, a lot of pathogens are not cultivable or they might have entered a state called viable but non-cultivable in response to different stresses such as low temperature, salt or UV radiation (Podolich et al.2015). Here, molecular markers and cultivation-independent methods become important for their detection. In addition, there are diseases for which, up until now, no pathogen could be identified or diseases, for which a microbiome imbalance (dysbiosis) of the soil microbiota is likely to play a role, e.g. replant diseases (Mazzola and Manici 2012; Yim et al.2013).
Pathogens can interact with a wide variety of plants independent of the mode of life (biotroph, necrotroph; obligate or facultative). The hosts may range from a few to several hundred plant species. In addition to an endophytic pathogenic lifestyle in a host plant, fungal pathogens can adopt to a contrasting non-pathogenic lifestyle when growing on various other non-host plant species (Freeman, Horowitz and Sharon 2001; Berg, Eberl and Hartmann 2005; Vilhelmsson et al.2016). The fungal plant pathogen Verticillium dahliae was identified as a frequent inhabitant of the endosphere in many plants (Götz et al.2006) and used for biological control (Tyvaert et al.2014; Postma and Goossen-van de Geijn 2016). Rhizoctonia is another interesting example; it can cause serious symptoms in many crops and has a certain degree of host specificity (Wibberg et al.2015).
Microbial diversity and disease outbreaks
The ubiquitous occurrence of potential pathogens on plants raises the question as to whether the outbreak of certain ‘microbiome diseases’ is not always dependent on microbial diversity. We know that the resistance of the microbial community to the invasion of pathogens is linked to its level of diversity (Jousset et al.2011; Van Elsas et al.2012; Mallon, Elsas and Salles 2015) and ‘missing microbes’ can support disease outbreaks (Blaser 2014). The microbiome of each plant forms a network; these networks can be visualized by co-occurrence patterns (Cardinale et al.2015). The strength and connection of the network is crucial for the invasion success of pathogens and also for the establishment of biocontrol agents. Microbial invasions can cause microbiome shifts, and endophytes can be involved in controlling pathogens in that same manner (Trivedi et al.2012; Erlacher et al.2014; Mercado-Blanco and Lugtenberg 2014; Podolich et al.2015). Mendes et al. (2007) observed the suppression of Fusarium moniliforme on sugarcane by endophytic isolates of the Burkholderia cepacia complex. Microbes can also inhibit the induction of pathogenic genes by impairing them with quorum sensing or otherwise the pathogens have to compete with microbes for plant resources (Friesen et al.2011). In addition, microbes can directly influence plant defense response by producing antagonistic molecules within plant tissue or on the surface of the plants (Fravel 1988). An indirect influence on a plant host's defensive response level is evidenced either as an alteration in defense pathways or as an improvement of plant vigor (e.g. via the production of hormones). We know that microorganisms can support the pathogens (Erlacher et al.2014). These are often members of the Enterobacteriacea family, known for their degrading capacity of plant tissues. Both responder groups, those who support and antagonize the pathogen, have to be considered as interesting targets for biocontrol approaches in the development of plant protection methods.
Interestingly, soil microbiota was identified as causal agent of suppressive soils. Historically, crop rotation is one of the oldest plant protection methods as cited in biblical teachings. In recent decades, crop rotations became shorter and in the USA there is a long tradition of monocultures. There is no doubt that crop rotation is a highly efficient method of controlling many diseases, and this can be explained by the great extent of specificity of the plant microbiome, which enhances the overall microbial diversity in soil. A multitude of pathogens existing as normal members of these communities have a low outbreak potential. In monocultures, an increase of pathogen density resulted in severe disease followed by an increase of antagonistic microorganisms which in a best-case scenario cause suppressiveness (Mazzola 2004). This phenomenon was intensively studied in wheat monocultures in the Pacific Northwest (Weller et al.2002) and was recently defined as soil immune response to pathogens (Raaijmakers and Mazzola 2016).
There are important future perspectives for linking plant microbiome and diseases by monitoring. Microbiome-wide association studies in human medicine revealed complex interactions among the microbiome and the environment as first keys for developing precision diagnostics and therapies that are based on the microbiome (Gilbert et al.2016). This can be endorsed as a worthy objective not only for the promotion of plant health but also for the promotion of soil health, which are closely connected.
MICROBIOME-BASED SOLUTIONS FOR PLANT PROTECTION
Microbiome and biocontrol strategies
Plant diseases are the cause of major economic losses for farmers worldwide. The FAO estimated that pests and diseases are responsible for about 25% of crop loss (Martinelli et al.2014). There are regional differences reported: it is estimated that diseases typically reduce crop yields by 10% every year in more developed settings, but yield loss due to diseases often exceeds 20% in less developed areas. Disease control is reasonably successful for most crops and is achieved by use of plants that have been bred for appropriate resistance against many diseases, by plant cultivation approaches such as crop rotation, use of pathogen-free seeds, appropriate planting date and plant density, and by chemical and biological control. Most of these plant protection methods can influence the plant microbiome and therefore new insights should be taken into consideration for the improvement of plant protection strategies. In many cases, diseases are associated with microbiome imbalances or shifts which makes exploitation of the entire microbiome a desirable objective. Analyzing the plant microbiome as well as the metabolic interplay with the host plant opens new doors for advanced biocontrol technologies (Berg 2015).
New insights reveal an impressive microbial diversity amongst all plants and novel antagonistic microorganisms towards phytopathogens. Despite the fact that plant microbial diversity depends on many factors, there were some facts reported which should be considered in future screening strategies. Mosses, the oldest land plants on Earth, are characterized by a unique microbial diversity (Bragina et al.2012; Bragina, Berg and Berg 2015) and they harbor an extraordinary potential of antagonists due to their ecology (Opelt et al.2007). Furthermore, medicinal plants have been evaluated as being an interesting source for biodiversity (Köberl et al.2013a). This originates from their rich secondary metabolism, which triggers the plant microbiome composition. Endemic plants were also identified as being unique sources for biodiversity and antagonists (Zachow et al.2014). Due to novel insights, we expect that endophytes, and in particular seed endophytes, can serve as sources for novel biocontrol agents. Up to now, mainly bacteria and fungi have been exploited for biocontrol purposes. Archaea were recently identified as plant microbiome members (Müller et al.2015); their functions on plants and their potential for biocontrol are completely unknown.
As mentioned above, all microorganisms associated with plants form a network which can be influenced by microbial invasion. These network models of soil and plant microbiomes provide new opportunities for enhancing disease management and can be deciphered for biocontrol. Poudel et al. (2016) suggested a framework for interpreting microbiome networks, illustrating how observed network structures can be used to generate testable hypotheses about candidate microbes affecting plant health. They presented four different types of network analyses: (i) general network analysis to identify candidate taxa for maintaining an existing microbial community, (ii) host-focused analysis to include plant responses, (iii) pathogen-focused analysis to identify taxa with direct or indirect associations with taxa known a priori as pathogens and (iv) disease-focused analysis to identify taxa associated with disease. Interestingly, there are a lot of parallels to manage invasive plants by symbiotic microorganisms (Kowalski et al.2015).
Most of the potential biocontrol agents were screened by in vitro antagonisms against the pathogen. Despite the fact that many of these screenings yielded in successful biocontrol agents, there is a long and intense debate about this screening assay. One reason is that many of the efficient plant growth promoting and biocontrol organisms in vivo show no or only modest in vitro antagonisms (Adesina et al.2009; Schreiter et al.2014a). High-throughput in planta assays were developed, but they also showed disadvantages because of their artificial character. For example, treatment of oilseed rape seeds with Paenibacillus showed a negative effect on the plant growth under gnotobiotic conditions, no effect in sterile soil, but plant growth promotion in natural soil (Rybakova et al.2016b). Thus, testing of the potential plant growth promoting and biocontrol strains needs to be done in soils and the effects of plant species and soil types need to be tested. Schreiter et al. (2014b) could show that similar genera, among them Pseudomonas, were enriched in the rhizosphere of lettuce grown under field conditions in three different soil types. This finding might explain that the soil types did neither influence the rhizo-competence nor the biocontrol activity. Recently, microbiome shifts were discussed as potential novel mechanisms for biocontrol (Schmid et al.2011; Grosch et al.2012; Erlacher et al.2014; Schreiter et al.2014a). The potential mechanisms of microbiome shifts are not well studied but it is assumed that they might include several direct interactions with plant pathogens as well as indirect interactions via the plant by stimulation of the plant immune system (Berg 2009; Lugtenberg and Kamilova 2009). Bacillus amyloliquiefaciens FZB42, which is a commercialized and efficient plant strengthener, is able to enhance overall microbial diversity (Erlacher et al.2014). Targeting enhanced diversity as biomarker for screening methods will be an interesting objective.
The development and application of biological control products is an increasingly attractive objective worldwide. While single organisms were mainly used in the past, often correlated with inconsistent effects, it is now possible to develop microbiome-based biocontrol strategies (Berg et al.2013). Emmert and Handelsman (1999) recommended Gram-positive bacteria as a good potential candidate for biocontrol applications. And indeed, looking on the registered products and those which are in the pipeline, most of the products are based on Bacillus (e.g. EU pesticides database). There are mainly technological reasons for the prevalent use of Bacillus as a base product: in comparison to Gram-negative bacteria, bacilli are able to develop spores to survive unfavorable conditions. These spores are easy to formulate and have a high shelf life. When we examined the plant-associated microorganisms which comprise thousands of bacterial species, we came to the realization that only a small proportion of their taxonomic diversity is currently being exploited for biocontrol purposes. Moreover, Firmicutes (e.g. Bacillus) normally present a small proportion of the plant microbiome; Janssen (2006) reported them as being contributors to a mean of only 2% (range 0%–8%) in the total bacterial soil community. Exceptions were published by Köberl et al. (2011) who found up to 37% of Firmicutes (Bacillus and Paenibacillus) in soils of arid environments. There is still an ongoing debate regarding the mode of action of Bacillus on plants as well as towards plant pathogens, and although induced resistance and plant growth hormones are reported to be involved in plant–microbe interaction, it is clear that all Bacillus and Paenibacillus strains are able to produce a long list of bioactive substances and antibiotics (Cawoy et al.2015; Rybakova et al.2016a). Due to the fact that Bacillus and Paenibacillus strains and spores are highly persistent in the environment, a reduction of plant-associated microbial diversity may be an outcome. Therefore, we would again like to emphasize our demand for more diversity among and within biocontrol products. New cultivation strategies should be developed and applied to exploit the entire antagonistic diversity associated with plants (Zachow et al.2013).
Antibiotic resistances are spreading globally, threatening our ability to treat common infectious diseases, resulting in prolonged illness, disability and death. Unfortunately, agricultural practices such as the use of antibiotics in the livestock industry lead to increased levels of resistance (Ferber 2002; Jechalke et al.2014). The spreading of manure can transfer resistances into plant production systems; due to their bioactive secondary metabolites plants often enriched resistant bacteria in their rhizosphere (Berg, Eberl and Hartmann 2005). Although antibiotic resistance is ancient and widespread in environmental bacteria, risk assessment studies to minimize the emergence and impact of resistance for the whole cycles are needed. Although many biocontrol agents showed low persistence on plants and in soil (Scherwinski, Grosch and Berg 2008), the massive application of spore-forming bacteria could change this picture. Moreover, as most soil bacteria, bacterial antagonists in general, and Paenibacillus in particular, do not only contain antibiotic production genes but show also high numbers of antibiotic resistance genes resulting from their ecological background and their antagonistic function within the microbiome. The potential mobilization of these resistance genes via different routes of horizontal gene transfer needs further investigation.
Future health trends
Targeted microbiome engineering for crops is a future trend. Biodiversity should be a biomarker for these microbiome modulations. Higher plant-associated diversity can be achieved not only through the implementation of biological control agents which shifts the microbiome (Erlacher et al.2014), but also by the application of microbial consortia. In this context, crop-specific biological consortia can be taken together from a pool of selected biocontrol agents. Unfortunately, the application of several microbial strains for biocontrol reasons is currently limited in the European Union by the necessity of registering each of the strains, which is a very long and expensive process.
David et al. (2014) showed that food-borne microbes from diet were able to survive and transiently colonized the gut. The plant-associated microbial diversity can be transferred to the gut microbiome because fruits and vegetables are the major components of a healthy diet. However, outbreaks are due to alterations of the gut microbial diversity and are associated with chronic disease, e.g. allergies, obesity and mental diseases (Gilbert et al.2016). Increasing chronic diseases in children can be explained by the ‘missing microbe theory’ published by Blaser (2014). Microbial diversity is already established as a key factor in preventing human diseases and should be implemented in plant protection measures.
Concluding remarks
Continued advances in phytopathology and microbial ecology are needed to improve disease control, and to keep pace with changes in disease pressure caused by the ongoing evolution and movement of plant pathogens and changes in agricultural practices. In order to effectively address this issue, all crop plants have to be recognized as holobionts. Plant-associated microbial diversity is a crucial factor for plant health. Although the task remains for us to clarify the question of what specifically constitutes a healthy microbiome, microbial diversity can be used as a biomarker for healthy plant microbiomes, e.g. in breeding and biocontrol strategies. Targeted and predictive biocontrol approaches are possible by taking a holistic approach and integrating microbiome-based solutions. Moreover, combined breeding and biocontrol strategies maintaining diversity and ecosystem health are required. These systemic approaches are required in order to avoid further losses of our biodiversity and to ensure sustainable agricultural practices.
Acknowledgments
We would like to thank Timothy Mark (Graz) Additionally, we thank Ilse-Marie Jungkurth (Braunschweig) for her endless – but unfortunately fruitless – ambition to teach us Latin.
FUNDING
This work was supported by the EU-Egypt Innovation Fund [RDI ENPI/2014/342-707] and by the European Union in frame of FP7-KBBE-2013-7-single-stage [BIOCOMES; No. 612713]. The cooperation of GB was funded by a project in the Austrian Centre of Industrial Biotechnology, which has been supported by the Austrian BMWFW, BMVIT, SFG, Standortagentur Tirol and ZIT through the Austrian FFG-COMET-Funding Program. MK acknowledges support by the Austrian Science Fund FWF [J 3638, T 847]. Funding of research on soil microbial diversity and impacts on plant health and growth is provided to KS through BMBF 031A560C, 031B0025B and EU Biofector 312117.
Conflict of interest. None declared.



