-
PDF
- Split View
-
Views
-
Cite
Cite
Dania Richter, Boris Schröder, Niklas K. Hartmann, Franz-Rainer Matuschka, Spatial stratification of various Lyme disease spirochetes in a Central European site, FEMS Microbiology Ecology, Volume 83, Issue 3, March 2013, Pages 738–744, https://doi.org/10.1111/1574-6941.12029
Close - Share Icon Share
Abstract
To determine whether the genospecies composition of Lyme disease spirochetes is spatially stratified, we collected questing Ixodes ricinus ticks in neighboring plots where rodents, birds, and lizards were present as reservoir host and compared the prevalence of various genospecies. The overall prevalence of spirochetes in questing ticks varied across the study site. Borrelia lusitaniae appeared to infect adult ticks in one plot at the same frequency as did Borrelia afzelii in the other plots. The relative density of questing nymphal and adult ticks varied profoundly. Where lizards were exceedingly abundant, these vertebrates seemed to constitute the dominant host for nymphal ticks, contributing the majority of infected adult ticks. Because lizards support solely B. lusitaniae and appear to exclude other genospecies, their narrow genospecies association results in predominance of B. lusitaniae in sites where lizards are abundant, while limiting its spread to the host's habitat range. To the extent that Central European B. lusitaniae strains are nonpathogenic, the presence of numerous lizards should locally decrease risk of infection for people. Evaluation of regional risk of infection by Lyme disease spirochetes should take the spatial effect of hosts into consideration, which stratify the distribution of specifically infected ticks on a small scale.
Introduction
In Europe, a variety of Lyme disease spirochetes are transmitted by Ixodes ricinus ticks. Although these vector ticks feed virtually indiscriminately on a wide array of vertebrates, each of the Lyme disease genospecies appears to be associated with a particular group of hosts. Whereas Borrelia afzelii spirochetes appear to be perpetuated by rodent hosts, Borrelia garinii and Borrelia valaisiana are considered to be associated mainly with birds (Kurtenbach et al., 2002; Hanincová et al., 2003). Hosts that are reservoir competent for one Lyme disease genospecies may even be zooprophylactic to another, because, for example, a tick that has acquired B. afzelii from a rodent during its larval blood meal may be cleared of these spirochetes when feeding as a nymph on a bird (Matuschka & Spielman, 1992). Borrelia burgdorferi s.s. spirochetes, on the other hand, are considered as generalists and may thrive in both rodents and birds (Richter et al., 2000, 2004a). Whereas B. afzelii, B. garinii, B. valaisiana, and B. burgdorferi s.s. are ubiquitously prevalent in questing ticks, others, such as Borrelia spielmanii and Borrelia lusitaniae, which are closely associated with certain dormice and lizards, respectively, may be limited in distribution to the habitats of their hosts (Richter et al., 2004b, 2006; Richter & Matuschka, 2006a). At least, four species of lizards support B. lusitaniae in nature. Sand lizards, Lacerta agilis, wall lizards, Podarcis muralis, green lizards, Lacerta viridis, and Algerian sand racer, Psammodromus algirus, serve as reservoir hosts in Europe and the latter species in Tunisia (Dsouli et al., 2006; Majláthová et al., 2006; Richter & Matuschka, 2006a; Amore et al., 2007). Whereas B. lusitaniae constitutes the major, and in some sites, sole genospecies of Lyme disease spirochetes in the Mediterranean basin, it is only focally distributed in Northern Europe (Richter & Matuschka, 2006a).
The composition of the diverse genospecies infecting vector ticks varies and depends on the local host composition. For relatively ubiquitous genospecies, such as B. afzelii, B. garinii, and B. valaisiana, the proportion of hosts competent for one or another of these genospecies likely may influence the relative genospecies mix in the tick population. A genospecies with a narrow host association, such as B. lusitaniae, which is perpetuated by nonubiquitous hosts, as are lizards in Germany, may only occur where its host is present. As observed in other host-parasite relationships (e.g. Heisswolf et al., 2009; Zurell et al., 2009), habitat parameters may particularly influence the distribution of those spirochetes that have a limited range of reservoir hosts.
To determine whether the various Lyme disease genospecies are stratified or distributed evenly across a study site, we compared the prevalence of each genospecies in questing ticks collected in three neighboring plots, one of which appeared to support lizard populations. In addition, we compared the relative density of nymphal and adult ticks.
Materials and methods
Our three plots were located at the top of a hill, 300 m above sea level, near the city of Heilbronn in the southwestern German state of Baden-Württemberg. The southern slope of the hill serves as vineyard. Plot A was a sun-exposed brushy ecotone, 2–3 m in width, between an agricultural road and the forest, where sand lizards, Lacerta agilis, and wall lizards, Podarcis muralis, were exceedingly abundant (Richter & Matuschka, 2006a). Plot B was located at the edge of a forestry road cutting through the forest consisting mainly of beech trees. The shadowy Plot C was characterized by an abandoned path into the forest that had been virtually overgrown by brushy vegetation. Each plot was about 100 m long and about 50 m distant from the other two plots.
Questing nymphal and adult ticks were collected monthly from April through October 2007 at all three plots by means of a flannel flag by the same person. Ticks were taken to the laboratory, preserved in 80% ethanol, and microscopically identified to stage and species. We determined the relative density of questing nymphal and adult ticks in each of our three plots by dragging a flannel flag (1.0 by 1.5 m) for a specified time span over the vegetation growing immediately adjacent to the road or path. Ticks were collected per unit time by recording the period of active flagging. Additional nymphal ticks collected at Plot A in April and May 2008 were included to obtain sufficient nymphal ticks to determine the prevalence of spirochetal infection in this stage.
To detect and identify the various spirochetes that may be present in the ticks, the opisthosoma of each was opened, and the contained mass of soft tissue dissected out in physiologic saline, transferred to a tube containing 180-μL lysis buffer (ATL Tissue Lysis Buffer, Qiagen, Hilden, Germany) and 20-μL proteinase K (600 mAU mg−1), and lysed at 56 °C overnight. DNA was extracted using the QIAamp DNA Mini Kit (Qiagen) according to the manufacturer's instructions, DNA of nymphal or adult ticks was eluted with 50- or 75-μL elution buffer, respectively, and stored at −20 °C until PCR was performed. Borrelia genospecies were characterized by amplifying and sequencing a 600-nucleotide fragment of the gene encoding the 16S rRNA. To increase the sensitivity for detection of spirochetal DNA in ticks, we used nested PCR as described previously (Richter & Matuschka, 2006a). Each PCR amplification product was purified using a QIAquick-Spin PCR column (Qiagen) according to the manufacturer's instructions. Amplified DNA fragments were directly sequenced in both directions using the inner primers by the dideoxynucleotide chain termination method on a Licor DNA4200 sequencer (Licor Biosciences, Bad Homburg, Germany). Each resulting sequence was compared with sequences of the same gene fragment representing various spirochetal genospecies. The following sequences served for comparison: Accession numbers X85196 and X85203 for B. burgdorferi s.s., X85190, X85192, and X85194 for B. afzelii, X85193, X85199, and M64311 for B. garinii, X98228 and X98229 for B. lusitaniae, X98232 and X98233 for B. valaisiana, AY147008 for B. spielmanii as well as AY253149 for B. miyamotoi. A match, permitting no more than two nucleotide changes, was required. Infections by more than one genospecies were recognized in the image of the sequencing gel when simultaneous bands occurred at all signature sequences that differentiate coinfecting genospecies from each other.
We used Fisher's exact test for independence in m × n contingency tables to assess whether the prevalence of each of the host-associated genospecies, B. afzelii,B. garinii,B. valaisiana, and B.lusitaniae, in questing nymphal or adult ticks is independent of the study plot (Fisher, 1935; Mehta & Patel, 1986).
We performed nine comparisons and to account for multiple comparisons, we report p-values adjusted by Holm's correction, which strictly controls the family-wise error-rate under arbitrary assumptions (Holm, 1979; Sonnemann, 2008). All calculations were performed with R (version 2.13.2; R Development Core Team, 2011, http://www.R-project.org).
Results
First, we determined the overall rate of infection in questing ticks collected in our three neighboring study plots. A quarter to a third of the nymphs and a third to half of the adult ticks in these three plots were infected by spirochetes (Table 1). Whereas infection rates in nymphal (26.1%) and adult ticks (28.4%) did not vary within Plot C, one and a half as many adults (49.3%) as nymphs (31%) and twice as many adults (49.3%) as nymphs (24.3%) were infected in Plots A and B, respectively. The non-Lyme disease spirochete, B. miyamotoi, was present in about 7.5% of all infected ticks (range 3.7–12.5%), independent of tick stage or plot. Whereas in Plots B and C, about 5% of all infected adult ticks harbored more than one kind of spirochete, twice as many adults were coinfected in Plot A. No nymphal tick was coinfected. In seven of the nine coinfected adult ticks, B. miyamotoi paired with a Lyme disease genospecies. In Plot A, two adults were coinfected by B. miyamotoi and B. valaisiana, one by B. miyamotoi and B. garinii, one by B. afzelii and SV1 as well as one by B. afzelii and B. garinii; in Plots B and C, two adults each were coinfected by B. miyamotoi and B. afzelii. The overall prevalence of spirochetes in questing ticks varied across the study site.
Prevalence of various spirochetes in questing Ixodes ricinus ticks collected at our study plots, displayed relative to other spirochetes and as absolute prevalence. Plot A was inhabited by numerous lizards, whereas none were observed in the neighboring Plots B and C
lus,lusitaniae;afz, afzelii;bur,burgdorferi s.s.; SV1,isolate SV1 (Casjens et al., 2011); gar,garinii;val,valaisiana;miy,miyamotoi.
Associated with lizards.
Associated mainly with rodents.
Associated with birds and rodents.
Associated mainly with birds.
Prevalence of various spirochetes in questing Ixodes ricinus ticks collected at our study plots, displayed relative to other spirochetes and as absolute prevalence. Plot A was inhabited by numerous lizards, whereas none were observed in the neighboring Plots B and C
lus,lusitaniae;afz, afzelii;bur,burgdorferi s.s.; SV1,isolate SV1 (Casjens et al., 2011); gar,garinii;val,valaisiana;miy,miyamotoi.
Associated with lizards.
Associated mainly with rodents.
Associated with birds and rodents.
Associated mainly with birds.
For each genospecies, we determined its proportion in infected ticks across the three plots of our study site. The proportion of infected adult ticks harboring B. lusitaniae was largest in the lizard-inhabited Plot A (57.8%), less than a seventh of that in Plot B (8.6%) and no B. lusitaniae-infected ticks were detected in Plot C (Fisher's exact test, Holm corrected: Pcorr. < 0.0001). Adult ticks in Plot A were almost eight times more likely to be infected by B. lusitaniae than were nymphal ticks (7.4%; Pcorr. = 0.0002), and infection of nymphs did not vary significantly between plots (Pcorr. = 1). Whereas B. afzelii infected a similar proportion of infected nymphal ticks in all three plots (averaging 75.6%; Pcorr. = 1), it was least abundant in adult ticks questing in Plot A. Less than a fifth of the infected adults (17.7%) there harbored B. afzelii, but about half of the adult ticks (48.6 and 55%) did so in the other plots (Pcorr. = 0.0043). The proportional prevalence of B. burgdorferi s.s. in adult ticks appeared somewhat similar to that of B. afzelii across the three plots. A spirochete, genotypically related to the recently described isolate SV1 (Casjens et al., 2011) was detected solely in adult ticks; its proportion was largest in Plot C, where it infected as much as an eighth of the infected adult ticks (12.5%) and smallest in Plot A infecting 4.4% of the infected adult ticks. The proportions of B. valaisiana and B. garinii (B. garinii serotype 4, now designated as B. bavariensis (Margos et al., 2009), was not detected in this study) infecting questing adult ticks appeared not to vary significantly across the study site (Pcorr. = 0.7289 for B. garinii in adults; Pcorr. = 1 for B. valaisiana in adults and both genospecies in nymphs). No ticks harbored B. spielmanii spirochetes. Thus, the proportions of at least two of the six different genospecies prevalent in our study site appeared to be distinctly stratified in questing adult ticks, with B. lusitaniae infecting adult ticks in Plot A at the same frequency as did B. afzelii in the other plots.
We determined the relative density of nymphal and adult ticks questing in our plots. Whereas seasonal averages of about 120 and 140 ticks were collected per hour in Plots A and B, respectively, more than 270 ticks were sampled per hour in the shadowy Plot C (Table 2, Fig. 1). Nymphal activity appeared low in all plots during June, while adult ticks were particularly abundant. In Plot A, almost five times more adult than nymphal ticks were found questing (21.3 nymphs vs. 102.2 adults per hour). In contrast, three times more nymphs than adults were collected in Plot B (106.6 nymphs vs. 38 adults per hour). Both stages were almost similarly abundant in Plot C. Of the few nymphs that were collected throughout the tick season in Plot A, three quarters (73%) were collected in April. During that month, only a third of all nymphs were collected in Plots B and C (34.7% and 29.8%, respectively). The relative density of questing nymphal and adult ticks varied in each of our three study plots.
Density of questing Ixodes ricinus ticks in our study plots determined by flagging the vegetation, averaged across the season, and the rate of nymphs questing during the month of April. Plot A was inhabited by numerous lizards, whereas none were observed in the neighboring Plots B and C
| Plot | Tick density per hour | % Nymphs questing in April | |
| Nymphs | Adults | ||
| A | 21.3 | 102.2 | 73.0 |
| B | 106.6 | 38.0 | 34.7 |
| C | 109.5 | 163.1 | 29.8 |
| Plot | Tick density per hour | % Nymphs questing in April | |
| Nymphs | Adults | ||
| A | 21.3 | 102.2 | 73.0 |
| B | 106.6 | 38.0 | 34.7 |
| C | 109.5 | 163.1 | 29.8 |
Density of questing Ixodes ricinus ticks in our study plots determined by flagging the vegetation, averaged across the season, and the rate of nymphs questing during the month of April. Plot A was inhabited by numerous lizards, whereas none were observed in the neighboring Plots B and C
| Plot | Tick density per hour | % Nymphs questing in April | |
| Nymphs | Adults | ||
| A | 21.3 | 102.2 | 73.0 |
| B | 106.6 | 38.0 | 34.7 |
| C | 109.5 | 163.1 | 29.8 |
| Plot | Tick density per hour | % Nymphs questing in April | |
| Nymphs | Adults | ||
| A | 21.3 | 102.2 | 73.0 |
| B | 106.6 | 38.0 | 34.7 |
| C | 109.5 | 163.1 | 29.8 |
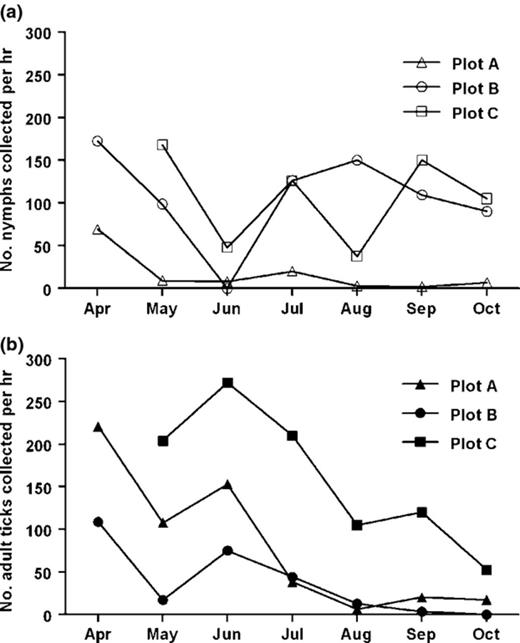
Seasonal activity of questing nymphal (a) and adult (b) Ixodes ricinus ticks in our study plots determined by flagging the vegetation. Plot A was inhabited by numerous lizards, whereas none were observed in the neighboring Plots B and C.
Discussion
The various genospecies of Lyme disease spirochetes appear not to be evenly distributed within our study site. Although our three study plots lie in close vicinity of each other in an area smaller than an acre, the ratio of particular genospecies infecting adult ticks varied significantly among them. Whereas B. lusitaniae predominated in questing adult ticks in Plot A, B. afzelii did so in the other plots. In contrast to Plots B and C, Plot A was inhabited by numerous lizards, considered to be the main reservoir host of B. lusitaniae (Dsouli et al., 2006; Majláthová et al., 2006; Richter & Matuschka, 2006a; Amore et al., 2007). Our observation that more than half of all infected adult ticks harbored this spirochete, suggests that at least this fraction of the nymphal population had completed their blood meal on lizards. Although host composition in a plot may vary between years, the rate of infected adults harboring B. lusitaniae did not differ significantly from that in the previous year (27 of 59 infected adults harbored B. lusitaniae in 2006 and 26 of 45 infected adults did so in 2007). In a site where lizards are exceedingly abundant, these vertebrates seem to constitute the dominant host for nymphal ticks, contributing the majority of infected adult ticks.
The relative density of questing nymphal and adult ticks appeared also to be spatially stratified in our study site. The presence or absence of lizards may similarly affect the relative abundance of tick stages questing there. In the lizard-inhabited Plot A, we found few nymphs questing and did so mainly when ticks first became active in early spring. At this time, few alternative hosts appeared to deplete the population of questing nymphs. Once lizards emerge from their hibernation and with their increasing activity throughout the spring and summer months, they may be the most readily available host in this plot. They appear to serve as hosts for at least a quarter of all questing nymphs, as inferred by the rate of infection with the lizard-associated B. lusitaniae in adult ticks. The relative abundance of questing tick stages may be influenced by various habitat conditions, such as available hosts and microclimate, and may vary between years.
Infection with a particular genospecies appears to be associated with the tick stage. Far more adult ticks harbored B. lusitaniae in our lizard plot than did nymphs, whereas B. afzelii was more likely to infect nymphal than adult ticks. This observation may also reflect the feeding preference of larval and nymphal ticks. Generally, larvae outnumber nymphs infesting rodents by a factor of 20 or more, whereas both stages appear to infest sand and wall lizards almost equally (Matuschka et al., 1991; Richter & Matuschka, 2006a; Amore et al., 2007). Larvae feeding on lizards sometimes fail to develop (Dsouli et al., 2006; nonpublished observation). This is paralleled by a similar observation for larvae feeding on pheasants (Kurtenbach et al., 1998). Sufficient larval ticks, however, appear to engorge successfully on lizards and acquire B. lusitaniae from them to subsequently transmit them in their nymphal stage to hitherto noninfected lizards. This together with these lizards' life expectancy ensures that three quarters of the lizard population in our study site are infected by B. lusitaniae (Richter & Matuschka, 2006a), thereby efficiently perpetuating this spirochete.
In contrast to B. lusitaniae, other Lyme disease genospecies are able to thrive in a wider host array. Borrelia burgdorferi s.s., for example, may be perpetuated in nature by both rodents and birds (Richter et al., 2000, 2004a). Whereas it is the sole Lyme disease genospecies in northeastern U.S., its prevalence in Central Europe is generally lower than that of other genospecies. We demonstrated experimentally that specialist spirochetes, such as B. afzelii, appear to be more readily transmitted between rodents and ticks and vice versa than are generalist spirochetes, such as B. burgdorferi s.s. (Richter et al., 2004a). Specialists appear to thrive efficiently where their closely associated host is abundant. One such specialist is B. lusitaniae (Richter & Matuschka, 2006a; Amore et al., 2007). Because lizards support solely B. lusitaniae and appear to exclude all other genospecies, their narrow genospecies association, and possibly also their life expectancy, result in predominance of B. lusitaniae in sites where lizards are abundant, while simultaneously limiting its spread to the habitat range of its host.
Because rodents and birds, serving as reservoir hosts for B. afzelii,B. burgdorferi and B. garinii, B. valaisiana, respectively, are ubiquitous, these spirochetes infect questing ticks throughout Northern and Central Europe. The genospecies composition varies somewhat with the preponderance of rodents or birds. In particular sites, however, the presence of hosts that are competent for B. spielmanii or B. lusitaniae profoundly affects the local composition of genospecies in questing ticks (Richter et al., 2004b; Richter & Matuschka, 2006a). Because lateral movement of questing Ixodes ricinus-like ticks rarely exceeds a few meters (Carroll & Schmidtmann, 1996), our observations in this study suggest that the presence of ticks infected with specialist spirochetes is restricted to the habitat range of its particular host animal. The specific habitat requirements of lizards, for example, sand and wall lizards, in Central European sites (Bischoff, 1984; Gruschwitz & Böhme, 1986) appear to result in a focal distribution of B. lusitaniae-infected ticks. Our observation that the genospecies composition significantly varies across distances as short as a few dozen meters suggests that the presence of hosts with a specific genospecies association impacts the composition of genospecies across a relatively small area. The distribution of particular Lyme disease genospecies is spatially stratified.
The proportion of B. lusitaniae relative to other spirochetes appears to vary in lizard-inhabited sites and may affect risk of infection. In Mediterranean sites, B. lusitaniae frequently constitutes the sole spirochete in questing vector ticks (De Michelis et al., 2000; Younsi et al., 2001; Sarih et al., 2003). But even in particular Central European sites, B. lusitaniae can largely displace other genospecies. In a German site, about 100 km south of our study site and inhabited by numerous sand lizards, B. lusitaniae infected more than two-thirds of the infected nymphal ticks and almost all infected adult ticks (nonpublished observation). Because lizards appear incompetent for all other Lyme disease spirochetes (Richter & Matuschka, 2006a), those appear to be displaced where lizards are the main hosts for vector ticks. Despite the high relative proportion of B. lusitaniae in particular Central Europe sites, it has not been associated with human cases in this region. Although two human cases apparently related to B. lusitaniae infection have been described in Portugal (Collares-Pereira et al., 2004; De Carvalho et al., 2008), they may be explained by variable pathogenicity, as has been observed for B. burgdorferi s.s. strains in Northern America (Wormser et al., 2008). An abundant tick host, which supports a nonpathogenic genospecies while excluding pathogenic genospecies, may enhance the proportion of its associated spirochete in the local tick population and may thereby act zooprophylactically. To the extent that Central European B. lusitaniae strains are nonpathogenic, the presence of numerous lizards should locally decrease risk of infection for people.
Our study demonstrates that ecological differences may induce spatial stratification in the relative prevalence of Lyme spirochete genospecies even on small spatial scales, in our case less than one acre. Although this study reports on observations in one site, it may serve to initiate more extensive studies on the stratification of the diverse Lyme disease genospecies in Central Europe. The genospecies stratification observed in our study site seemed to parallel a linear trend from a sun exposed to a shadowy location, from Plot A to Plot C, in accordance with the habitat choice of lizards. The diversity of bacterial communities infecting I. ricinus ticks varies between and even within particular forest habitat types (van Overbeek et al., 2008) and is likely associated with the composition and abundance of different tick hosts which in turn depend on the local habitat structure. In contrast, the non-Lyme disease spirochete, B. miyamotoi, which is vertically transmitted (Richter et al., 2012) and appears to be independent of reservoir hosts, is evenly distributed across our study site and thus may serve to demonstrate the uniformity of our sample. Concurrent and detailed characterization of local habitat conditions and host arrays may further strengthen subsequent studies on the stratifying associations of Lyme disease genospecies with particular ecological parameters. However, the comparison of abundances of tick hosts of different taxonomic groups, such as rodents, birds and lizards, is impeded by incommensurable trapping methods and may only be approximated.
The local abundance of tick hosts that support particular Lyme disease spirochetes appears to influence the genospecies mix. We suspect that the presence of zooprophylactic hosts, being incompetent for all genospecies, such as ruminants (Richter & Matuschka, 2006b, 2010, 2011), affects the overall prevalence of infected ticks on a similarly short distance. Therefore, evaluation of regional risk of infection by Lyme disease spirochetes should take the spatial effect of hosts into consideration, which stratify the distribution of specifically infected ticks on a small scale.
Acknowledgements
We thank Andrea Schäfer for excellent technical assistance. This study was partially supported by the Baden-Württemberg-Stiftung. None of the authors has a conflict of interest to declare.
References
Author notes
Landschaftsökologie, Department für Ökologie und Ökosystemmanagement, Technische Universität München, Freising, Germany
Lancaster Environment Centre, Lancaster University, Lancaster, UK
Editor: Julian Marchesi



