-
PDF
- Split View
-
Views
-
Cite
Cite
Emily Levin-Edens, Olusegun O. Soge, David No, Amy Stiffarm, J. Scott Meschke, Marilyn C. Roberts, Methicillin-resistant Staphylococcus aureus from Northwest marine and freshwater recreational beaches, FEMS Microbiology Ecology, Volume 79, Issue 2, February 2012, Pages 412–420, https://doi.org/10.1111/j.1574-6941.2011.01229.x
Close - Share Icon Share
Abstract
The aim of the study was to determine the spatial distribution of methicillin-resistant Staphylococcus aureus (MRSA) at two marine and one freshwater recreational beaches in the Seattle area. Fifty-six marine water, 144 freshwater, and 96 sand samples were collected from June through August 2010. Isolates were biochemically verified as MRSA. Staphylococcal cassette chromosome mec (SCCmec) typing, multilocus sequence typing (MLST), pulse field gel electrophoresis and the presence of other antibiotic resistance genes were determined. Twenty-two freshwater (15.3%; n = 144), one dry sand (1.9%; n = 53), six wet sand (14%; n = 43), and two marine water samples (3.6%; n = 56) were MRSA positive. Of the 27 freshwater stream sites sampled multiple times, 37% of the sites were positive for MRSA and/or S. aureus ≥ 2 times. Twenty-one (67.7%) of 31 MRSA were SCCmec type IV, 15 (48.4%) of the isolates had MLST types not previously associated with humans, and 29 (93.5%) of the isolates carried other antibiotic resistance genes. This study is the first to report and characterize repeated MRSA-positive samples from freshwater drainages and creeks surrounding popular recreational beaches.
Introduction
Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has become a major cause of skin and soft tissue infection in the general population, which do not have classic risk factors such as healthcare exposure (King et al., 2006) with the morbidity and mortality per 100 000 people estimated at 4.6 and 0.5, respectively (Klevens et al., 2007). Contact with seawater has been associated with a fourfold increase in the risk of S. aureus skin infections in children (Charoenca & Fujioka, 1995), suggesting that recreational marine beaches may be potential sources of MRSA exposure in the community. Several potential sources of S. aureus and MRSA contamination in the marine environment have been examined including shedding from recreational bathers, untreated wastewater, and urban runoff (Selvakumar & Borst, 2006; Elmir et al., 2007; Börjesson et al., 2009; Plano et al., 2011).
Over the past several years S. aureus and MRSA have been isolated from marine water, stream water, and intertidal sand samples from beaches in California (Goodwin & Pobuda, 2009), Florida (Abdelzaher et al., 2010), Hawaii (Tice et al., 2010; Viau et al., 2011), and the Pacific Northwest (Soge et al., 2009). MRSA spatial distribution or strain characterization was not addressed in the studies by Goodwin & Pobuda (2009) in California and Tice et al. (2010) in Hawaii. A more recent study sampled freshwater streams draining into coastal beaches on O'ahu Hawaii found that 63.6% of the stream water sites (n = 22) repeatedly sampled were positive for S. aureus (Viau et al., 2011). Temporal and spatial distribution of pathogenic microorganisms including S. aureus was examined in a Florida study (Abdelzaher et al., 2010); however, none of the presumptive S. aureus isolates were biochemically confirmed as S. aureus. The study by Soge et al. (2009) identified MRSA and/or S. aureus from six of 10 marine beaches from the Pacific Northwest; however, the number of samples taken was limited. That study found that the MRSA isolates were related to strains previously associated with hospital-acquired MRSA, and the S. aureus isolates were related to widespread clones.
In the current study, we conduct multiple sampling of two marine water beaches and one freshwater beach in the Seattle metro area to assess the spatial distribution of MRSA between June and August 2010. All MRSA isolates were molecularly characterized using SCCmec typing, pulse field gel electrophoresis (PFGE), and multilocus sequence typing (MLST) to determine the potential sources of the isolates.
Materials and methods
Site descriptions
Water and sand samples were collected from two marine water beaches (A and B) and one freshwater beach (C) in the Seattle WA area. Marine beach A is a public beach located in a 0.36-km2 park along the east side of the Puget Sound. The marine water on beach A is the receiving water for several upland drainage streams and a natural creek that traverse a steep slope boarding the beach to the west. At the top of this slope is an 8903-m2 off-lease dog park and immediately south of the beach is a full-service marina and boat launch. There are two combined sewer overflows (CSO) one 1.6 km south of beach A and one between Beach A and Beach B. Marine beach B, 3 km north of beach A, is also a public beach located in a 0.9-km2 park and is the receiving water for a natural stream, a county CSO, and a pump station. Despite the numerous potential point and nonpoint sources of fecal contamination, beaches A and B have been compliant with seasonal regulatory monitoring criteria for enterococci from 2004 to 2010 (WA Dept. of Ecology, 2011). Beach C is a freshwater beach located on the west side of Lake Washington in an 8903-m2 park. This beach is the receiving point in Lake Washington for a natural creek and a county CSO. Beach C intermittent high fecal coliform counts are believed to be caused by bacterial contamination from the natural creek. During summer 2010, beach C did not exceed seasonal regulatory monitoring criteria for fecal coliform counts (King County, 2011).
Sample collection
A total of 296 samples of marine water (n = 56), freshwater from the drainage and natural creeks at beaches A and B and Lake Washington water at beach C (n = 144), and sand samples (n = 96) were collected from June 24 to August 31, 2010 (Table 0001). The MRSA distribution over time was assessed by repeatedly sampling 30 sites over six sampling events, 19 sites over four sampling events, and nine sites over three sampling events from beach A, B and C, respectively (Table 0001, Fig. 0001). Differences in the number of sampling events at the three sites were because of additional convenient sampling at site A. For beaches A and B, marine water samples were taken at ankle level (5 cm) at regular intervals along the beach. Wet sand samples from the waterline and dry sand samples from the high tideline were collected along a vertical transect from the location of the marine water sample collection. The locations of the wet and dry samples varied based on the tideline; however, the location of the vertical transect was consistent between sampling events. Freshwater samples from the natural stream and various drainage points were also collected. From beach C, water samples were taken at 5-cm and 1-m-deep depths and sand was collected along a horizontal mid-beach transect. Water and sand samples were collected in sterile 1-L bottles and 50-mL tubes, respectively, stored at 4 °C and processed within 24 h of collection.
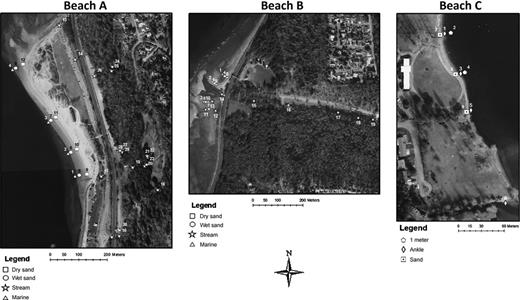
Freshwater, marine water and sand sampling locations and numeric designation of sites repeatedly surveyed between June and August, 2010 at beaches A, B, and C. Aerial photography courtesy of the City of Seattle (1993).
Sampling distribution of water and sand samples in beaches A, B, and C
| No. of sampling events | Water | Sand | Total | |||
| Marine | Fresh | Wet | Dry | |||
| Beach A | 6 | 38 | 87 | 24 | 27 | 176 |
| Beach B | 4 | 18 | 37 | 19 | 15 | 89 |
| Beach C | 3 | NA | 20 | 0 | 11 | 31 |
| Total | 13 | 56 | 144 | 43 | 53 | 296 |
| No. of sampling events | Water | Sand | Total | |||
| Marine | Fresh | Wet | Dry | |||
| Beach A | 6 | 38 | 87 | 24 | 27 | 176 |
| Beach B | 4 | 18 | 37 | 19 | 15 | 89 |
| Beach C | 3 | NA | 20 | 0 | 11 | 31 |
| Total | 13 | 56 | 144 | 43 | 53 | 296 |
NA, not applicable.
For beaches A and B, freshwater refers to samples collected from natural streams and drainage points. For beach C, freshwater refers to samples collected from Lake Washington.
Wet: sand collected along the waterline.
Dry: sand collected along the high tideline.
Sampling distribution of water and sand samples in beaches A, B, and C
| No. of sampling events | Water | Sand | Total | |||
| Marine | Fresh | Wet | Dry | |||
| Beach A | 6 | 38 | 87 | 24 | 27 | 176 |
| Beach B | 4 | 18 | 37 | 19 | 15 | 89 |
| Beach C | 3 | NA | 20 | 0 | 11 | 31 |
| Total | 13 | 56 | 144 | 43 | 53 | 296 |
| No. of sampling events | Water | Sand | Total | |||
| Marine | Fresh | Wet | Dry | |||
| Beach A | 6 | 38 | 87 | 24 | 27 | 176 |
| Beach B | 4 | 18 | 37 | 19 | 15 | 89 |
| Beach C | 3 | NA | 20 | 0 | 11 | 31 |
| Total | 13 | 56 | 144 | 43 | 53 | 296 |
NA, not applicable.
For beaches A and B, freshwater refers to samples collected from natural streams and drainage points. For beach C, freshwater refers to samples collected from Lake Washington.
Wet: sand collected along the waterline.
Dry: sand collected along the high tideline.
Sample processing
Prior to processing, marine and freshwater samples were vigorously shaken for 30 s, and a 25-mL sample was transferred to a sterile glass bottle. Water samples were diluted 1 : 1 with 1.5× Bacto® m Staphylococcus broth (Difco Laboratories, Becton Dickinson & Co, Sparks, MD), supplemented with a final concentration of 75 µg mL−1 polymyxin B (Sigma, St. Louis, MO) and 0.01% potassium tellurite (Sigma). For each sand sample, 10 g (wet weight) was transferred to a sterile 50-mL tube. Twenty-five milliliters of supplemented 1.0× m Staphylococcus broth was added to each of the sand samples, vigorously shaken for 2 min, and decanted into a sterile glass bottle. After initial processing, both water and sand broth mixtures were incubated at 36.5 °C with 5% CO2 until the samples became turbid with black precipitate indicating Staphylococcus spp. growth (Soge et al., 2009). Samples that were not turbid or did not have a black precipitate were considered negative for Staphylococcus spp. after 7 days. Sampling events on August 2 (marine water n = 4; freshwater n = 21) and August 30 (marine water n = 4; freshwater n = 18; dry sand n = 4; wet sand n = 4) for beach A and July 22 (marine water n = 3; freshwater n = 9; wet sand n = 4; dry sand n = 3) freshwater and for August 16 (marine water n = 3; freshwater n = 8; wet sand n = 4; dry sand n = 3) for beach B were quantified using the most-probable number (MPN) technique as previously described (Levin-Edens et al., 2011a).
Staphylococcusaureus and MRSA biochemical verification
Positive enrichments were serially diluted and plated onto mannitol salt agar (Difco) and incubated at 36.5 °C with 5% CO2 for 48 h. Yellow colonies from the mannitol salt agar were considered presumptive positive for S. aureus, and a maximum of 10 yellow colonies were streaked onto Brucella agar (Difco) supplemented with 5% sterile sheep blood to test for β-hemolysis. Positive isolates were coagulase tested using the Remel Staphaurex rapid latex kit (Thermo Fisher Scientific Remel Products, Lenexa, KS). Isolates that were both β-hemolytic and coagulase positive were confirmed S. aureus and subsequently streaked onto Bacto®Staphylococcus Medium 110 (Difco) supplemented with 0.01% potassium tellurite and 10 µg mL−1 methicillin (Sigma) and incubated at 36.5 °C with 5% CO2 for a maximum of 7 days to screen for MRSA. β-Hemolytic, coagulase-positive S. aureus that grew on Bacto®Staphylococcus Medium 110 supplemented with methicillin were considered presumptive MRSA, and the presence of the mecA gene was determined by PCR (see next section). For the sampling events that were quantitatively processed, presumptive MRSA isolates were isolated as previously described (Levin-Edens et al., 2011a).
PCR assay for mecA, SCCmec typing, MLST, and PFGE
All presumptive MRSA isolates were confirmed using a mecA PCR assay, as previously described (Soge et al., 2009). Isolates that were mecA negative were labeled S. aureus. SCCmec typing was done as previously described by multiplex PCR assay for types I–V using positive controls (Soge et al., 2009). Isolates that were negative were categorized as nontypeable (NT). MLST was determined with previously described primers and PCR conditions for seven housekeeping genes: arc, carbamate kinase; aro, shikimate dehydrogenase; glp, glycerol kinase; gmk, guanylate kinase; pta, phosphate acetyltransferase; tpi, triosephosphate isomerase; and ygi, acetyl coenzyme A acetyltransferase (Enright et al., 2000), and bi-directionally sequenced at the University of Washington High Throughput Sequencing Facility. For each locus, alleles were characterized based on comparison with previously described alleles in the S. aureus MLST database (www.mlst.net). New alleles were confirmed unique by bi-directionally re-sequencing three times and assigned a new identification number and new MLST type.
Nineteen selected isolates were analyzed by PFGE, and the 14 different PFGE patterns were labeled A–N as previously described (Soge et al., 2009). Isolates were considered to be identical if the banding patterns differed by ≤ 3 bands (Roberts et al., 2009). The percent similarity of each isolate analyzed by PFGE was compared against a clinical USA300 control strain to calculate the percent similarity using UPGMA method and DICE cluster analysis on GelCompar II software (Applied Maths, Inc., Austin, TX). PCR assays for tetracycline resistance genes, tet(M), tet(K), and macrolide resistance genes erm(A), erm(B) and erm(C), and msr(A), were performed as previously described (Soge et al., 2009). The presence of the aminoglycoside resistance gene aadD was determined by PCR assay as previously described (van Asselt et al., 1992).
Statistical analysis
The chi-squared test was used to test the strength of association between sample type and MRSA and was calculated in Stata11 I.C (StataCorp LP, College Station, TX). P < 0.05 was considered statistically significant.
Results
MRSA and S. aureus distribution and quantification
From the 296 water and sand samples collected, 31 (10.5%) samples were MRSA positive (Table 0002). For marine beaches A and B, freshwater refers to samples collected from natural streams and drainage points. The highest percentage of MRSA-positive samples (19.4%) was from the freshwater recreational beach, while the marine beaches ranged between 5.6% and 11.4%. Twenty-two (15.3%) of the 144 freshwater samples, one (1.9%) of the 53 dry sand, six (14%) of the 43 wet sand, and two (3.6%) of the 56 marine water samples were MRSA positive. MRSA was dependent on sample type, and freshwater and wet sand samples were the most frequently contaminated (P = 0.01). Twenty MRSA strains were isolated from beach A and were from sand (n = 4), marine water (n = 2), and freshwater (n = 14). All five of the MRSA isolates were isolated from freshwater samples from beach B. Six MRSA isolates were found from sand (n = 3) and from the fresh lake water (n = 3) from beach C. This study was focused on the identification of MRSA and as a result most likely underestimated the frequency and distribution of S. aureus in the three recreational beaches surveyed.
Overall distribution of MRSA and Staphylococcus aureus isolates by beach and sample type
MRSA isolates are mecA positive; S. aureus isolates are mecA negative.
Wet sand includes sand collected at the waterline at marine beaches A (n = 3) and sand collected from freshwater beach C (n = 3).
Freshwater refers to water collected from the streams and drainage points on beaches A and B and lake water collected from beach C.
Lower 95% confidence interval truncated at 0.0.
Overall distribution of MRSA and Staphylococcus aureus isolates by beach and sample type
MRSA isolates are mecA positive; S. aureus isolates are mecA negative.
Wet sand includes sand collected at the waterline at marine beaches A (n = 3) and sand collected from freshwater beach C (n = 3).
Freshwater refers to water collected from the streams and drainage points on beaches A and B and lake water collected from beach C.
Lower 95% confidence interval truncated at 0.0.
Ninety-two samples, 55 samples from beach A (August 2 and 30) and 37 samples from beach B (July 22 and August 16), were quantitatively processed. Six (6.5%) of the samples were positive for MRSA and as defined by a positive mecA PCR assay with the median MRSA MPN 100 mL−1 of 3.5 with a range between 2.0 and 66.2 MPN 100 mL−1.
Fourteen (4.7%) of the 296 samples were S. aureus positive, nine from marine beach A, and five from marine beach B. This included one (1.8%) S. aureus-positive marine water sample, one (1.9%) S. aureus-positive dry sand sample and 12 (8.3%) S. aureus-positive samples from the freshwater streams draining into the marine beaches. No S. aureus samples were identified from the freshwater beach C (Table 0002).
Sites repeatedly sampled for MRSA and/or S. aureus
There were 30 marine water, freshwater, and sand sampling sites on beach A, 19 marine water, freshwater, and sand sampling sites on beach B, and nine freshwater and sand sites from beach C that were repeatedly sampled during six, four and three sampling events, respectively. On beach A, two marine water sites (sites 2 and 4) and three sand sites (sites 6, 11 and 12) were MRSA or S. aureus positive once. There was no positive marine water or sand sites for beach B. In contrast, there were 27 freshwater stream sites samples repeatedly from marine beaches A and B of which 10 (37%) sites, six on beach A (sites 16, 17, 27, 28, 29 and 30) and four (sites 12, 14, 17, and 19) on beach B, were MRSA or MRSA/S. aureus positive between two and four different sampling events. Of the six freshwater sites and three sand sites repeatedly sampled at beach C, one sand site was MRSA positive twice (site 7) and three sites (sites 1, 5 and 9) were MRSA positive once over the four sampling events (Figs 0001 and 0002).
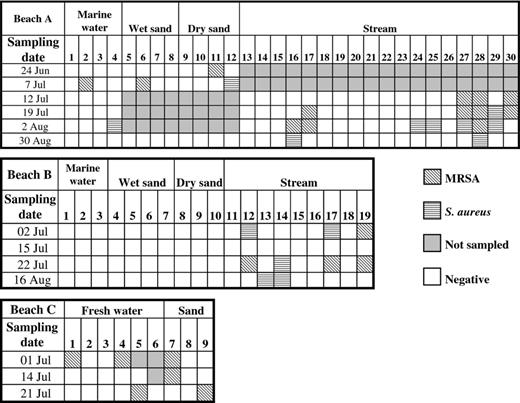
Distribution of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) isolates in beaches A, B, and C. Site numbering corresponds to Fig. 0001. Sites positive for MRSA/MSSA ≥ 2 days are sites 16, 17, 27, 28, 29, and 30 for beach A, sites 12, 14, 17, and 19 for beach B, and site 7 for beach C.
MRSA molecular characterization
Thirty-one MRSA isolates were characterized and included 21 isolates that were SCCmec type IV (67.7%), three were SCCmec type I (9.7%), one was SCCmec type II (3.2%), and six were NT (19.4%). All nine MRSA strains isolated from marine (n = 1) and sand (n = 8) samples were SCCmec type IV, while the 22 freshwater samples were a mixture of SCCmec type IV (n = 12), SCCmec type I (n = 3), SCCmec type II (n = 1), and SCCmec type NT (n = 6) (Table 0003). None of the SCCmec type IV isolates were related to USA300, the most common CA-MRSA strain associated with outbreaks in the USA, by PFGE (homologies < 50%; data not shown).
Genotypic characterization of 31 MRSA isolates collected during summer 2010
Isolates with the same SCCmec type, MLS and PFGE type were considered to be genetically related and based on these the 31 isolates represent 21 different strains.
ST5, ST6, ST8, ST15, ST30, ST45, ST88, ST97, ST109 have all been found in humans and animals; ST133 has been found in livestock; ST1956 has been found in squirrels; ST1875 and ST2049 are new MLST types isolated in the study.
Site number corresponds with Fig. 0001; NA, not applicable.
SCCmec type: I–V; NT, nontypeable. No SCCmec type IV related to USA300 (homology < 50%).
MLST type determined by the allelic profile of seven housekeeping genes arc, aro, glp, gmk, pta, tpi, and ygi.
Genotypic characterization of 31 MRSA isolates collected during summer 2010
Isolates with the same SCCmec type, MLS and PFGE type were considered to be genetically related and based on these the 31 isolates represent 21 different strains.
ST5, ST6, ST8, ST15, ST30, ST45, ST88, ST97, ST109 have all been found in humans and animals; ST133 has been found in livestock; ST1956 has been found in squirrels; ST1875 and ST2049 are new MLST types isolated in the study.
Site number corresponds with Fig. 0001; NA, not applicable.
SCCmec type: I–V; NT, nontypeable. No SCCmec type IV related to USA300 (homology < 50%).
MLST type determined by the allelic profile of seven housekeeping genes arc, aro, glp, gmk, pta, tpi, and ygi.
Fourteen different MLST types were identified including two new ST types, ST1875 and ST2049. Five of the ST types were found once (ST 8, 30, 45, 88, and 97), while ST133 was identified from five different freshwater samples from marine beach A (n = 4) and beach B (n = 1) and three freshwater samples and one sand sample from beach C (Table 0003). Eighteen (58.1%) of the isolates were ST types that had not been previously associated with humans including ST133 (n = 9) which have previously been isolated in cows, sheep, and goats (Smyth et al., 2009), ST1956 (n = 3) isolated from squirrels (www.mlst.net) and the new ST1875 (n = 4) and ST2049 (n = 2) (Table 0003).
Fourteen distinct PFGE patterns (A–N) were identified from the 19 isolates analyzed. Nine isolates represented four PFGE groups (patterns A, B, D, and F) with each group having the same ST type and identical or very similar combination of antibiotic resistance genes (Table 0003). Among the ST133 isolates, three isolates (125, 512, and 824) gave different PFGE patterns and carried a different number and combination of the antibiotic resistance genes. Isolates 909 and 910, isolated from different samples taken the same day, had indistinguishable PFGE patterns (D), were SCCmec IV and carried the same antibiotic resistance genes that were most likely genetically related. Isolates 244 and 248 carried SCCmec type IV, had a common PFGE pattern (A) and had similar but not identical antibiotic resistance genes and may be related to each other but not the other ST133 isolates in the study. The isolates 1112(1) and 252, both ST133 had indistinguishable PFGE pattern (B) but carried different SCCmec elements (I vs. IV) and different antibiotic resistance gene carriage patterns. Both isolates were from the freshwater samples isolated in beach A in August [1112(1)] and beach B in July [252]; however, their genetic relationship to each other is unclear.
MRSA isolates 308 and 361, isolated from marine water on beach A on the same date from different samples, had the same ST type, were SCCmec type IV, had a common PFGE pattern (F), and carried the same combination of other antibiotic resistance genes. Thus, they most likely represent a single strain. The isolate 302 collected from sand on beach A on the same date as isolates 308 and 361 was also ST109, SCCmec type IV and PFGE pattern F, but differed in some of the antibiotic resistance genes carried. These three isolates most likely are genetically related and represent a single clone.
In addition to ST133 and ST109, four MRSA isolates collected from beach A on two different dates were ST1875, SCCmec type IV, and carried identical or very similar antibiotic resistance genes. Isolates 1012 and PC3, isolated on different dates from beach C, were both SCCmec NT and ST6 with the same antibiotic resistance gene combination. Two MRSA isolates collected from beach B on the same date from two different freshwater sites carried the new MSLT ST2049 and SCCmec NT and may be genetically related. One freshwater sample from beach A had two different MRSA strains isolated, 1112(1) and 1112(2), which differed in all measured characteristics and were genetically unrelated (Table 0003).
Twenty-nine (93.5%) of the MRSA strains carried ≥ 1 of the other antibiotic resistance genes with 24 (77.4%) carrying one or both of the tetracycline resistance genes [tet(K), tet(M)], 28 (90.3%) carrying ≥ 1 of the macrolide resistance genes [erm(A), erm(C), msr(A)], and 29 (93.5%) carrying the aminoglycoside resistance gene (aadD) (Table 0003).
Discussion
In the current study, 31 MRSA isolates were characterized with the majority (71%) isolated from the freshwater drainages and creeks surrounding marine beach A and B and/or freshwater beach C. The concentration of MRSA in the positive samples ranged from 2.0 to 66.2 MPN 100 mL−1; however, the level of risk to beach visitors at these levels is unknown. In addition, 37% of the freshwater sites at beach A and B sampled repeatedly were positive for MRSA and/or MRSA/S. aureus ≥ 2 times. There was no clear indication why these sites were consistently positive compared to other freshwater sites that were positive once for MRSA and S. aureus negative. Freshwater drainages sites were not sampled in our previous study (Soge et al., 2009) or in most other studies making it difficult to directly compare the current study to these studies (Goodwin & Pobuda, 2009; Abdelzaher et al., 2010; Tice et al., 2010). The recent study by Viau et al. (2011) repeatedly surveyed freshwater drainage sites from O'ahu and found that 14 (64%) of 22 streams were positive for S. aureus at least twice but only one (4.5%) of the 22 sites was MRSA positive from the December sampling. Differences in MRSA detection frequency between the Viau et al., 2011 study and the current study may be due to differences in sampling frequency, isolation methodology, and/or extrinsic factors such as water and air temperature differences found between Hawaii and Washington State.
Based on the combination of the SCCmec type, MLST type, and PFGE pattern, the 31 MRSA isolates most likely represent 21 different strains. Some of the related isolates from a single strain were isolated from different samples on the same day, collected on different days and/or identified on different beaches, suggesting that certain strains persisted in this environment. These results support our sterile laboratory microcosm studies which found that MRSA had a 1-log drop at 5–10 days in both marine and freshwater at 13 °C (Levin-Edens et al., 2011b). It is also possible that some of these strains, such as ST133, are widely distributed because they are associated with the wildlife found in this urban environment.
In the current study, MRSA isolates were more often (67.7%) SCCmec IV, while in our previous study, 83.3% of the isolates were SCCmec type I (Soge et al., 2009). This difference could be due to small sample sizes in the first study, changes to the community/environmental MRSA between 2008 and 2010, or the addition of freshwater samples in the current study which yield the majority (71%) of the MRSA isolates in the current study. The high prevalence of SCCmec type IV was also found in a recent study that found the SCCmec type IV predominated among MRSA from untreated wastewater (Börjesson et al., 2009). MRSA isolates from other recreational beaches studies have not been molecularly characterized, and it is therefore difficult to know how the MRSA strains in the current study compare to other locations such as California and Hawaii (Goodwin & Pobuda, 2009; Tice et al., 2010) where the beach environment is significantly warmer than in the Washington State. Characterization of MRSA from recreational beach environments from a variety of geographical locations is needed to verify whether the MRSA distribution and strains found in the Pacific Northwest is representative of the broader environment.
Two novel MLST types ST1875 and ST2049 were identified from samples taken from freshwater drainage and creek samples at beaches A and B. Including the novel ST types, > 50% of the isolates have not been previously associated with humans (Smyth et al., 2009; www.mlst.net). It was of interest that the most common ST type was ST133 which have been previously associated with cows, sheep and goats, animals not normally part of the Seattle urban environment. However, it is possible that MRSA ST133 is adaptive to wildlife such as squirrels or seagulls and/or with other unknown environmental reservoirs. Three isolates with ST1959 were taken from beach A and have been previously found in squirrels from the UK, and all three beaches have indigenous squirrel populations. The remaining isolates have been previously found in humans (ST109), humans and animals (ST15, ST45, ST88, ST97), or humans and meat products (ST5, ST6, ST8, ST30) (Armand-Lefevre et al., 2005; Collery et al., 2008; Boyl-Vavra & Daum, 2010; Cuny et al., 2010; Deleo et al., 2010; Graveland et al., 2010; Hata et al., 2010; Kawaguchiya et al., 2011; Lin et al., 2011; Waters et al., 2011).
This study found MRSA most often contaminating samples from freshwater streams running into the marine beaches where we frequently observed children playing during sampling and at the freshwater beach on Lake Washington. The diversity of MRSA isolates found in this study concurs with the previous hypothesis that MRSA is increasingly disseminated throughout the environment via multiple potential sources including humans and wildlife (Seifried et al., 2007).
This study is the first to isolate and molecularly characterize repeated MRSA-positive samples from freshwater drainages and creeks surrounding popular recreational marine beaches and from a freshwater recreational beach. Approximately half of the MRSA strains from the study have MLST types traditionally associated with humans and the remaining associated with animal sources suggesting multiple reservoirs may be important in the Pacific Northwest. The distribution of MLST types may have important implications for recreational beach visitors in colder climates. Further studies are needed to determine the source of MRSA contamination and potential differences in reservoirs for MRSA in colder vs. warmer climates for both marine and freshwater beaches.
Acknowledgements
The work was funded in part by NIH National Institute of Environmental Health and Science (NIEHS) 5 R25 ES016150-03 REVISED. A.S. was a UW STAR Program. NHLBI- Grant # 1 R25HL103180-01.
References
Author notes
Editor: Kornelia Smalla



