-
PDF
- Split View
-
Views
-
Cite
Cite
Sam Bretherton, George M. Tordoff, T. Hefin Jones, Lynne Boddy, Compensatory growth of Phanerochaete velutina mycelial systems grazed by Folsomia candida (Collembola), FEMS Microbiology Ecology, Volume 58, Issue 1, October 2006, Pages 33–40, https://doi.org/10.1111/j.1574-6941.2006.00149.x
Close - Share Icon Share
Abstract
Phanerochaete velutina is a major agent of wood decomposition in temperate forests. It grows out of woody resources in search of other resources and is then vulnerable to grazing by invertebrates. The aim of this study was to determine how continuous grazing and grazing for only 2 days by different densities of collembola, Folsomia candida, affect mycelial development (radial extension, hyphal coverage and fractal dimension) of P. velutina growing across non-sterile soil. High density (80 collembola) continuous grazing resulted in different mycelial foraging patterns compared to controls and lower density (20 and 40 collembola) continuous grazing: radial extension rate was reduced from 8.4 mm day−1 (control) to 6.9 mm day−1 (80 collembola), hyphal coverage was reduced to 81% of controls and mass fractal dimension increased from 1.68 (control) to 1.72 (80 collembola). There was evidence of over-compensatory growth: when high density grazing ceased the new growth was considerably greater (38%) than in controls. Grazing also resulted in growth stimulation: at low density continuous grazing (20 collembola) hyphal coverage was 15.6% greater than in controls. The ecological implications of compensatory and stimulatory growth in fungal-invertebrate interactions are considered.
Introduction
Mycelia of many fungal species that decompose wood and leaf litter are able to grow out of one resource in search of others. In doing so they may cross territory that is inhospitable in terms of available nutrients and microclimate, encounter antagonistic microorganisms and be grazed upon by invertebrates, including collembola, flies, nematodes and, in some localities, termites (Maraun, 2003). Grazing on fungal mycelia can result in marked changes in mycelial morphology (Hedlund, 1991;Kampichler, 2004;Harold, 2005;Tordoff, 2006) and enzyme production (Hedlund, 1991; Dyer, 1992), and increases in fungal respiratory activity (Hanlon & Anderson, 1979;Hanlon, 1981;Visser, 1981;Bengtsson & Rundgren, 1983;Bengtsson, 1993). In some cases greater growth (termed compensatory or ‘catch-up’ growth) has occurred following grazing, e.g. with the zygomycete Mortierella isabellina and the ascomycetes Penicillium spinulosum and Verticillium bulbillosum (Bengtsson & Rundgren, 1983; Bengtsson, 1993). Compensatory growth is a well-studied phenomenon across a wide range of macroorganisms, including herbivores grazing on plants and vertebrates whose initial growth has been slow, for example due to suboptimal time of birth, poor prenatal or early post-natal nutrition (Trlica & Rittenhouse 1993; Agrawal, 2000; Metcalfe & Monaghan, 2001). Essentially, the organism experiences retarded growth and then enters a phase of accelerated growth once conditions improve. The present study investigates possible compensatory growth of Phanerochaete velutina following removal of grazing pressure.
There have been a great many studies on the effects of grazing on microfungi and mycorrhizal-formers (e.g. Finlay, 1985;McGonigle, 1995;Gange, 2000;Maraun, 2003), but until recently few studies have centred on saprotrophic cord-forming basidiomycetes (Dyer, 1992;Kampichler, 2004;Harold, 2005;Tordoff, 2006). This is surprising considering the crucial central role that these fungi play in decomposition processes and nutrient cycling (Boddy, 1993; Boddy & Watkinson, 1994). Existing studies on saprotrophic basidiomycetes show that continuous collembola grazing can result in dramatic changes to mycelial morphology and foraging patterns, and the extent of these changes depends upon species of fungi, inoculum resource status, grazing intensity (density) and collembola species (Kampichler, 2004;Harold, 2005;Tordoff, 2006). Further, selective grazing can influence the vertical distribution of fungi in soil (Newell 1984a, b). In the field, grazing pressure will vary in time and space, but little if anything is known about the behaviour of mycelia following removal of grazing pressure (i.e. resilience). This paper investigates: (1) the density dependent effects of grazing by the collembola Folsomia candida on mycelia of the cord-forming basidiomycete P. velutina in soil microcosms in the laboratory; and (2) recovery of the mycelium when grazing ceases (i.e. when collembola are removed). Previous studies (Kampichler, 2004; Tordoff, 2006) suggest that heavy grazing will have the greatest effects in terms of reduction in hyphal coverage and changes to mycelial morphology (fractal dimension). It is hypothesized that high intensity grazing will reduce radial extension rate and hyphal coverage, and that when grazing stops, heavily grazed mycelia will take longer to recover than lightly grazed mycelia.
Materials and methods
Fungal isolate and inoculum preparation
Phanerochaete velutina (Cardiff University culture collection; Dowson, 1986) was subcultured on 2% malt extract agar (MEA: 20 g L−1 Munton & Fison spray malt, 15 g L−1 Lab M agar no. 2). Petri dishes (14 cm diameter) were colonized with P. velutina for 12 days before 15 beech (Fagus sylvatica) wood blocks (2 × 2 × 1 cm) were added, and incubated for 3 months in the dark at 20°C. The wood blocks had been cut from a freshly-felled tree and stored at −18°C until required. Prior to use, the blocks were soaked overnight in deionized water to defrost and autoclaved at 121°C for 20 min in sealed autoclave bags, three times at 24 h intervals.
Culturing and extraction of F. candida
Folsomia candida (supplied by Centre for Ecology and Hydrology, Lancaster, UK) were reared on a 90% plaster of Paris (Minerva Dental Ltd, Cardiff, UK): 10% charcoal (Sigma, UK) substrate in 0.9 L plastic containers, at room temperature. Culture boxes had holes in the lid to provide aeration and collembola were fed weekly with dried baker's yeast (Saccharomyces cerevisiae; Spice of Life, Cardiff, UK) and the substrate kept moist with deionized water.
Collembola were extracted from cultures using a series of stacked metal sieves (Nickel-Electro Ltd., Weston-super-Mare, UK) of progressively smaller pore size. Those of the required size (250–400 μm) were transferred to new culture boxes and starved for 24 h prior to use in the experiments. Individuals were collected for introduction into each microcosm using an electrical aspirator (pooter).
Preparation of soil microcosms
Soil (0–20 cm depth) was collected from mixed deciduous woodland in the Coed Beddick Inclosure, Tintern, UK. Wood and leaf litter were removed and the soil was sieved through a 10 mm mesh. The soil was air-dried for 14 days, sieved firstly through ≤4 mm mesh then through ≤2 mm mesh, and frozen (−18°C) for 24 h to defaunate. Soil was then thoroughly mixed with an appropriate volume of de-ionized water to achieve a matric potential of −0.012 MPa. Wet soil (200 g) was added to 24 × 24 cm lidded bioassay trays (Nunc-Gibco, Paisley, UK), and compacted evenly and smoothly to approximately 5 mm depth.
Soil trays (n=70) were inoculated by positioning a colonized wood block, which had been first scraped free of adhering agar and mycelium using a scalpel, at the centre of each tray. The weight of each soil tray, including inoculum, was recorded; every 7 days, trays were re-weighed and re-moistened by evenly spraying a mist of deionized water onto uncolonized regions of soil. Trays were kept individually in sealed polythene bags (to reduce moisture loss and prevent collembola escaping) and stacked randomly in the dark at 20±1°C.
Experimental design
Seven treatments with 10 replicates each were carried out, each treatment assigned randomly amongst the 70 trays: ungrazed control; 20, 40 or 80 collembola added at 10 days (by which time mycelia were at least 8 cm diameter) and allowed to continue grazing for the duration of the experiment; 20, 40 or 80 collembola added at 10 days and then removed 2 days later. Collembola were removed using an electrical aspirator taking care not to damage the mycelium. Although 80 collembola per tray represents only 1566 m−2 soil, which is less than usual field densities (104–105 m−2) (Petersen & Luxton, 1982), this is appropriate since the collembola are restricted to two dimensions in the microcosms, whereas the field figures quoted are for soil cores of three dimensions.
Image capture and analysis
Digital images of experimental systems were captured immediately prior to collembola addition and then after 2, 3, 4, 5, 7, 9 and 12 days, with a Sony Cyber Shot digital still camera positioned 60 cm above the microcosm, with natural lighting. Saved JPEG images were processed using IMAGEJ 1.33u software (National Institute of Health, USA) in a Microsoft Windows XP environment. Tray margins and wood inocula were electronically removed from all digital images prior to conversion to greyscale (8-bit). Images were then subject to manual thresholding: any pixels with a grey value less than the threshold were converted to black to represent soil, and pixels with values greater than the threshold were converted to white, representing mycelium. Radial extension measurements were obtained by electronically measuring eight lines radiating at 45° angles from the position of the wood block inocula to the mycelial margin. The pixel length of a 22.6 cm line (the internal length of the side of a bioassay tray) was used for calibration. Determination of radial extent ceased when mycelia reached the edges of the trays. Hyphal coverage was determined as the number of white pixels in a binary image, converted by IMAGEJ to cm2. Fractal dimension provides a good measure of space filling. Phanerochaete velutina is approximately mass fractal (i.e. the whole mycelium, not just the border, is fractal; Boddy, 1999) and hence only the mass fractal dimension (DBM) was determined in IMAGEJ by the box-counting method, using the Fractional Dimension and Lacunarity Plug-in.
As well as analysis of each entire image, images at 12 days after collembola addition were divided electronically into two parts – the area bounded by the mycelial front at 2 days (i.e. when collembola were removed from 30 trays), and that between the line of this front and the mycelial front at 12 days (Fig. 1). Hyphal coverage was determined for the outer zone.
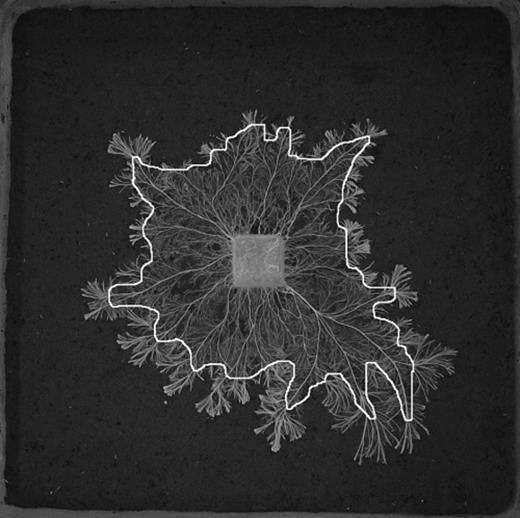
A binary image of a mycelial system of Phanerochaete velutina grazed for 12 days. Line indicates the position of the mycelial front after 2 days of grazing. Hyphal coverage was determined for the entire system and separately for the outer zone that had developed between 2 and 12 days.
Statistical analyses
Radial extension, hyphal coverage and DBM were analyzed by one-way Repeated Measures Analysis of Variance (rmanova; SPSS, release 12), using grazing treatments as the main effect and time as a sub-factor. Data were normally distributed (Kolmogorov-Smirnov test), had equal variance (Levene's test) and displayed sphericity (Mauchly's test of sphericity), and therefore met assumptions for rmanova. Significant results were explored further using the Tukey's pairwise comparison to determine significant differences between means at individual time points. Data are presented with standard error of the mean.
Results
Grazing for 2 days caused dramatic differences in mycelial morphology, especially in the high density (80 collembola) grazing treatment, where mycelium was denser in central regions and there was prolific fanning at the mycelial margin (Fig. 2). Grazing was largely at the mycelial margin and, to a lesser extent, close to the inoculum. When grazing pressure was removed from these systems, subsequent growth looked similar to the control, but was sometimes quantitatively different (see below).
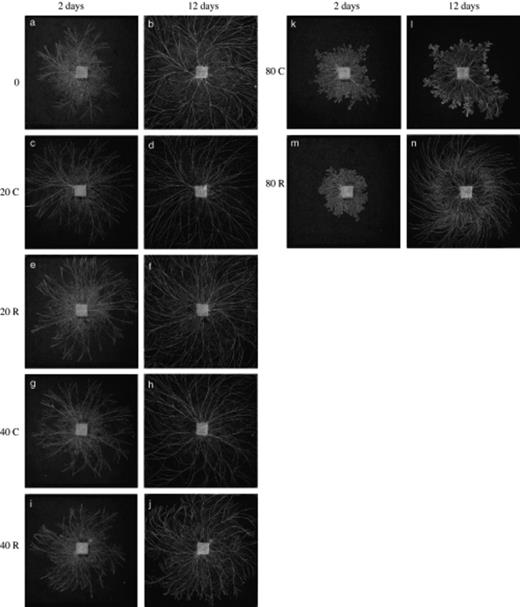
Digital images of mycelial systems of Phanerochaete velutina (in 24 × 24 cm trays of compressed non-sterile soil) that had been ungrazed (a, b), continuously grazed (C: c, d, g, h, k, l) or grazed for 2 days then grazing pressure removed (R: e, f, i, j, m, n). Images captured 2 days (a, c, e, g, i, k, m) and 12 days (b, d, f, h, j, l, n) after adding collembola (time of image capture indicated at head of column of images). Collembola were added at different densities (indicated in left margin): 0 (a, b), 20 (c–f), 40 (g–j) or 80 (k–n) per tray.
Radial extension
Grazing did not significantly (P>0.05) affect extension rate relative to ungrazed systems (Fig. 3). The extension rate of mycelia continuously grazed by 40 or 80 collembola was less than in those that were only grazed for 2 days, though not significantly (P>0.05). The extension rate of systems grazed continuously by 20 collembola was, however, significantly (P≤0.05) faster than in systems only grazed for 2 days.
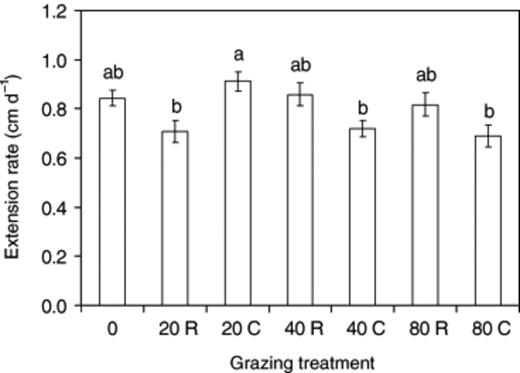
Extension rate of Phanerochaete velutina mycelium subject to different grazing intensity (0, 20, 40, 80 collembola per tray). Grazing was for only 2 days in half of the treatments (indicated by R) or continued (indicated by C) for the duration of the experiment (12 days). Error bars are the standard error of the mean. Bars with the same letter are not significantly (P>0.05) different.
Hyphal coverage
In all treatments, hyphal coverage increased linearly until 5 days after collembola addition (when sides of trays were reached) and then did not rise (Fig. 4a–c). By 12 days after collembola addition there was, however, an increase in hyphal coverage in systems continuously grazed by 20 collembola and systems which had been grazed by 80 collembola for 2 days. Hyphal coverage was similar for all treatments except for that continuously grazed for 80 days, which was significantly (P=0.02) lower.
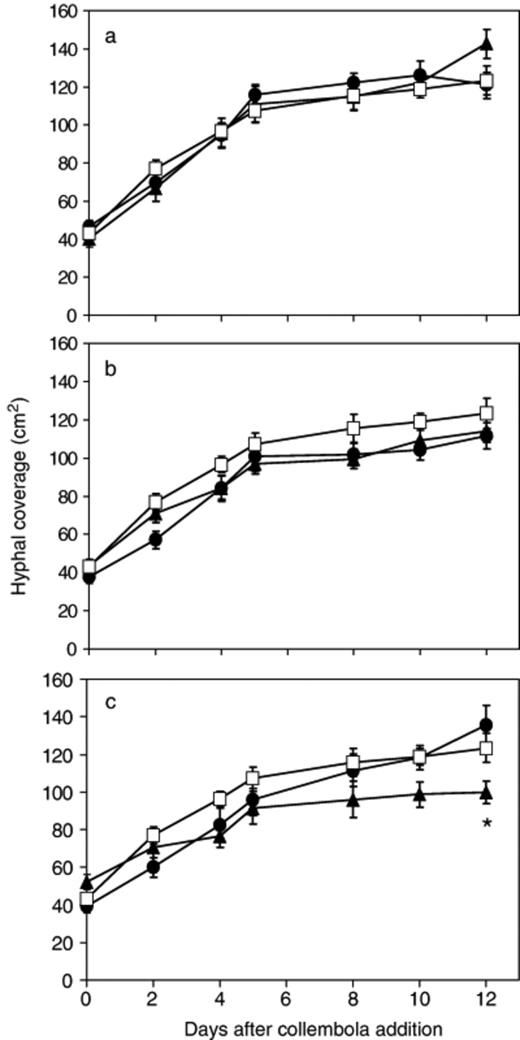
Change in Phanerochaete velutina hyphal coverage with time in mycelial systems grazed by 20 (a), 40 (b) or 80 (c) collembola compared with ungrazed systems. ◻ ungrazed; ▲ continuously grazed; ● grazed for 2 days then grazing ceased. Repeated measures anova revealed a significant (F36,378=2.871, P<0.001) time × treatment interaction, but no overall treatment effect (i.e. irrespective of time) (F6,63=1.897, P=0.095). *Significant difference (P=0.002) between continuously grazed and grazed for 2 days.
Hyphal coverage in the area which developed after 2 days was significantly (P≤0.05) less in systems continuously grazed by 80 collembola than in all other systems (Fig. 5). In systems which had been grazed for 2 days by 80 collembola or continuously grazed by 20 collembola, hyphal coverage was significantly (P≤0.05) greater than in ungrazed controls. Other grazing treatments were not significantly (P>0.05) different from ungrazed controls.
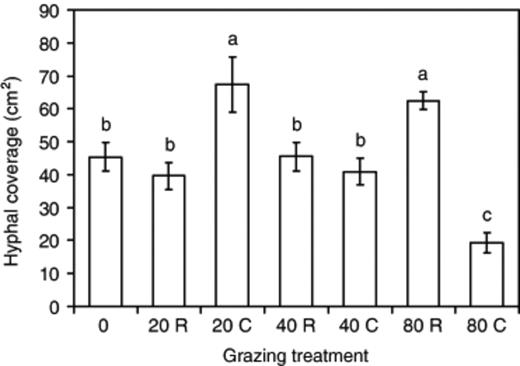
Hyphal coverage of Phanerochaete velutina mycelium subject to different grazing intensity (0, 20, 40, 80 collembola per tray) in the zone that developed after grazing (see Fig. 1). Grazing was for only 2 days in half of the treatments (indicated by R) or continued (indicated by C) for 12 days until the end of the experiment. Error bars are the standard error of the mean. Bars with the same letter are not significantly (P>0.05) different.
Mass fractal dimension
DBM was at a maximum at about 5 days and then decreased (Fig. 6). Systems that were only grazed for 2 days were not significantly (P>0.05) different from ungrazed controls. Continuously grazed systems had consistently higher DBM than other systems, though this was not significantly (P>0.05) different.
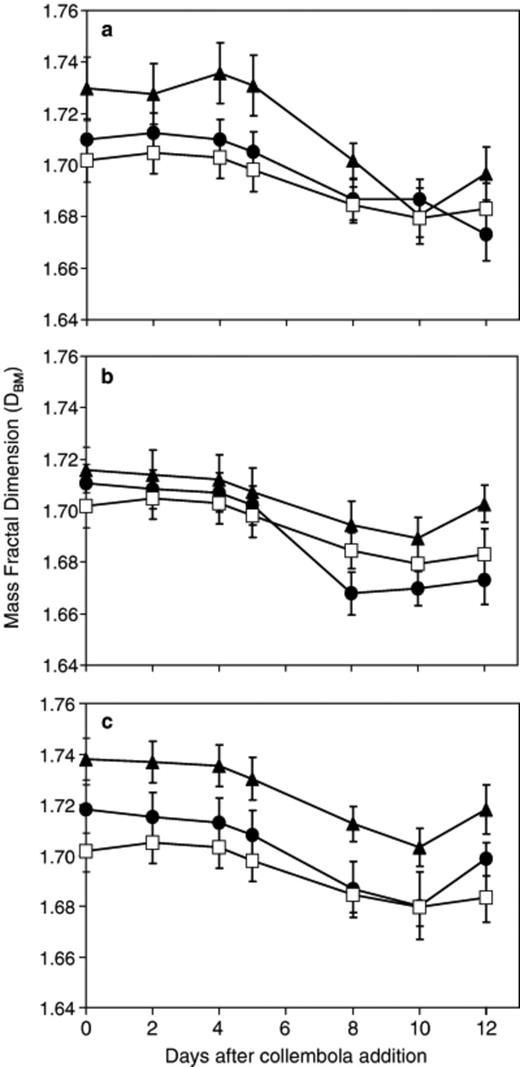
Change in Phenerochaete velutina mass fractal dimension (DBM) with time in mycelial systems grazed by 20 (a), 40 (b) or 80 (c) collembola compared with ungrazed. ◻ ungrazed; ▲ continuously grazed; ● grazed for 2 days then grazing ceased. Repeated measures anova revealed no significant (F36,378=0.851, P=0.716) time × treatment interaction, but a significant (F6,63=2.884, P=0.015) overall treatment effect (i.e. irrespective of time).
Discussion
High intensity grazing (at least in the context of this experiment) dramatically affected fractal dimension, hyphal coverage and overall morphology, but not the extension rate, of mycelium of the cord-forming basidiomycete P. velutina, only partly confirming the hypothesis that high intensity grazing will reduce radial extension rate and hyphal coverage. This study did not support earlier work which found that the extension rate of P. velutina was dependent on the density of grazing F. candida, and that there was no effect on hyphal coverage (Tordoff, 2006). This may have been due to the use of smaller trays in the earlier study. Grazing intensity was also important in systems of Hypholoma fasciculare and Resinicium bicolor (Tordoff, 2006).
The second hypothesis, that heavily grazed systems will take longer to recover than those grazed more lightly, was rejected. Mycelial systems rapidly recovered from 2 days high intensity grazing, and hyphal coverage, in the region of mycelium that developed after removal of grazers, was greater in the high intensity grazing (80 collembola) systems than in controls. Equally significantly, systems subject to low intensity (20 collembola) continuous grazing exhibited greater hyphal coverage (compared to ungrazed controls) in the mycelial area that developed following addition of collembola, i.e. compensatory growth. Often compensatory growth is incomplete, with deprived organisms being unable to reach the size of unaffected controls but over-compensation, such as that in the present study, does sometimes occur, with deprived/stressed individuals becoming larger than controls (Detling & Painter, 1983; Hayward, 1997). Determining a mechanism by which a heavily grazed organism will eventually grow larger than ungrazed ones is difficult (McNaughton, 1983;Paige, 1992;Trumble, 1993;Callaway, 2001). For plants it has been suggested that removal of apical dominance and/or growth promoters produced by the herbivores may be a means by which plants benefit from grazing, producing, for example, multiple flowering stalks (Paige & Whitham, 1987;Paige, 1992;Dyer, 1995;Tiffin, 2000). Similar increased branching occurred in the present study. A further compensatory mechanism in plants results from increased photosynthesis, possibly due to increased light penetration in grazed areas resulting in decreased ‘competition’ by leaves for light, and also decreased competition for water and nutrients (McNaughton, 1983; Dyer, 1995). Decreased competition for water and nutrients may also apply to the grazed mycelia in the microcosms.
Stimulatory effects of grazing have never been reported for saprotrophic basidiomycetes (Kampichler, 2004;Harold, 2005;Tordoff, 2006). Indeed, in previous studies continuous grazing has resulted in decreases in hyphal coverage and radial extension rates rather than the increased hyphal coverage observed with the low density grazing (20 collembola) in the present study. Why growth of P. velutina should be stimulated in this study and not in the previous study with similar grazing pressure (Tordoff, 2006) is not clear, but it may relate to differences in the size of the soil trays used, which were smaller previously.
Compensatory growth of plants often carries a cost. For example, storage reserves may be utilized, and growth of replacement tissues may be at the expense of other growth and metabolic centres, or of reproduction (Tiffin, 2000; Trumble et al., 2003; Pratt, 2005). Such costs can be paid over a range of time scales (Metcalfe & Monaghan, 2001). Mycelia could operate a trade-off between extension rate of mycelia and hyphal coverage, though there is no evidence of that in this study. Here the trade-off for increased coverage is probably the more rapid use of the wood inoculum reducing the length of time that the fungus could utilize the resource.
A variety of evolutionary responses to herbivory have been noted for plants, including resistance, mutualism, over-compensation, phenological escape and tolerance (Agrawal, 2000). Of these, resistance, phenological escape and tolerance are all defensive mechanisms that increase fitness by reducing grazing. With fungi, resistance to grazing could be effected by the production of allelopathic chemicals on the surface of individual hyphae and cords, or as volatiles or secondary metabolites produced when hyphae are damaged. Mycelial cords of some basidiomycetes have crystals, especially of calcium oxalate, on their surface, and these may inhibit invertebrate grazing (Connolly & Jellison, 1995). Phenological escape (i.e. plants not being available when herbivores are most active) may have an analogue with fungi: although basidiomycete cord-formers produce extensive perennial systems (Boddy, 1993; Cairney, 2005), they are dynamic with new outgrowth from extending foraging fronts and from newly colonized organic resources (Cairney, 2005). There is evidence that during winter months, mycelium of P. velutina not interconnecting with resources dies back and new growth from a resource only then occurs when conditions improve (L. Boddy, R. G. Bolton & S. H. Abdallah, pers. commun.). If renewed outgrowth were timed to coincide with times of reduced grazer activity, this might be considered phenological escape. Tolerance implies little or no reduction in fitness as a result of grazing; in the present study, hyphal coverage of systems grazed by 40 collembola was not significantly different from that of ungrazed controls.
Grazing resulting in stimulation/over-compensation of mycelial growth, as seen in the present study, or collembola recycling nutrients through production of faeces, and removal of competing microbes from the soil surface may be regarded as mutualism. Increased mycelial growth, however, should only be regarded as beneficial if more resources are obtained to compensate for the more rapid use of the organic resource from which the fungus is obtaining its carbon and mineral nutrients for mycelial extension. Since compensatory growth is presumably largely fed by decomposition of organic resources interconnected to the mycelium in soil, it is likely that mycelia will compensate to different extents depending on the quality and quantity of resources available, and the relative size of the mycelium. Similarly, in plants utilization of stored reserves is likely to be an important mechanism of tolerance to grazing, and some studies have shown a positive correlation between root-shoot ratios and regrowth following defoliation (Tiffin, 2000).
Having found evidence of dramatic density dependent grazing effects in fungi, including compensatory and stimulatory growth, we now need to know (1) how widespread this phenomenon is in fungi, (2) whether other types of mechanical damage have similar effects to invertebrate grazing, and (3) whether grazing and mechanical damage affect mycelia in the field to the same extent as in the laboratory.
Acknowledgements
We thank the Natural Environmental Research Council for provision of a studentship (GMT).
References
Author notes
Editor: Jim Prosser


