-
PDF
- Split View
-
Views
-
Cite
Cite
Michiei Sho, Chantal Hamel, Charles W. Greer, Two distinct gene clusters encode pyrene degradation in Mycobacterium sp. strain S65, FEMS Microbiology Ecology, Volume 48, Issue 2, May 2004, Pages 209–220, https://doi.org/10.1016/j.femsec.2004.01.011
Close - Share Icon Share
Abstract
A pyrene degrading bacterium, identified as Mycobacterium sp. strain S65, was isolated from a jet-fuel contaminated site in Quebec, Canada. Strain S65 utilized pyrene, phenanthrene, and fluoranthene as sole carbon and energy sources, but did not mineralize naphthalene, anthracene, or fluorene. Pyrene mineralization was enhanced by adding benz[a]anthracene, benzo[a]pyrene, or phenanthrene as co-substrates. Southern hybridization using the naphthalene inducible pyrene dioxygenase gene (nidA), from Mycobacterium vanbaalenii strain PYR-1, indicated that nidA homologues were found in two separate loci in strain S65. Each locus encoded two large subunit ring-hydroxylating dioxygenase genes (designated pdoA/X, and nidA/X), two alcohol dehydrogenase genes (designated pdoC/H and nidC/H) and one unknown orf (orfP6 and orfN4). The pdo locus also includes pdoB, which was predicted to function as a small subunit ring-hydroxylating dioxygenase gene. RT-PCR analyses showed that both nidA homologues, which were 99% and 89% identical to nidA from PYR-1, as well as the other genes in the two clusters, were induced during growth on pyrene and phenanthrene but not on glucose as sole carbon and energy source. The pdo locus also encoded for an IS3 like transposase upstream of pdoB. This transposase has a stop codon within its coding sequence indicating that it has lost functionality. Its' presence, however, suggests that there was a duplication of pyrene degrading genes within the genome. The presence of two separate pyrene degradation gene clusters in strain S65 may provide opportunities to explore how bacteria develop the abilities to degrade high-molecular-weight polycyclic aromatic hydrocarbons.
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous organic pollutants from anthropological sources such as fossil fuel refining, timber products processing, iron and steel manufacturing, textile mills, and vehicle exhausts, and from natural sources such as forest fires and volcanoes. PAHs with three fused-benzene rings or less are considered low-molecular-weight (LMW) PAHs, and those with four or more fused-benzene rings are high-molecular-weight (HMW) PAHs. Polycyclic aromatic hydrocarbons are considered as human health hazards because some of them possess mutagenic, genotoxic, and/or carcinogenic activities [1–3]. The degree of toxicity of PAHs is suspected to increase proportionally to the number of aromatic rings [1,3].
A number of studies have reported the successful degradation of HMW-PAHs by different bacteria, and the microbial degradation of pyrene has been elucidated [4–11]. Most of the reported HMW-PAH degrading bacteria are Gram-positive bacteria, which have shown the capacity to degrade a variety of different PAHs. Mycobacterium vanbaalenii strain PYR-1 could mineralize naphthalene, phenanthrene, and fluoranthene in addition to pyrene in the presence of low concentrations of supplemental organic nutrients [6,12]. Mycobacterium sp. strain CH1 has been reported to utilize PAHs including phenanthrene, fluoranthene, and pyrene, as well as a wide range of alkanes as sole carbon and energy sources [4]. Stenotrophomonas maltophilia strain VUN10,003 [8], is one of only a few Gram-negative bacteria able to degrade HMW-PAHs. In studies using high cell density inocula, this strain utilized fluoranthene, pyrene, benz[a]anthracene, benzo[a]pyrene, dibenz[a,h]anthracene, and coronene.
Enzymes and pathways involved in PAH catabolism have been extensively studied and reviewed in the past several decades. However, our understanding of the bacterial degradation of PAHs mainly came from studies done on the bacterial degradation of LMW-PAHs [13–20]. The enzymes and genes involved in HMW-PAHs degradation have not yet been fully elucidated. This has been made difficult due to the low aqueous solubility of HMW-PAHs and only a few microorganisms have been isolated as potential HMW-PAH degraders. Recently, Khan et al. [21] reported the successful cloning of an initial pyrene dioxygenase gene, nidA, from a pyrene degrading bacterium, M. vanbaalenii strain PYR-1. The complete sequence of the gene revealed that it was distantly related to classic bacterial ring-hydroxylating genes such as the nahAc, ndoB, and phnAc genes from Pseudomonas and Burkholderia species. It clustered with a high degree of similarity to the newly described dioxygenase genes, nidA and phdA from the Gram-positive bacteria, Rhodococcus sp. strain I24 and Nocardioides sp. strain KP7, respectively.
Mycobacterium sp. strain S65 was isolated from a site contaminated with jet-fuel in northern Quebec, Canada. In this study, the identity of the strain was confirmed by 16S rDNA gene sequencing, and the PAH degradation capabilities were characterized. In addition, the genes responsible for pyrene degradation were identified and their expression under different growth conditions was investigated.
2 Materials and methods
2.1 Bacterial strain isolation and culture conditions
A HMW-PAH degrading bacterium was isolated from a soil contaminated with jet fuel in Sept-Iles, northern Quebec, Canada by Jamshid Jazestani. A serial dilution of the soil in 0.1% sodium pyrophosphate buffer, pH 7.0, was plated on YTS250 (0.25 g each of yeast extract, tryptone, and starch per litre, pH 7.0) plates, overlaid with 0.5 ml of a 2.5 g/l solution of phenanthrene in acetone [22], and incubated at room temperature. A colony producing a clearing zone on the phenanthrene plate was selected and designated as S65. Its potential PAH degradation capacity was determined by mineralization as described below. Growth of strain S65 was also evaluated in YTS1000 (YTS as above but with 1 g/l of yeast extract, tryptone and starch) at different temperatures.
2.2 Mineralization of PAHs
The mineralization of various PAHs by strain S65 was performed in triplicate serum bottles each containing 20 ml mineral salts medium (MSM) at 30 °C as previously described [23]. A culture grown in YTS1000 without pyrene and washed twice with an equal volume (v/v) of MSM, was inoculated at a final concentration of 1.0×107 cfu/ml to each bottle. A heat-killed culture was used as a negative control. The 14C-labelled substrates (Sigma, St. Louis, Mo.) (>95% purity by HPLC), and the concentrations used in this assay, were: [1,2,3,4,4a,9a-14C]anthracene (specific activity, 11.2 mCi/mmol; concentration, 358 μg/l), [9-14C]fluorene (specific activity, 14.55 mCi/mmol; concentration, 225 μg/l), [3-14C]fluoranthene (specific activity, 45 mCi/mmol; concentration, 101 μg/l), [1-14C]naphthalene (specific activity, 8.1 mCi/mmol; concentration, 356 μg/l), [9-14C]phenanthrene (specific activity, 46.9 mCi/mmol; concentration, 358 μg/l), and [4,5,9,10-14C]pyrene (specific activity, 58.7 mCi/mmol; concentration, 78 μg/l); 100,000 dpm of each 14C-labelled substrate was added to each serum bottle.
Competitive mineralization of pyrene by strain S65 was also carried in the presence of unlabelled PAH co-substrates (phenanthrene, fluoranthene, benzo[a]pyrene, and benz[a]anthracene). In addition to radiolabeled pyrene each co-substrate was added at a concentration of 1 mg/l, either individually (pyrene plus one co-substrate) or as a mixture of all four co-substrates to determine the impact of the different substrates on pyrene degradation. Microcosms that received only radiolabelled pyrene served as the reference. The culture was either pre-grown in YTS1000 as a non-induced culture, or in MSM supplemented with 10 mg/l pyrene as an induced culture.
2.3 Genomic DNA extraction
Genomic DNA of strain S65 was prepared using the Ausubel method [24] with the addition of a physical lysis step prior to extraction, since the general chemical/enzymatic treatments did not lyse the cells. One millilitre of cell suspension was lysed using 0.5 g zirconium/silica beads (0.1 mm diameter) in a Mini Bead-Beater 8 (BioSpec Products, Bartlesville, OK) for two minutes. After cells were disrupted, cell debris was separated by centrifugation and the lysates were further processed to extract genomic DNA according to the Ausubel method.
2.4 Identification of strain S65
The genus of strain S65 was identified using direct sequencing of the 16S rDNA. The polymerase chain reaction (PCR) was performed using universal primers with the following sequences: 5′-GAGTTTGATCCTGGCTACG-3′ (11–29, Escherichia coli numbering) as a forward primer and 5′-AGAAAGGAGGTGATCCAGCC-3′ (1525–1544, E. coli numbering) as a reverse primer [25]. The PCR conditions used were described previously [26] except each cycle consisted of 1 min at 94 °C, 1 min at 62 °C, and 1 min at 72 °C, with 1.25 U of Taq polymerase. The PCR product was purified using the QIAQuick Purification Kit (Qiagen, Mississauga, ON) and sequenced using the ABI Prism dye terminator cycle sequencing ready reaction kit and the ABI Prism 377 automated fluorescence sequencer (Applied Biosystems, Foster City, CA). The sequence was submitted to the GenBank database to search for similarity with sequences of other bacteria by using the Blast alignment tool [27].
2.5 Determination of PAH degradation genes
Strain S65 was screened for the presence of three known bacterial ring-hydroxylating dioxygenase genes that degrade naphthalene (ndoB) [15], phenanthrene (phnAc) [16], and pyrene (nidA) [21] by PCR amplification. The primers and expected sizes of the PCR amplicons are summarized in Table 1. The PCR conditions were as previously described [26] at various annealing temperatures (50–70 °C).
| Target gene | Primer sequences | Amplicon size (bp) |
| ndoBa | Forward 5′-CACTCATGATAGCCTGATTCCTGCCCCCGGCG-3′ | 642 |
| Reverse 5′-CCGTCCCACAACACACCCATGCCGCTGCCG-3′ | ||
| phnAcb | Forward 5′-CCATTACGGTGATTTCGTGACC-3′ | 462 |
| Reverse 5′-ACAAAATTCTCTGACGGCGC-3′ | ||
| nidAc | Forward 5′-ATCTTCGGGCGCGGCTGGGTGTTTCTCGG-3′ | 508 |
| Reverse 5′-AATTGTCGGCGGCTGTCTTCCAGTTCGC-3′ |
| Target gene | Primer sequences | Amplicon size (bp) |
| ndoBa | Forward 5′-CACTCATGATAGCCTGATTCCTGCCCCCGGCG-3′ | 642 |
| Reverse 5′-CCGTCCCACAACACACCCATGCCGCTGCCG-3′ | ||
| phnAcb | Forward 5′-CCATTACGGTGATTTCGTGACC-3′ | 462 |
| Reverse 5′-ACAAAATTCTCTGACGGCGC-3′ | ||
| nidAc | Forward 5′-ATCTTCGGGCGCGGCTGGGTGTTTCTCGG-3′ | 508 |
| Reverse 5′-AATTGTCGGCGGCTGTCTTCCAGTTCGC-3′ |
| Target gene | Primer sequences | Amplicon size (bp) |
| ndoBa | Forward 5′-CACTCATGATAGCCTGATTCCTGCCCCCGGCG-3′ | 642 |
| Reverse 5′-CCGTCCCACAACACACCCATGCCGCTGCCG-3′ | ||
| phnAcb | Forward 5′-CCATTACGGTGATTTCGTGACC-3′ | 462 |
| Reverse 5′-ACAAAATTCTCTGACGGCGC-3′ | ||
| nidAc | Forward 5′-ATCTTCGGGCGCGGCTGGGTGTTTCTCGG-3′ | 508 |
| Reverse 5′-AATTGTCGGCGGCTGTCTTCCAGTTCGC-3′ |
| Target gene | Primer sequences | Amplicon size (bp) |
| ndoBa | Forward 5′-CACTCATGATAGCCTGATTCCTGCCCCCGGCG-3′ | 642 |
| Reverse 5′-CCGTCCCACAACACACCCATGCCGCTGCCG-3′ | ||
| phnAcb | Forward 5′-CCATTACGGTGATTTCGTGACC-3′ | 462 |
| Reverse 5′-ACAAAATTCTCTGACGGCGC-3′ | ||
| nidAc | Forward 5′-ATCTTCGGGCGCGGCTGGGTGTTTCTCGG-3′ | 508 |
| Reverse 5′-AATTGTCGGCGGCTGTCTTCCAGTTCGC-3′ |
2.6 Cloning and sequencing of the pyrene catabolic genes
Genomic DNA of strain S65 was digested with BamHI, EcoRI, EcoRV, HindIII, SacI, SacII, SalI, SpeI, or XbaI (New England Biolabs, Mississauga, ON) and transferred onto a membrane using a protocol described by Sambrook et al. [28] for Southern hybridization. A nidA PCR amplicon produced using S65 template DNA and the primers identified above (Table 1) was used as a probe. The PCR amplicon was labelled with a DIG labelling system according to the manufacturer's instructions (Roche–Boehringer–Mannheim, Quebec).
Two size fractions (∼7 and 11 kb) of EcoRV digested genomic DNA that hybridized to the nidA probe were separately purified and ligated into the EcoRV site of pBluescriptIIKS in E. coli DH10B transformants. The transformants with inserts were selected with 100 μg/ml of ampicillin on LB medium overlaid with X-Gal and IPTG and checked for a positive nidA gene by colony hybridization. Two clones, one from each size fraction, with strong signals against the nidA probe were selected and sequenced using primers designed from the KS (5′-CGAGGTCGACGGTATCG-3′) and SK (5′-TCTAGAACTAGTGGATC-3′) ends of the BluescriptIIKS plasmid, prepared according to Hanahan [29]. To complete gaps, primers were designed from both ends of the assembled sequences. Sequences were manually assembled and edited using Gap4 from the Staden Sequence Analysis software package. The assembled sequences were submitted to the GenBank database to search for similarity using the Blast alignment tool [27].
2.7 Differentiation of the pyrene degrading genes encoded in the two gene clusters
Total RNA was extracted from strain S65 grown in MSM containing 50 mg/l pyrene, 50 mg/l phenanthrene, or 3 g/l glucose using a method modified from Fonzi and Sypherd [30]. The cell pellet was suspended in lysis buffer (0.05 M sodium acetate, pH 4.5, 1% SDS, 1 mM EDTA, pH 8.0), preheated to 65 °C, and immediately lysed using 0.5 g of zirconium/silica beads (0.1 mm diameter) in a Bead-Beater 8 for 1.5 min. RNA was further extracted from the lysate twice with an equal volume of acid phenol (pH 4.5) at 65 °C and once with chloroform/isoamyl alcohol (24:1) at room temperature. The RNA extract was precipitated with 1/10 volume of 3 M sodium acetate (pH 5.0) and v/v of isopropanol, and kept at −80 °C until use. The purified RNA was resuspended in DEPC treated (or HPLC grade) water and treated with RNase-free DNase (Ambion, Austin, TX) to remove DNA contaminants. PCR without reverse transcription was performed with the DNase-treated RNA to ensure the complete elimination of DNA. The sequences of AF546904 and AF546905 were used to design unique primers for RT-PCR of the nid and pdo genes, respectively.
RT-PCR was carried out using the QIAGEN One-Step RT-PCR kit (Qiagen, Mississauga, ON) according to the manufacturer's instructions. Primers unique for individual pyrene degrading genes (Table 2) were designed and tested for specificity and optimal conditions by PCR prior to RT-PCR. Each primer set was tested on both clones to avoid cross-amplification of nid genes with pdo primers, and vice versa. The conditions of the RT-PCR were as follows: 100–150 ng of total RNA; 50 °C for 30 min; 95 °C for 15 min; followed by 30 cycles of 94 °C for 1 min, 60–65 °C for 1 min, 72 °C for 1 min, and a final extension at 72 °C for 10 min. As a control for DNA contamination, PCR amplification with an equal amount of total RNA was performed without reverse transcription in each experiment. As a positive control for PCR amplification, 10 ng of genomic DNA from strain S65 was used as a template.
| Fragment numbera | Target gene | Primer sequence | |
| nid locus (AF546904) | |||
| 1. | nidAF | 5′-AGCTGACGCGACCGATCC-3′ | |
| 1. | nidAR | 5′-GGGCGCCATGCCTAGCTCG-3′ | |
| 2. | nidCF | 5′-TCATCCCACTCCTGCGCC-3′ | |
| 2. | nidCR | 5′-ATCCTCGGCGTGCGGGTG-3′ | |
| 3. | nidHF | 5′-GCCAGACCCGGCGCCGCAGGAC-3′ | |
| 3. | nidHR | 5′-GCGCTGCCCGCCACATGGAAGACC-3′ | |
| 4. | nidHF2 | 5′-CGCGCCGTCAAGGAGGGATTGGG-3′ | |
| 4. | orf4R | 5′-GTCAGTCCAGTGGTCTGCGCCCGCGGTGG-3′ | |
| 5. | orf4F | 5′-ATTTACCGCAGCACCGAC-3′ | |
| 5 | orf4R2 | 5′-AACAGGCCGCGGTC-3′ | |
| 6. | nidXF | 5′-AGTGAAGCGTGGACCGAC-3′ | |
| 6. | nidXR | 5′-AGTCGCGCAACGGATCC-3′ | |
| 7. | orf4F2 | 5′-CCACCGCGGGCGCAGACCACTGGACTGAC-3′ | |
| 7. | nidXR2 | 5′-CGGCTGTATGTGGTCGAACTCCAGC-3′ | |
| pdo locus (AF546905) | |||
| 8. | pdoAF | 5′-ATGACCACCGAAACAACCGAGACCACCG-3′ | |
| 8. & 9. | pdoAR | 5′-CCAGTTCGCAGACATAGT-3′ | |
| 9. | pdoBF | 5′-TCTACAGCGGAGGACTTATC-3′ | |
| 10. | pdoAF2 | 5′-CTACATGAGCCAACCTGCCCAG-3′ | |
| 10. | pdoR1 | 5′-ACTTCGATAATCACCTCACCAGG-3′ | |
| 11. | pdoHF2 | 5′-AGTGCGGTGGAGGAGGGGTTGAA-3′ | |
| 11. & 12. | orf6R | 5′-TCAGTGAGCTCGCCTTCCAG-3′ | |
| 12. | orf6F | 5′-ACCGGGGACAGACATGGG-3′ | |
| 13. | pdoXF | 5′-TCTGGAACAGCGCCGATGCG-3′ | |
| 13. | pdoXR | 5′-CGGCGAACGGTGGCG-3′ | |
| 14. | orf6F2 | 5′-GTACCGCGGGTGCTAACTGCTGGACTGGG-3′ | |
| 14. | pdoXR2 | 5′-AGCCTGGACATGGTCGAATTCCAGG-3′ | |
| Fragment numbera | Target gene | Primer sequence | |
| nid locus (AF546904) | |||
| 1. | nidAF | 5′-AGCTGACGCGACCGATCC-3′ | |
| 1. | nidAR | 5′-GGGCGCCATGCCTAGCTCG-3′ | |
| 2. | nidCF | 5′-TCATCCCACTCCTGCGCC-3′ | |
| 2. | nidCR | 5′-ATCCTCGGCGTGCGGGTG-3′ | |
| 3. | nidHF | 5′-GCCAGACCCGGCGCCGCAGGAC-3′ | |
| 3. | nidHR | 5′-GCGCTGCCCGCCACATGGAAGACC-3′ | |
| 4. | nidHF2 | 5′-CGCGCCGTCAAGGAGGGATTGGG-3′ | |
| 4. | orf4R | 5′-GTCAGTCCAGTGGTCTGCGCCCGCGGTGG-3′ | |
| 5. | orf4F | 5′-ATTTACCGCAGCACCGAC-3′ | |
| 5 | orf4R2 | 5′-AACAGGCCGCGGTC-3′ | |
| 6. | nidXF | 5′-AGTGAAGCGTGGACCGAC-3′ | |
| 6. | nidXR | 5′-AGTCGCGCAACGGATCC-3′ | |
| 7. | orf4F2 | 5′-CCACCGCGGGCGCAGACCACTGGACTGAC-3′ | |
| 7. | nidXR2 | 5′-CGGCTGTATGTGGTCGAACTCCAGC-3′ | |
| pdo locus (AF546905) | |||
| 8. | pdoAF | 5′-ATGACCACCGAAACAACCGAGACCACCG-3′ | |
| 8. & 9. | pdoAR | 5′-CCAGTTCGCAGACATAGT-3′ | |
| 9. | pdoBF | 5′-TCTACAGCGGAGGACTTATC-3′ | |
| 10. | pdoAF2 | 5′-CTACATGAGCCAACCTGCCCAG-3′ | |
| 10. | pdoR1 | 5′-ACTTCGATAATCACCTCACCAGG-3′ | |
| 11. | pdoHF2 | 5′-AGTGCGGTGGAGGAGGGGTTGAA-3′ | |
| 11. & 12. | orf6R | 5′-TCAGTGAGCTCGCCTTCCAG-3′ | |
| 12. | orf6F | 5′-ACCGGGGACAGACATGGG-3′ | |
| 13. | pdoXF | 5′-TCTGGAACAGCGCCGATGCG-3′ | |
| 13. | pdoXR | 5′-CGGCGAACGGTGGCG-3′ | |
| 14. | orf6F2 | 5′-GTACCGCGGGTGCTAACTGCTGGACTGGG-3′ | |
| 14. | pdoXR2 | 5′-AGCCTGGACATGGTCGAATTCCAGG-3′ | |
Fragment number corresponds to the regions shown in Fig. 4.
| Fragment numbera | Target gene | Primer sequence | |
| nid locus (AF546904) | |||
| 1. | nidAF | 5′-AGCTGACGCGACCGATCC-3′ | |
| 1. | nidAR | 5′-GGGCGCCATGCCTAGCTCG-3′ | |
| 2. | nidCF | 5′-TCATCCCACTCCTGCGCC-3′ | |
| 2. | nidCR | 5′-ATCCTCGGCGTGCGGGTG-3′ | |
| 3. | nidHF | 5′-GCCAGACCCGGCGCCGCAGGAC-3′ | |
| 3. | nidHR | 5′-GCGCTGCCCGCCACATGGAAGACC-3′ | |
| 4. | nidHF2 | 5′-CGCGCCGTCAAGGAGGGATTGGG-3′ | |
| 4. | orf4R | 5′-GTCAGTCCAGTGGTCTGCGCCCGCGGTGG-3′ | |
| 5. | orf4F | 5′-ATTTACCGCAGCACCGAC-3′ | |
| 5 | orf4R2 | 5′-AACAGGCCGCGGTC-3′ | |
| 6. | nidXF | 5′-AGTGAAGCGTGGACCGAC-3′ | |
| 6. | nidXR | 5′-AGTCGCGCAACGGATCC-3′ | |
| 7. | orf4F2 | 5′-CCACCGCGGGCGCAGACCACTGGACTGAC-3′ | |
| 7. | nidXR2 | 5′-CGGCTGTATGTGGTCGAACTCCAGC-3′ | |
| pdo locus (AF546905) | |||
| 8. | pdoAF | 5′-ATGACCACCGAAACAACCGAGACCACCG-3′ | |
| 8. & 9. | pdoAR | 5′-CCAGTTCGCAGACATAGT-3′ | |
| 9. | pdoBF | 5′-TCTACAGCGGAGGACTTATC-3′ | |
| 10. | pdoAF2 | 5′-CTACATGAGCCAACCTGCCCAG-3′ | |
| 10. | pdoR1 | 5′-ACTTCGATAATCACCTCACCAGG-3′ | |
| 11. | pdoHF2 | 5′-AGTGCGGTGGAGGAGGGGTTGAA-3′ | |
| 11. & 12. | orf6R | 5′-TCAGTGAGCTCGCCTTCCAG-3′ | |
| 12. | orf6F | 5′-ACCGGGGACAGACATGGG-3′ | |
| 13. | pdoXF | 5′-TCTGGAACAGCGCCGATGCG-3′ | |
| 13. | pdoXR | 5′-CGGCGAACGGTGGCG-3′ | |
| 14. | orf6F2 | 5′-GTACCGCGGGTGCTAACTGCTGGACTGGG-3′ | |
| 14. | pdoXR2 | 5′-AGCCTGGACATGGTCGAATTCCAGG-3′ | |
| Fragment numbera | Target gene | Primer sequence | |
| nid locus (AF546904) | |||
| 1. | nidAF | 5′-AGCTGACGCGACCGATCC-3′ | |
| 1. | nidAR | 5′-GGGCGCCATGCCTAGCTCG-3′ | |
| 2. | nidCF | 5′-TCATCCCACTCCTGCGCC-3′ | |
| 2. | nidCR | 5′-ATCCTCGGCGTGCGGGTG-3′ | |
| 3. | nidHF | 5′-GCCAGACCCGGCGCCGCAGGAC-3′ | |
| 3. | nidHR | 5′-GCGCTGCCCGCCACATGGAAGACC-3′ | |
| 4. | nidHF2 | 5′-CGCGCCGTCAAGGAGGGATTGGG-3′ | |
| 4. | orf4R | 5′-GTCAGTCCAGTGGTCTGCGCCCGCGGTGG-3′ | |
| 5. | orf4F | 5′-ATTTACCGCAGCACCGAC-3′ | |
| 5 | orf4R2 | 5′-AACAGGCCGCGGTC-3′ | |
| 6. | nidXF | 5′-AGTGAAGCGTGGACCGAC-3′ | |
| 6. | nidXR | 5′-AGTCGCGCAACGGATCC-3′ | |
| 7. | orf4F2 | 5′-CCACCGCGGGCGCAGACCACTGGACTGAC-3′ | |
| 7. | nidXR2 | 5′-CGGCTGTATGTGGTCGAACTCCAGC-3′ | |
| pdo locus (AF546905) | |||
| 8. | pdoAF | 5′-ATGACCACCGAAACAACCGAGACCACCG-3′ | |
| 8. & 9. | pdoAR | 5′-CCAGTTCGCAGACATAGT-3′ | |
| 9. | pdoBF | 5′-TCTACAGCGGAGGACTTATC-3′ | |
| 10. | pdoAF2 | 5′-CTACATGAGCCAACCTGCCCAG-3′ | |
| 10. | pdoR1 | 5′-ACTTCGATAATCACCTCACCAGG-3′ | |
| 11. | pdoHF2 | 5′-AGTGCGGTGGAGGAGGGGTTGAA-3′ | |
| 11. & 12. | orf6R | 5′-TCAGTGAGCTCGCCTTCCAG-3′ | |
| 12. | orf6F | 5′-ACCGGGGACAGACATGGG-3′ | |
| 13. | pdoXF | 5′-TCTGGAACAGCGCCGATGCG-3′ | |
| 13. | pdoXR | 5′-CGGCGAACGGTGGCG-3′ | |
| 14. | orf6F2 | 5′-GTACCGCGGGTGCTAACTGCTGGACTGGG-3′ | |
| 14. | pdoXR2 | 5′-AGCCTGGACATGGTCGAATTCCAGG-3′ | |
Fragment number corresponds to the regions shown in Fig. 4.
3 Results
3.1 Physiological and biochemical characteristics of strain S65
A strain capable of utilizing phenanthrene and pyrene as sole carbon and energy sources was isolated from an airport in Sept-Iles, northern Quebec, Canada. The strain was found to be Gram-variable, catalase-positive, with a rod-coccal morphology, and produced creamy yellow circular colonies. Strain S65 did not grow on rich media, such as trypticase soy broth (TSB) and Luria–Bertani (LB). The growth temperatures ranged from 10 to 30 °C: growth of strain S65 took 22 days to reach the stationary phase at 10 °C, compared to 6 days at 30 °C, the optimum temperature. No growth was observed at 35 °C. A large plasmid was detected in strain S65, but since hybridization to the nidA probe was not detected, it does not appear to be associated with pyrene degradation, and was not studied further (data not shown). Direct sequencing of the 16S rDNA revealed that this strain belongs to the genus Mycobacterium. The 16S rDNA sequence was 99% identical to the toluene degrading bacteria, Mycobacterium spp. strains T104 and T103 [31]. The 16S rDNA sequence of strain S65 was deposited in GenBank as Accession No. AF544230.
3.2 Mineralization of PAHs
To screen for the ability of strain S65 to degrade PAHs, mineralization of PAHs with varying numbers of aromatic rings (2–4 rings) and structures (linear, angular, and cluster) was monitored over a 2-week period in MSM containing different PAHs as sole carbon sources. Pyrene mineralization began immediately upon inoculation reaching 45% within the first 24 h, which eventually reached almost 60% within 96 h (Fig. 1). No significant mineralization of phenanthrene was observed for the first 72 h, yet the mineralization extent eventually reached almost 60%. The LMW-PAHs (anthracene, naphthalene, and fluorene) were not mineralized by this strain. Fluoranthene was mineralized without a lag phase but a maximum extent of only 20% was attained.
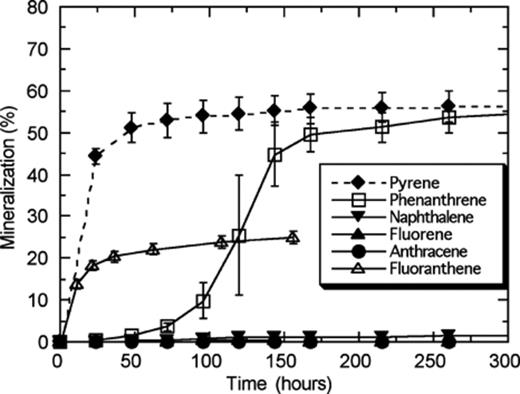
Time course for mineralization of various PAHs by Mycobacterium sp. strain S65. Strain S65 was pre-grown in a low nutrient broth to the mid-log phase and the culture was washed with MSM, to remove residual nutrients, prior to inoculation. The ability of strain S65 to mineralize PAHs was tested on anthracene, naphthalene, fluorene, phenanthrene, and pyrene.
The influence of other PAHs on the mineralization of pyrene by strain S65 was examined by adding unradiolabelled PAHs (phenanthrene, fluoranthene, benzo[a]pyrene, or benz[a]anthracene, or a mixture of all four PAHs) into the microcosms containing radiolabelled pyrene (Fig. 2(a) and (b)). Under the non-induced condition, initiation of pyrene mineralization was observed immediately after strain S65 was introduced into the microcosms containing benzo[a]pyrene, benz[a]anthracene, or fluoranthene in spite of pre-growing strain S65 in the absence of pyrene (Fig. 2(a)). The extent of pyrene degradation in the presence of benzo[a]pyrene, and benz[a]anthracene, was higher than 30%, whereas it was only 10% with fluoranthene in the first 15 h. Pyrene mineralization with benzo[a]pyrene reached a maximum extent of 57%. In the presence of phenanthrene, pyrene mineralization reached the second highest extent (54%) but a long lag phase was observed, as was previously seen during phenanthrene mineralization. A lag phase was also observed when a mixture of these PAH substrates was present, and mineralization reached 45%. Pyrene was mineralized the least in the presence of fluoranthene.
![Time course of pyrene mineralization in the presence of various PAHs by Mycobacterium sp. strain S65 under non-induced (a) and induced (b) conditions. Mineralization of pyrene in the presence of unlabelled phenanthrene, fluoranthene, benz[a]anthracene, or benzo[a]pyrene, added to a concentration of 1 mg/l in each microcosm. The mineralization of a mixture of these PAHs and no unlabelled PAH substrates was also measured in this experiment. Mineralization of pyrene in the absence of co-substrates was used as a reference. Data points are the average of triplicate samples and error bars represent one standard deviation.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsec/48/2/10.1016_j.femsec.2004.01.011/1/m_FEM_209_f2.jpeg?Expires=1750135454&Signature=3eh0bEOhjr062Si5G8bZclS9HYq-hfGZRDR5SWp55MOVQ2DAUhM~MH86ajxvO6zwTvOG~0x8ic8bZ0ZJMVBSibXTOhSlF2ZHvElrS0MGLzaBuaAsTPvvfVRvcqPVnjrezwnVNIwq-32~vkZHKBMk5zkoNSH42YQjTSkJFNeDW~YU~ELmRVbOzqH2~cn78MGRx0k3mjosMmg5F~h8ImD3xFDAfK3hCt19cofxV-jAT4uv4UXUvQPf0r60jnZv811KKaKEvAJLKiT~jb3B2zNOKEZNdjh9XH1WkJNwTMw1Tf7ImQS6pcIEGFRXZxDq8377NjoZGcgj97Jc1A1A-GlyLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Time course of pyrene mineralization in the presence of various PAHs by Mycobacterium sp. strain S65 under non-induced (a) and induced (b) conditions. Mineralization of pyrene in the presence of unlabelled phenanthrene, fluoranthene, benz[a]anthracene, or benzo[a]pyrene, added to a concentration of 1 mg/l in each microcosm. The mineralization of a mixture of these PAHs and no unlabelled PAH substrates was also measured in this experiment. Mineralization of pyrene in the absence of co-substrates was used as a reference. Data points are the average of triplicate samples and error bars represent one standard deviation.
Significant differences in pyrene mineralization were observed in microcosms containing phenanthrene, fluoranthene, or the PAH mixture under the induced condition. There was no lag phase during pyrene mineralization in the presence of these PAHs (Fig. 2(b)). A 25% increase in pyrene mineralization in the first 15 h was observed in the presence of benz[a]anthracene with the induced culture. Microcosms containing benzo[a]pyrene or the PAH mixture also showed a 20% increase. This increase with the PAH mixture was significant compared to that observed under the non-induced condition. The mineralization exceeded the reference (microcosms with radiolabelled pyrene only) and reached 67%, which was equivalent to the highest mineralization (68%) observed with benzo[a]pyrene, in spite of the presence of fluoranthene in the mixture. The overall extent of pyrene mineralization was also greatly enhanced by addition of other unradiolabelled PAHs. However, in microcosms containing only unradiolabelled fluoranthene, pyrene mineralization was not significantly different from that observed under the non-induced condition.
3.3 PCR screening of strain S65 for PAH catabolic dioxygenase genes
No PCR amplification of the ndoB- or phnAc-like genes was detected in strain S65, but a strong product was obtained with primers targeting nidA-like genes. Therefore the nidA PCR fragment from strain S65 (508 bp) was used as a probe for further analyses.
The digested genomic DNA of strain S65 was screened for PYR-1 nidA homologues by Southern hybridization. Putative nidA homologues were identified in several sites of the strain S65 genome (Fig. 3). Multiple hybridization bands appeared in the genomic DNA digested with BamHI, EcoRI, EcoRV, SacI, and SacII. The EcoRV digested DNA gave strong positive hybridization signals at estimated molecular sizes of 7 and 11 kb, which were further purified and cloned. Following PCR and RFLP analyses, two clones, representing one from each fragment size, were selected and sequenced for pyrene degradation genes. The actual lengths of the clones determined following sequencing were 5606 and 8722 bp. The 5606 bp locus was designated as the nid locus and the 8722 bp locus was designated as the pdo locus. Sequences of the pyrene degradation genes in the two clones were deposited in the GenBank database under Accession Nos: AF546904 and AF546905, respectively. The genetic organizations of pyrene degradation genes in both loci are shown in comparison to those in PYR-1 in Fig. 4, and the functional description and homology between the genes found in this study and representative homologues are presented in Table 3.
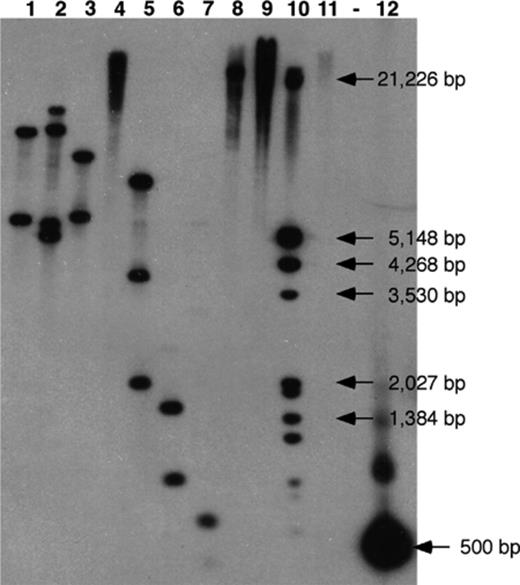
Southern hybridization of S65 genomic DNA using the pyrene catabolic gene probe, nidA, derived from S65. Genomic DNA of strain S65 was digested with various restriction enzymes and was tested for the presence of the nidA gene identified in Mycobacterium sp. strain PYR-1. S65 genomic DNA was digested with lane1, BamHI; lane 2, EcoRI; lane 3, EcoRV; lane 4, HindIII; lane 5, SacI; lane 6, SacII; lane 7, SalI; lane 8, SpeI; lane 9, XbaI; lane 10, DIG marker; lane 11, S65 plasmid; (-) blank lane; lane 12, nidA PCR probe.
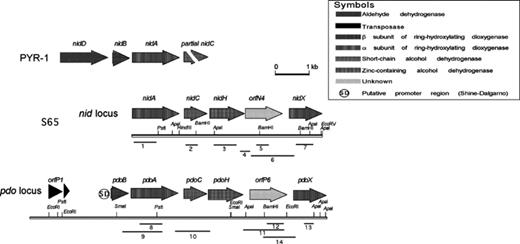
Genetic organization of the loci encompassing the genes identified in this study. The positions and orientations of the putative genes are shown by shaded arrows. The positions of the primers and the fragments they amplify are indicated by numbered lines (see Table 2).
| Gene | Length n.t. | Functiona | Homology (%)b | Reference (GenBank no.)c | |
| n.t. | a.a. | ||||
| nidA | 1368 | α Subunit pyrene dioxygenase | 99 | 99 | nidA, M. vanbaalenii strain PYR-1, AF249301 |
| 89 | 93 | pdoA, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| nidC | 681 | cis-4,5-Dihydroxy 4,5-dihydropyrene dehydrogenase | 16 | 0 | putative, Sinorhizobium meliloti pSymA, AAK64840 |
| 90 | 90 | pdoC, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| nidH | 1026 | Zinc-containing dehydrogenase | 10 | 0 | 2,3-Butanediol dehydrogenase, Pseudomonas putida, AAB58982 |
| 87 | 90 | pdoH, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| orfN4 | 941 | Unknown | 87 | 40 | orfP6, Mycobacterium sp. strain S65 (this study), AF546905 |
| nidX | 1187 | α Subunit dioxygenase | 44 | 47 | orfG1Sphingomonas sp. strain RW1, AJ223219 |
| 94 | 95.5 | pdoX, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| orfP1 | 987 | Transposase (pseudo) | 78 | 82 | Transposase-like protein, Mycobacterium abscessus, AAN38731 |
| pdoB | 510 | β Subunit dioxygenase | 88 | 93 | nid B, Mycobacterium vanbaalenii strain PYR-1, AF249302 |
| pdoA | 1376 | α Subunit pyrene dioxygenase | 89 | 93 | nidA, Mycobacterium vanbaalenii strain PYR-1, AF249301 |
| 89 | 93 | nidA, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| pdoC | 681 | cis-4,5,-Dihydroxy-4,5-dihydropyrene | 19 | 0 | putative, Sinorhizobium meliloti pSymA, AAK64840 |
| 90 | 90 | nidC, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| pdoH | 1026 | Dehydrogenase | 8.5 | 0 | 2,3-butanediol dehydrogenase Pseudomonas putida, AAB58982 |
| 87 | 90 | nidH, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| orfP6 | 942 | Unknown | 87 | 40 | orfN4, Mycobacterium sp. strain S65 (this study), AF546904 |
| pdoX | 1180 | α Subunit pyrene dioxygenase | 46 | 40 | orfG1, Sphingomonas sp. strain RW1, AJ223219 |
| 94 | 95.5 | nidX, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| Gene | Length n.t. | Functiona | Homology (%)b | Reference (GenBank no.)c | |
| n.t. | a.a. | ||||
| nidA | 1368 | α Subunit pyrene dioxygenase | 99 | 99 | nidA, M. vanbaalenii strain PYR-1, AF249301 |
| 89 | 93 | pdoA, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| nidC | 681 | cis-4,5-Dihydroxy 4,5-dihydropyrene dehydrogenase | 16 | 0 | putative, Sinorhizobium meliloti pSymA, AAK64840 |
| 90 | 90 | pdoC, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| nidH | 1026 | Zinc-containing dehydrogenase | 10 | 0 | 2,3-Butanediol dehydrogenase, Pseudomonas putida, AAB58982 |
| 87 | 90 | pdoH, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| orfN4 | 941 | Unknown | 87 | 40 | orfP6, Mycobacterium sp. strain S65 (this study), AF546905 |
| nidX | 1187 | α Subunit dioxygenase | 44 | 47 | orfG1Sphingomonas sp. strain RW1, AJ223219 |
| 94 | 95.5 | pdoX, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| orfP1 | 987 | Transposase (pseudo) | 78 | 82 | Transposase-like protein, Mycobacterium abscessus, AAN38731 |
| pdoB | 510 | β Subunit dioxygenase | 88 | 93 | nid B, Mycobacterium vanbaalenii strain PYR-1, AF249302 |
| pdoA | 1376 | α Subunit pyrene dioxygenase | 89 | 93 | nidA, Mycobacterium vanbaalenii strain PYR-1, AF249301 |
| 89 | 93 | nidA, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| pdoC | 681 | cis-4,5,-Dihydroxy-4,5-dihydropyrene | 19 | 0 | putative, Sinorhizobium meliloti pSymA, AAK64840 |
| 90 | 90 | nidC, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| pdoH | 1026 | Dehydrogenase | 8.5 | 0 | 2,3-butanediol dehydrogenase Pseudomonas putida, AAB58982 |
| 87 | 90 | nidH, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| orfP6 | 942 | Unknown | 87 | 40 | orfN4, Mycobacterium sp. strain S65 (this study), AF546904 |
| pdoX | 1180 | α Subunit pyrene dioxygenase | 46 | 40 | orfG1, Sphingomonas sp. strain RW1, AJ223219 |
| 94 | 95.5 | nidX, Mycobacterium sp. strain S65 (this study), AF546904 | |||
The putative function of these genes was predicted based on the presence of a consensus domain obtained from PROSITE at http://ca.expasy.org and Pfam at http://ncbi.nim.nih.gov/Structure/cdd.
Homology of pyrene degrading genes in this study to the corresponding closest genes was determined at the nucleotide and amino acid levels using PHYLIP software.
GenBank (http://ncbi.nim.nih).
| Gene | Length n.t. | Functiona | Homology (%)b | Reference (GenBank no.)c | |
| n.t. | a.a. | ||||
| nidA | 1368 | α Subunit pyrene dioxygenase | 99 | 99 | nidA, M. vanbaalenii strain PYR-1, AF249301 |
| 89 | 93 | pdoA, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| nidC | 681 | cis-4,5-Dihydroxy 4,5-dihydropyrene dehydrogenase | 16 | 0 | putative, Sinorhizobium meliloti pSymA, AAK64840 |
| 90 | 90 | pdoC, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| nidH | 1026 | Zinc-containing dehydrogenase | 10 | 0 | 2,3-Butanediol dehydrogenase, Pseudomonas putida, AAB58982 |
| 87 | 90 | pdoH, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| orfN4 | 941 | Unknown | 87 | 40 | orfP6, Mycobacterium sp. strain S65 (this study), AF546905 |
| nidX | 1187 | α Subunit dioxygenase | 44 | 47 | orfG1Sphingomonas sp. strain RW1, AJ223219 |
| 94 | 95.5 | pdoX, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| orfP1 | 987 | Transposase (pseudo) | 78 | 82 | Transposase-like protein, Mycobacterium abscessus, AAN38731 |
| pdoB | 510 | β Subunit dioxygenase | 88 | 93 | nid B, Mycobacterium vanbaalenii strain PYR-1, AF249302 |
| pdoA | 1376 | α Subunit pyrene dioxygenase | 89 | 93 | nidA, Mycobacterium vanbaalenii strain PYR-1, AF249301 |
| 89 | 93 | nidA, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| pdoC | 681 | cis-4,5,-Dihydroxy-4,5-dihydropyrene | 19 | 0 | putative, Sinorhizobium meliloti pSymA, AAK64840 |
| 90 | 90 | nidC, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| pdoH | 1026 | Dehydrogenase | 8.5 | 0 | 2,3-butanediol dehydrogenase Pseudomonas putida, AAB58982 |
| 87 | 90 | nidH, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| orfP6 | 942 | Unknown | 87 | 40 | orfN4, Mycobacterium sp. strain S65 (this study), AF546904 |
| pdoX | 1180 | α Subunit pyrene dioxygenase | 46 | 40 | orfG1, Sphingomonas sp. strain RW1, AJ223219 |
| 94 | 95.5 | nidX, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| Gene | Length n.t. | Functiona | Homology (%)b | Reference (GenBank no.)c | |
| n.t. | a.a. | ||||
| nidA | 1368 | α Subunit pyrene dioxygenase | 99 | 99 | nidA, M. vanbaalenii strain PYR-1, AF249301 |
| 89 | 93 | pdoA, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| nidC | 681 | cis-4,5-Dihydroxy 4,5-dihydropyrene dehydrogenase | 16 | 0 | putative, Sinorhizobium meliloti pSymA, AAK64840 |
| 90 | 90 | pdoC, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| nidH | 1026 | Zinc-containing dehydrogenase | 10 | 0 | 2,3-Butanediol dehydrogenase, Pseudomonas putida, AAB58982 |
| 87 | 90 | pdoH, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| orfN4 | 941 | Unknown | 87 | 40 | orfP6, Mycobacterium sp. strain S65 (this study), AF546905 |
| nidX | 1187 | α Subunit dioxygenase | 44 | 47 | orfG1Sphingomonas sp. strain RW1, AJ223219 |
| 94 | 95.5 | pdoX, Mycobacterium sp. strain S65 (this study), AF546905 | |||
| orfP1 | 987 | Transposase (pseudo) | 78 | 82 | Transposase-like protein, Mycobacterium abscessus, AAN38731 |
| pdoB | 510 | β Subunit dioxygenase | 88 | 93 | nid B, Mycobacterium vanbaalenii strain PYR-1, AF249302 |
| pdoA | 1376 | α Subunit pyrene dioxygenase | 89 | 93 | nidA, Mycobacterium vanbaalenii strain PYR-1, AF249301 |
| 89 | 93 | nidA, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| pdoC | 681 | cis-4,5,-Dihydroxy-4,5-dihydropyrene | 19 | 0 | putative, Sinorhizobium meliloti pSymA, AAK64840 |
| 90 | 90 | nidC, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| pdoH | 1026 | Dehydrogenase | 8.5 | 0 | 2,3-butanediol dehydrogenase Pseudomonas putida, AAB58982 |
| 87 | 90 | nidH, Mycobacterium sp. strain S65 (this study), AF546904 | |||
| orfP6 | 942 | Unknown | 87 | 40 | orfN4, Mycobacterium sp. strain S65 (this study), AF546904 |
| pdoX | 1180 | α Subunit pyrene dioxygenase | 46 | 40 | orfG1, Sphingomonas sp. strain RW1, AJ223219 |
| 94 | 95.5 | nidX, Mycobacterium sp. strain S65 (this study), AF546904 | |||
The putative function of these genes was predicted based on the presence of a consensus domain obtained from PROSITE at http://ca.expasy.org and Pfam at http://ncbi.nim.nih.gov/Structure/cdd.
Homology of pyrene degrading genes in this study to the corresponding closest genes was determined at the nucleotide and amino acid levels using PHYLIP software.
GenBank (http://ncbi.nim.nih).
3.4 Sequence analysis of pyrene degradation genes
3.4.1 Bacterial ring hydroxylating dioxygenase genes
The genes nidA, nidX, pdoA, and pdoX, encode the α subunits of bacterial ring-hydroxylating dioxygenase genes. The consensus sequence Cys-X1-His-X16-Cys-X2-His of a Rieske-type [2Fe-2S] cluster binding site was found in their deduced amino acid (aa) sequences. The nucleotide sequence of nidA (1368 bp) was 99.5% identical to the nidA (AF249301) of M. vanbaalenii strain PYR-1, and pdoA (1380 bp) was 89% identical to the PYR-1 nidA, with clear differences at the 5′ end. The putative function of nidX and pdoX was predicted from the BlastX and TblastX searches since there was no match found using their nucleotide sequences in the database. Both searches indicated a high match with the hypothetical α subunit dioxygenase from a dioxin degrading bacterium, Sphingomonas sp. strain RW1 [32]. A match was also found with dfdA1, a gene responsible for the angular dioxygenation of dibenzofuran in actinomyces and for the oxidation of various PAHs by Terrabacter sp. strain YK3 [33]. A β subunit dioxygenase gene, found in orfP2 (pdoB) had 89% identity to PYR-1 nidB.
3.4.2 Alcohol dehydrogenase genes
A short-chain alcohol dehydrogenase gene, nid/pdoC, and a zinc-containing alcohol dehydrogenase gene, nid/pdoH, were predicted just downstream of nidA and pdoA. Amino acid consensus sequences, obtained from the deduced a.a. sequences, were: S-X(12)-YSASKGGV-X(2)-L-X(3)-MA (http://ca.expasy.org/cgi-bin/nicesite.pl?PS00061) for Nid/PdoC, and G-H-E-x(2)-G-x(5)-G-x(2)-V for NidH or G-H-E-x(2)-G-x(5)-G-x(2)-I for PdoH (http://ca.expasy.org/cgi-bin/nicesite.pl?PS00059), respectively. As with nid/pdoX, no significant match to the nid/pdoC and nid/pdoH genes was found using BlastN. In TblastX, the translated a.a.s of nid/pdoC were closest to the small molecule metabolic enzyme from the Sinorhizobium meliloti pSymA plasmid (GenBank No. AAK64840) and those of nid/pdoH were closest to 2,3-butanediol dehydrogenase from Pseudomonas putida strain PpG2 (GeneBank No. AAB58982). There was no close match found to known PAH alcohol dehydrogenases.
3.4.3 Other genes found in the pyrene catabolic gene cluster
Open reading frames, orf's, N4 and P6 were identified between nidH and nidX, and pdoH and pdoX, respectively. No matches were found at the nucleotide level. The deduced a.a. sequences of orfN4/P 6 were closest to VPS10 of Bacillus anthracis strain A2012 (GenBank No. NP655627). However, it is difficult to determine whether orfN4/P6 truly belong to the VPS10 family since these orf's lack the conserved consensus domain (CCD) of VPS10 (http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?uid=smart00602&version=v1.63). Both orf's N4/P6 and VPS10, however, have bacterial neuramidase (BNA) repeats ( rotein
rotein  ilies database of alignments and HMMs, Pfam, no. PF02012.) These repeats are mainly found in glycosyl hydrolases and extracellular proteins. The functions of these orf's have not been determined.
ilies database of alignments and HMMs, Pfam, no. PF02012.) These repeats are mainly found in glycosyl hydrolases and extracellular proteins. The functions of these orf's have not been determined.
The deduced a.a. sequence of orfP1 exhibited the DDE motif, a highly conserved motif in the IS3 family [34] and showed a close relationship to a putative tranposase-like protein of Mycobacterium abscessus strain 390R (GenBank No. AF513500). The activity of orfP1, however, has likely been lost because an in-frame stop codon was found in the middle of the coding region. Truncated transposase-like proteins were also seen in M. abscessus strain 390R [35].
3.4.4 Putative promoter
A putative promoter region (TCTCTCTTGACGCGCAAG TGAGATCTGGCTAACATTCTCT CAGCGGAGG), with the underlined base as an initiation point, was determined using Neural Network Promoter Prediction (NNPP) software available at the Berkeley Drosophila Genome Project (BDGP) web site (http://www.fruitfly.org/seq-tools/promoter.html) [36]. With a possible maximum score of 1.0, the putative promoter 57 bp upstream of the pdoB gene scored 0.97. A putative Shine-Dalgarno sequence, GGAG, was also found 10 bp upstream of the putative initiation codon of pdoB.
CAGCGGAGG), with the underlined base as an initiation point, was determined using Neural Network Promoter Prediction (NNPP) software available at the Berkeley Drosophila Genome Project (BDGP) web site (http://www.fruitfly.org/seq-tools/promoter.html) [36]. With a possible maximum score of 1.0, the putative promoter 57 bp upstream of the pdoB gene scored 0.97. A putative Shine-Dalgarno sequence, GGAG, was also found 10 bp upstream of the putative initiation codon of pdoB.
3.5 Expression analysis of the pyrene catabolic genes using RT-PCR
PCR was first performed using the RT-PCR primers specifically designed to differentiate genes from the nid and pdo loci (Table 2). Fig. 5 shows nidA was amplified using DNA extracted from the nid and pdo clones, S65, and PYR-1 at an annealing temperature of 65 °C. With nidA primers, a strong product of the expected size was seen when nidA clone DNA was used as a template, but only a very faint product was seen when the pdoA clone DNA was the template. With pdoA primers, a strong product was seen from the pdoA clone DNA template, but not from the nidA clone, or from PYR-1 DNA. Total RNA extracted from S65 cultures grown on pyrene, phenanthrene, or glucose were used to examine expression of the putative pyrene catabolic genes found in the two loci by RT-PCR. Analysis of the pyrene catabolic genes using specific primers showed that all the genes in both loci were transcribed under pyrene and phenanthrene induced conditions but not when strain S65 was grown on glucose (Table 4). Polycistronic transcription primer sets for the pdo locus (pdoB–pdoA, pdoA–pdoH, pdoH–orfP6, and orfP6–pdoX) showed that these genes are transcribed in one operon. For the nid locus, it was more difficult to design polycistronic transcription primers specific for the amplification of nidA C, and H. Therefore, transcription of the nidA, C, and H genes was determined individually. However, polycistronic transcripts of nidH and orfN4, and orfN4 and nidX were detected by RT-PCR, along with transcripts containing the individual genes.
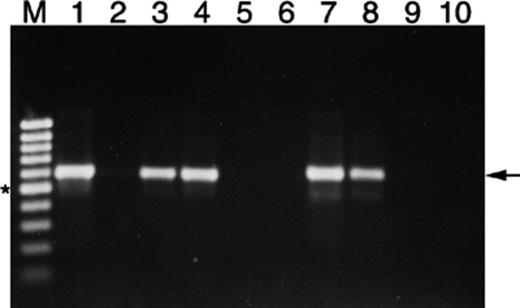
PCR amplification at an annealing temperature of 65 °C of plasmid DNA from the nid and pdo clones derived from Mycobacterium sp. S65 using primers designed specifically from the nidA gene. Lane M is a 100 bp molecular weight ladder, lanes 1–5 are the nid clone (1), the pdo clone (2), S65 (3), PYR-1 (4) and negative control (5), all amplified using the nidAF/nidAR primers, and lanes 6–10 are the nid clone (6), the pdo clone (7), S65 (8), PYR-1 (9) and negative control (10), amplified with the pdoAF/pdoAR primers. The star indicates the position of the 500 bp fragment in the molecular marker, and the arrow indicates the position of the expected amplicon.
| Amplicon numbera | Target gene | Expected amplicon size (bp) | Growth conditions | ||
| Pyrene | Phenanthrene | Glucose | |||
| nid locus (AF546904) | |||||
| 1. | nidA | 697 | +b | + | –c |
| 2. | nidC | 397 | + | + | NAd |
| 3. | nidH | 671 | + | + | NA |
| 4. | nidH/orf4 | 272 | + | + | NA |
| 5. | orf4 | 314 | + | + | NA |
| 6. | nidX | 545 | + | + | NA |
| 7. | orf4/nidX | 977 | + | + | NA |
| pdo locus (AF546905) | |||||
| 8. | pdoA | 644 | + | + | – |
| 9. | pdoB/pdoA | 1324 | + | + | – |
| 10. | pdoA/pdoH | 1039 | + | + | – |
| 11. | pdoH/orf6 | 1187 | + | + | – |
| 12. | orf6 | 487 | + | + | NA |
| 13. | pdoX | 215 | + | + | NA |
| 14. | orf6/pdoX | 977 | + | + | NA |
| Amplicon numbera | Target gene | Expected amplicon size (bp) | Growth conditions | ||
| Pyrene | Phenanthrene | Glucose | |||
| nid locus (AF546904) | |||||
| 1. | nidA | 697 | +b | + | –c |
| 2. | nidC | 397 | + | + | NAd |
| 3. | nidH | 671 | + | + | NA |
| 4. | nidH/orf4 | 272 | + | + | NA |
| 5. | orf4 | 314 | + | + | NA |
| 6. | nidX | 545 | + | + | NA |
| 7. | orf4/nidX | 977 | + | + | NA |
| pdo locus (AF546905) | |||||
| 8. | pdoA | 644 | + | + | – |
| 9. | pdoB/pdoA | 1324 | + | + | – |
| 10. | pdoA/pdoH | 1039 | + | + | – |
| 11. | pdoH/orf6 | 1187 | + | + | – |
| 12. | orf6 | 487 | + | + | NA |
| 13. | pdoX | 215 | + | + | NA |
| 14. | orf6/pdoX | 977 | + | + | NA |
Fragment number corresponds to the regions shown in Fig. 4.
Expected fragment produced.
No fragment produced.
NA, not analyzed.
| Amplicon numbera | Target gene | Expected amplicon size (bp) | Growth conditions | ||
| Pyrene | Phenanthrene | Glucose | |||
| nid locus (AF546904) | |||||
| 1. | nidA | 697 | +b | + | –c |
| 2. | nidC | 397 | + | + | NAd |
| 3. | nidH | 671 | + | + | NA |
| 4. | nidH/orf4 | 272 | + | + | NA |
| 5. | orf4 | 314 | + | + | NA |
| 6. | nidX | 545 | + | + | NA |
| 7. | orf4/nidX | 977 | + | + | NA |
| pdo locus (AF546905) | |||||
| 8. | pdoA | 644 | + | + | – |
| 9. | pdoB/pdoA | 1324 | + | + | – |
| 10. | pdoA/pdoH | 1039 | + | + | – |
| 11. | pdoH/orf6 | 1187 | + | + | – |
| 12. | orf6 | 487 | + | + | NA |
| 13. | pdoX | 215 | + | + | NA |
| 14. | orf6/pdoX | 977 | + | + | NA |
| Amplicon numbera | Target gene | Expected amplicon size (bp) | Growth conditions | ||
| Pyrene | Phenanthrene | Glucose | |||
| nid locus (AF546904) | |||||
| 1. | nidA | 697 | +b | + | –c |
| 2. | nidC | 397 | + | + | NAd |
| 3. | nidH | 671 | + | + | NA |
| 4. | nidH/orf4 | 272 | + | + | NA |
| 5. | orf4 | 314 | + | + | NA |
| 6. | nidX | 545 | + | + | NA |
| 7. | orf4/nidX | 977 | + | + | NA |
| pdo locus (AF546905) | |||||
| 8. | pdoA | 644 | + | + | – |
| 9. | pdoB/pdoA | 1324 | + | + | – |
| 10. | pdoA/pdoH | 1039 | + | + | – |
| 11. | pdoH/orf6 | 1187 | + | + | – |
| 12. | orf6 | 487 | + | + | NA |
| 13. | pdoX | 215 | + | + | NA |
| 14. | orf6/pdoX | 977 | + | + | NA |
Fragment number corresponds to the regions shown in Fig. 4.
Expected fragment produced.
No fragment produced.
NA, not analyzed.
4 Discussion
Mycobacterium sp. strain S65 has 99% rDNA nucleotide identity to Mycobacterium sp. strains T103 and T104 [31]. However, strain S65 can be distinguished from these strains by its ability to degrade HMW-PAHs. Strain S65 is also closely related to Mycobacterium sp. RJGII-135 (PAH-135) (98% identity) [37,38], and Mycobacterium vanbaalenni strain PYR-1 (97.8% identity) and both are HMW-PAH degrading bacteria, like S65. Strain S65 is different from strains RJGII-135 and PYR-1 in that it is able to grow at temperatures ranging from 10 °C to 30 °C (data not shown) but not at 35 °C, whereas strains RJGII-135 and PYR-1 are able to grow at 37 °C. Also, strain S65 does not grow in rich media such as TSB as do the other mycobacteria. Most importantly, the mineralization kinetics of strain S65 are distinctive to those of strains RJGII-135 and PYR-1 (discussed below). These differences suggest that strain S65 may be a new member of the PAH-degrading mycobacteria.
Mycobacterium sp. strain S65 was able to utilize phenanthrene and pyrene (60% mineralization), as sole carbon and energy sources, and to metabolize fluoranthene (20% mineralization). The pyrene degrading bacterium, M. vanbaalenii strain PYR-1 requires a low concentration of nutrient supplements in order to degrade pyrene and other LMW- and HMW-PAHs [12]. S65, however, does not require additional nutrients and is not able to degrade LMW-PAHs (Fig. 1). Usually, LMW-PAHs are more rapidly biodegraded than HMW-PAHs because of their lower hydrophobicity and lower electrochemical stability [39,40]. Also, because an increase in the molecular size and angularity of a PAH makes ring cleavage more difficult, HMW-PAHs are not usually as accessible as substrates. These general statements do not fit the substrate specificity seen in strain S65. Strain S65 has a preference for degrading cluster (pyrene) and angular PAH (phenanthrene and fluoranthene) molecules rather than linear (naphthalene, anthracene, and fluorene) PAH molecules. This substrate specificity of strain S65 is very similar to that of Mycobacterium flavenscens[5] and Mycobacterium sp. strain AP1 [10]. Strain S65, however, degrades pyrene, phenanthrene, and fluoranthene to a greater extent than M. flavenscens. One hypothesis is that strain S65 preferentially attacks a bay-region and a K-region of PAH molecules as the initial ring-cleavage site. Various proposed pyrene degradation pathways in a number of Mycobacterium strains support this hypothesis [4,6,10]. All the pathways reported in the literature agree that an initial hydroxylation of pyrene occurs at the C-4, C-5 positions, located in the K-region. A 3-day lag phase was observed in phenanthrene degradation by strain S65 (Fig. 1) despite the fact that phenanthrene was mineralized to the same extent as was pyrene. Because the same pyrene degradation genes were expressed during growth on phenanthrene and pyrene, the lag phase suggests that degradation of henanthrene may require additional enzyme induction in strain S65. It is possible that phenanthrene can be broken down by strain S65 because it has a structure similar to 4-phenanthroic acid, one of the intermediate metabolites of pyrene degradation. NidA, essential for the initial hydroxylation of pyrene, is predicted to be a transmembrane protein [21]. Since PAHs are hydrophobic and would tend to partition into cell membranes, it is likely that both NidA and PdoA are located within the membrane of S65. This kind of uptake of PAHs and the involvement of membrane-bound enzymes were also seen in Pseudomonas fluorescens LP6a [41].
The interaction of pyrene with other PAHs was investigated, under induced and non-induced conditions, in double and multiple substrate systems. From previous studies on the behaviour of multisubstrate PAH degradation [42], the biodegradation of the more degradable and abundant LMW-PAHs is reduced due to the presence of other PAHs (inhibitors), but the biodegradation of more recalcitrant HMW-PAHs is increased due to the simultaneous growth of the biomass on multiple substrates (enhancers). Under the non-induced condition, a lag phase was observed when phenanthrene was added as a co-substrate (Fig. 2(a)). With fluoranthene in the PAH mixture, pyrene mineralization did not reach the same extent as pyrene mineralization alone, suggesting that fluoranthene is acting as an inhibitor. Under the induced condition, the PAH mixture did not affect pyrene mineralization and the mineralization extent was as high as that observed during pyrene mineralization in the presence of benz[a]anthracene, benzo[a]pyrene, or phenanthrene (Fig. 2(b)). This suggests that the synergistic effects of other PAHs in the mixture overcame the inhibition of fluoranthene under the induced condition. These results support the study done by Bouchez et al. [42] that showed that co-metabolism of other PAHs is commonly inhibited when the added PAHs are more water-soluble than the test PAH. When less water-soluble PAHs, i.e., benz[a]anthracene or benzo[a]pyrene, were added as co-substrates, pyrene was mineralized to a greater extent than when more water-soluble PAHs, i.e., fluoranthene, were added.
Multiple signals in Southern hybridization of S65 genomic DNA with nidA of PYR-1 indicated that at least two loci encoding nidA or nidA homologues are present in the genome. As seen in most catabolic pathways in bacteria, the genes for pyrene degradation are also clustered (Fig. 4). Interestingly, the organization of the pyrene degrading genes is identical in both loci, suggesting that the two loci may be evolutionarily related. The horizontal transfer of catabolic genes (or functions) among different bacteria, or the duplication and movement of genes within the same bacterium, facilitated by transposable elements, is common in nature [43]. The presence of a transposase gene upstream of the pyrene catabolic genes in the pdo locus supports this hypothesis. This hypothesis is further supported by the high degree of nucleotide sequence similarity of the catabolic genes in the pdo and nid loci. The difference between the genes in the pdo locus and their corresponding genes in the nid locus is consistent at both the nucleotide and amino acid levels, except orfN4 and orfP6. The organization of the pyrene degrading genes in strain S65 is unique in comparison to what was previously reported [13–16,18,44]. In the pdo locus, a putative promoter site was found, but no nidD homologue was located upstream of the pdoB gene, as was found upstream of nidB in PYR-1. What is most intriguing and unique is the arrangement of two alcohol dehydrogenase genes adjacent to each other.
The localization of two alcohol dehydrogenase genes adjacent to each other and clustered within the pyrene catabolic genes created some confusion as to which gene may be involved in the dehydrogenation of cis-4,5-dihydroxy-4,5-dihydropyrene. Because most dehydrogenase genes involved in the initial dehydrogenation of aromatic rings belong to the short-chain alcohol dehydrogenase family (PROSITE accession number PDOC00060), nidC and pdoC are more likely to be cis-4,5-dihydroxy-4,5-dihydropyrene dehydrogenase genes. A function of the zinc-containing alcohol dehydrogenase gene, nidH and pdoH, was more difficult to predict because there are not many PAH degradation pathways that use a zinc-containing dehydrogenase. The only reference found was to xylB, involved in the dehydrogenation of benzyl alcohol to benzaldehyde in Pseudomonas putida[17]. Rehmann et al. [9] identified 2-carboxy-benzaldehyde as one of the intermediate metabolites in pyrene degradation by Mycobacterium sp. strain KR2. It is worth mentioning that an alcohol group attached directly to aromatic rings is typically dehydrogenated by enzymes in the short-chain alcohol dehydrogenase family, whereas alcohol groups attached to a substituent group on aromatic rings is dehydrogenated by enzymes in the long-chain alcohol dehydrogenase family. The second putative α subunit dioxygenases, NidX and PdoX were the closest to the hypothetical α subunit dioxygenase (ORFG1) in Sphingomonas sp. strain RW1 [32]. Strain RW1 is a dioxin-degrading bacterium and its dioxin dioxygenase gene, dxnA1, is capable of attacking the aromatic ring of dibenzofuran at a bridge position, resulting in the production of the metabolite, 2,2′,3-trihydroxybiphenyl (THB). This metabolite has a similar structure to the recently identified pyrene metabolite, 6,6′-dihydroxy-2,2′-biphenyl dicarboxylic acid, found in Mycobacterium sp. strain AP1 [10]. Therefore, NidX and PdoX may play a similar role in the pyrene degradation pathway to that of DxnA1 in the dioxin degradation pathway.
Expression studies using RT-PCR showed that the putative pyrene catabolic genes in both loci were expressed during growth on pyrene and phenanthrene, but not during growth on glucose, indicating that these genes were induced by PAH substrates. The possession of two PAH-degrading gene clusters (nid and pdo) may contribute to more efficient PAH degradation.
In this study, the pyrene degradation potential of Mycobacterium sp. strain S65 was characterized and genetic elements of the pathway for pyrene catabolism were identified. The pyrene catabolic genes, some of which appear to be novel, were identified in two distinct loci in the genome of S65. Two distinct transcripts were amplified from pyrene and phenanthrene grown cells indicating that both loci are induced during growth on PAH substrates. The pdoA gene had 89% sequence identity to the nidA gene but could be distinguished from nidA using a PCR primer designed from unique regions of the pdoA gene. Mycobacterium sp. strain PYR-1 did not appear to possess a pdoA gene as demonstrated by PCR analysis using the pdoA primers, although a recent study suggested the possibility that PYR-1 may contain another nidA-like homologue [45]. Having both the nidA and pdoA genes may explain why strain S65 appears to be an efficient pyrene degrader. The isolation and identification of pyrene metabolites will help shed light on the functions of these genes and the potential roles of these two distinct pyrene degradation loci.
Acknowledgements
We thank Dr. C. Cerniglia for supplying Mycobacterium vanbaalenni strain PYR-1, and Jamshid Jazestani for isolating and supplying strain S65. We also thank D. Labbé for technical support and D. Dignard for help using the Staden software.
References



