-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Müller, Marina Müller, Undine Behrendt, Leucine arylamidase activity in the phyllosphere and the litter layer of a Scots pine forest, FEMS Microbiology Ecology, Volume 47, Issue 2, February 2004, Pages 153–159, https://doi.org/10.1016/S0168-6496(03)00258-7
Close - Share Icon Share
Abstract
The activity of the leucine arylamidase (EC 3.4.11.2) was measured in washings of green needles from the canopy and dead needles from the litter of Scots pine throughout one year. It was highest in the litter and markedly higher in 2-year-old needles than in young ones, which were colonized by only a few bacteria. The leucine arylamidase activity largely arose from microbial epiphytes. Screenings for the potential of the enzyme activity among strains of a collection of phyllosphere microorganisms isolated from forest trees revealed that the leucine arylamidase activity was more abundant among bacteria (79%) and yeasts (57%) than among filamentous fungi, whereas the opposite was true in degrading complex proteins by proteinases.
1 Introduction
The phyllosphere is the external surface of the green physiologically active leaf acting as an environment for microorganisms [1]. It is colonized by communities of bacteria, yeasts and filamentous fungi [2]. During the last three decades, research on microbiology of phyllosphere comprised a broad spectrum of issues, e.g., physical and chemical conditions of microbial life on above-ground parts of plants, movement of microbes on the leaves, microbial population dynamics, interactions between microorganisms and their hosts, interactions among microorganisms on plant surfaces etc. (for reviews see the symposium books edited by Andrews and Hirano [3], Morris et al. [4] and Lindow et al. [5]). The phyllosphere represents the largest terrestrial habitat [6]. Nevertheless, it is still rather neglected with regard to ecosystem processes, such as nutrient cycling or energy flow.
A significant proportion of nitrogen in forest ecosystems arises from the canopy and is transported to soil via precipitation throughfall [7]. Throughfall (canopy drip plus stemflow) is an important source of dissolved organic nitrogen (DON) [8]. Annual DON fluxes in the throughfall of deciduous and coniferous forest ecosystems vary slightly between 3 and 4 kg N ha−1 yr−1[7,9]. DON fluxes in throughfall, however, have a high temporal and spatial variability even within one site [7] and little is known about the origin of DON in throughfall [10]. One suggestion is leaching from leaves and needles, another release by phyllosphere microorganisms. The main components of DON are amino N and amino sugar N. Most of the DON is fixed in amide bonds of proteins, peptides and amino acids, which are found in plant or microbial biomass and also, to a lesser extent, in amino sugars originating exclusively from microorganisms [11,12]. Free amino acids constitute only a small portion of total N in the solution [13]. However, there is no information on whether the DON runs through the canopy in a stable composition or is variable due to transformation processes. It is very likely that phyllosphere microorganisms use parts of DON for assimilation and metabolic processes. However, bacteria, the most numerous group of microorganisms on leaf surfaces, cannot assimilate proteins directly [14]. They depend on extracellular and/or cell-associated enzymes to liberate amino acids from polymeric, high-molecular-mass compounds [15]. These aminopeptidases may arise from the microorganisms themselves or from plant tissues.
Therefore, the aim of this study was to show that such enzyme activity is potentially present in the canopy of forest trees. The leucine arylamidase, which was demonstrated to be active in soils [16], was chosen as an example of aminopeptidases. Its activity was detected on green needles of Scots pine and compared with that on dead needles of pine litter. Additional tests were carried out to show that this enzyme activity is mainly due to phyllosphere microorganisms.
2 Materials and methods
2.1 Study site and sampling
The study site is a typical forest of the Mark Brandenburg with mainly Scots pine (Pinus sylvestris), scattered with beech (Fagus sylvatica) and oak (Quercus petraea) on sandy soil in the northeast German lowlands. The pine stand used for the investigations was approximately 25 years old. Branches from the lower strata and the periphery of the canopies of three trees were cut with pruning shears once a month from the emergence of new shoots in the middle of June 2001 until the end of June 2002. The trees were randomly distributed in the forest with distances of 30–50 m from each other. Additionally, dead needles from the litter layer underneath the canopies were sampled. Twigs of the current and the previous year as well as litter material from each tree were separately stored in sterile plastic bags at −20°C until laboratory processing. They were thawed at 5°C throughout the night before analyses.
2.2 Microbiological analyses
Needles of P. sylvestris were carefully ripped out of the twigs. Five grams of each sample of green needles of the twigs and 5 g of dead needles from the litter material was blended using a Stomacher lab blender in 145 ml sterile distilled water for 2 min. Leaf washings were logarithmically diluted in quarter-strength Ringer's solution (Merck) and spread-plated onto one-tenth-strength Tryptic soy agar (Merck; pH 7.2) supplemented with 0.4 g l−1 cycloheximide (Merck) to enumerate the number of aerobic heterotrophic bacteria in the sample, while suppressing the growth of fungi. Yeasts and filamentous fungi were grown on a Sabouraud–1% dextrose–1% maltose agar (Merck; pH 5.5) with 0.4 g l−1 chloramphenicol (Berlin-Chemie) to suppress bacterial growth. All plates were incubated at 25°C for 5 days.
2.3 Determination of leucine arylamidase activity on the surface of needles
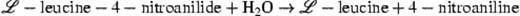
Its activity was determined in the washings of intact Scots pine green needles and dead needles from litter: 5 g (fresh matter) of green needles were incubated in 45 ml of a 0.14 M NaCl solution [17] containing 1.3 mM of the substrate L-leucine-4-nitroanilide (Merck), whilst 2 g (fresh matter) of dead needles were incubated in 58 ml of a 0.14 M NaCl solution with the same concentration of L-leucine-4-nitroanilide for 6 h at 30°C in a shaking incubator (120 rpm). Sample material was incubated in the NaCl solution without substrate in order to determine the blank values. Blanks of the substrate were measured after the incubation of 1.3 mM L-leucine-4-nitroanilide in the NaCl solution without sample material. The reaction was stopped by adding 7.7 ml of 10% (w/v) trichloroacetic acid to vessels with green needles and 10 ml of the same solution to those with dead needles. Solid particles were separated from the reaction solution by filtration through filter paper of moderate density. The extinction of free 4-nitroaniline was measured colorimetrically (405 nm) in eight repetitions on PS microplates (96 wells, U-shape; Greiner bio-one) using a microplate spectrophotometer (Spectramax Plus; Molecular Devices, Sunnyvale, CA, USA) and the software SOFTmaxPRO 3.12. The absorbances were related to a standard curve with the enzyme amino acid arylamidase M (EC 3.4.11.2) from porcine kidney (Boehringer, cat. no. 102 768) in the range of 0.01–3 mU. Results are expressed in mU g−1 related to the dry matter of needles. One unit was defined as follows: it hydrolyzes 1.0 μmol of L-leucine-4-nitroanilide to L-leucine and 4-nitroaniline within 6 h at 30°C.
2.4 Determination of leucine arylamidase activity in the tissue of needles
The surface of green and dead needles was sterilized as described by Araújo et al. [18]. For the preparation of needle extracts, 5 g (fresh matter) of green needles and 2 g of dead needles in about 20 ml of filter-sterilized 0.14 M NaCl solution containing 1.3 mM of the substrate L-leucine-4-nitroanilide were disintegrated with pestle and mortar for 5 min under sterile conditions. The same was done without substrate in the NaCl solution to determine the blank values of the samples. Then the suspensions were transferred into Erlenmeyer flasks and filled up to 45 ml (green needles) or 58 ml (dead needles) with the NaCl solution either with or without the substrate. The incubation and determination of enzyme activity followed as described in Section 2.3. The experiment was repeated in two independent trials.
2.5 Determination of leucine arylamidase activity in cultures of phyllosphere microorganisms
An enzyme assay with cultures of typical representatives of phyllosphere microorganisms should clarify which group is mainly responsible for this activity. For that purpose, 126 cultures of bacteria, 60 of yeasts and 51 cultures of filamentous fungi, which had been previously isolated from the phyllosphere of forest trees, were randomly chosen from our Institute's collection of microorganisms. Most aerobic heterotrophic bacteria were Gram-negative belonging to the genera Pseudomonas, Pantoea, Rahnella, Stenotrophomonas, Serratia and Enterobacter. Gram-positive bacteria belonged to the genera Clavibacter and Curtobacterium, to coryneform bacteria, aerobic spore formers and Micrococcaceae. Yeast representatives were pink-pigmented Sporobolomyces and Rhodotorula species and species of the genus Cryptococcus, while filamentous fungi were members of the genera Cladosporium, Alternaria, Stemphylium, Trichoderma, Fusarium, Aspergillus, Penicillium, Stachybotrys, Eurotium and Aureobasidium.
Since the photometric method, described in Section 2.3, was not sensitive enough for this purpose, we used the fluorogenic substrate L-leucine-4-methyl-7-coumarinylamide hydrochloride (leu-MCA; Fluka) for the fluorometric determination of enzyme activity in microbial cultures. The principle of this method is described by Hoppe [19].
Cultures of bacteria and yeasts were spread-plated on Standard II nutrient agar (Merck) and Sabouraud–1% dextrose–1% maltose agar (Merck), respectively, to form a cell lawn within 2 days at 25°C. The cells were picked up with a sterile cottonwool pad and transferred into a 0.14 M NaCl solution. The cell densities were adjusted according to McFarland standard #5 (BioMerieux). Additional tests were carried out with cell-free supernatants of liquid cultures of bacteria and yeasts to clarify whether or not the enzyme activities are cell-associated. Cells were removed by centrifugation (10 000×g for 10 min) and filter sterilization (0.25 μm).
Filamentous fungi were grown on potato dextrose agar (Merck) until a strong formation of spores. One milliliter of spore suspension in sterile distilled water was inoculated into 200 ml of dextrose–peptone–yeast extract–malt extract broth, pH 5.5 [20]. The cultures were grown in the liquid medium in a shaking incubator (60 rpm) at 25°C in darkness for about 5 days until strong production of fungal mass. Thereafter, the fungal biomass was separated by filtration through filter paper and filter sterilization (0.25 μm).
Stock solutions of the substrate leu-MCA were prepared to a concentration of 5 mM in deionized autoclaved water according to Chróst [15] and stored at −25°C. The stock solution was thawed at room temperature and diluted 1:10 with deionized sterile water to prepare an assay solution before use. Clear bottom and black polystyrene Microtiter plates (Dynex Technologies, Chantilly, VA, USA) were used for the incubation and measurement of the sample preparations. The wells of the plates were filled as follows: 150 μl of cell suspensions or cell-free supernatants of cultures, plus 100 μl of leu-MCA assay solution. The end concentration of the substrate was 200 μM. Each combination was pipetted in eight repetitions. After incubation for 3 h at 30°C in darkness, the fluorescence of 7-amino-4-methylcoumarin (AMC) separated from leucine was measured with a microplate fluorometer (Spectramax Gemini XS; Molecular Devices, Sunnyvale, CA, USA) and the software SOFTmaxPRO 3.12. The AMC fluorescence was determined at 350 nm for excitation and 445 nm for emission, corrected by culture and substrate blanks and related to a standard curve with the enzyme amino acid arylamidase M (EC 3.4.11.2) from porcine kidney (Boehringer, cat. no. 102 768) in the range of 0.01–0.5 mU. One unit was defined as follows: it hydrolyzes 1.0 μmol of L-leucine-4-methyl-7-coumarinylamide to L-leucine and 4-methyl-7-coumarinylamide within 3 h at 30°C. Results are expressed in mU ml−1 cell culture suspension or cell-free supernatant.
2.6 Detection of proteinase activity in bacterial and fungal cultures
Strains of bacteria, yeasts and filamentous fungi, which had also been used for the determination of leucine arylamidase activity (Section 2.5), were screened for proteinase activity on casein in skim milk agar [21]. Bacterial cultures grown overnight in Standard II nutrient broth (Sifin) and yeasts in yeast nitrogen broth (Difco) were streaked on the agar. Colonies of filamentous fungi were transplanted from potato dextrose agar (Merck) onto the skim milk agar. Clear zones in the white-colored casein indicated proteinase activity after 1–2 days (bacteria) or 3–5 days (fungi) of incubation at 25°C.
2.7 Statistical analyses
The numbers of microbial populations (colony-forming units, CFU) on needles were related to the needles’ dry matter (DM) before being log-transformed. Statistical analysis was performed using the SPSS software 8.0.0 (SPSS, Chicago, IL, USA). Differences in microbial colonization and leucine arylamidase activity were tested for significance by the non-parametric test Tamahane. The correlation coefficients were computed according to Pearson product–moment correlations.
3 Results
3.1 Microbial colonization of the needles and leucine arylamidase activity in needle washings
The colonization of the needles by bacteria, yeasts and filamentous fungi varied throughout the year. Compared with green needles the litter was always more heavily colonized (Fig. 1) (P<0.05), and the differences were most pronounced in the group of aerobic heterotrophic bacteria. Bacteria on green needles of the previous year had higher cell densities than those of the current year (P<0.05).
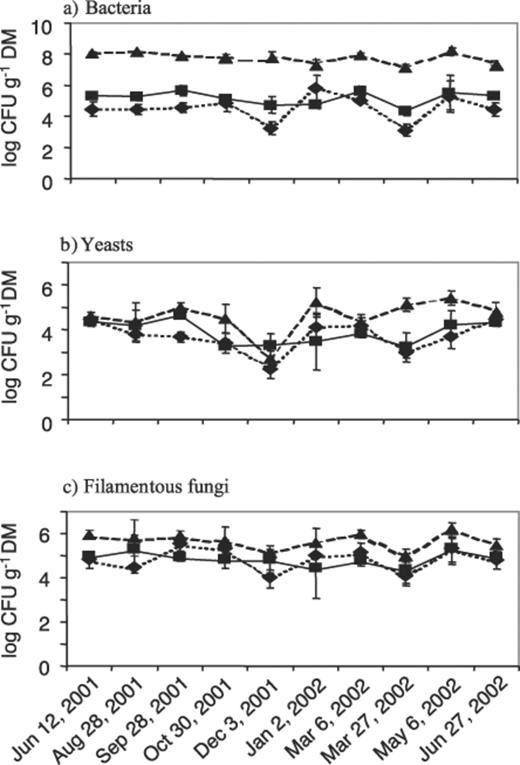
Cell densities of culturable microorganisms on green needles of P. sylvestris of the previous (▪, continuous line) and the current year (♦, dotted line) and on dead needles from the litter layer (▲, dashed line). Each point is the mean of three samples taken from separate trees, error bars indicate the standard deviations.
The activity of the leucine arylamidase was measurable throughout the year in washings of both the green needles and litter (Fig. 2). The highest activity of this enzyme was determined in litter, while the arylamidase on green needles of the previous year was more active than that on young needles (P<0.05), i.e., the relations here are similar to those of bacterial colonization (Fig. 1a). Correlations between the number of culturable microorganisms on the needles and enzyme activity were only found for bacteria (r2=0.402, P<0.01).
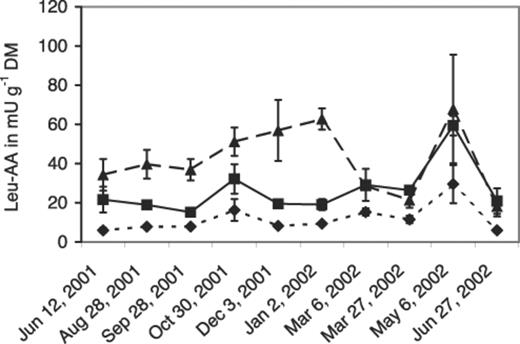
Activities of the leucine arylamidase (leu-AA) in washings of green needles of P. sylvestris of the previous (▪, continuous line) and the current year (♦, dotted line) and of dead needles from the litter layer (▲, dashed line). Each point is the mean of three samples taken from separate trees, error bars indicate the standard deviations.
The leucine arylamidase in green needles of the previous and the current year followed the same trend throughout the year with peaks in late autumn (time of strongest fall of dead needles) and much more pronounced peaks in spring (emergence of new shoots). In litter, an increase of enzyme activity was noticed from autumn until winter, before a drastic decrease and a new peak that appeared during the emergence of new shoots.
3.2 Leucine arylamidase activity in the tissue of the needles
The removal of epiphytic microorganisms by surface sterilization resulted in a loss of about 75% of the enzyme activity in the washings of green needles and of about 80% in dead needles from litter compared with the activity on untreated needles (Fig. 3). The additional disintegration of the green needles’ tissue led to similar results, whereas disintegrated dead needles lost 65% of the activity. This indicates that leucine arylamidase largely originated from microbial epiphytes and not from the plant tissue.
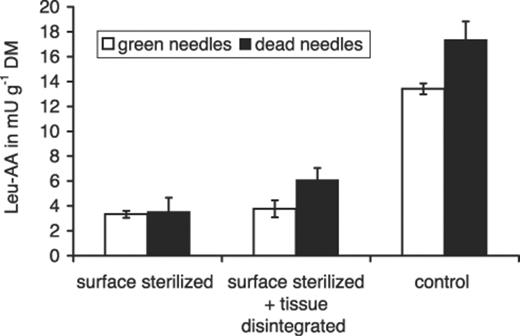
Leucine arylamidase activity (leu-AA) in washings of green needles from the canopy of P. sylvestris and dead needles from the litter layer that were surface-sterilized, surface-sterilized and tissue-disintegrated with pestle and mortar and not treated (control). Each column is the mean of two independent trials. Error bars show the highest and lowest values.
3.3 Proteolytic activity in cultures of phyllosphere microorganisms
Strains of bacteria and yeasts isolated from leaves of forest ecosystems were tested for their potential leucine arylamidase activity and their capability to degrade a complex protein (casein) in vitro. About 80% of all bacterial strains and more than half of the yeast strains were arylamidase-positive (Fig. 4). However, only 53% of the bacterial strains and only 12% of the yeasts were able to degrade casein. A combination of both traits was found in 42% of bacterial strains and in 3% of yeast strains.
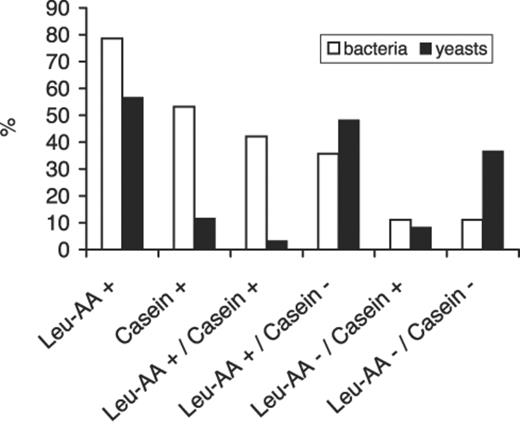
Strains of bacteria (n=126) and yeasts (n=60) isolated from leaves and needles of forest trees in tests for leucine arylamidase activity (leu-AA+/−) and for degrading casein in skim milk agar (casein+/−).
The mean cell-associated activity of leucine arylamidase-positive bacteria of 2.26±1.39 mU ml−1 was higher than that of the yeasts (1.99±1.40 mU ml−1). However, the difference was not significant because of high variations.
Due to the morphologic structure of filamentous fungi, they could only be tested for leucine arylamidase activity by means of the cell-free filtrates of their liquid cultures. One third of all tested strains showed leucine arylamidase activity (mean of 0.75±0.32 mU ml−1). Seventy-one percent of the isolates of filamentous fungi degraded casein in skim milk agar.
A mean activity of 0.87±0.69 mU ml−1 was determined in the cell-free filtrates of 26 out of 28 bacterial cultures that were arylamidase-active in the cell-associated assay. The same was found in four out of 15 cultures of yeasts with a mean activity of 1.24±0.30 mU ml−1. This indicates that with regard to bacteria and yeasts, the activity of leucine arylamidase in the phyllosphere is mainly cell-associated.
4 Discussion
Processes of N cycling in the soil and litter of forest ecosystems are well investigated [22]. The canopy as a habitat for microorganisms colonizing leaves has hardly been considered in this connection so far. However, Papen et al. [23] have recently detected an appreciable number of cells of chemolithoautotrophic ammonia- and nitrite-oxidizing bacteria in the phyllosphere of spruce trees. These bacteria have high substrate turnover rates, increase mineral atmospheric nitrogen uptake and, hence, the total nitrogen supply of the trees. This clearly indicates that microbial nitrogen cycling also takes place in the canopy of a forest.
The activity of the leucine arylamidase on needles of Scots pine might be another indication for nitrogen transformations in the phyllosphere. In this case, the enzyme is analogous to the leucine aminopeptidase (EC 3.4.11.1) that is used as an indicator for organic nitrogen transformations in aquatic microbial ecology [19,24–26]. According to the definition given by Tabatabai et al. [16], we called our enzyme leucine arylamidase, since p-leucine-nitroanilide was the applied substrate.
The activity of leucine arylamidase as measured in the washings of pine needles indicates the potential activity of an exopeptidase in the final part of the so-called proteolytic cascade, in which complex proteins are broken into smaller fragments by proteinase enzymes (endopeptidases). Furthermore, these peptides serve as substrates for exopeptidases that release terminal amino acids [27]. It was shown in our study that this exopeptidase activity is due to epiphytic microorganisms on the needles. The enzyme tests with microbial strains had no reference to the assay with leaf washings and could only show the potential of the microbial groups for the corresponding activity. However, they also confirmed the finding that the enzyme activity in needles’ washings correlated best with the abundance of bacteria on the needles. The quantification of microorganisms in ecosystems is preferentially determined by molecular-biological methods that are independent of previous cultivation [28]. However, Lindow [29] concluded from a few studies that the state of viable but nonculturable cells is much less pronounced in the phyllosphere than in water or soil. Furthermore, Grimes et al. [30] pointed out that about two thirds of all proteobacteria actively involved in geochemical processes are culturable. Hence, it seems legitimate that the quantification of microbial populations in connection with nutrient cycling in the phyllosphere is based on cultivation methods. Furthermore, it seems legitimate to conclude from the present results that the leucine arylamidase activity in the phyllosphere of Scots pine is mainly a result of bacterial metabolism.
The bacterial enzyme activity was predominantly cell-associated. It could also be detected in most cell-free filtrates of bacterial cultures, however, the mean activity was only 38.5% compared with that in cell suspensions. This is in accordance with Chróst [31] and Worm and Nybroe [32] who found that most of the leucine aminopeptidase activity in aquatic environments is associated with cells of heterotrophic bacteria.
In accordance with data from freshwater studies of Worm et al. [26], a little more than half of the bacterial isolates were also proteinase-active on skim milk agar. The most important step in the proteolytic cascade is the proteinase reaction by endopeptidases that provides substrates for further microbial degradation and utilization [33]. Therefore, microorganisms that only have exopeptidase but no proteinase activity are poor degraders of protein [34]. As demonstrated in this study, bacteria are more involved in proteinase processes in the phyllosphere than yeasts (Fig. 4). However, the portion of proteinase-active isolates was highest among filamentous fungi. Hence, fungi might be the pioneers with respect to proteolytic processes in the phyllosphere just as they are with respect to C cycling in the litter of leaves [35]. Otherwise, filamentous fungi are considered transient inhabitants of leaf surfaces being present predominantly as inactive spores [36].
Temporal dynamics of leucine arylamidase were recorded throughout the year with partly different trends in green needles and litter. Since aminopeptidase activity generally indicates N limitation in microbial communities [37], it could mean that, in our case, this nutrient is less available for microorganisms in litter during wintertime and on green needles in May. On the other hand, DON was detected in relatively high concentrations in forest floor solutions of a Norway spruce stand in winter and in the throughfall in summer [7], but nitrogen in high-molecular-mass compounds of DON is not available for bacteria [14]. This is probably the reason for the enhanced aminopeptidase activity in the litter and on the needles as it has already been observed in aquatic environments when DON occurred in higher concentrations [15,25]. It remains the aim of further studies to investigate the relations between seasonal leucine arylamidase activity and the flux of organic nitrogen in the canopy's throughfall of forest trees.
Acknowledgements
This study was supported by the Bundesministerium für Verbraucherschutz, Ernährung und Landwirtschaft and the Ministerium für Landwirtschaft, Umweltschutz und Raumordnung des Landes Brandenburg. We thank Mrs. Liane Lingk for excellent technical assistance and Dr. Jakob Worm, Royal Veterinary and Agricultural University, Frederiksberg, Denmark, for special advice.
References



