-
PDF
- Split View
-
Views
-
Cite
Cite
Dimitry Yu. Sorokin, Andreas Teske, Lesley A. Robertson, J. Gijs Kuenen, Anaerobic oxidation of thiosulfate to tetrathionate by obligately heterotrophic bacteria, belonging to the Pseudomonas stutzeri group, FEMS Microbiology Ecology, Volume 30, Issue 2, October 1999, Pages 113–123, https://doi.org/10.1111/j.1574-6941.1999.tb00640.x
Close - Share Icon Share
Abstract
A number of strains of heterotrophic bacteria were isolated from various environments on the basis of their potential to oxidize inorganic sulfur compounds to tetrathionate. The isolates were screened for the ability to oxidize thiosulfate under denitrifying conditions. Many of them could grow anaerobically with acetate and nitrate, and eight strains could oxidize thiosulfate to tetrathionate under the same conditions. In batch cultures with acetate as carbon and energy source, most active anaerobic thiosulfate oxidation occurred with N2O as electron acceptor. The level of anaerobic thiosulfate-oxidizing activity in cultures and cell suspensions supplied with nitrate correlated with the activity of nitrite reductase in cell suspensions. Some strains converted thiosulfate to tetrathionate equally well with nitrite, nitrate and N2O as electron acceptors. Others functioned best with N2O during anaerobic thiosulfate oxidation. The latter strains appeared to have a lower level of nitrite reductase activity. Thiosulfate oxidation under anaerobic conditions was much slower than in the presence of oxygen, and was obviously controlled by the availability of organic electron donor. The strains had DNA-DNA similarity levels higher than 30%. Sequence analysis of the 16S rRNA gene of four selected isolates showed their affiliation to specific genomovars of Pseudomonas stutzeri and the proposed new species, Pseudomonas balearica. As shown by 16S rRNA sequence analysis and DNA-DNA hybridization, the previously misnamed ‘Flavobacterium lutescens’ (ATCC 27951) is also a P. stutzeri strain which can oxidize thiosulfate to tetrathionate aerobically and anaerobically in the presence of N2O. The data suggest that tetrathionate-forming heterotrophic bacteria, in particular those belonging to the P. stutzeri‘superspecies’, can play a much more significant role in the biogeochemical cycles than was previously recognized.
1 Introduction
Heterotrophic bacteria able to oxidize thiosulfate to tetrathionate are widely distributed in soil and natural water [1–5]. Such bacteria dominate among sulfur-oxidizing communities in stratified bodies of water where there are low sulfide concentrations in the interface layer, such as the Black Sea. Large numbers of tetrathionate-forming bacteria have been found in freshwater lakes and specialized environments such as soda lakes and sulfide-oxidizing bioreactors ([6]; D. Sorokin, unpublished data). Most of the strains of tetrathionate-forming heterotrophic bacteria thus far investigated were able to reduce tetrathionate to thiosulfate under anaerobic conditions at the expense of organic electron donors [1,7,8]. Moreover, some marine isolates could grow anaerobically using tetrathionate as electron acceptor [9].
Despite numerous examples of the presence of such bacteria in various sulfide-containing environments, the reason for tetrathionate production by heterotrophic bacteria has, until recently, remained obscure. However, research on the physiology of some marine isolates has shown that tetrathionate-producing heterotrophs can benefit from thiosulfate oxidation when grown under organic carbon limitation [10]. Moreover, biological tetrathionate production also can drive chemical sulfide oxidation [10]. Heterotrophic tetrathionate-producers may thus be important in the natural sulfur cycle in some environments.
Denitrification among the known colorless sulfur bacteria is not common. Thiosulfate- or sulfide-dependent denitrification to N2 is best known in the obligately autotrophic Thiobacillus denitrificans and Thiomicrospira denitrificans[11], although it has been reported for the facultatively autotrophic Paracoccus pantotrophus (formerly Thiosphaera pantotropha[12,13]). Other colorless sulfur bacteria such as Thiobacillus thioparus can carry out partial denitrification, often to nitrite, while oxidizing thiosulfate [11].
Preliminary indications that some tetrathionate-forming heterotrophs were able to denitrify while oxidizing thiosulfate were of obvious interest. In particular, heterotrophic bacteria from marine sulfide-containing environments formed gas when grown anaerobically in sea water medium with thiosulfate and nitrate [2,3,14].
This paper describes thiosulfate-dependent denitrification by strains of tetrathionate-forming heterotrophic bacteria from different environments. These bacteria represent a compact phylogenetic group affiliated with Pseudomonas stutzeri.
2 Materials and methods
2.1 Strains
Forty-five strains of obligately heterotrophic, tetrathionate-forming bacteria isolated from sea and fresh water, and from a sulfide-oxidizing bioreactor [4], were screened for the ability to grow anaerobically with acetate and nitrate. Nineteen of the positive strains were then examined for the ability to oxidize thiosulfate while growing anaerobically with acetate as carbon and energy source, and nitrate, nitrite or N2O as electron acceptors. Of these, the eight strains shown in Table 1 were able to oxidize thiosulfate anaerobically, and were selected for further investigation. Table 1 also shows the depth and sulfide concentration in the water from which the isolates were taken.
| Strain | Source of inoculum | Depth (m) | Sulfide (μM) |
| ChG 4-1 | Black Sea, eastern halistase | 140 | traces |
| ChG 5-1 | Black Sea, western halistase | 120 | 1.2 |
| ChG 5-2 | Black Sea, south-west | 120 | 9.6 |
| ChG 5-3 | Black Sea, south | 120 | 0.9 |
| ChG 6-1 | Black Sea, western halistase | 140 | 20.5 |
| ChG 7-4 | Black Sea, south east | 220 | 62 |
| TG 31 | Volcanic Green Lake, Raul Island, Kermadek Archipelago, New Zealand | 6 | 17 |
| BG 2 | Sulfide-oxidizing bioreactor | – | up to 100 |
| Strain | Source of inoculum | Depth (m) | Sulfide (μM) |
| ChG 4-1 | Black Sea, eastern halistase | 140 | traces |
| ChG 5-1 | Black Sea, western halistase | 120 | 1.2 |
| ChG 5-2 | Black Sea, south-west | 120 | 9.6 |
| ChG 5-3 | Black Sea, south | 120 | 0.9 |
| ChG 6-1 | Black Sea, western halistase | 140 | 20.5 |
| ChG 7-4 | Black Sea, south east | 220 | 62 |
| TG 31 | Volcanic Green Lake, Raul Island, Kermadek Archipelago, New Zealand | 6 | 17 |
| BG 2 | Sulfide-oxidizing bioreactor | – | up to 100 |
| Strain | Source of inoculum | Depth (m) | Sulfide (μM) |
| ChG 4-1 | Black Sea, eastern halistase | 140 | traces |
| ChG 5-1 | Black Sea, western halistase | 120 | 1.2 |
| ChG 5-2 | Black Sea, south-west | 120 | 9.6 |
| ChG 5-3 | Black Sea, south | 120 | 0.9 |
| ChG 6-1 | Black Sea, western halistase | 140 | 20.5 |
| ChG 7-4 | Black Sea, south east | 220 | 62 |
| TG 31 | Volcanic Green Lake, Raul Island, Kermadek Archipelago, New Zealand | 6 | 17 |
| BG 2 | Sulfide-oxidizing bioreactor | – | up to 100 |
| Strain | Source of inoculum | Depth (m) | Sulfide (μM) |
| ChG 4-1 | Black Sea, eastern halistase | 140 | traces |
| ChG 5-1 | Black Sea, western halistase | 120 | 1.2 |
| ChG 5-2 | Black Sea, south-west | 120 | 9.6 |
| ChG 5-3 | Black Sea, south | 120 | 0.9 |
| ChG 6-1 | Black Sea, western halistase | 140 | 20.5 |
| ChG 7-4 | Black Sea, south east | 220 | 62 |
| TG 31 | Volcanic Green Lake, Raul Island, Kermadek Archipelago, New Zealand | 6 | 17 |
| BG 2 | Sulfide-oxidizing bioreactor | – | up to 100 |
1grew at 50°C
‘Flavobacterium lutescens’ LMD 95.190 was obtained from the Culture Collection of the Kluyver Laboratory for Biotechnology, Delft University of Technology, Delft, The Netherlands.
2.2 Growth experiments
For batch cultivation, all media contained the following (g l−1): NH4Cl 0.5, KH2PO4 2, K2HPO4 5, MgSO4·7H2O 0.4, CaCl2·2H2O 0.1, NaCl (for marine strains) 15, trace elements solution [15] 1 ml, yeast extract 0.1. Unless otherwise mentioned, 10–20 mM acetate, 10 mM thiosulfate and 10–20 mM of nitrite or nitrate were used. Anaerobiosis was achieved by flushing the medium with sterile argon for 10–15 min. When N2O was the electron acceptor, the gas phase in the flask was first displaced with argon before the liquid was flushed with sterile N2O for 2 min. Autotrophic growth was tested using the mineral medium without organic additions. The pH of the medium was adjusted to 7.8 with NaHCO3. The results presented are averages of at least five sets of data, with a variation of 5–10%.
Anaerobic continuous cultivation of strain TG 3 was performed in a laboratory fermenter with a pH and dO2 Biocontroller (Applicon, The Netherlands) and a 1.5-l working volume. The mineral base used for continuous culture was the same as for batch cultivation, except that the magnesium and calcium concentrations were halved. The pH was maintained at 7.5 by auto-titration with 1 M HCl. The medium bottles and the gas phase of the fermenter were kept under a gentle argon flow. Measurements began after 5–6 volume changes when a steady state had become established, and there was less than 1% difference between measurements made on different days. At least two sets of measurements were made per steady state, with an interval of at least 1 volume change between measurements. The results are presented as averages of the two measurements.
2.3 Experiments with washed cells
Cells were collected by centrifuging anaerobic cultures grown to stationary phase with acetate, thiosulfate and either nitrate, nitrite or nitrous oxide, as indicated in the text. Cells were washed with anaerobic 0.05 M potassium phosphate buffer, pH 7.5, containing 15 g l−1 NaCl, and resuspended in the same buffer at a concentration of about 2 mg protein per ml.
Experiments with washed cells were done in 40-ml flasks containing 5 ml of cell suspension and sealed with butyl rubber stoppers. To allow the cells to use the final traces of oxygen, flasks were incubated for 20 min after flushing with argon before the reaction was started by adding an anaerobic thiosulfate solution (10 mM). Nitrate and nitrite concentrations were 10–12 mM unless otherwise stated. 1-ml samples of the suspension were taken by syringe at intervals of 0.5–1 h. Bacteria were removed by rapid centrifuging, and the concentrations of sulfur and nitrogen compounds in the supernatant were analysed as described below. Subsequently, cultures were fixed with formaldehyde (3% v/v final concentration), and the flasks were then used for gas analysis. Aerobic thiosulfate and sulfide oxidation by washed cells was assayed by measuring the decrease of thiosulfate and sulfide concentrations, and with an oxygen electrode (Yellow Spring Instrument Co., Yellow Spring, OH, USA). Each experiment was repeated at least three times. The data in the tables represent averages of the results.
2.4 Cytochrome spectra
Extracts for cytochrome spectra were prepared from cells grown aerobically in batch culture with acetate and thiosulfate, or anaerobically with acetate, thiosulfate and nitrate. Cells were centrifuged, washed and resuspended in 0.05 M potassium phosphate buffer (pH 7.5) with 15 g l−1 NaCl and disrupted by sonication. Unbroken cells and cells debris were removed by additional centrifugation at 15 000×g for 10 min. Solid dithionite was used for the complete reduction of cytochromes, and bubbling with air for their oxidation. CO difference spectra were made by comparing the results from dithionite-reduced preparations with those obtained after pure CO had been bubbled through the preparations in the cuvettes. The difference cytochrome spectra were recorded with a Pye-Unicam 1800 (UK) spectrophotometer.
2.5 Chemical analysis
Nitrite was assayed with N-(1-naphthyl) ethylene diamine dihydrochloride [16], and nitrate by the micro-salicylate method [17] after removal of nitrite by addition of solid sulfaminic acid. Thiosulfate and tetrathionate were measured by cyanolysis [18]. In separate experiments, it was found that nitrite did not interfere with the cyanolytic determination of sulfur compounds. Sulfide was precipitated with 2% zinc acetate, the sediment was removed by centrifuging, rinsed with distilled water to remove nitrite, and then assayed by the methylene blue method [19]. The N2O concentration in the gas phase was analyzed by gas chromatography (Fison Instruments, Poropaq Q column, Ni-63 electron-capture detector). Total N2O production per flask was calculated assuming a gas–liquid partition coefficient of 1.875.
2.6 DNA-DNA hybridization
DNA was isolated and purified according to the standard protocol described by Marmur et al. [20]. DNA-DNA hybridization was performed by two independent procedures. Most of the hybridization and G+C mol% measurements were done using the thermal denaturation technique [21]. Where there was a low similarity level (less than 50%), the nick translation technique with 3H-labelled DNA was also used [22].
2.7 Phylogenetic analysis
For 16S rRNA sequencing and subsequent phylogenetic analysis, DNA was isolated from pure cultures by phenol extraction [23] and used for PCR amplification of 16S rRNA genes as described previously [24]. PCR products were sequenced directly using the non-radioactive Taq Dyedeoxy Terminator Cycle Sequencing Kit and Applied Biosystems 373 sequencer (Applied Biosystems, Foster City, CA). In addition, the 3′ ends of the 16S rDNA amplificates were re-sequenced manually, as described previously [24], to check the often minute sequence differences between the strains. The Ribosomal Database Project [25] and GenBank [26] were searched for sequences of closely related strains. Sequences were aligned according to secondary structure in the sequence editor SeqPup [27]. Jukes-Cantor distance trees were calculated with the programs DNADIST and FITCH, as implemented in the phylogeny package PHYLIP 3.5c [28]. The sequences have GenBank accession numbers AF054933 (ChG 5-3), AF054934 (BG-2), AF054935 (ChG 5-2) and AF054936 (TG-3).
3 Results
3.1 Thiosulfate and sulfide oxidation by cultures and cell suspensions
All selected tetrathionate-forming heterotrophic isolates (Table 1) could be grown as denitrifiers on media containing acetate (±thiosulfate) as well as nitrate, nitrite or N2O, resulting in vigorous production of nitrogen gas which was clearly visible in cultures grown in media including 0.5% agar. In the presence of nitrate or N2O, thiosulfate was oxidized exclusively to tetrathionate (Table 2). If nitrite was the electron acceptor, active growth was observed, but thiosulfate was not converted. When acetate or the electron acceptors were not provided, the bacteria did not grow or oxidize thiosulfate. Although tetrathionate was also the sole product of thiosulfate oxidation in aerobic cultures, the extent of thiosulfate oxidation was significantly higher than in the absence of oxygen (Table 2).
Oxidation of thiosulfate in batch cultures of tetrathionate-forming heterotrophic bacteria grown with different electron acceptors
| Strain | Product formation (mM) | ||||||
| 1 | 2 | 3 | 4 | 5 | |||
| NO2− | S4O62− | NO2− | S4O62− | S4O62− | S4O62− | S4O62− | |
| BG 2 | 0 | 1.0 | 0 | 2.0 | 0 | 4.5 | 9.5 |
| TG 3 | 1–3 | 4–5 | 2–4 | 4.2–5.5 | 0.5 | 4.1 | 9.2 |
| ChG 5-2 | 0 | 2.1 | 0.1 | 5.8 | 0 | 5.5 | 9.5 |
| ChG 6-1 | 1.2 | 1.5 | 6.7 | 3.9 | 0.9 | 4.1 | 9.0 |
| ChG 5-1 | – | – | 4.8 | 2.1 | – | 5.1 | 9.6 |
| ChG 5-3 | – | – | 1.2 | 2.9 | – | 3.8 | 9.6 |
| ChG 4-1 | – | – | 1.4 | 3.1 | – | 4.0 | 8.4 |
| ChG 7-4 | – | – | 0.5 | 3.5 | – | 4.8 | 8.5 |
| Electron donors: acetate 10 mM; thiosulfate: anaerobic 12 mM, aerobic 20 mM; incubation 48 h at 30°C. Column 1: NO3−, 10 mM; column 2: NO3−, 20 mM; column 3: NO2−, 10–20 mM; column 4, N2O; column 5, O2. | |||||||
| Strain | Product formation (mM) | ||||||
| 1 | 2 | 3 | 4 | 5 | |||
| NO2− | S4O62− | NO2− | S4O62− | S4O62− | S4O62− | S4O62− | |
| BG 2 | 0 | 1.0 | 0 | 2.0 | 0 | 4.5 | 9.5 |
| TG 3 | 1–3 | 4–5 | 2–4 | 4.2–5.5 | 0.5 | 4.1 | 9.2 |
| ChG 5-2 | 0 | 2.1 | 0.1 | 5.8 | 0 | 5.5 | 9.5 |
| ChG 6-1 | 1.2 | 1.5 | 6.7 | 3.9 | 0.9 | 4.1 | 9.0 |
| ChG 5-1 | – | – | 4.8 | 2.1 | – | 5.1 | 9.6 |
| ChG 5-3 | – | – | 1.2 | 2.9 | – | 3.8 | 9.6 |
| ChG 4-1 | – | – | 1.4 | 3.1 | – | 4.0 | 8.4 |
| ChG 7-4 | – | – | 0.5 | 3.5 | – | 4.8 | 8.5 |
| Electron donors: acetate 10 mM; thiosulfate: anaerobic 12 mM, aerobic 20 mM; incubation 48 h at 30°C. Column 1: NO3−, 10 mM; column 2: NO3−, 20 mM; column 3: NO2−, 10–20 mM; column 4, N2O; column 5, O2. | |||||||
Oxidation of thiosulfate in batch cultures of tetrathionate-forming heterotrophic bacteria grown with different electron acceptors
| Strain | Product formation (mM) | ||||||
| 1 | 2 | 3 | 4 | 5 | |||
| NO2− | S4O62− | NO2− | S4O62− | S4O62− | S4O62− | S4O62− | |
| BG 2 | 0 | 1.0 | 0 | 2.0 | 0 | 4.5 | 9.5 |
| TG 3 | 1–3 | 4–5 | 2–4 | 4.2–5.5 | 0.5 | 4.1 | 9.2 |
| ChG 5-2 | 0 | 2.1 | 0.1 | 5.8 | 0 | 5.5 | 9.5 |
| ChG 6-1 | 1.2 | 1.5 | 6.7 | 3.9 | 0.9 | 4.1 | 9.0 |
| ChG 5-1 | – | – | 4.8 | 2.1 | – | 5.1 | 9.6 |
| ChG 5-3 | – | – | 1.2 | 2.9 | – | 3.8 | 9.6 |
| ChG 4-1 | – | – | 1.4 | 3.1 | – | 4.0 | 8.4 |
| ChG 7-4 | – | – | 0.5 | 3.5 | – | 4.8 | 8.5 |
| Electron donors: acetate 10 mM; thiosulfate: anaerobic 12 mM, aerobic 20 mM; incubation 48 h at 30°C. Column 1: NO3−, 10 mM; column 2: NO3−, 20 mM; column 3: NO2−, 10–20 mM; column 4, N2O; column 5, O2. | |||||||
| Strain | Product formation (mM) | ||||||
| 1 | 2 | 3 | 4 | 5 | |||
| NO2− | S4O62− | NO2− | S4O62− | S4O62− | S4O62− | S4O62− | |
| BG 2 | 0 | 1.0 | 0 | 2.0 | 0 | 4.5 | 9.5 |
| TG 3 | 1–3 | 4–5 | 2–4 | 4.2–5.5 | 0.5 | 4.1 | 9.2 |
| ChG 5-2 | 0 | 2.1 | 0.1 | 5.8 | 0 | 5.5 | 9.5 |
| ChG 6-1 | 1.2 | 1.5 | 6.7 | 3.9 | 0.9 | 4.1 | 9.0 |
| ChG 5-1 | – | – | 4.8 | 2.1 | – | 5.1 | 9.6 |
| ChG 5-3 | – | – | 1.2 | 2.9 | – | 3.8 | 9.6 |
| ChG 4-1 | – | – | 1.4 | 3.1 | – | 4.0 | 8.4 |
| ChG 7-4 | – | – | 0.5 | 3.5 | – | 4.8 | 8.5 |
| Electron donors: acetate 10 mM; thiosulfate: anaerobic 12 mM, aerobic 20 mM; incubation 48 h at 30°C. Column 1: NO3−, 10 mM; column 2: NO3−, 20 mM; column 3: NO2−, 10–20 mM; column 4, N2O; column 5, O2. | |||||||
The most active anaerobic oxidation of thiosulfate occurred with N2O as electron acceptor, with more than 90% of the thiosulfate being converted to tetrathionate. When nitrate was supplied in excess, more thiosulfate was oxidized, but nitrite accumulation prevented complete conversion. If the nitrate concentration was limiting, acetate was preferentially oxidized. The most active anaerobic thiosulfate oxidation with nitrate as electron acceptor was found with strain TG 3. The formation of tetrathionate proceeded at the same time as growth and nitrate reduction (Fig. 1), and was highest at a nitrate:acetate ratio of 2:1. Less nitrite accumulated in nitrate-reducing cultures containing thiosulfate and acetate, presumably because of the changed electron donor:acceptor ratio.
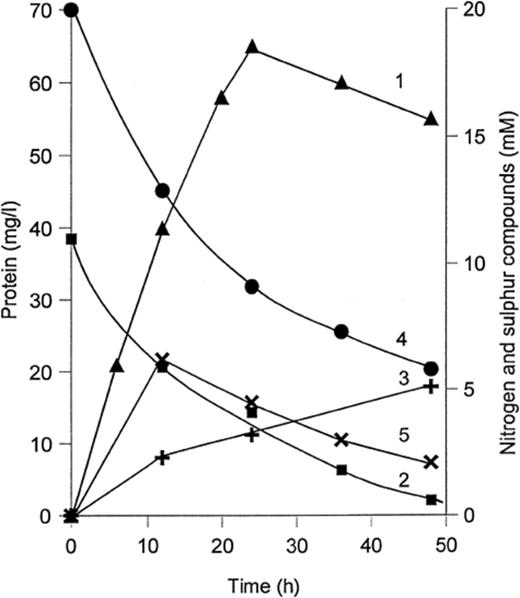
Growth and thiosulfate oxidation in anaerobic batch culture of strain TG 3 with acetate(10 mM) and nitrate (20 mM). 1: growth, mg protein l−1; 2: thiosulfate; 3: tetrathionate; 4: nitrate; 5: nitrite.
As might be expected, there was a clear relationship between the amount of available electron acceptor and tetrathionate production in acetate-limited anaerobic continuous cultures of strain TG 3 (Table 3). These experiments also demonstrated that the culture could not gain sufficient energy from thiosulfate oxidation to increase the growth yield observed with acetate alone.
Anaerobic growth and thiosulfate oxidation in acetate-limited continuous culture of strain TG 3 (D=0.1 h−1, temp. 30°C, pH 7.5)
| Influent concentration (mM) | Biomass (mg protein l−1) | Concentration in steady-state culture (mM) | |||||
| Acetate | NO3− | S2O32− | NO3− | NO2− | S2O32− | S4O62− | |
| 10 | 10.8 | 0 | 56 | 0 | 0 | 0 | 0 |
| 10 | 10.8 | 5.9 | 64 | 0 | 0 | 2.6 | 1.5 |
| 10 | 15.3 | 5.9 | 55 | 1.5 | 1.5 | 0.3 | 2.6 |
| 10 | 15.3 | 10.8 | 54 | 5.0 | 5.0 | 0.5 | 4.9 |
| 15 | 15.3 | 10.8 | 70 | 0 | 0 | 4.4 | 3.0 |
| Influent concentration (mM) | Biomass (mg protein l−1) | Concentration in steady-state culture (mM) | |||||
| Acetate | NO3− | S2O32− | NO3− | NO2− | S2O32− | S4O62− | |
| 10 | 10.8 | 0 | 56 | 0 | 0 | 0 | 0 |
| 10 | 10.8 | 5.9 | 64 | 0 | 0 | 2.6 | 1.5 |
| 10 | 15.3 | 5.9 | 55 | 1.5 | 1.5 | 0.3 | 2.6 |
| 10 | 15.3 | 10.8 | 54 | 5.0 | 5.0 | 0.5 | 4.9 |
| 15 | 15.3 | 10.8 | 70 | 0 | 0 | 4.4 | 3.0 |
Anaerobic growth and thiosulfate oxidation in acetate-limited continuous culture of strain TG 3 (D=0.1 h−1, temp. 30°C, pH 7.5)
| Influent concentration (mM) | Biomass (mg protein l−1) | Concentration in steady-state culture (mM) | |||||
| Acetate | NO3− | S2O32− | NO3− | NO2− | S2O32− | S4O62− | |
| 10 | 10.8 | 0 | 56 | 0 | 0 | 0 | 0 |
| 10 | 10.8 | 5.9 | 64 | 0 | 0 | 2.6 | 1.5 |
| 10 | 15.3 | 5.9 | 55 | 1.5 | 1.5 | 0.3 | 2.6 |
| 10 | 15.3 | 10.8 | 54 | 5.0 | 5.0 | 0.5 | 4.9 |
| 15 | 15.3 | 10.8 | 70 | 0 | 0 | 4.4 | 3.0 |
| Influent concentration (mM) | Biomass (mg protein l−1) | Concentration in steady-state culture (mM) | |||||
| Acetate | NO3− | S2O32− | NO3− | NO2− | S2O32− | S4O62− | |
| 10 | 10.8 | 0 | 56 | 0 | 0 | 0 | 0 |
| 10 | 10.8 | 5.9 | 64 | 0 | 0 | 2.6 | 1.5 |
| 10 | 15.3 | 5.9 | 55 | 1.5 | 1.5 | 0.3 | 2.6 |
| 10 | 15.3 | 10.8 | 54 | 5.0 | 5.0 | 0.5 | 4.9 |
| 15 | 15.3 | 10.8 | 70 | 0 | 0 | 4.4 | 3.0 |
The experiments with washed cells aimed to eliminate any potential influence of organic electron donors, and to clarify the roles of the different stable nitrogen oxides in anaerobic thiosulfate oxidation. In general, the strains could be divided into two main groups on the basis of their response to nitrite. Group 1 includes strains ChG 4-1, ChG 5-2, ChG 5-3 and ChG 7-4, all of which had highly active cytochrome cd1 nitrite reductase (see below). Group 2 (strains ChG 5-1, ChG 6-1 and BG 2) is characterized by relatively lower nitrite reductase activity, and no detectable cytochrome cd1. The behavior of the two groups during experiments with washed cells was quite different.
Group 1 strains (an example is given in Table 4) did not accumulate nitrite during the anaerobic reduction of nitrate, but produced substantial amounts of N2O when incubated anaerobically with nitrite and thiosulfate. The most favorable electron acceptors for anaerobic thiosulfate oxidation by these strains were nitrite (washed cells) and N2O (batch cultures). Acetate inhibited anaerobic thiosulfate oxidation by washed cells from this group. Group 2 strains (Table 5) accumulated substantial amounts of nitrite when growing anaerobically with acetate and nitrate, but did not produce N2O when washed cells were incubated anaerobically with nitrite and thiosulfate. N2O was the best electron acceptor for anaerobic thiosulfate oxidation by this group, and acetate did not inhibit anaerobic thiosulfate oxidation by the washed cells. In some experiments it even stimulated the reaction. Strain TG 3 combined properties from both groups.
Changes in the concentrations (mM) of tetrathionate and nitrogen oxides during anaerobic incubation of washed cells of strain ChG 5-2 (group 1) grown with different electron acceptors, and with either thiosulfate or a mixture of thiosulfate and acetate as electron donors
| Electron donor/acceptor | Cells grown with NO3− | Cells grown with NO2− | Cells grown with N2O | |||||||||||
| ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | |||
| S2O32−: | ||||||||||||||
| NO3− | +1.9 | −3.5 | 0 | +0.35 | +1.0 | −0.5 | 0 | +0.08 | +0.3 | −0.9 | 0 | +0.05 | ||
| NO2− | +3.2 | nd | −3.8 | +1.6 | +4.1 | nd | −4.9 | +2.43 | +0.5 | nd | −0.05 | +0.20 | ||
| N2O | +1.0 | nd | nd | nd | +1.2 | nd | nd | nd | +2.8 | nd | nd | nd | ||
| O2 | +4.5 | nd | nd | nd | +5.0 | nd | nd | nd | +3.4 | nd | nd | nd | ||
| S2O32−+acetate: | ||||||||||||||
| NO3− | +0.5 | −6.9 | 0 | +0.05 | +0.2 | −2.1 | 0 | +0.42 | +0.2 | −3.8 | 0 | +0.01 | ||
| NO2− | +1.2 | nd | −7.8 | +0.06 | +0.2 | nd | −11.5 | +0.11 | +0.2 | nd | −0.5 | +0.15 | ||
| N2O | +0.2 | nd | nd | nd | +0.2 | nd | nd | nd | +0.2 | nd | nd | nd | ||
| Experimental conditions: buffer, 0.05 M potassium phosphate+15 g l−1 NaCl, pH 7.5; biomass, 0.25 mg protein ml−1; incubation time, 2 h (with O2 1 h); S2O32−, 10.5 mM; NO3−, 11 mM; NO2−, 11.5 mM; N2O, 20 mM (saturation); acetate, 10 mM. nd=not detected; −=consumed; +=produced. | ||||||||||||||
| Electron donor/acceptor | Cells grown with NO3− | Cells grown with NO2− | Cells grown with N2O | |||||||||||
| ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | |||
| S2O32−: | ||||||||||||||
| NO3− | +1.9 | −3.5 | 0 | +0.35 | +1.0 | −0.5 | 0 | +0.08 | +0.3 | −0.9 | 0 | +0.05 | ||
| NO2− | +3.2 | nd | −3.8 | +1.6 | +4.1 | nd | −4.9 | +2.43 | +0.5 | nd | −0.05 | +0.20 | ||
| N2O | +1.0 | nd | nd | nd | +1.2 | nd | nd | nd | +2.8 | nd | nd | nd | ||
| O2 | +4.5 | nd | nd | nd | +5.0 | nd | nd | nd | +3.4 | nd | nd | nd | ||
| S2O32−+acetate: | ||||||||||||||
| NO3− | +0.5 | −6.9 | 0 | +0.05 | +0.2 | −2.1 | 0 | +0.42 | +0.2 | −3.8 | 0 | +0.01 | ||
| NO2− | +1.2 | nd | −7.8 | +0.06 | +0.2 | nd | −11.5 | +0.11 | +0.2 | nd | −0.5 | +0.15 | ||
| N2O | +0.2 | nd | nd | nd | +0.2 | nd | nd | nd | +0.2 | nd | nd | nd | ||
| Experimental conditions: buffer, 0.05 M potassium phosphate+15 g l−1 NaCl, pH 7.5; biomass, 0.25 mg protein ml−1; incubation time, 2 h (with O2 1 h); S2O32−, 10.5 mM; NO3−, 11 mM; NO2−, 11.5 mM; N2O, 20 mM (saturation); acetate, 10 mM. nd=not detected; −=consumed; +=produced. | ||||||||||||||
Changes in the concentrations (mM) of tetrathionate and nitrogen oxides during anaerobic incubation of washed cells of strain ChG 5-2 (group 1) grown with different electron acceptors, and with either thiosulfate or a mixture of thiosulfate and acetate as electron donors
| Electron donor/acceptor | Cells grown with NO3− | Cells grown with NO2− | Cells grown with N2O | |||||||||||
| ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | |||
| S2O32−: | ||||||||||||||
| NO3− | +1.9 | −3.5 | 0 | +0.35 | +1.0 | −0.5 | 0 | +0.08 | +0.3 | −0.9 | 0 | +0.05 | ||
| NO2− | +3.2 | nd | −3.8 | +1.6 | +4.1 | nd | −4.9 | +2.43 | +0.5 | nd | −0.05 | +0.20 | ||
| N2O | +1.0 | nd | nd | nd | +1.2 | nd | nd | nd | +2.8 | nd | nd | nd | ||
| O2 | +4.5 | nd | nd | nd | +5.0 | nd | nd | nd | +3.4 | nd | nd | nd | ||
| S2O32−+acetate: | ||||||||||||||
| NO3− | +0.5 | −6.9 | 0 | +0.05 | +0.2 | −2.1 | 0 | +0.42 | +0.2 | −3.8 | 0 | +0.01 | ||
| NO2− | +1.2 | nd | −7.8 | +0.06 | +0.2 | nd | −11.5 | +0.11 | +0.2 | nd | −0.5 | +0.15 | ||
| N2O | +0.2 | nd | nd | nd | +0.2 | nd | nd | nd | +0.2 | nd | nd | nd | ||
| Experimental conditions: buffer, 0.05 M potassium phosphate+15 g l−1 NaCl, pH 7.5; biomass, 0.25 mg protein ml−1; incubation time, 2 h (with O2 1 h); S2O32−, 10.5 mM; NO3−, 11 mM; NO2−, 11.5 mM; N2O, 20 mM (saturation); acetate, 10 mM. nd=not detected; −=consumed; +=produced. | ||||||||||||||
| Electron donor/acceptor | Cells grown with NO3− | Cells grown with NO2− | Cells grown with N2O | |||||||||||
| ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | |||
| S2O32−: | ||||||||||||||
| NO3− | +1.9 | −3.5 | 0 | +0.35 | +1.0 | −0.5 | 0 | +0.08 | +0.3 | −0.9 | 0 | +0.05 | ||
| NO2− | +3.2 | nd | −3.8 | +1.6 | +4.1 | nd | −4.9 | +2.43 | +0.5 | nd | −0.05 | +0.20 | ||
| N2O | +1.0 | nd | nd | nd | +1.2 | nd | nd | nd | +2.8 | nd | nd | nd | ||
| O2 | +4.5 | nd | nd | nd | +5.0 | nd | nd | nd | +3.4 | nd | nd | nd | ||
| S2O32−+acetate: | ||||||||||||||
| NO3− | +0.5 | −6.9 | 0 | +0.05 | +0.2 | −2.1 | 0 | +0.42 | +0.2 | −3.8 | 0 | +0.01 | ||
| NO2− | +1.2 | nd | −7.8 | +0.06 | +0.2 | nd | −11.5 | +0.11 | +0.2 | nd | −0.5 | +0.15 | ||
| N2O | +0.2 | nd | nd | nd | +0.2 | nd | nd | nd | +0.2 | nd | nd | nd | ||
| Experimental conditions: buffer, 0.05 M potassium phosphate+15 g l−1 NaCl, pH 7.5; biomass, 0.25 mg protein ml−1; incubation time, 2 h (with O2 1 h); S2O32−, 10.5 mM; NO3−, 11 mM; NO2−, 11.5 mM; N2O, 20 mM (saturation); acetate, 10 mM. nd=not detected; −=consumed; +=produced. | ||||||||||||||
Anaerobic thiosulfate oxidation by washed cells of strain BG 2 (group 2) grown anaerobically with acetate, thiosulfate and nitrate
| Electron | Changes in tetrathionate and nitrogen oxide concentration (mM) during incubation of cell acceptor suspension | |||||||
| no acetate | +10 mM acetate | |||||||
| ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62 | ΔNO3− | ΔNO2− | ΔN2O | |
| NO3− | 1.3 | −1.0 | +2.6 | 0.01 | 1.1 | −8.2 | +7.8 | 0.03 |
| NO2− | 0.25 | nd | −1.5 | 0.02 | 0.9 | nd | −4.6 | 0.06 |
| N2O | 2.0 | nd | nd | nd | 1.3 | nd | nd | nd |
| O2 | 4.1 | nd | nd | nd | 4.0 | nd | nd | nd |
| Experimental conditions as indicated in Table 4. nd=not detected; −=consumed; +=produced. | ||||||||
| Electron | Changes in tetrathionate and nitrogen oxide concentration (mM) during incubation of cell acceptor suspension | |||||||
| no acetate | +10 mM acetate | |||||||
| ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62 | ΔNO3− | ΔNO2− | ΔN2O | |
| NO3− | 1.3 | −1.0 | +2.6 | 0.01 | 1.1 | −8.2 | +7.8 | 0.03 |
| NO2− | 0.25 | nd | −1.5 | 0.02 | 0.9 | nd | −4.6 | 0.06 |
| N2O | 2.0 | nd | nd | nd | 1.3 | nd | nd | nd |
| O2 | 4.1 | nd | nd | nd | 4.0 | nd | nd | nd |
| Experimental conditions as indicated in Table 4. nd=not detected; −=consumed; +=produced. | ||||||||
Anaerobic thiosulfate oxidation by washed cells of strain BG 2 (group 2) grown anaerobically with acetate, thiosulfate and nitrate
| Electron | Changes in tetrathionate and nitrogen oxide concentration (mM) during incubation of cell acceptor suspension | |||||||
| no acetate | +10 mM acetate | |||||||
| ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62 | ΔNO3− | ΔNO2− | ΔN2O | |
| NO3− | 1.3 | −1.0 | +2.6 | 0.01 | 1.1 | −8.2 | +7.8 | 0.03 |
| NO2− | 0.25 | nd | −1.5 | 0.02 | 0.9 | nd | −4.6 | 0.06 |
| N2O | 2.0 | nd | nd | nd | 1.3 | nd | nd | nd |
| O2 | 4.1 | nd | nd | nd | 4.0 | nd | nd | nd |
| Experimental conditions as indicated in Table 4. nd=not detected; −=consumed; +=produced. | ||||||||
| Electron | Changes in tetrathionate and nitrogen oxide concentration (mM) during incubation of cell acceptor suspension | |||||||
| no acetate | +10 mM acetate | |||||||
| ΔS4O62− | ΔNO3− | ΔNO2− | ΔN2O | ΔS4O62 | ΔNO3− | ΔNO2− | ΔN2O | |
| NO3− | 1.3 | −1.0 | +2.6 | 0.01 | 1.1 | −8.2 | +7.8 | 0.03 |
| NO2− | 0.25 | nd | −1.5 | 0.02 | 0.9 | nd | −4.6 | 0.06 |
| N2O | 2.0 | nd | nd | nd | 1.3 | nd | nd | nd |
| O2 | 4.1 | nd | nd | nd | 4.0 | nd | nd | nd |
| Experimental conditions as indicated in Table 4. nd=not detected; −=consumed; +=produced. | ||||||||
If cultures were grown aerobically, they could not denitrify with thiosulfate as the electron donor, even though low levels of the nitrate and nitrite reductases could be detected if acetate was supplied. Cells grown under denitrifying conditions with acetate and thiosulfate were capable of active aerobic thiosulfate oxidation. 10 mM nitrite inhibited aerobic thiosulfate oxidation.
Diethyldithiocarbamate (DDC), an inhibitor of Cu-containing NO2− reductase, did not influence anaerobic thiosulfate oxidation by washed cells from either group in the presence of nitrate or nitrite. Acetylene (an inhibitor of N2O reductase; 5% v/v in the gas phase) did not influence anaerobic thiosulfate oxidation by washed cells with nitrate, slightly inhibited the process in the presence of nitrite, and completely stopped thiosulfate oxidation with N2O as electron acceptor.
Washed cells of strain ChG 5-2, grown anaerobically with acetate, thiosulfate and nitrate, were able to oxidize sulfide aerobically to tetrathionate. Under anaerobic conditions, the rate of sulfide oxidation was very low. The highest rate (10 nmol mg protein−1 min−1) was observed in the presence of nitrite, when the distinctive yellow color associated with the accumulation of polysulfide was transiently evident.
3.2 Cytochromes
Difference spectra of cell-free extracts prepared from strains from group 1 showed the presence of cytochrome cd1. The absorption maxima typical of this nitrite reductase (γ 460–470 nm; α 610–615 and 660–670 nm) were not observed in spectra made on similar protein samples from the group 2 isolates. While this might indicate the presence of a copper-containing nitrite reductase, rather than cytochrome cd1, this seems unlikely in view of the failure of DDC to inhibit nitrite reduction. It is possible that the cytochrome cd1 in such strains was simply below the detection limit of the method.
Cytochromes c and b were detectable in cell-free extracts of all eight isolates when they were grown aerobically with acetate and thiosulfate. Cytochrome cd1 (nitrite reductase) was not expressed under aerobic conditions in any of the isolates from either group. Cytochrome b was present at relatively low concentrations, compared to the level of cytochrome c. CO difference spectra of dithionite-reduced extracts had the maxima and minima in the γ region (415 and 432 nm respectively), and minima in the α region (556–558 nm) typical of cytochrome oxidase type o.
3.3 Taxonomy
All of the isolates were Gram-negative, oxidase- and catalase-positive, curved, motile rods with polar flagella when grown anaerobically in liquid culture. Two colony types were formed. R-type colonies were yellowish or pinkish, spreading, skin-like and with a very complex surface. S-types were small, smooth, soft, slimy and colorless. The R-type dominated when plated on dry agar surfaces and when incubated under aerobic conditions. The S-type predominated on wet surfaces and during anaerobic incubation. When sub-cultured, the R-type was stable, but the S-type produced both types under appropriate conditions. The morphology of the cells within the R- and S-type colonies was sufficiently different to suggest two different species. However, DNA-DNA hybridization on R and S clones from each of three of the strains (TG 3, BG 2 and ChG 5-2) confirmed that in each case, the clones were the same organism (hybridization level 99–100%). Cells from S-colonies and anaerobic liquid cultures were usually motile, curved rods, while cells from R-colonies were mostly coccoid and non-motile, being embedded in a slime matrix that was hard to homogenize. Motile cells were only found at the very edge of such colonies.
All of the isolates were obligately heterotrophic. They did not grow in mineral medium with only thiosulfate or H2 as the sole electron donor. Among the organic compounds utilized as carbon and energy sources were: organic acids including acetate, succinate, malate, α-ketoglutarate, citrate, fumarate, lactate, pyruvate, propionate, butyrate, gluconate, glyoxylate; alcohols including ethanol, propanol, glycerol; hexoses –d-glucose, d-fructose and d-maltose; amino acids and amines including i-alanine, l-glutamate, l-glutamine, l-aspartate, l-asparagine, l-proline and l-leucine. Strains ChG 6-1 and ChG 5-3 differed from the others by their ability to utilize d-galacturonic and d-glucuronic acids. Sugars were not fermented.
The bacteria described here have a relatively narrow range of GC mol% values (60–63.5). Direct DNA-DNA hybridization between all isolates was therefore possible. As can be seen in Fig. 2, there was DNA homology of more than 30% among the assorted isolates. The levels of hybridization with a non-specific control (Escherichia coli) were very low (0.2%). Within the group were four clusters. Strain TG 3, from the Pacific Ocean, was sufficiently different from the Black Sea isolates to be considered a different species by current standards for DNA-DNA-hybridization data [29].
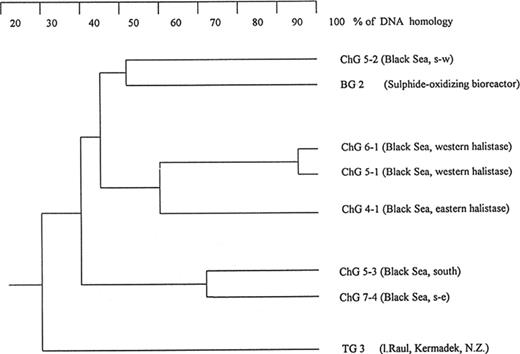
Dendrogram of the DNA-DNA homology among strains capable of thiosulfate-dependent denitrification. DNA from E. coli gave less than 0.1% homology with any of these samples.
3.4 Phylogeny
16S rRNA sequence analysis showed that isolates ChG 5-2, ChG 5-3, BG 2 and TG 3 were new Pseudomonas stutzeri strains. P. stutzeri has been subdivided into eight different genomic groups, genomovars, defined by DNA-DNA hybridization, G+C% values and 16S rRNA sequences [30–33]. This taxonomic category allows phenotypically variable strains of broadly defined species to be sorted into genetically distinct and stable groups [30]. On the basis of 16S rRNA sequence distances, isolates ChG 5-2, ChG 5-3, BG 2 and TG 3 were affiliated with genomovars 5, 3, 4, and 6 (Fig. 3). The isolates also matched all P. stutzeri genomovar signature nucleotides [32], with the exception of an adenosine replaced by a guanosine in E. coli position 1036 of strain ChG 5-3 and in E. coli position 278 of strain BG 2. As can be seen from Fig. 3, the only other organism in the clump with ChG5.2 and P. stutzeri was ‘Flavobacterium lutescens’, and its taxonomic position was therefore given some attention.
![Phylogenetic tree based on 16S rRNA sequence positions 34–1461 for P. stutzeri and related strains, including the new thiosulfate-oxidizing isolates described in this study. The numbers 1–8 indicate genomovars [32,33]. The tree was rooted with P. aeruginosa strain as outgroup. The scale bar corresponds to 5 substitutions per 1000 nucleotides.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsec/30/2/10.1111_j.1574-6941.1999.tb00640.x/1/m_FEM_113_f3.jpeg?Expires=1749904834&Signature=OYLbPMR~6R5mTb8zShY1ILnBugXxYXzxeMtT2xB8kmsJArARCHcgcPuAQfHkb8Nt1G11fgLkW~PQXENNoWx8MqFW~zamkhdHmyGIlMjY5jzbDAysVaQ-mrk6B-jqgLQR0j9SPBbuYlel6ot~ReiIqURTGiDqk~zl5mknMu8ovF41IsC~hXG~PvNEieOZXimmwt4A34s55B9OkNmhOQ7JlRgKwCd-cHrwaY7u1J~zS6viTyp7pMBKE14cQYy7uGGtn8byPVlCXnMWACqOc9gm~M8G0YmR5w1YxbIh5ufs52V5qlQoXN6KVX0XYSWkUlp1uYtM8wctVad~3S2S3kWWrQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Phylogenetic tree based on 16S rRNA sequence positions 34–1461 for P. stutzeri and related strains, including the new thiosulfate-oxidizing isolates described in this study. The numbers 1–8 indicate genomovars [32,33]. The tree was rooted with P. aeruginosa strain as outgroup. The scale bar corresponds to 5 substitutions per 1000 nucleotides.
Strain TG 3 was separated from the P. stutzeri isolates by lower DNA-DNA hybridization and 16S rRNA similarity (30% and 97%, respectively). These molecular differences exclude strain TG 3 from the species [29]. Strain TG 3 fell into genomovar 6, which has recently been proposed as a separate species, Pseudomonas balearica[32].
3.5 ‘Flavobacterium lutescens’
According to 16S rRNA analysis, ‘Flavobacterium lutescens’ appeared to be related to P. stutzeri, especially to genomovars 1 and 5, and to the isolate ChG 5-2 (Fig. 3). By DNA-DNA hybridization, ‘F. lutescens’ showed 60–70% homology with strains ChG 5-2 and BG 2, and is therefore placed within the species P. stutzeri. The physiological properties of the strain confirm this classification. ‘Flavobacterium lutescens’ was able to grow anaerobically with acetate and nitrate, nitrite or N2O as electron acceptors. It oxidized thiosulfate to tetrathionate in batch cultures in the presence of acetate with oxygen or N2O, but not with nitrate or nitrite as electron acceptors. Washed cells of ‘F. lutescens’ grown anaerobically with acetate, thiosulfate and either nitrate or N2O could oxidize thiosulfate anaerobically, but were totally inactive with nitrite as electron acceptor. Cytochrome cd1 nitrite reductase was not spectroscopically detected in cell-free extracts prepared from cells grown anaerobically with acetate and nitrate. ‘F. lutescens’ also formed two colony types on dry agar. However, the R-type was smoother, practically colorless, and grew down into the agar.
4 Discussion
This study provides the first detailed and direct evidence of the ability of obligately heterotrophic bacteria to oxidize thiosulfate under denitrifying conditions. The isolates described here differ from the chemolithotrophic sulfur bacteria in that they produce tetrathionate rather than sulfate. The oxidation of thiosulfate to tetrathionate (yielding one electron) instead of sulfate (yielding eight electrons) generates insufficient energy to be able to support significant autotrophic growth, and it is therefore not surprising that all of the tetrathionate-forming isolates are obligately heterotrophic. However, some of them appear to be chemolithoheterotrophs, since the provision of thiosulfate increased their growth yield in acetate-limited continuous cultures [10]. The synthesis of ATP during the oxidation of thiosulfate to tetrathionate in washed suspensions of cells from several strains has been reported [34].
Under anaerobic conditions, thiosulfate oxidation should be even less favorable because of, among other factors, the more reduced state of the respiratory chain. It is therefore not surprising that denitrifying thiosulfate oxidation was much less active than in the presence of oxygen. The observed preference for N2O as an electron acceptor for anaerobic thiosulfate oxidation to tetrathionate during organotrophic growth could be explained by the high redox potential of N2O/N2, compared with the nitrate/nitrite and nitrite/N2O couples (ΔE0′=1.36, 0.43 and 0.36 V, respectively), and assuming a relatively high value for the S2O32−/S4O62− pair (about 0.1 V). There was no evidence for anaerobic chemolithoheterotrophy. For example, during the anaerobic growth of strain TG 3 with nitrate and acetate, there was no evidence of biomass increase when thiosulfate was added, even under acetate limitation in continuous culture (Table 3). Further research will be necessary to understand the details of the respiratory chains of these organisms, and to establish their modes of energy generation. It may also help to clarify the different behavior of strains with high and low nitrite oxidase activities.
The ability of the heterotrophic isolates described here to oxidize thiosulfate under denitrifying conditions is important for the understanding of their functioning in natural ecosystems. Most of the strains were isolated from the interface micro-aerobic layer of the Black Sea where they represent the dominant sulfur-oxidizing community [2,4]. This habitat is characterized by the coexistence of sulfide and oxygen at relatively low concentrations. Both of these compounds are known to inhibit N2O reductase in many organisms [35], and therefore N2O should accumulate at the interface. Thiosulfate is produced by the chemical oxidation of sulfide at the low oxygen concentrations to be found in seawater at these interfaces [36]. The isolates described here would be capable of converting thiosulfate to tetrathionate in the presence of the available N2O. Taking into account that tetrathionate can drive very efficient anaerobic sulfide oxidation with cyclic regeneration of thiosulfate [10], denitrifying tetrathionate-forming heterotrophs may be considered an important component in sulfide oxidation, in both natural and artificial sulfide-rich environments. Moreover, the ability to denitrify might also explain why some of the strains were isolated from the deep, anaerobic, sulfide-containing layers of the Black Sea [4].
The P. stutzeri‘superspecies’ is considered to be one of the most active denitrifying groups of heterotrophic bacteria [37]. It is to be expected, as techniques are refined, that other strains will be classified, upon closer examination, as P. stutzeri. In addition to ‘F. lutescens’, several marine isolates are good candidates. The hydrothermal vent isolate NF-13 has been described as able to oxidize thiosulfate to polythionate under aerobic conditions, and to produce gas when incubated anaerobically in sea water medium with thiosulfate and nitrate [14]. The fragmentary 16S rRNA sequence of NF-13, while indicating that this organism is a member of the genus Pseudomonas, did not allow reliable species or genomovar identification. Two new denitrifying strains (HTA208 and MT-1) [38,39] isolated from the deepest sediment of the Mariana Trench at 10897 m depth are, on the basis of 16S rRNA sequencing, also Ps. stutzeri strains, but not affiliated with one of the known genomovars (Fig. 3).
The ability of P. stutzeri to oxidize thiosulfate to tetrathionate both aerobically and anaerobically was not previously known. This widespread bacterium could be important in the turnover of thiosulfate in marine environments, and possibly compete with thiosulfate disproportionation and reduction by sulfate-reducing bacteria [40].
Acknowledgements
This work received financial support from the Max-Planck-Gesellschaft, Munich to A.T.
References



