-
PDF
- Split View
-
Views
-
Cite
Cite
Kiwamu Minamisawa, Yoko Nakatsuka, Tsuyoshi Isawa, Diversity and field site variation of indigenous populations of soybean bradyrhizobia in Japan by fingerprints with repeated sequences RSα and RSβ, FEMS Microbiology Ecology, Volume 29, Issue 2, June 1999, Pages 171–178, https://doi.org/10.1111/j.1574-6941.1999.tb00608.x
Close - Share Icon Share
Abstract
Two hundred and thirteen isolates of soybean bradyrhizobia indigenous to six field sites in Japan were characterized using hybridization probes RSα, RSβ, nifDK and hupSL from Bradyrhizobium japonicum and indole-3-acetic acid production to clarify diversity and endemism of their population structures. Significant diversities and site-dependent variations were observed in terms of Bradyrhizobium species, hup genotype and fingerprints with repeated sequences RSα and RSβ. The fingerprints at one site with no history of soybean cultivation were less diverse than those at other sites. Even within the populations of B. japonicum hup− isolates, which were commonly indigenous to the six field sites, several RSα copies clustering around nif genes were highly conserved. The results suggest that soybean bradyrhizobia may be diversified in individual fields as associated with host plants and local soil conditions.
1 Introduction
One of the major agronomic problems of applying superior strains of soybean bradyrhizobia as inoculants is that indigenous soil populations of (brady)rhizobia are often more competitive than the inoculant strains [1]. The failure of inoculant bradyrhizobia to overcome the dominance of indigenous strains reminds us of a fundamental question in microbial ecology: that is, how microbial communities are structured in space and time.
Soybean bradyrhizobia, which are composed of two species, B. japonicum and B. elkanii[2], are slow-growing, Gram-negative, heterotrophic bacteria which have the ability to form root nodules on several leguminous plants and to fix atmospheric nitrogen. Free-living soybean bradyrhizobia are also members of oligotrophic bacteria in soil [3]. The diversity of indigenous populations of soybean bradyrhizobia has been evaluated by various methods: serology [4], protein banding patterns [5], intrinsic antibiotic resistance [6,7], fatty acid composition [6], numerical taxonomy [8] and molecular genetic techniques [6,9,10]. Fingerprints of hybridization with repeated sequences RSα and RSβ seem most discriminative among these methodologies [9,10]. In a previous paper [9], 49 isolates of soybean bradyrhizobia indigenous to a Nakazawa field where soybeans had been cultivated for 45 years without receiving inoculants were characterized using nifDK-, hupSL-, RSα- and RSβ-specific hybridization probes of B. japonicum. RSα- and RSβ-specific fingerprints (RS-fingerprint) demonstrated hierarchical diversities among the population. The highly distinct diversity in RS-fingerprints (33 distinct RS-fingerprints from 41 soybean nodules) suggests that the indigenous populations of soybean bradyrhizobia are composed of bacteria possessing extremely heterogeneous genomic structures. Moreover, cluster analysis revealed that the RS-fingerprints were correlated with Bradyrhizobium species and hup genotypes, suggesting that they reflect the evolutionary history and genetic background of soybean bradyrhizobia [9].
The objectives of this work were to determine whether the diversity in RS-fingerprints observed in bradyrhizobia at the Nakazawa field site extends to other fields in Japan and to determine whether this variation is dependent on individual field sites.
2 Materials and methods
2.1 Isolation of B. japonicum from fields
Seeds of soybean (Glycine max) cultivar Enrei were surface-sterilized by immersion in 0.5% of sodium hypochlorite for 5 min, followed by several washes with sterilized water. Seeds were sown in sterile vermiculite, and inoculated with moist soil samples (1 g), which were collected from the plough layer of field sites. The field sites chosen were the Tokachi field at Tokachi Prefectural Agricultural Experimental Station (Memuro, Tokachi, Hokkaido, Japan), the Nagakura fields at Niigata Agricultural Experiment Station (Nagaoka, Niigata, Japan), the Ami field at the experimental farm of Ibaraki University (Ami, Ibaraki, Japan), the Fukuyama field at the Experimental Farm of Hiroshima University (Fukuyama, Hiroshima, Japan) and the Ishigaki field at the experimental field of the Ishigaki Island Branch of the Tropical Agriculture Research Center (Ishigaki, Okinawa, Japan). There was no history of soybean cultivation at the field site on Ishigaki Island, whereas the remaining field sites had been cultivated with soybeans. The inoculation procedure and plant cultivation have been described previously [9].
Nodules, which were randomly excised from host plants 40 days after germination, were rinsed, and surface-sterilized in an acidic mercuric chloride solution (0.1%, w/v) for 5 min. An inoculation needle was inserted into the cut surface of the nodule, and the cells adhering to the needle were streaked onto yeast extract-mannitol (YM) agar plates [11,12]. Isolates were maintained on YM agar slants at 4°C after single colony isolations.
2.2 Bacterial strains and media
B. japonicum strain USDA110, obtained from H.H. Keyser of the US Department of Agriculture (Beltsville, MD, USA), was used as a standard strain. Two hundred and thirteen soybean bradyrhizobia were isolated from the six field sites described above. The prefixes A, T, NC, NK, F and I are used for field isolates from the Ami, Tokachi, Nakazawa [9], Nagakura, Fukuyama and Ishigaki sites, respectively. B. japonicum strains were grown aerobically at 30°C in YM medium [11,12]. A previous set of isolates obtained from the Nakazawa field site [9] was used. Tris-YMRT broth medium [13] supplemented with 0.3 mM tryptophan was used for the indole-3-acetic acid (IAA) production assay. Escherichia coli HB101 (recA−, hsdR, hsdM, pro, leu, Strr) containing plasmids pRJ676 [14] and pHU52 [15,16] was grown in Luria-Bertani medium supplemented with appropriate antibiotics [17] for plasmid preparations.
2.3 DNA isolation and hybridization
Total DNA isolation and hybridization were carried out as described previously [9]. Total DNAs from soybean bradyrhizobia were digested with HindIII for nifDK- and hupSL-specific hybridization, and with XhoI for RSα- and RSβ-specific hybridization. Hybridization probes were prepared from plasmids pRJ676 [14] and pHU52 [15,16] as described previously [9,12]. The 3.5-kb BglII fragment, 0.2-kb HindIII-ClaI fragment and 0.25-kb XhoI-BglII fragment from pRJ676 were used as probes for nifDK, RSα and RSβ, respectively. The 2.2-kb SstI fragment from pHU52 was used as a probe for structural genes of uptake hydrogenase [16].
2.4 Analysis of IAA
The presence of IAA in the culture supernatant was used to classify the field isolates into B. japonicum and B. elkanii. IAA levels were determined colorimetrically as described previously [13].
2.5 Cluster analysis
To evaluate the relatedness of RS-fingerprints, similarity coefficients (SAB) were calculated by RSα- and RSβ-specific hybridization profiles. Cluster analysis was carried out using a matrix of SAB as described previously [9].
3 Results
3.1 Grouping of field isolates into three categories of B. japonicum hup+, B. japonicum hup− and B. elkanii
Soybean bradyrhizobia, formerly named Bradyrhizobium japonicum, are composed of two species, japonicum and elkanii, based on various criteria [2,6,11–13]. Previous work [9,12] demonstrated that RS-fingerprints were correlated with the hydrogenase (Hup) trait as well as with Bradyrhizobium species. Therefore, the isolates tested in this work, in advance, were classified into three groups of B. japonicum hup+, B. japonicum hup− and B. elkanii (Fig. 2) according to the following criteria: (1) B. japonicum excretes no IAA in culture and exhibits an intense hybridization band (normally 9.5 kb in size) with the nifDK genes from B. japonicum USDA110; (2) B. elkanii produces IAA in culture, and shows a weak hybridization signal (normally 27 kb in size) with the nifDK genes under a high stringency condition [12]; (3) hydrogen uptake-positive B. japonicum strains consistently show an intense hybridization signal (normally 5.9 kb in size) under a high stringency condition with the hupSL gene probe isolated from B. japonicum USDA122 [12]. Among 213 isolates tested, the isolates showing hupSL-specific hybridization consistently fell into the B. japonicum grouping. Thus, the categories for hupSL-positive and -negative isolates were expressed as B. japonicum hup+ and B. japonicum hup−, respectively.
![Examples of RSα- and RSβ-specific fingerprints of field isolates of soybean bradyrhizobia at three sites, Fukuyama (A), Ishigaki (B) and Nagakura (C). ‘M’ and ‘e’ indicate lanes loaded with DNAs from B. japonicum USDA110 used as a marker and from B. elkanii isolates identified by IAA production and nif hybridization (see text), respectively. ‘H’ and unmarked lane show HRS isolates and normal isolates of B. japonicum[20]. After complete digestion with XhoI, total DNA from each isolate (3 μg/lane) was subjected to electrophoresis in horizontal 0.8% agarose-TAE [7], blotted onto a nylon filter (Hybond-N, Amersham, Japan), and hybridized with radioactive RSα- and RSβ-specific probes from pRJ676 [14](see text).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsec/29/2/10.1111_j.1574-6941.1999.tb00608.x/1/m_FEM_171_f2.jpeg?Expires=1750291035&Signature=nJtJ7EK5JLxx51XPJYyk6NbYBfE97kzIPXgQkOzfuhR6RuSWcjDPNHOFPkdPgEGHcOA-medNL6OvLqd0WOLSAi06AkhTlCjQwWJtrJGCVOX1kCyfILd47iJOPJG6UjzSo~dihdxEY8XcEuKw0zOtZY4R2SzoagWjFhOL9GVm~vyyCPcuYxIu2twcXKDv8lVvuAILKhJCPwzkp1cZ7axN2Jv7pk2QqrQpHdXkfhB8ViJUpl-jQtE5eC-NOPGqcpCNKRVNRxaHtrVZ7oARRcBlJDsqwaQINYDoNLHEJNzj5qYHRjzXrZninyVk8XqoQGw1WJRYPhaWHzK8g1b4Qv~3mg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Examples of RSα- and RSβ-specific fingerprints of field isolates of soybean bradyrhizobia at three sites, Fukuyama (A), Ishigaki (B) and Nagakura (C). ‘M’ and ‘e’ indicate lanes loaded with DNAs from B. japonicum USDA110 used as a marker and from B. elkanii isolates identified by IAA production and nif hybridization (see text), respectively. ‘H’ and unmarked lane show HRS isolates and normal isolates of B. japonicum[20]. After complete digestion with XhoI, total DNA from each isolate (3 μg/lane) was subjected to electrophoresis in horizontal 0.8% agarose-TAE [7], blotted onto a nylon filter (Hybond-N, Amersham, Japan), and hybridized with radioactive RSα- and RSβ-specific probes from pRJ676 [14](see text).
Fig. 1 shows the incidence of B. japonicum hup+, B. japonicum hup− and B. elkanii from the various field sites. B. elkanii was founded in half of the sites, Nakazawa, Fukuyama and Ishigaki. B. japonicum hup+ appeared at four field sites, Nagakura, Nakazawa, Fukuyama and Ishigaki. All isolates from the Ami and Tokachi sites belonged to the B. japonicum hup− group. These results indicate that field-dependent variation exists in terms of Bradyrhizobium species and the hup genotype among the isolates investigated in this study. This type of variation has also been reported by other workers [18,19].
![Incidence of B. japonicum hup+ and hup− and B. elkanii from different field sites. Field isolates of soybean bradyrhizobia were grouped into three categories: B. japonicum hup+ and hup−, and B. elkanii, according to nifDK- and hupSL-specific hybridizations and production of indole-3-acetic acid in culture (see text). Total numbers of isolates tested were 22 for Ami, 24 for Tokachi, 26 for Nagakura, 41 for Nakazawa, 26 for Fukuyama, and 17 for Ishigaki. Previous data were used for the Nagakura field [9]. Dark shaded box, B. japonicum hup+; light shaded box, B. japonicum hup−; clear box, B. elkanii.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsec/29/2/10.1111_j.1574-6941.1999.tb00608.x/1/m_FEM_171_f1.jpeg?Expires=1750291035&Signature=wzXqLp7XwWqLDgryAX9t8mp~PItDKM-poF97-ZcM3IZm-lnEPonhP6NA2ElHNjUG~oVx1kbTRchqFFuOalW0f1tDb-wff6yXhiPLQpwJbrmGtK9aIzu-cztmXjq52gsoip5YWTuM9CGAFomyzuBhdDLpti3fnunT8hx1Wb3Uf-iBipSrySab7cItJ9XUnYJ5Mil5IfJyEXbzQWrOxWo0a~KO5ISIL89v9K8YvaQyRrDApZP~PSrKT0zRi0nfg0HyYSx7Grkqpk5wgAcqQuAwOlsnIv8AYORQevyKpnaw1-armEhCz--5OTCqKfWb6yfe8iRd9mTFz7yfrfAlmGVStg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Incidence of B. japonicum hup+ and hup− and B. elkanii from different field sites. Field isolates of soybean bradyrhizobia were grouped into three categories: B. japonicum hup+ and hup−, and B. elkanii, according to nifDK- and hupSL-specific hybridizations and production of indole-3-acetic acid in culture (see text). Total numbers of isolates tested were 22 for Ami, 24 for Tokachi, 26 for Nagakura, 41 for Nakazawa, 26 for Fukuyama, and 17 for Ishigaki. Previous data were used for the Nagakura field [9]. Dark shaded box, B. japonicum hup+; light shaded box, B. japonicum hup−; clear box, B. elkanii.
3.2 Diversity in RS-fingerprints of soybean bradyrhizobia from six field sites
Examples of RS-fingerprints are shown in Fig. 2 to see at a glance quite different RS-fingerprints depending on the field sites. At the Fukuyama site (Fig. 2A), a significant diversity in RS-fingerprints was observed: 20 distinct RS-fingerprints from 26 nodules, which is comparable to that in Nakazawa site reported previously [9]. On the other hand, simple and dominant RS-fingerprints appeared at the Ishigaki site (Fig. 2B). The difference in the diversity of RS-fingerprint between the two sites was attributed to that within B. japonicum isolates (unlabelled lanes). Most B. elkanii isolates (labeled ‘e’ in Fig. 2A,B) showed a few bands of RSα- and RSβ-specific hybridization and similar diversity between the two sites.
At the Nagakura site (Fig. 2C), approximately half of the isolates (labelled ‘H’) exhibited extremely numerous RSα- and RSβ-specific hybridization bands. This is not due to overloading on the agarose gel or partial digestion of total DNA as described previously [9]. Such isolates have been phenotypically and genetically characterized as extra-slow growers subjected to DNA rearrangements, and designated B. japonicum HRS (highly reiterated sequence-possessing) strains [20]. The remaining normal isolates of B. japonicum (unlabelled lanes in Fig. 2C) also showed a significant diversity in RS-fingerprints (11 distinct RS-fingerprints from 12 nodules) at the Nagakura site (Fig. 2C).
To evaluate quantitatively diversities of RS-fingerprints of soybean bradyrhizobia from the six sites, we calculated dominance and diversity indices [21,22](Table 1), where HRS strains were eliminated because highly multiple bands could not be counted such as lanes labelled H in Fig. 2C[9]. Significant diversities of RS-fingerprints were observed at the Ami, Tokachi, Nagakura, Nakazawa and Fukuyama sites. On the other hand, RS-fingerprints at the Ishigaki site distinctively showed high dominance and poor diversity, suggesting that the soybean bradyrhizobium population was not diverse at the site. There is no history of soybean cropping only at the Ishigaki site, where sugarcane had been mainly cropped. Thus, the cultivation of host plants seemed to enhance the diversity of bacteria at the remaining field sites. The Ishigaki site is probably suitable for investigating the effects of host plant cultivation on the diversification of indigenous soybean bradyrhizobia.
Dominance and diversity indexes of RSα- and RSβ-specific fingerprints of the indigenous populations of soybean bradyrhizobia at different field sitesa
| Site | Index | |
| Dominance | Diversity | |
| Ami | 8.6 | 78 |
| Tokachi | 5.9 | 85 |
| Nagakura | 3.3 | 85 |
| Nakazawa | 7.0 | 87 |
| Fukuyama | 6.2 | 77 |
| Ishigaki | 17.6 | 59 |
| Site | Index | |
| Dominance | Diversity | |
| Ami | 8.6 | 78 |
| Tokachi | 5.9 | 85 |
| Nagakura | 3.3 | 85 |
| Nakazawa | 7.0 | 87 |
| Fukuyama | 6.2 | 77 |
| Ishigaki | 17.6 | 59 |
Dominance and diversity indexes were determined based on the combination of RSα- and RSβ-specific hybridization profiles. The indexes were calculated from the following equations where S, N and ni are the number of patterns, the number of isolates tested and the number of isolates belonging to a certain pattern, respectively. Dominance index=∑(ni/N)2×100 [22]. Diversity index=(S/N)×100 [21].
Dominance and diversity indexes of RSα- and RSβ-specific fingerprints of the indigenous populations of soybean bradyrhizobia at different field sitesa
| Site | Index | |
| Dominance | Diversity | |
| Ami | 8.6 | 78 |
| Tokachi | 5.9 | 85 |
| Nagakura | 3.3 | 85 |
| Nakazawa | 7.0 | 87 |
| Fukuyama | 6.2 | 77 |
| Ishigaki | 17.6 | 59 |
| Site | Index | |
| Dominance | Diversity | |
| Ami | 8.6 | 78 |
| Tokachi | 5.9 | 85 |
| Nagakura | 3.3 | 85 |
| Nakazawa | 7.0 | 87 |
| Fukuyama | 6.2 | 77 |
| Ishigaki | 17.6 | 59 |
Dominance and diversity indexes were determined based on the combination of RSα- and RSβ-specific hybridization profiles. The indexes were calculated from the following equations where S, N and ni are the number of patterns, the number of isolates tested and the number of isolates belonging to a certain pattern, respectively. Dominance index=∑(ni/N)2×100 [22]. Diversity index=(S/N)×100 [21].
3.3 Site-dependent variations of RS-fingerprints
To evaluate the relatedness of RS-fingerprints, we constructed a dendrogram by SAB matrix in all pairwise combinations of the profiles of hybridization with RSα and RSβ. HRS isolates of B. japonicum were also eliminated because their RS-fingerprints were too dense to be compared. The dendrogram shows a primary division at 22% similarity, which was in accord with a division of Bradyrhizobium species between japonicum and elkanii (Fig. 3). Within the B. japonicum cluster, hup+ isolates formed two clusters, a major cluster containing hup+ isolates derived from four sites and a minor cluster exclusively from the Nagakura site. Theses correlation of RS-fingerprints with Bradyrhizobium species and hup genotype were almost compatible with the previous results from the Nakazawa field site [9]. Cluster analysis (Fig. 3) also demonstrated that the variations of RS-fingerprints depended on sites for isolation of soybean bradyrhizobia. In other words, the RS-fingerprints of soybean bradyrhizobium populations from each site occupied unique positions in the dendrogram. For example, RS-fingerprints of hup− isolates from the Tokachi (T), Nakazawa (NC) and Ami (A) sites tended to belong to different clusters. Within the B. elkanii cluster, the isolates from Nakazawa (NC) and Fukuyama (F) did not seem to share the same cluster at a lower level as each other. The RS-fingerprints at the Ishigaki site (I) were scattered in various clusters irrespective of their poor diversity.
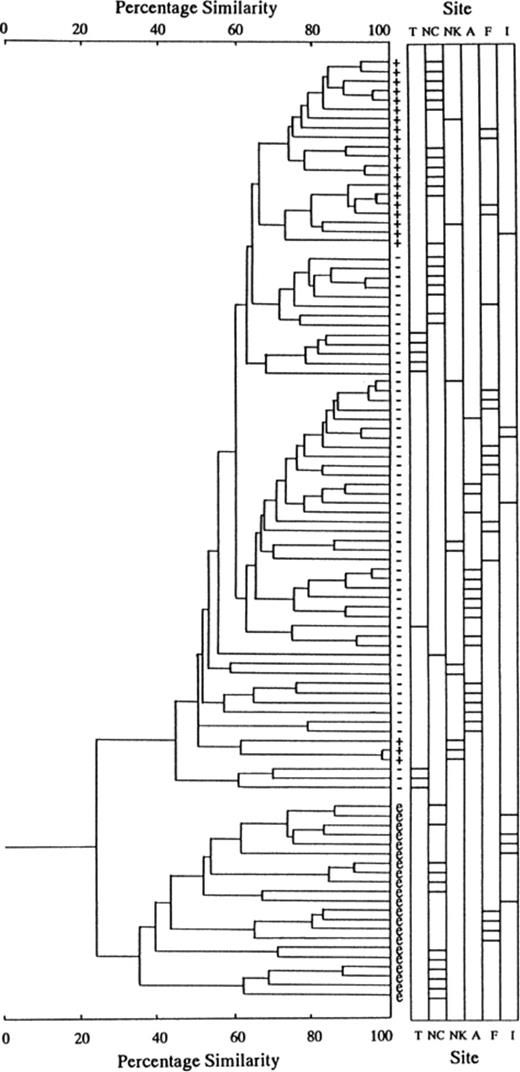
Dendrogram depicting relatedness among RSα- and RSβ-specific fingerprints of soybean bradyrhizobia from six field sites, Tokachi (T), Nakazawa (NC), Nagakura (NK), Ami (A), Fukuyama (F) and Ishigaki (I). ‘+’, ‘−’ and ‘e’ indicate B. japonicum hup+, B. japonicum hup− and B. elkanii, respectively. The bar in each column (labelled ‘Site’) to the right of the dendrogram indicates from which field the fingerprinted strain came.
3.4 Conservation of RSα copies around the nif region
Since all six field sites commonly harbored B. japonicum hup− isolates (Fig. 1), we compared RS-fingerprints of B. japonicum hup− isolates from all fields including the Nakazawa site previously reported [9](Fig. 4). As a result, unique and common features of RS-fingerprint profiles were observed. Several RSα-specific bands (α1, α3, α4, α8, α9, α12), which cluster around nif genes of B. japonicum[23], were highly conserved, while the remaining RSα-specific bands were generally diverse and site-dependent.
![Conservation of RSα copies around the nif region of B. japonicum hup− isolates from the six field sites. Site prefixes are as in Fig. 3. Migration distances of the bands of RSα-specific hybridization were normalized as compared with the profiles of B. japonicum USDA110 [14]. Numbers below the prefix are isolate numbers. Previous data were used for the Nagakura field site [9]. Bands α1 (13 kb), α3 (6 kb), α4 (5.2 kb), α8 (3.1 kb), α9 (2.5 kb) and α12 (1.2 kb) of RSα-specific hybridization were well conserved, which corresponded to RSα1, RSα3, RSα4, RSα8, RSα9 and RSα12 [23]. Total DNA of each isolate was digested with XhoI.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsec/29/2/10.1111_j.1574-6941.1999.tb00608.x/1/m_FEM_171_f4.jpeg?Expires=1750291035&Signature=1i8yJ4D3b3kN5-MHbjKZR7WC7WunC6ZX2rfYxVOwZgtYAWYqwtKcmR6nnd7xg0bLHuEa-8Ws8jKbwZAUn1O~xAGp9fpGSTw8~KTCNqQrGCud22hEuchZTfQBaiHMrplQzWK9sORsuz6ZUs8r67R0GaEGywr6YmQAN6pY1W6gAMvamNfbyzG3AOj7l3lPsDz6jaEFFDdmUmF~mS5HIxevfs2oZr29U7omS6IXqWM5A3e-uYrEWHDHXae6IhxFMMzDffrohz2IVmZWUOW4JCtKlizTD0tr1PuwedHCl5EPRP9o4rB~wtslMXi020DmQebK8I2F~Y5o4duMhW-3pM7iOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Conservation of RSα copies around the nif region of B. japonicum hup− isolates from the six field sites. Site prefixes are as in Fig. 3. Migration distances of the bands of RSα-specific hybridization were normalized as compared with the profiles of B. japonicum USDA110 [14]. Numbers below the prefix are isolate numbers. Previous data were used for the Nagakura field site [9]. Bands α1 (13 kb), α3 (6 kb), α4 (5.2 kb), α8 (3.1 kb), α9 (2.5 kb) and α12 (1.2 kb) of RSα-specific hybridization were well conserved, which corresponded to RSα1, RSα3, RSα4, RSα8, RSα9 and RSα12 [23]. Total DNA of each isolate was digested with XhoI.
4 Discussion
To our knowledge, this is the first report dealing with extensive comparisons of DNA fingerprints of indigenous populations of soybean bradyrhizobia from various field sites using repeated sequences RSα and RSβ as indicators of diversity. Consequently, the diversity in RS-fingerprints observed at the Nakazawa field site [9] can be extended to other fields where soybeans have been cultivated. Moreover, the variations of RS-fingerprints were partially dependent upon individual field sites. Diversities and their site-dependent variations were also observed in terms of species, hup genotype and the incidence of HRS strains (Fig. 2) [20]. Since the diversities of RS-fingerprints were partially dependent upon the field sites, it is possible that the genomes of soybean bradyrhizobia may have diversified in association with various factors found in individual fields. Data at the Ishigaki site strongly supported the idea that soybean cultivation is one of the factors enhancing the diversity of soybean bradyrhizobia. However, the history of soybean cultivation by itself did not explain the site-dependent variation of RS-fingerprints. They may be fluctuated by the selection of soybean cultivars, domestic other legumes, indigenous microbial community and unknown soil conditions around the individual fields.
The six RSα bands clustering around nif genes of B. japonicum[23] were highly conserved (Fig. 4), while the positions of the remaining RSα-specific bands were diverse and site-dependent. This result indicates that symbiotic regions around nif genes are most likely to be well conserved regardless of the geographic origins of the isolates, whereas the remainder of the genome is less conserved. The symbiotic gene cluster (approximately 380 kb) represents less than 5% of the whole genome of B. japonicum (8.7 Mb) [24]. There are two possibilities to explain the conservation of symbiotic regions: (1) mutations in and around symbiotic genes are rejected or eliminated by the failure of nitrogen-fixing symbiosis; (2) a symbiotic gene cluster is exchanged or transferred among symbiotic and ‘non-symbiotic’ bradyrhizobia with different backgrounds of RS-fingerprints.
The genomic positions of RSα and RSβ have been shown to be stable in B. japonicum under laboratory conditions and nodule bacteroids [9,23]. Nevertheless, RSα and RSβ sequences possess structural features of insertion sequences (IS), a mobile element in prokaryotes. Indeed, RSα and RSβ are homologous to Shigella sonnei IS630[25] and Shigella dysenteriae IS911[26], respectively ([23], unpublished data for RSβ). It is possible that IS-mediated DNA rearrangements contribute to the generation of diversities of RS-fingerprints. In particular, this may be true for the generation of B. japonicum HRS strains that possess large numbers of RSα copies in the genome.
The indigenous populations are probably highly adapted to the local soil conditions including host plant cultivation, which possibly makes the introduction of Bradyrhizobium inoculants difficult. Although the diversities of RS-fingerprints have not yet been proven to be involved in phenotypic diversity, they might interfere with successful nodulation by the introduced inoculant.
Acknowledgements
This work was supported in part by grants from the Ministry of Education, Science and Culture of Japan (Nos. 02455007 and 07660077), and the Joint Research Program of the Institute of Genetic Ecology, Tohoku University (Nos. 942206 and 953009). The authors gratefully acknowledge Dr. T. Asami, Dr. M. Kubota (Ibaraki University) and Dr. T. Hattori (Tohoku University) for their continuing interest.
References



