-
PDF
- Split View
-
Views
-
Cite
Cite
Francesco Paolocci, Andrea Rubini, Bruno Granetti, Sergio Arcioni, Rapid molecular approach for a reliable identification of Tuber spp. ectomycorrhizae, FEMS Microbiology Ecology, Volume 28, Issue 1, January 1999, Pages 23–30, https://doi.org/10.1111/j.1574-6941.1999.tb00557.x
Close - Share Icon Share
Abstract
Protocols for isolating and amplifying fungal DNA from ectomycorrhizae, overcoming the presence of PCR inhibitors, were established to ensure a fast and reliable truffle mycorrhizal identification. By using species-specific ITS primers in multiplex PCR, morphologically very similar Tuber melanosporum, Tuber indicum and Tuber brumale ectomycorrhizae were identified in a single step of amplification, irrespective of their physiological stage. The molecular method assures a more accurate and reliable monitoring of nursery-inoculated plants both before and after their in-field planting, than any morphological or ITS/RFLP analyses so far performed. It should also prevent frauds, already a problem, and the potential for environmental damage due to the incorrect typing of the allochthonous species T. indicum as European truffle species, when the identity of ectomycorrhizae is based solely on morphology.
1 Introduction
The beneficial effects of ectosymbiotic agents on growth rate and survival of transplanted trees, in addition to a flourishing worldwide market of truffle ascocarps, have, during the last two decades, encouraged the large production and commercialization of trees artificially inoculated with Tuber species in southern European countries both within and outside reforestation projects. However, the biology and ecology of truffle species remains insufficiently understood [1]. Also, long-term monitoring of the development of the fungal species in host root systems is difficult because of the persisting difficulty in characterizing Tuber species ectomycorrhizae. Truffle identification is based on morphological analysis, which generally allows Tuber fruit bodies to be typed but may fail to distinguish their ectomycorrhizae, as reliable anatomical traits are few and highly susceptible to environmental conditions [2–4]. Since species with widely diverse ecological requirements and commercial value, such as within black truffles: Tuber melanosporum Vitt., Tuber indicum Cooke and Massee and Tuber brumale Vitt., and within white truffles: T. magnatum Pico and T. borchii Vitt., exhibit mycorrhizae with very similar morphological traits [2–6], there is a pressing need for techniques that reliably identify these fungi across their entire symbiotic life cycle. A correct species typing will prevent economic frauds and facilitate the cultivation and dissemination of only ecologically adapted truffle species. In this context, several molecular approaches have been proposed for Tuber species typing based on rDNA internal transcribed spacer (ITS) and intergenic spacer (IGS) markers, random amplified polymorphic DNA (RAPD) markers and/or species-specific probes [7–12]. However, in order to design a fast, inexpensive method for analyzing ectomycorrhizae, the problems inherent in isolating and amplifying the DNA need to be overcome. Direct PCR amplification is highly affected by the tissue assayed. Also, DNA isolation and amplification can be dramatically affected by various inhibitors, e.g., heavy metal ions, humic and fulvic acids, polysaccharides, phenolic and oxidized phenolic compounds [13–16].
The recent massive and increasing importation into Europe of low-quality and, hence, low-priced ascocarps of the Asiatic truffle species T. indicum[17], which are morphologically very similar to those of T. melanosporum[18,19], and their worldwide remarketing, is pushing the European countries to seek a method that can clearly differentiate fruit bodies and ectomycorrhizae of this species from native T. melanosporum and T. brumale species [12, 19, 20]. Failure to differentiate these black truffle species produces considerable economical consequences, and the ecological impact of uncontrolled planting of T. indicum-inoculated plants where other truffle species are indigenous is unpredictable.
This paper presents the PCR characterization, by using species-specific ITS primers, of mycorrhizae of the most economically important and widespread black truffle species, which are T. melanosporum, T. brumale and T. indicum, irrespective of the physiological stage. The method used for isolation and amplification of DNA from single ectomycorrhizae is simple and overcomes the presence of PCR inhibitors in root samples. The approach, which proved more sensitive and powerful than morphological and molecular analyses so far performed, is proposed for typing ectomycorrhizae as well as for monitoring and certifying nursery mycorrhizal plants. The results are discussed in connection with ecological aspects of truffle cultivation.
2 Materials and methods
2.1 Sample source
The collection sites for fruit bodies of the truffle species used are listed in Table 1. The truffle-host plant associations considered are listed in Table 2 and, as controls, roots collected from uninoculated host plant species were also processed. Nursery-inoculated plants were prepared by spore inoculation as described by Bencivenga [21] and Chevalier et al. [22]. As spore donors, French and Italian T. melanosporum and T. brumale fruit bodies from different geographical locations were used to produce hundreds of artificially inoculated plants. For each of the other Tuber species in Table 1, ectomycorrhizae were produced by using truffles collected in different Italian regions, with the exception of T. indicum. The Asiatic truffles used for artificial plant inoculation were imported into Italy and France in 1995 and 1996.
List of ascocarps of Tuber spp. and their collection sitesa
| T. melanosporum | ||
| Meuse | (2) | France |
| Dordogne | (9) | France |
| unknown | (8) | Spain |
| Abruzzo | (13) | Italy |
| Umbria | (11) | Italy |
| Lazio | (9) | Italy |
| Toscana | (3) | Italy |
| Marche | (2) | Italy |
| T. indicum | ||
| imported in France in 1995 | (13) | |
| imported in Italy in 1995 | (14) | |
| imported in France in 1996 | (18) | |
| imported in Italy in 1996 | (34) | |
| imported in Italy in 1997 | (22) | |
| T. brumale | ||
| Vaucluse | (4) | France |
| Meuse | (5) | France |
| Abruzzo | (2) | Italy |
| Marche | (3) | Italy |
| Lazio | (7) | Italy |
| Umbria | (18) | Italy |
| T. magnatum | ||
| Molise | (4) | Italy |
| Marche | (3) | Italy |
| Piemonte | (5) | Italy |
| Lazio | (4) | Italy |
| Umbria | (7) | Italy |
| T. borchii | ||
| Umbria | (9) | Italy |
| Basilicata | (6) | Italy |
| T. rufum | ||
| Meuse | (1) | France |
| Umbria | (2) | Italy |
| T. macrosporum | ||
| Marche | (2) | Italy |
| Toscana | (3) | Italy |
| Umbria | (5) | Italy |
| T. aestivum | ||
| Molise | (5) | Italy |
| Emilia-Romagna | (3) | Italy |
| Abruzzo | (11) | Italy |
| T. mesentericum | ||
| Abruzzo | (4) | Italy |
| T. melanosporum | ||
| Meuse | (2) | France |
| Dordogne | (9) | France |
| unknown | (8) | Spain |
| Abruzzo | (13) | Italy |
| Umbria | (11) | Italy |
| Lazio | (9) | Italy |
| Toscana | (3) | Italy |
| Marche | (2) | Italy |
| T. indicum | ||
| imported in France in 1995 | (13) | |
| imported in Italy in 1995 | (14) | |
| imported in France in 1996 | (18) | |
| imported in Italy in 1996 | (34) | |
| imported in Italy in 1997 | (22) | |
| T. brumale | ||
| Vaucluse | (4) | France |
| Meuse | (5) | France |
| Abruzzo | (2) | Italy |
| Marche | (3) | Italy |
| Lazio | (7) | Italy |
| Umbria | (18) | Italy |
| T. magnatum | ||
| Molise | (4) | Italy |
| Marche | (3) | Italy |
| Piemonte | (5) | Italy |
| Lazio | (4) | Italy |
| Umbria | (7) | Italy |
| T. borchii | ||
| Umbria | (9) | Italy |
| Basilicata | (6) | Italy |
| T. rufum | ||
| Meuse | (1) | France |
| Umbria | (2) | Italy |
| T. macrosporum | ||
| Marche | (2) | Italy |
| Toscana | (3) | Italy |
| Umbria | (5) | Italy |
| T. aestivum | ||
| Molise | (5) | Italy |
| Emilia-Romagna | (3) | Italy |
| Abruzzo | (11) | Italy |
| T. mesentericum | ||
| Abruzzo | (4) | Italy |
The numbers in parentheses are the truffles processed for each collection site.
List of ascocarps of Tuber spp. and their collection sitesa
| T. melanosporum | ||
| Meuse | (2) | France |
| Dordogne | (9) | France |
| unknown | (8) | Spain |
| Abruzzo | (13) | Italy |
| Umbria | (11) | Italy |
| Lazio | (9) | Italy |
| Toscana | (3) | Italy |
| Marche | (2) | Italy |
| T. indicum | ||
| imported in France in 1995 | (13) | |
| imported in Italy in 1995 | (14) | |
| imported in France in 1996 | (18) | |
| imported in Italy in 1996 | (34) | |
| imported in Italy in 1997 | (22) | |
| T. brumale | ||
| Vaucluse | (4) | France |
| Meuse | (5) | France |
| Abruzzo | (2) | Italy |
| Marche | (3) | Italy |
| Lazio | (7) | Italy |
| Umbria | (18) | Italy |
| T. magnatum | ||
| Molise | (4) | Italy |
| Marche | (3) | Italy |
| Piemonte | (5) | Italy |
| Lazio | (4) | Italy |
| Umbria | (7) | Italy |
| T. borchii | ||
| Umbria | (9) | Italy |
| Basilicata | (6) | Italy |
| T. rufum | ||
| Meuse | (1) | France |
| Umbria | (2) | Italy |
| T. macrosporum | ||
| Marche | (2) | Italy |
| Toscana | (3) | Italy |
| Umbria | (5) | Italy |
| T. aestivum | ||
| Molise | (5) | Italy |
| Emilia-Romagna | (3) | Italy |
| Abruzzo | (11) | Italy |
| T. mesentericum | ||
| Abruzzo | (4) | Italy |
| T. melanosporum | ||
| Meuse | (2) | France |
| Dordogne | (9) | France |
| unknown | (8) | Spain |
| Abruzzo | (13) | Italy |
| Umbria | (11) | Italy |
| Lazio | (9) | Italy |
| Toscana | (3) | Italy |
| Marche | (2) | Italy |
| T. indicum | ||
| imported in France in 1995 | (13) | |
| imported in Italy in 1995 | (14) | |
| imported in France in 1996 | (18) | |
| imported in Italy in 1996 | (34) | |
| imported in Italy in 1997 | (22) | |
| T. brumale | ||
| Vaucluse | (4) | France |
| Meuse | (5) | France |
| Abruzzo | (2) | Italy |
| Marche | (3) | Italy |
| Lazio | (7) | Italy |
| Umbria | (18) | Italy |
| T. magnatum | ||
| Molise | (4) | Italy |
| Marche | (3) | Italy |
| Piemonte | (5) | Italy |
| Lazio | (4) | Italy |
| Umbria | (7) | Italy |
| T. borchii | ||
| Umbria | (9) | Italy |
| Basilicata | (6) | Italy |
| T. rufum | ||
| Meuse | (1) | France |
| Umbria | (2) | Italy |
| T. macrosporum | ||
| Marche | (2) | Italy |
| Toscana | (3) | Italy |
| Umbria | (5) | Italy |
| T. aestivum | ||
| Molise | (5) | Italy |
| Emilia-Romagna | (3) | Italy |
| Abruzzo | (11) | Italy |
| T. mesentericum | ||
| Abruzzo | (4) | Italy |
The numbers in parentheses are the truffles processed for each collection site.
| Ectomycorrhiza | Plant growing conditions | |
| Greenhouse | Field | |
| T. melanosporum×Corylus avellana | (10) | (2) |
| T. melanosporum×Corylus colurna | – | (3) |
| T. melanosporum×Ostrya carpinifolia | (5) | – |
| T. melanosporum×Quercus pubescens | (5) | (4) |
| T. brumale×Corylus avellana | (5) | – |
| T. brumale×Quercus pubescens | (10) | (2) |
| T. brumale×Quercus ilex | – | (2) |
| T. borchii×Corylus avellana | (2) | (5) |
| T. borchii×Pinus pinea | (2) | – |
| T. borchii×Populus alba | – | (1) |
| T. magnatum×Corylus avellana | (3) | (1) |
| T. aestivum×Quercus cerris | (2) | – |
| T. indicum×Quercus cerris | (9) | – |
| T. indicum×Ostrya carpinifolia | (5) | – |
| T. indicum×Corylus colurna | (3) | – |
| T. indicum×Corylus avellana | (5) | – |
| Ectomycorrhiza | Plant growing conditions | |
| Greenhouse | Field | |
| T. melanosporum×Corylus avellana | (10) | (2) |
| T. melanosporum×Corylus colurna | – | (3) |
| T. melanosporum×Ostrya carpinifolia | (5) | – |
| T. melanosporum×Quercus pubescens | (5) | (4) |
| T. brumale×Corylus avellana | (5) | – |
| T. brumale×Quercus pubescens | (10) | (2) |
| T. brumale×Quercus ilex | – | (2) |
| T. borchii×Corylus avellana | (2) | (5) |
| T. borchii×Pinus pinea | (2) | – |
| T. borchii×Populus alba | – | (1) |
| T. magnatum×Corylus avellana | (3) | (1) |
| T. aestivum×Quercus cerris | (2) | – |
| T. indicum×Quercus cerris | (9) | – |
| T. indicum×Ostrya carpinifolia | (5) | – |
| T. indicum×Corylus colurna | (3) | – |
| T. indicum×Corylus avellana | (5) | – |
The number of plants for each fungus-host plant association is in parentheses.
| Ectomycorrhiza | Plant growing conditions | |
| Greenhouse | Field | |
| T. melanosporum×Corylus avellana | (10) | (2) |
| T. melanosporum×Corylus colurna | – | (3) |
| T. melanosporum×Ostrya carpinifolia | (5) | – |
| T. melanosporum×Quercus pubescens | (5) | (4) |
| T. brumale×Corylus avellana | (5) | – |
| T. brumale×Quercus pubescens | (10) | (2) |
| T. brumale×Quercus ilex | – | (2) |
| T. borchii×Corylus avellana | (2) | (5) |
| T. borchii×Pinus pinea | (2) | – |
| T. borchii×Populus alba | – | (1) |
| T. magnatum×Corylus avellana | (3) | (1) |
| T. aestivum×Quercus cerris | (2) | – |
| T. indicum×Quercus cerris | (9) | – |
| T. indicum×Ostrya carpinifolia | (5) | – |
| T. indicum×Corylus colurna | (3) | – |
| T. indicum×Corylus avellana | (5) | – |
| Ectomycorrhiza | Plant growing conditions | |
| Greenhouse | Field | |
| T. melanosporum×Corylus avellana | (10) | (2) |
| T. melanosporum×Corylus colurna | – | (3) |
| T. melanosporum×Ostrya carpinifolia | (5) | – |
| T. melanosporum×Quercus pubescens | (5) | (4) |
| T. brumale×Corylus avellana | (5) | – |
| T. brumale×Quercus pubescens | (10) | (2) |
| T. brumale×Quercus ilex | – | (2) |
| T. borchii×Corylus avellana | (2) | (5) |
| T. borchii×Pinus pinea | (2) | – |
| T. borchii×Populus alba | – | (1) |
| T. magnatum×Corylus avellana | (3) | (1) |
| T. aestivum×Quercus cerris | (2) | – |
| T. indicum×Quercus cerris | (9) | – |
| T. indicum×Ostrya carpinifolia | (5) | – |
| T. indicum×Corylus colurna | (3) | – |
| T. indicum×Corylus avellana | (5) | – |
The number of plants for each fungus-host plant association is in parentheses.
Mycorrhizal root tips were collected from pot-grown plants 6 months, 1 and 3 years after inoculation and from three truffle plantations located in Umbria (Central Italy) 3, 5 and 10 years after planting. Mycorrhizal roots were washed with tap water to remove soil and major contaminants and checked for the presence of corresponding ectomycorrhizae on the basis of morphological, structural and biometric traits, such as the outer cells of the mantle, cystidia and emanating hyphae [2,4–6]. The mycorrhizae were then frozen in liquid nitrogen and immediately used for DNA isolation or stored at −70°C.
2.2 DNA isolation
Genomic DNA was isolated from freeze-dried and fresh ascocarps (1–5 mg), mycelia, root tips (1–5 mm long) of host plants and single and/or pooled mycorrhizal root tips (1–5 mm long). To ensure that ectomycorrhizae of each black truffle species were represented across the full range of developmental stages, they were collected both at the active growth phase, when emanating hyphae and cystidia are present, and at the quiescent stage when root tips show few or none of these taxonomically important structures. Pools containing up to 50 mycorrhizal root tips were collected from: (a) different plants inoculated with a given Tuber spp.; (b) plants inoculated with different Tuber species; (c) single plants inoculated with a given truffle species. DNA was isolated as described by Edwards et al. [23] with some modifications. The tissue was crushed in liquid nitrogen in a 2-ml microcentrifuge tube using a sterilized micropestle. The ground tissue was suspended in 100–300 μl buffer containing 200 mM Tris-HCl pH 7.5, 250 mM NaCl, 25 mM EDTA and 0.5% SDS, vortexed for 10 s and kept at room temperature until all samples were extracted. The extracts were centrifuged for 10 min at 10 000×g, the supernatants were transferred to a new microcentrifuge tube and precipitated in an equal volume of isopropanol for 15–30 min at −20°C. The DNA was pelleted by centrifugation for 15 min, vacuum-dried and resuspended in 10–200 μl double-distilled nuclease-free water. Resuspension of the pellet was occasionally facilitated by warming at 65°C for 2–5 min. When clumps were present, a further 2–5-min centrifugation was performed and the supernatant carefully transferred to a new tube. The DNA concentration was evaluated against Quantitation Standards Phage λ DNA (Life Technologies, Glasgow, UK) on an 0.8% agarose gel. DNA was visualized by staining with ethidium bromide.
DNA isolation and PCR amplification from single or pooled ectomycorrhizae, ascocarps and host plants were carried out at least in triplicate, unless specified.
2.3 PCR amplification
Forward black truffle species-specific ITS primers ITSML (5′-TGGCCATGTGTCAGATTTAGTA-3′) specific to T. melanosporum, ITSB (5′-CAATGTCAGAGCCAATCTAATGC-3′) specific to T. brumale, ITSCHCH (5′-AACAACAGACTTTGTAAAGGGTTG-3′) specific to T. indicum and the backward ITS4LNG (5′-TGATATGCTTAAGTTCAGCGGG-3′) common to the three black species were derived by Paolocci et al. ([20], Rubini and Paolocci, unpublished results). PCR amplification with these primers was carried out in an OMNI-E (Hybaid, UK) thermal cycler using 0.3–5 ng of target DNA isolated from ectomycorrhizae, control plants and ascocarps of truffle species. The cycling parameters were: an initial denaturation step at 95°C for 3 min; 23 cycles consisting of 30 s at 94°C, 30 s at 63°C and 45 s at 72°C; and a final extension step for 7 min at 72°C. Three different amplification buffers (A, B and C) and different concentrations (0, 0.5, 1,3.5,7, 15 and 25 mg ml−1) of bovine serum albumin (BSA, Sigma, Italy) were tested. The composition of the three buffers was: (A) 75 mM Tris (pH 9), 50 mM KCl and 0.01% Tween 20; (B) 75 mM Tris (pH 9), 20 mM (NH4)SO4 and 0.01% Tween 20; (C) 10 mM Tris (pH 9) and 50 mM KCl. All the amplification buffers contained a final MgCl2 concentration of 4 mM, 20 pmol of each specific forward primer, 20 pmol of the backward primer, 200 mM of each dNTP in a final volume of 50 μl, in the presence of 2.5 units of Taq polymerase (Pharmacia Biotech, Uppsala, Sweden). PCR products (20 μl) were size-fractionated on 1% or 2% agarose gels for distinguishing products of the multiplex PCR.
PCR amplification with universal ITS1 and ITS4 primers [24] on host plants and ectomycorrhizal fungi was performed as above, but the annealing temperature was decreased to 55°C. Positive and negative controls (no DNA template) were included in all experiments.
3 Results
3.1 DNA isolation and PCR conditions for ectomycorrhizal analyses
With the DNA isolation protocol used in this study, a single quiescent ectomycorrhizal root tip (10 young ectomycorrhizae of a weight of about 1.5 mg) yielded sufficient DNA for at least one PCR reaction, while on average 15–20 ng DNA was obtained from actively growing mycorrhizae for multiple reactions. DNA isolated from 1–5 mg of ascocarp tissue was also enough for at least 100 PCR reactions. DNAs isolated from ascocarps of all the species under scrutiny, as well as from roots of host plants, were amplified with or without BSA in the presence of specific primers. BSA was not essential for amplification of the ITS region on DNA isolated from mycelia and fruit bodies (see below).
DNA isolated from pools of 50 quiescent mycorrhizae of T. melanosporum on Corylus avellana and T. brumale on Quercus pubescens were amplified in duplicate samples with the three amplification buffers in the presence of different concentrations of BSA (0, 0.5, 1, 3.5, 7, 15 and 25 mg ml−1), both with the ITS1/ITS4 primer pair and with all the black truffle species-specific ITS primers. Amplification was obtained in DNA isolated from both ectomycorrhizal species with all buffers tested only in the presence of high concentrations (7, 15 and 25 mg ml−1) of BSA (data not shown). In terms of presence and intensity of the amplified PCR fragments, buffer C gave the best results and DNA amplification was easily detectable on ectomycorrhizae with 7, 15 and 25 mg ml−1 of BSA. In contrast, none of the ectomycorrhizal samples showed the expected amplicons with a BSA concentration of less than 3.5 mg ml−1, giving rise to false-negative amplifications (data not shown). Therefore, all the amplifications reported were performed with buffer C and 7 mg ml−1 of BSA was added when single or pooled ectomycorrhizae were processed.
3.2 Ectomycorrhizal identification by multiplex PCR analysis
Multiplex PCR experiments carried out in the presence of the three specific primers plus the backward ITS4LNG on DNA targets isolated from samples of different sizes (3–30 apical root tips) of ectomycorrhizae of T. melanosporum, T. brumale and T. indicum on hazel, mixed in a ratio of 1:1:1, revealed the three Tuber spp. These were also unambiguously distinguished by their different ITS lengths: about 440 bp in T. melanosporum, 700 bp in T. brumale and 140 bp in T. indicum. DNA from T. melanosporum, T. brumale and T. indicum ascocarps yielded amplicons with lengths perfectly matching those of their relative ectomycorrhizae, while host plant species did not display any amplification products (Fig. 1).
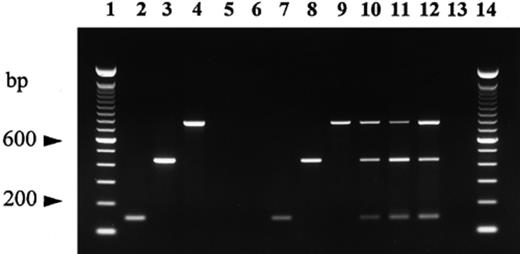
Multiplex PCR amplification of DNA isolated from ascocarps of T. melanosporum, T. brumale and T. indicum, their relative quiescent ectomycorrhizae and host plants using the primers ITS4LNG, ITSB, ITSML, ITSCHCH. Lanes 1 and 14: 100-bp ladder (Gibco BRL); lane 2: T. indicum ascocarp 20; lane 3: T. melanosporum ascocarp; lane 4: T. brumale ascocarp; lane 5: oak root; lane 6: hazel root; lane 7: T. indicum mycorrhiza; lane 8: T. melanosporum mycorrhiza; lane 9: T. brumale mycorrhiza; lanes 10–12: mixtures of T. indicum, T. melanosporum and T. brumale mycorrhizae at a ratio of 1:1:1 with 3 (lane 10), 9 (lane 11) and 30 (lane 12) root tips; lane 13: negative control (no DNA template).
To ascertain whether PCR amplification was affected by the relative amount of DNA targets, duplicate samples were prepared by mixing T. indicum and T. melanosporum ectomycorrhizae in ratios of 1:1, 1:3, 1:5, 1:10, 1:25 and 1:50. For each sample of mixed mycorrhizae, DNA was isolated and then amplified. The specific primers ITSCHCH and ITSML together with the common backward primer ITS4LNG allowed easy detection of the contaminating fungus (T. indicum) in all samples (Fig. 2). DNA isolated from mixtures of ectomycorrhizae of T. melanosporum, T. brumale and T. indicum gave similar results (data not shown).
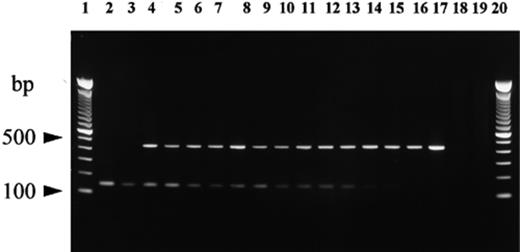
PCR amplification of DNA isolated from mixtures of actively growing ectomycorrhizae of T. melanosporum and T. indicum on potted grown hazel plants using the primers ITS4LNG, ITSB, ITSML and ITSCHCH. Lanes 1 and 20: 100-bp ladder (Gibco BRL); lane 2: T. indicum ascocarp; lane 3: T. indicum ectomycorrhizae; lanes 4–15: mixtures of T. indicum and T. melanosporum ectomycorrhizae at ratios of 1:1 (lanes 4 and 5), 1:3 (lanes 6 and 7), 1:5 (lanes 8 and 9), 1:10 (lanes 10 and 11), 1:25 (lanes 12 and 13), 1:50 (lanes 14 and 15); lane 16: T. melanosporum ectomycorrhizae; lane 17: T. melanosporum ascocarp; lane 18: hazel roots; lane 19: negative control (no DNA template).
When PCR was carried out with species-specific primers, amplicons were visible only on DNA tested from T. melanosporum, T. brumale and T. indicum mycorrhizae, irrespective of fungal genotypes used as spore donors, host plant species and plant growing conditions (see Figs. 1,2,3). In contrast, the above mentioned primers did not produce amplicons on DNA isolated from host root tips and ectomycorrhizae of both other truffle species (Table 2) and other symbiotic fungi, such as Hebeloma and Cenococcum spp. (Fig. 3). For control purposes all of the target DNAs were amplified using the universal ITS1/ITS4 primers, and all DNAs, isolated either from fruit bodies or from ectomycorrhizae, displayed PCR products (Fig. 3).
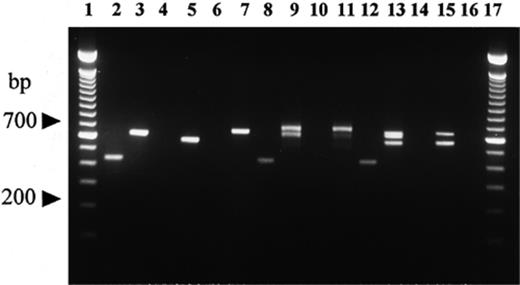
Comparison of products resulting from PCR amplification using species-specific ITS primers vs. universal ITS primers ITS1 and ITS4, on DNA isolated from fruit bodies and mycorrhizal root tips from field-grown plants. Lanes 1 and 17: 100-bp ladder (Gibco BRL); lanes 2, 4, 6, 8, 10, 12 and 14: amplification with ITSB, ITSM, ITSCHCH and ITS4LNG primers; lanes 3, 5, 7, 9, 11, 13, 15 and 16: amplification with ITS1/ITS4 primers. Lanes 2 and 3: T. melanosporum ascocarp; lanes 4 and 5: T. borchii ascocarp; lanes 6 and 7: T. magnatum ascocarp; lanes 8 and 9: non-truffle-forming ectomycorrhizal root tips mixed with a young T. melanosporum mycorrhiza; lanes 10 and 11: non-truffle-forming ectomycorrhizal root tips; lanes 12 and 13: mixture of single young mycorrhizae of T. magnatum, T. borchii and T. melanosporum; lanes 14 and 15: mixture of T. magnatum and T. borchii young mycorrhizae; lane 16: negative control (no DNA template).
4 Discussion
The new procedure for DNA isolation from Tuber spp. described in this study minimizes the amount of ectomycorrhizal tissue required for molecular analysis in comparison with protocols reported so far [3,7,11,12]. In fact this protocol reduces the number of steps and avoids the use of organic solvents such as phenol, chloroform and benzyl chloride for DNA purification. Although the yield of nucleic acids is highly dependent on the physiological stage and/or age of ectomycorrhizae, this method is able to isolate intact DNA from as little as 1–2 mm long ectomycorrhizae and to process numerous samples in a short time. When BSA was added to the PCR reaction mixture in the range of 7–25 mg ml−1, the expected fungal ITS fragment was always amplified from root tip DNA isolated with the simple and rapid protocol adopted. None of the DNA targets were amplified with BSA concentrations of 0.5 mg ml−1 and 1 mg ml−1, or without BSA, while only few samples showed the expected amplicons at a concentration of 3.5 mg ml−1 of BSA. As DNA isolated from fruit bodies was amplified without the addition of BSA as a competitor protein [16,25], the inhibitors must be either highly concentrated or peculiar to host roots, irrespective of whether they are mycorrhizal or not.
This PCR approach produced reliable results in multiplex PCR in all fungus-host plant associations tested and permitted ectomycorrhizae of Tuber species to be identified on different host plants, and even on a single plant when it was simultaneously colonized by more than one of the black truffles under investigation. The simultaneous presence on a single host plant of more than one black truffle species even in combination with non-truffle-forming colonizing fungi, such as Cenococcum and Hebeloma spp. and other non-truffle forming undetermined species, never affected Tuber species identification (Fig. 3). In contrast, the replacement of species-specific primers with universal ITS1/ITS4 primers produced an electrophoretic pattern difficult to interpret (Fig. 3). Moreover, these results did not allow a clear fungal typing by RFLP analyses of amplified ITS, as previously suggested for truffle typing [7,11,12].
The relative technical simplicity of the DNA isolation protocol and multiplex PCR amplification reported here makes the method of practical value. The results are obtained within a few hours and this molecular approach is particularly suitable for resolving those cases where species cannot be identified on the basis of morphological criteria, for instance in discriminating the T. melanosporum mycorrhizae from those of T. brumale or from those of the Asiatic species T. indicum. The same approach could be applied to identifying other Tuber spp., e.g. distinguishing Tuber magnatum from Tuber borchii mycorrhizae. As truffles are not produced until 8–10 years after the planting of nursery-inoculated plants, growers consider the identification of fungal species present on host plant roots an essential prerequisite for guaranteeing the future production of the desired truffle species. The molecular approach proposed here would give growers a clearer answer regarding truffle species identification than any visual or molecular analysis so far performed.
The test we propose offers a reliable, relatively easy to perform and inexpensive tool for typing morphologically similar truffle ectomycorrhizae. Moreover, this method is useful for tracking the fate of mycorrhizal seedlings and monitoring the interaction of introduced truffle species with preexisting ectomycorrhizal symbionts.
Acknowledgements
The authors thank: Mr. F. Calderini for technical assistance and Mr. A. Bolletta for photographic plates; the Ispettorato Prevenzioni e Repressione Frodi Agroalimentari in Perugia and Rome, Dr. J.C. Raquillet (Modene, France) and Gruppo Urbani Tartufi for providing some ascocarps used in this study. This research was supported by The National Research Council Italy, Progetto Strategico ‘Biotecnologia della micorrizazione’.
References



