-
PDF
- Split View
-
Views
-
Cite
Cite
Kuangyi Xu, The coevolution of flower longevity and self-fertilization in hermaphroditic plants, Evolution, Volume 75, Issue 8, 1 August 2021, Pages 2114–2123, https://doi.org/10.1111/evo.14303
Close - Share Icon Share
Abstract
Self-fertilization, prevalent in plants, is typically divided into three modes – prior, competing, and delayed selfing – based on the timing in which it occurs. Flower longevity affects both the opportunity for pollination and the resources allocated for fertility, and thus may influence the selection on different modes of self-fertilization. Additionally, selfing causes fertilization to depend less on pollinators, which may also influence the evolution of flower longevity. Using game-theoretical models, I investigate how inbreeding depression and the pollination environment influences the coevolution of the three modes of self-fertilization with flower longevity. Invasion of prior selfing allows the subsequent evolution of shorter flower longevity, and thus is favored over competing selfing. Prior selfing can also invade even under high inbreeding depression when the pollen deposition rate is low, but is inhibited by a higher level of delayed selfing. In general, the evolution of selfing decreases flower longevity, and reveals asymmetric effects of pollen deposition and removal on flower longevity. This study suggests considering realization of selfing and outcrossing as concrete processes by incorporating flower reproductive strategies (e.g., flower longevity) and pollination ecology (e.g., accrual rate) may offer better understanding of the evolution of mating systems and flower reproductive traits.
The evolution of mating systems, particularly, outcrossing and self-fertilization, constitutes one of the major evolutionary transitions in angiosperms (Stebbins 1974; Barret 2008; Barret 2013). Based on the timing of self-pollination relative to cross-pollination, Lloyd and Schoen (1992) classified self-fertilization into three modes: prior, competing, and delayed selfing. Prior selfing happens when anthers dehisce and stigmas become receptive before anthesis (here defined as the period when a flower is fully functional to allow outcross-pollination to happen), and the two pollinating surfaces are oriented to make contacts. Competing selfing refers to self-fertilization that occurs during anthesis when cross-pollination is happening simultaneously. It can be either autonomous, which does not require pollinators, or pollinator-dependent, which may result from geitonogamy (pollination between flowers within the same individual) or facilitated selfing (Schoen and Lloyd 1992). Delayed selfing happens after opportunities for outcrossing or pollen dispersal have passed, and can be accomplished through mechanisms like corolla abscission, style curvature, reduced herkogamy, or transient self-incompatibility (Goodwillie and Weber 2018).
From a genetic aspect, selfing automatically enjoys the advantage of transmitting 50% more alleles than outcrossing, since it transmits genes through both selfed and outcrossed offspring (Fisher 1941). However, it is opposed by inbreeding depression, which is the fitness reduction in selfed offspring compared to outcrossed offspring, as selfing exposes more recessive deleterious mutations in homozygotes. Classical models have shown that a higher level of competing selfing is favored when inbreeding depression is lower than 0.5, while the condition for prior selfing to invade depends on the outcrossing rate (Lloyd 1979; Lande and Schemske 1985). A more recent model shows that fluctuating pollination visitation rates can select for an intermediate level of prior selfing even under high inbreeding depression (Morgan and Wilson 2005). Unlike the other two types of selfing, delayed selfing can always invade (Lloyd 1979), because it happens after pollination and does not restrict the opportunity for ovules to be outcrossed. Moreover, delayed selfing can provide reproductive assurance when pollinators are lacking. Therefore, it is often regarded as a “best-of-both-worlds” strategy that allows plants to maximize their reproductive fitness (Becerra and Lloyd 1992; Lloyd 1992; Kalisz and Vogler 2003).
However, researchers rarely consider the ecological processes that affect how outcrossing and selfing are implemented and their influences on resource-allocation in floral reproductive strategies. Regarding the ecological aspect, one of the most important factors that affect the reproductive outcome is flower longevity, which has been found to have important effects on mating outcomes (Primack 1985; Sato 2002; Ashman 2004; Roddy et al. 2020). Since the arrival of pollinators is often unpredictable, an outcrossing flower needs to remain open for a certain period to allow the possibility of pollen deposition and removal. Although greater flower longevity can increase pollination opportunities for outcrossing, it imposes a cost because more resources and energy will be spent on the daily maintenance of flowers. Previous models and empirical studies have shown that greater flower longevity is selected for when the rates of pollen deposition and removal are low (Ashman and Schoen 1994; Schoen and Ashman 1995; Ashman 2004). However, these studies focus on predominantly outcrossing populations, and it is not clear how flower longevity interacts with the evolution of mating systems. For example, the evolution of selfing may select for shorter flower longevity because autonomous selfing does not require pollinators and it thus becomes less economical to maintain the flowers for a long period. An extreme case may be cleistogamy, that is, production of non-opening, self-pollinating flowers (Culley and Klooster 2007). On the other hand, decreased flower longevity may allow more energy to be allocated to seed reproduction, which may compensate for the fitness cost of selfing due to inbreeding depression. Indeed, selfing and flower longevity may coevolve with each other. For example, in Leptosiphon jepsonii, a population that evolved a higher level of delayed selfing also shows increased flower longevity (Weber and Goodwillie 2009).
Although the evolution of mating systems has been intensively studied, the effects of floral reproductive strategies and pollination environment are rarely considered (reviewed in Goodwillie et al. 2005, but see Sakai 1995). Therefore, here I use the evolutionary game theory (Maynard Smith 1982) to study the coevolution of flower longevity and the three modes of self-fertilization. In the model, the pollination process is explicitly divided into two aspects: the rate of pollen deposition and ovule fertilization (female fitness accrual rate), and the rate at which pollen is disseminated and enters into the pollen pool to fertilize ovules of other individuals (male fitness accrual rate). The effects of inbreeding depression, and female and male fitness accrual rates on the coevolutionary pattern are investigated.
Model
I assume a hermaphroditic population, where each flower produces equal amounts of pollen and numbers of ovules. Female and male fitness accrual rates are denoted as and . It should be noted that female fitness accrual rate in the model includes both self-pollination (geitonogamy, facilitated selfing and autonomous competing selfing) and outcrossing during anthesis. Throughout the paper, I assume both female and male accrual rates do not change over time.
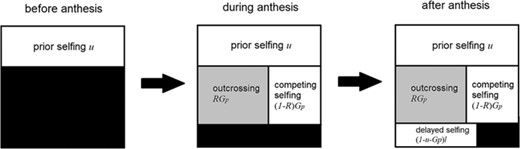
A schematic plot that shows how ovules in a flower will be fertilized. Ovules remaining unfertilized are colored black, and ovules that are selfed and outcrossed are colored white and grey, respectively.
Results
Here, I first show the conditions for the evolution of competing, prior and delayed selfing, and then investigate how selfing influences the evolution of flower longevity. The key results are summarized in Table 1.
Summary of the key model results. is the proportion of prior selfing, and is the proportion of outcrossed ovules during anthesis, and measures the efficacy of delayed selfing. Note that there is no distinguish between prior, competing and delayed selfing when {}
| inbreeding depression . | inbreeding depression . | ESS flower longevity . | selfing rate at the ESS state . |
|---|---|---|---|
| The ESS is complete prior selfing, non-opening flowers{} (cleistogamy). However, this ESS may not directly invade into an outcrossing population, but {} will first invade and then the ESS {} can invade into {}. | When male fitness accrual rate is low and female fitness accrual rate is high, the ESS is , especially when is small; otherwise, the ESS is outcrossing, open flowers {}. | 1. A larger decreases 2. is determined more by the male fitness accrual rate 3. When is large, may increase with the female fitness accrual rate. | Selfing rate at the ESS state increases with the male fitness accrual rate, decreases with the female fitness accrual rate |
| inbreeding depression . | inbreeding depression . | ESS flower longevity . | selfing rate at the ESS state . |
|---|---|---|---|
| The ESS is complete prior selfing, non-opening flowers{} (cleistogamy). However, this ESS may not directly invade into an outcrossing population, but {} will first invade and then the ESS {} can invade into {}. | When male fitness accrual rate is low and female fitness accrual rate is high, the ESS is , especially when is small; otherwise, the ESS is outcrossing, open flowers {}. | 1. A larger decreases 2. is determined more by the male fitness accrual rate 3. When is large, may increase with the female fitness accrual rate. | Selfing rate at the ESS state increases with the male fitness accrual rate, decreases with the female fitness accrual rate |
Summary of the key model results. is the proportion of prior selfing, and is the proportion of outcrossed ovules during anthesis, and measures the efficacy of delayed selfing. Note that there is no distinguish between prior, competing and delayed selfing when {}
| inbreeding depression . | inbreeding depression . | ESS flower longevity . | selfing rate at the ESS state . |
|---|---|---|---|
| The ESS is complete prior selfing, non-opening flowers{} (cleistogamy). However, this ESS may not directly invade into an outcrossing population, but {} will first invade and then the ESS {} can invade into {}. | When male fitness accrual rate is low and female fitness accrual rate is high, the ESS is , especially when is small; otherwise, the ESS is outcrossing, open flowers {}. | 1. A larger decreases 2. is determined more by the male fitness accrual rate 3. When is large, may increase with the female fitness accrual rate. | Selfing rate at the ESS state increases with the male fitness accrual rate, decreases with the female fitness accrual rate |
| inbreeding depression . | inbreeding depression . | ESS flower longevity . | selfing rate at the ESS state . |
|---|---|---|---|
| The ESS is complete prior selfing, non-opening flowers{} (cleistogamy). However, this ESS may not directly invade into an outcrossing population, but {} will first invade and then the ESS {} can invade into {}. | When male fitness accrual rate is low and female fitness accrual rate is high, the ESS is , especially when is small; otherwise, the ESS is outcrossing, open flowers {}. | 1. A larger decreases 2. is determined more by the male fitness accrual rate 3. When is large, may increase with the female fitness accrual rate. | Selfing rate at the ESS state increases with the male fitness accrual rate, decreases with the female fitness accrual rate |
ESS OF OUTCROSSING PROPORTION () DURING COMPETING SELFING
ESS OF THE PROPORTION OF PRIOR SELFING ()
Recalling that when , a higher level of competing selfing will also invade, it is interesting to note that because the evolution of prior selfing () will cause all of the ovules to be self-fertilized before anthesis, the evolution of competing selfing will become irrelevant in these cases. When over time this mutant with complete prior selfing dominates the population, a shorter flower longevity (a smaller value of ) will invade due to its lower maintenance cost. As a result, when , the ESS strategy will be {}. Biologically, it means complete prior selfing with a flower longevity of 0, that is, cleistogamy.
Nevertheless, even under low inbreeding depression (), it should be emphasized that a cleistogamous mutant with strategy {} will not necessarily invade a predominantly outcrossing population. For example, consider that a cleistogamous mutant arises in a completely outcrossing population with longevity (where T > 0). Based on equations (6), (7a) and (7b), the relative fitness of the mutant is . Even under low inbreeding depression (e.g., ), as long as the maintenance cost is small and fitness accrual rate is high enough (e.g., , , ), the mutant cannot invade as the relative fitness . Therefore, evolution toward cleistogamy may often be step-wise. Initially, individuals with a higher level of prior selfing will invade, provided that they have a similar level of flower longevity, which allows the pollen disseminated by the plant to fertilize outcrossing residents; only through pollen dissemination does the mutant enjoy the “transmission advantage” of self-fertilization (Fisher 1941). After individuals with a high prior selfing rate dominates the population, a shorter level of flower longevity can easily invade.
In the above analyses, the ESS does not depend on the order of invasion. In other words, the ESS will be the same no matter whether each phenotype evolves to its ESS before another evolves or not. This is because the invasion conditions (equations (9), (10) and (12)) do not depend on the value of the other phenotypes. For example, for the invasion condition of in equation (9), the term that involves and is always positive for all values of and , so whether a larger will invade only depend on .
COEVOLUTION OF FLOWER LONGEVITY WITH SELF-FERTILIZATION
When delayed selfing can fertilize all remaining ovules ()
In the previous section, it has been shown that when and , a higher rate of prior and competing selfing cannot invade. In other words, the ESS strategy is , so the phenotypic space {} is reduced to a one-dimensional space of longevity . Here, I show how delayed selfing can influence the evolution of flower longevity. For the special case when inbreeding depression , no selfed seeds can survive to the next generation, so effectively, there is no delayed selfing. Since the population is effectively outcrossing, we return to the previous results obtained by Ashman and Schoen (1994), and as shown in Fig. 2A, the effects of female or male fitness accrual rates on flower longevity are symmetrical, and a higher female or male fitness accrual rate selects for a shorter flower longevity.
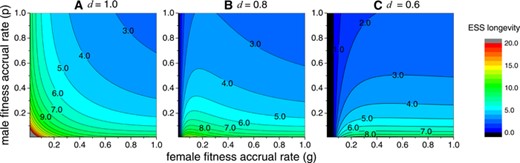
ESS flower longevity under different female and male fitness accrual rates when delayed selfing fertilizes all the remaining ovules (). The three panels (A), (B), and (C), respectively, show results under different levels of inbreeding depression , and . The color bar shows different levels of ESS longevity, and 20 is colored grey. For all panels, .
When , delayed selfing becomes effective. Given the same level of female and male fitness accrual rates, a lower inbreeding depression always results in a shorter flower longevity, which can be seen by comparing panel (A) with panels (B) and (C) in Fig. 2. This is easy to understand—when inbreeding depression is lower, selfing is more advantageous, and thus an individual is less likely to maintain flowers longer to wait for outcrossing.
Moreover, the incorporation of delayed selfing also changes the effects of the fitness accrual rate on flower longevity. When inbreeding depression is moderately high (), a higher female fitness accrual rate may even increase the ESS longevity. In Fig. 2B, for a fixed male fitness accrual rate, ESS flower longevity first increases with female fitness accrual rate but later decreases when gets large. Moreover, as inbreeding depression becomes lower () in Fig. 2C, flower longevity generally monotonically increases with female fitness accrual rate and converges to a certain value. In contrast, flower longevity is more sensitive to the male fitness accrual rate and decreases as the male fitness accrual rate gets larger. It should be noted that in panels (b) and (c), the ESS longevity is when the female fitness accrual rate is quite small. In this case, there is no distinction between prior, competing and delayed self-fertilization.
The effect of delayed selfing on reducing flower longevity even under high inbreeding depression () can be explained by performing some analysis on the relative fitness. Suppose a mutant has a shorter flower longevity than the residents (). Certainly, since the residents open their flowers for a longer time, the female and male fitness accrued by the mutant will always be lower than those of the resident, irrespective of whether there is self-fertilization or not. However, compared to the case of no delayed selfing, the relative female and male fitness of the mutant individual will be larger when there is delayed selfing. Moreover, when female fitness accrual rate is high so that only few ovules will remain unfertilized at the time of flower senescence, the beneficial effects from delayed selfing become less prominent since the female fitness and is already large. As a result, in Fig. 2, delayed self-fertilization becomes less efficient in lowering ESS flower longevity when the female fitness accrual rate is high.
Delayed selfing and flower longevity co-determine the selfing rate of the flower, here defined as the proportion of self-fertilized seeds out of the total fertilized seeds. When there is delayed selfing, the selfing rate is determined by both flower longevity and the female fitness accrual rate. As shown in Fig. 3, generally, a high selfing rate is found when female fitness accrual rate is low while male fitness accrual rate is high, which corresponds to the condition when delayed selfing results in a short flower longevity in Fig. 2. On the other hand, most ovules will be outcrossed under high female fitness accrual rate and low male fitness accrual rate, and this is the situation when a longer flower life span is favored in Fig. 2. However, selfing rate is more sensitive to the rate of pollen deposition (female fitness accrual rate), rather than the rate of pollen removal (male fitness accrual rate). Moreover, comparing Fig. 3A and B, it can be seen that lower inbreeding depression will increase selfing rate. This is because lower inbreeding depression lowers the ESS flower longevity, so there will be fewer outcrossed ovules.
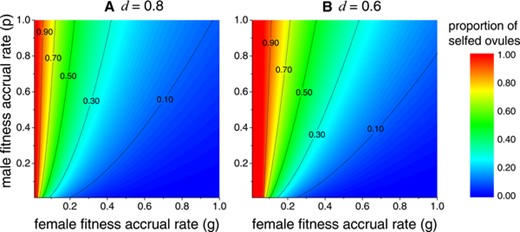
Proportion of selfed ovules under ESS flower longevity at different female and male fitness accrual rates when delayed selfing fertilizes all the remaining ovules (). Panels (A) and (B) respectively show results when inbreeding depression and . The color bar shows different level of selfing rate, which is the proportion of selfed ovules out of total fertilized ovules. For all panels, .
When delayed selfing cannot fertilize all remaining ovules ()
When delayed selfing cannot fertilize all the remaining ovules () and , a higher outcrossing proportion will always invade (see equations (8a) and (8b)), irrespective of the values of and . Therefore, I can always set , so that the phenotypic space of is reduced to two dimensions, that is, and . Equation (9) shows that given a certain value of , the ESS prior selfing rate can only be either or . Therefore, I can first find the ESS flower longevity conditioned on based on equation (8), denoted as (the subscription indicates it is conditioned on ), and then set and apply the invasion condition given by equation (9) to see whether a higher prior selfing rate will invade or not. If a higher is not favored at , then the ESS strategy is {, }. If a higher is favored, the ESS strategy is {}.
Figure 4 shows ESS flower longevity at different levels of inbreeding depression and proportions of delayed self-fertilization . Generally, {} will be the ESS when female fitness accrual rate is low while male fitness accrual rate is high (the black area). In this case, due to rare pollen deposition, outcrossing is inefficient to set seeds. On the other hand, a high selfing rate is more advantageous when pollen removal is frequent because selfing individuals can disseminate more pollen to fertilize outcrossing individuals (i.e., a greater transmission advantage).
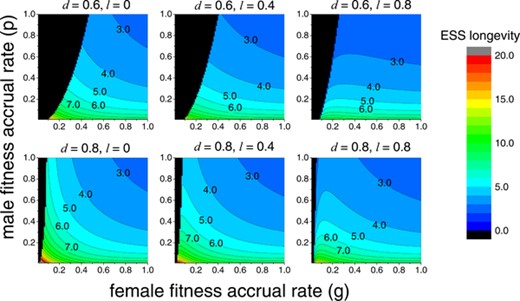
Effects of female and male fitness on ESS flower longevity under coevolution of self-fertilization and flower longevity. The ESS in the black area is , and is in the colored area. Panels show results at different levels of inbreeding depression () and delayed selfing rates (). For all panels, .
To examine the effects of inbreeding depression, by comparing panels with the same value of , it can be seen that the strategy {} is more likely to be ESS under a lower level of inbreeding depression, since self-fertilization should enjoy more benefits when inbreeding depression is low. Moreover, comparing panels with different values of , it can be seen that {} is less likely to be an ESS when delayed selfing is able to fertilize a larger proportion of the remaining ovules. Furthermore, within the parameter space in which {} is not an ESS (colored area), a higher value of decreases ESS flower longevity, because a stronger ability of fertilizing ovules through delayed selfing should select for a shorter flower life span spent waiting for outcrossing pollination.
Discussion
How the evolution of different modes of selfing (i.e., prior, competing, and delayed selfing) interacts with floral reproductive strategies and the pollination environment is often poorly understood. This study examines the coevolution of the three modes of self-fertilization with flower longevity, and explores how they are regulated by genetic and ecological factors including inbreeding depression and the rate of pollen deposition and removal. Unlike previous models that separately consider the evolution of different modes of selfing (Lloyd 1979; Morgan and Wilson 2005), the coevolutionary model shows that the evolution of one mode of selfing may prevent the evolution of other modes. When inbreeding depression is low, evolution of prior selfing will replace competing selfing, as the latter requires daily flower maintenance; while when inbreeding depression is high, a higher level of delayed selfing will prevent the evolution of prior selfing. On the other hand, the incorporation of selfing in the model unveils the asymmetric effects of pollen deposition and removal on the evolution of flower longevity, which is not apparent in previous models that assume an outcrossing population (Ashman and Schoen 1994). Specifically, flower longevity is much more sensitive to the rate of pollen removal than to the rate of pollen deposition, and may increase, rather than decrease, with the rate of pollen deposition.
This study also bridges factors that initially seem unrelated. Although the rates of pollen deposition and removal directly affect the evolution of flower longevity, the model shows they can indirectly regulate the evolution of different modes of self-fertilization (Figs. 2 and 4), as well as the selfing rate, which increases with the pollen deposition rate but decreases with the pollen removal rate (Fig. 3). Therefore, it may be valuable to divide the measurements of pollinator visitation rates into the rate of pollen deposition and removal in empirical studies. Similarly, although inbreeding depression directly affects the evolution of selfing, it also indirectly affects flower longevity (Fig. 4).
EVOLUTION OF CLEISTOGAMY
The current model shows that under low inbreeding depression, although both prior and competing selfing are favored, the evolution of prior selfing allows for the later evolution of shorter flower longevity, and finally reach an ESS with complete selfing and 0 flower longevity. Biologically, this ESS state is similar to cleistogamy, that is, the production of permanently closed, self-pollinated flowers. However, it is shown that cleistogamy sometimes cannot directly invade an outcrossing population because of pollen discounting. However, note that pollen discounting in the current model is not due to the consumption of pollen by self-fertilization, but due to short flower longevity.
Cleistogamy has been shown to be present across more than 50 families (Culley and Klooster 2007). Although previous study found cleistogamous flowers appear when pollinators are less frequent (Bennington and McGraw 1995), the current model indicates that cleistogamy is most likely to evolve under a low pollen deposition rate and a high rate of pollen dissemination (Fig. 3). Therefore, a future study that explicitly distinguishes between the two components of pollination may offer better understanding of the evolution of cleistogamy.
The prediction that cleistogamy will be favored under lower inbreeding depression is consistent with the results of previous models (Schoen and Lloyd 1984; Masuda et al. 2001). However, in populations with low inbreeding depression, complete cleistogamy is not common; instead many populations can produce both cleistogamous and open-pollinated flowers (Culley and Klooster 2007; Koontz et al. 2017). This can be due to several factors. First, populations may not be at an evolutionary equilibrium. As discussed previously, cleistogamy often cannot directly invade into an open-pollinated population, but populations will first evolve a higher selfing rate before flower longevity decreases. Second, spatial or temporal variation in pollinator visitation may favor the maintenance of open-pollinated flowers to set more outcrossed seeds and avoid inbreeding depression (Lloyd 1984; Schoen and Lloyd 1984).
COEVOLUTION OF FLOWER LONGEVITY AND SELF-FERTILIZATION
The model predicts that selfing will always decrease flower longevity, which is supported by meta-analysis. Using data from 52 species of annual crucifers, Kunin and Shmida (1997) found outcrossing species generally had a flower life span 25% longer than inbreeding species. Under low inbreeding depression in which outcrossing is favored, it is expected that unavoidable competing selfing due to geitonogamy in self-compatible species would select for shorter-lived flowers (Primack 1985).
The finding that prior self-fertilization can be ESS even under high levels of inbreeding depression when female fitness accrual rate is low may help explain high levels of inbreeding depression in some populations with intermediate or high selfing rates (Husband and Schemske 1996; Winn et al. 2011). A test of this prediction requires more detailed measurements of different modes of self-fertilization, pollination history, flower longevity and inbreeding depression. Moreover, due to the purging of deleterious alleles and thus lower inbreeding depression, prior selfing may finally dominate a population.
Although flower longevity has been frequently studied, there is not much direct evidence regarding how different modes of self-fertilization coevolve with flower longevity. In the annual plant species Leptosiphon jepsonii, individuals usually perform delayed selfing through transient self-incompatibility, in which flowers initially reject self-pollen but become self-compatible 1 or 2 days later. However, Goodwillie and Ness (2005) found variation in the timing of self-compatibility within 17 populations, where some individuals were fully self-compatible at the initial stage of flower anthesis, thus allowing for prior selfing. Moreover, the frequency of these initially self-compatible individuals varied among populations, ranging from 3.2% to 71.0%. Results from the current model suggest that the invasion of early self-compatibility may be associated with the pollination environment leading to a low female fitness accrual rate, or because delayed selfing through transient self-incompatibility does not allow complete reproductive assurance.
The model prediction in Fig. 3 that a higher rate of pollen deposition will inhibit the evolution of prior selfingseems to be supported by previous empirical evidence. A following study by Weber and Goodwillie (2009) simultaneously measured autofertility, pollination visitation rate, and flower longevity in three populations of L. jepsonii with different frequencies of initially self-compatible (ISC) individuals. Consistent with model predictions that prior selfing is favored when the accrual rate is low (Fig. 3), the population with 71% ISC individuals had a low pollinator visiting rate of 0.035 visits per flower per day, a high selfing rate of 0.94, and a short flower longevity of 1.55 days. In another population with a higher visitation rate of 0.78, it had a much lower ISC of only 16% with most individuals were self-compatible in the late stage of anthesis, and also a longer flower life span of 2.51 days, Moreover, as expected from the model prediction (Fig. 2), this population has a much lower selfing rate of 0.31.
LIMITATIONS OF THE MODEL
Obviously, some factors are not incorporated in the current model. For example, it is assumed that the cost of construction of a single flower is constant irrespective of selfing rate. However, a higher selfing rate is often correlated with changes in floral morphology, such as reduced flower size and increased ovule numbers, referred as the “selfing syndrome” (Sicard and Lenhard 2011). Therefore, the current model may underestimate the advantage of prior selfing as it can save even more reproductive energy for flower construction. The selfing syndrome also suggests that the prior selfing rate , outcrossing proportion , and delayed selfing rate may not evolve independently. For example, increase of (e.g., reduced anther-stigma distance) may inevitably lower , which may inhibit the evolution of prior selfing. Complete prior selfing and outcrossing may not be obtained due to constraints in mechanisms or pollination ecology, and the population may finally evolve to an intermediate ESS. In the model, pollen discounting is caused by flower longevity rather than the consumption of pollen by self-fertilization. However, pollen discounting due to selfing should not affect the ESS, since the ESS is either (no pollen will be dispersed) or (no prior or competing selfing). Also, it is assumed that delayed selfing will fertilize a constant proportion of unfertilized ovules, , irrespective of the absolute number of ovules, which may happen when there is pollen competition. However, if pollen competition is weak, delayed selfing may fertilize a fixed number of ovules (rather than proportion). In this case, will increase with flower longevity because there will be fewer unfertilized ovules, so I expected larger ESS flower longevity than the current model predicts. Spatial and temporal variation in female and male fitness accrual rates is neglected, and it has been shown that a fluctuating pollination visiting rate can select for a mixed strategy of outcrossing and prior selfing under high inbreeding depression (Morgan and Wilson 2005).
Conclusions
All in all, the current study suggests that the evolution of mating systems may be better understood by considering their coevolution with floral reproductive strategies (e.g., flower longevity in the current study; a negative association between selfing and seed dispersal found in Cheptou and Massol 2009). Specifically, the incorporation of the latter makes the realization of self- and outcrossed- fertilization into a concrete process, which helps to reveal the effects of both genetic (inbreeding depression) and ecological factors (fitness accrual rate) on plant reproduction.
ACKNOWLEDGMENTS
I would like to thank Maria Servedio and Brian Lerch for very useful comments on an earlier draft of this paper.
AUTHOR CONTRIBUTIONS
K.X. conceived of the project, built the model, conducted the analyses, and wrote the manuscripts.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
LITERATURE CITED
Associate Editor: P.-O. Cheptou
Handling Editor: A. McAdam



