-
PDF
- Split View
-
Views
-
Cite
Cite
Mary Jean Brown, Gerry McWeeney, Rokho Kim, Ardita Tahirukaj, Petar Bulat, Skender Syla, Zoran Savic, Yona Amitai, Timothy Dignam, Dorit Nitzan Kaluski, Lead poisoning among internally displaced Roma, Ashkali and Egyptian children in the United Nations-Administered Province of Kosovo, European Journal of Public Health, Volume 20, Issue 3, June 2010, Pages 288–292, https://doi.org/10.1093/eurpub/ckp164
Close - Share Icon Share
Abstract
Background: This study assessed the association between lead poisoning prevention activities and blood lead levels (BLLs) among children living in lead-contaminated camps for internally displaced persons in the United Nations-Administered Province of Kosovo. Methods: We conducted a population-based study to examine the relationship among geometric mean BLLs in children (i) born before any lead poisoning prevention activities were instituted, (ii) born when specific interim interventions were instituted and (iii) born after relocation and medical therapy were available. The study population consisted of 145 of the 186 children born in the camps between December 1999 and July 2007. Results: Lower mean BLLs were found in children born following implementation of the interventions as compared with the children born before the interventions. However, this decrease in mean BLLs was attenuated in children born into families suspected of informal lead smelting. Conclusion: Despite lower BLLs following interventions, children living in these camps have BLLs that remain unacceptably high. Further efforts are urgently needed to control or eliminate lead exposure in this population. Continued blood lead monitoring of the population is also warranted.
Introduction
Elevated blood lead levels (BLLs) cause severe and chronic adverse health effects in children and adults.1,2 These effects include liver, kidney and brain damage; decreased growth, intelligence and fertility; developmental delay; behavioural deficits, especially poor impulse control; hearing loss; motor function problems; and high blood pressure.3 Very high BLLs, such as >75 µg/dl, can cause convulsions, coma and death; however, at lower levels, the health effects of lead poisoning are often asymptomatic.1 The adverse health effects can persist after exposure is eliminated and BLLs decrease.
No BLL threshold has been identified for adverse health effects in children.4 Children are more vulnerable than adults to the effects of elevated BLLs because children's exposure,1 absorption and metabolism of heavy metals is much higher than that of adults.5 Absorption of lead into the body is affected by many factors, including age, nutritional status and lead particulate size.6 Lead is poorly excreted, and most lead is sequestered in bone.7 As a result, elevated BLLs take months to years to decrease, even in cases where external exposures have been well controlled and chelation therapy has been instituted, because bone stores are mobilized into blood.8–10
Common lead exposure sources include industrial emissions and occupational exposures, leaded paint, lead gasoline exhaust, consumer products, food2 and water carried in lead pipes.11 Removing lead from gasoline has had an abrupt and sustained impact on BLLs, which have declined in every country where lead is no longer used in gas.12 However, in the USA, after leaded gasoline was banned, the risk for lead poisoning became disproportionately high among children living in dilapidated housing with lead paint hazards.13 The risk for lead poisoning also is disproportionate for communities exposed to lead left behind at closed mining and smelting facilities, communities living close to active mining and smelting sites, and families who engage in informal smelting of car batteries and electronic wastes.14,15 Such exposures are common in developing countries.
The Mitrovica/ë region in the United Nations (UN)-Administered Province of Kosovo had the largest lead production industry in Europe, which caused a legacy of widespread environmental pollution with heavy metals. The United Nations Interim Administration Mission in Kosovo (UNMIK) suspended lead production in 2000 due to health concerns. The World Health Organization Regional Office for Europe (WHO-EURO) assessed in 2004 that 25% of children aged 2–3 years in the general population in the area had elevated (≥10 µg/dl) BLLs (WHO unpublished data).
WHO-EURO also found that BLLs were even higher among the Roma, Ashkali and Egyptian (RAE) communities living in three camps for internally displaced persons (IDPs) established in the highly lead-contaminated area of Northern Mitrovica/ë. The camps were located within 3 km of the Trepča lead smelter and within 500 m of mine tailing sites. Blood lead testing conducted in 2004 in children from the three camps; Cesmin Lug, Kablar and Zitkovac, indicated that all children had BLLs ≥65 µg/dl,3 the highest value reported by the handheld LeadCare analyzer (WHO unpublished data).
WHO-EURO, in consultation with the U.S. Centers for Disease Control and Prevention (CDC) and the United Nations Children's Fund (UNICEF), recommended that an interim package of lead poisoning intervention services be provided to the RAE IDPs while a sustainable approach was developed. These services included environmental hotspot cleanup, ongoing BLL surveillance and medical monitoring, health education campaigns on lead and its health effects and prevention, improved water and sanitation, hygiene packs, and the distribution of specifically tailored food baskets.6 These services were provided to all RAE IDPs living in the camps for the entire study period and thus available to children born into the camps after their inception.
In 2006 many but not all families were relocated, as an interim solution, to Osterode, a former barracks of the North Atlantic Treaty Organization (NATO)-led Kosovo Force (KFOR). Although not lead-free, Osterode had a much lower ambient level of lead contamination than the three IDP camps. When the families were relocated to Osterode, items were added to the intervention package and included chelation therapy for children with BLLs ≥45 µg/dl or who were symptomatic, and an early childhood education enrichment program for all children. As described in Table 1, each component was designed to meet specific behavioural or learning objectives. Many of the families who did not relocate spent time at Osterode, and some of their young children also attended an indoor early childhood enrichment program established at Osterode. In addition to its other benefits the education program limited the children's access to lead contaminated dust and soils which they were otherwise exposed to, as it is customary for RAE children to spend large amounts of time outside, even when they are very young. Moreover, all housing units in Cesmin Lug. Zitkovic and Kablar camps had some dirt floors which were contaminated by the adjacent mine tailings, while the Osterode facility had wood or metal floors covered with carpeting.7 All families, regardless of whether they relocated, continued to receive the package of interim intervention services and their children were welcome to attend the special education unit. These additional interventions were implemented beginning in March of 2006.
Components and objectives of interim lead poisoning intervention services provided to IDPs in Kosovo
| Components instituted August 2004 . | Objectives . |
|---|---|
| Surveillance | 1. To activate community and to improve knowledge |
| 2. To identify individuals for chelation treatment | |
| 3. To identify clusters of family members for risk assessment and health education | |
| Education | 1. To create social norms and expectations |
| 2. To encourage community to change behaviours (e.g. relocate to Osterode camp, abandon unhealthy smelting and other practices) | |
| Community organization and | 1. To catalyse inclusion and integration of all people in the area |
| partnerships | 2. To ensure accessibility to quality and tailored health services |
| 3. To provide the tools required for capacity development of the local health workers | |
| 4. To ensure public health services | |
| 5. To create a lever for the community to initiate and intervene in policy change (bottom-up approach) | |
| Additional components instituted March 2006 | Objectives |
| Relocation | To provide shelter in an area with lower ambient lead contamination |
| Chelation | To provide treatment for lead poisoning and decrease health effects |
| Early childhood education enrichment | 1. To provide enrichment education for children with elevated BLLs |
| 2. To provide a lead-safe play environment during the day | |
| Components instituted August 2004 . | Objectives . |
|---|---|
| Surveillance | 1. To activate community and to improve knowledge |
| 2. To identify individuals for chelation treatment | |
| 3. To identify clusters of family members for risk assessment and health education | |
| Education | 1. To create social norms and expectations |
| 2. To encourage community to change behaviours (e.g. relocate to Osterode camp, abandon unhealthy smelting and other practices) | |
| Community organization and | 1. To catalyse inclusion and integration of all people in the area |
| partnerships | 2. To ensure accessibility to quality and tailored health services |
| 3. To provide the tools required for capacity development of the local health workers | |
| 4. To ensure public health services | |
| 5. To create a lever for the community to initiate and intervene in policy change (bottom-up approach) | |
| Additional components instituted March 2006 | Objectives |
| Relocation | To provide shelter in an area with lower ambient lead contamination |
| Chelation | To provide treatment for lead poisoning and decrease health effects |
| Early childhood education enrichment | 1. To provide enrichment education for children with elevated BLLs |
| 2. To provide a lead-safe play environment during the day | |
Components and objectives of interim lead poisoning intervention services provided to IDPs in Kosovo
| Components instituted August 2004 . | Objectives . |
|---|---|
| Surveillance | 1. To activate community and to improve knowledge |
| 2. To identify individuals for chelation treatment | |
| 3. To identify clusters of family members for risk assessment and health education | |
| Education | 1. To create social norms and expectations |
| 2. To encourage community to change behaviours (e.g. relocate to Osterode camp, abandon unhealthy smelting and other practices) | |
| Community organization and | 1. To catalyse inclusion and integration of all people in the area |
| partnerships | 2. To ensure accessibility to quality and tailored health services |
| 3. To provide the tools required for capacity development of the local health workers | |
| 4. To ensure public health services | |
| 5. To create a lever for the community to initiate and intervene in policy change (bottom-up approach) | |
| Additional components instituted March 2006 | Objectives |
| Relocation | To provide shelter in an area with lower ambient lead contamination |
| Chelation | To provide treatment for lead poisoning and decrease health effects |
| Early childhood education enrichment | 1. To provide enrichment education for children with elevated BLLs |
| 2. To provide a lead-safe play environment during the day | |
| Components instituted August 2004 . | Objectives . |
|---|---|
| Surveillance | 1. To activate community and to improve knowledge |
| 2. To identify individuals for chelation treatment | |
| 3. To identify clusters of family members for risk assessment and health education | |
| Education | 1. To create social norms and expectations |
| 2. To encourage community to change behaviours (e.g. relocate to Osterode camp, abandon unhealthy smelting and other practices) | |
| Community organization and | 1. To catalyse inclusion and integration of all people in the area |
| partnerships | 2. To ensure accessibility to quality and tailored health services |
| 3. To provide the tools required for capacity development of the local health workers | |
| 4. To ensure public health services | |
| 5. To create a lever for the community to initiate and intervene in policy change (bottom-up approach) | |
| Additional components instituted March 2006 | Objectives |
| Relocation | To provide shelter in an area with lower ambient lead contamination |
| Chelation | To provide treatment for lead poisoning and decrease health effects |
| Early childhood education enrichment | 1. To provide enrichment education for children with elevated BLLs |
| 2. To provide a lead-safe play environment during the day | |
This report describes the impact of these population-based interventions on BLLs of children who were born after December 1999 when the three IDP camps in North Mitrovica/ë were established. We compare the BLLs of children born prior to implementation of specific interim interventions, to those born after the interim interventions (i.e. those implemented in 2004), and to those of children born after families were relocated to Osterode (i.e. those services implemented in 2006).
Methods
Study population
The study population consisted of children who were born to families living in the three IDP camps and who were born and tested for blood lead between December 1999 and July 2007.
The children were categorized into three age groups. Group 1 included children born and tested for blood lead between 2 December 1999 and 31 July 2004, before lead poisoning prevention activities were instituted. Group 2 consisted of children born and tested for blood lead between 1 August 2004 and 28 February 2006, when several interim lead poisoning prevention activities were instituted. In Group 3, children were born and tested for blood lead between 1 March 2006 and July 2007, after the Osterode facility had opened and more items were added to the intervention package (table 1). Chelation was performed on children who had BLL >4 µg/dl with the consent of the parents.5
Laboratory methods
Blood lead analysis was performed in the field using capillary samples tested by anodic stripping voltammetry (ASV) using Lead Care II® machines. The highest reported level of lead quantified by this analyzer is 65 µg/dl. Higher levels register as ‘HI’.3 The reference laboratories of RIVM in the Netherlands and CDC in Atlanta, Georgia, performed venous blood lead testing. Venous samples were taken, with every 10th capillary sample or where capillary BLLs were >15 µg/dl, when permitted by the parents. The correlation between split venous and capillary samples was r2 = 0.9.
WHO and the local health authorities collected BLLs, and the staff of the lead unit established at the Osterode barracks collected follow-up blood samples.
Statistical methods
Because BLLs follow a log normal distribution, geometric mean BLLs were calculated across variables of interest. Analysis of variance tests were performed to assess the statistical significance of these variables in predicting BLLs after a logarithmic scale conversion because the distribution of BLLs was left skewed (skewness = 0.44, kurtosis = −1.11). We tested the hypothesis that children in Group 3, who received health interventions, had significantly lower BLLs when separately compared with children in Groups 1 and 2, who did not receive health interventions, after adjusting for important covariates. Variables significant at the 0.10 level were assessed in multivariate linear regression models, and a final model was determined. Statistical analyses were conducted by using SAS for Windows (version 8.02; SAS Institute Inc., Cary, North Carolina, United States).
Demographic data
Norwegian Church Aid (NCA), who managed the Osterode camp, provided demographic characteristics of the RAE families. Camp management and medical personnel provided information on families suspected of informal smelting. Lead unit medical personnel provided the chelation data.
Results
Of the 186 children known to have been born into the camps between December 1999 and July 2007, 145 (78%) had a BLL test. The geometric mean initial BLL was 45.7 ± 21.4 µg/dl. For 139 children (96%), the initial BLL was ≥10 µg/dl. The mean age of the children at initial BLL was 2.7 ± 1.6 years. Boys tended to have a higher mean initial BLL than girls (48.2 and 43.2 µg/dl, respectively), but this difference was not significant (P > 0.30). Ninety-six percent of children were born in Mitrovica/ë, 53% were male, 12% had chelation therapy, 16% lived in families suspected of participating in informal smelting activities and 53% lived in families who were originally relocated to the Cesmin Lug camp.
The children's initial BLL test result differed significantly by group (P < 0.001). Mean initial BLL results were highest among children in Groups 1 (50.3 µg/dl) and 2 (41.2 µg/dl) when compared with children in Group 3 (25.6 µg/dl). Not surprisingly, children's participation in chelation therapy also significantly differed by group (P < 0.05) (table 2). After we adjusted for group, children living in families suspected of informal smelting activities and children from camp Zitkovic were more likely to have higher BLLs than the other children (table 3). After the final predictive model was calculated, children in Groups 1 and 2 and children living in families suspected of informal smelting activities had the highest BLLs (table 3).
Comparison of characteristics of internally displaced children in Kosovo by group (N = 145)
| Characteristic . | Group 1 (born and tested December 1999 to July 2004 (n = 98) . | Group 2 (born and tested August 2004 to February 2006) (n = 31) . | Group 3 (born and tested March 2006 to July 2007) (n = 16) . |
|---|---|---|---|
| Mean age in years (range) | 3.4 (0.3–6.6) | 1.5 (0–2.5) | 0.6 (0–1.2) |
| Gender | |||
| Male | 55 (56%) | 14 (45%) | 7 (44%) |
| Female | 43 (44%) | 17 (55%) | 8 (50%) |
| Missing | 0 | 0 | 1 (6%) |
| Birthplace | |||
| Mitrovica/ë | 89 (91%) | 21 (68%) | 10 (63%) |
| Other | 9 (9%) | 10 (32%) | 6 (37%) |
| Suspected smelter | |||
| Yes | 16 (16%) | 4 (13%) | 3 (19%) |
| No | 80 (82%) | 26 (84%) | 12 (75%) |
| Missing | 2 (2%) | 1 (3%) | 1 (6%) |
| Camp | |||
| Cesmin Lug | 51 (52%) | 13 (42%) | 4 (25%) |
| Kablar | 20 (20%) | 4 (13%) | 2 (13%) |
| Zitkovic | 23 (24%) | 6 (19%) | 4 (25%) |
| Missing/other | 4 (4%) | 8 (26%) | 6 (37%) |
| Year of BLL9 | |||
| 2004 | 40 (41%) | 0 | 0 |
| 2005 | 11 (11%) | 4 (13%) | 0 |
| 2006 | 37 (38%) | 13 (42%) | 3 (19%) |
| 2007 | 9 (9%) | 14 (45%) | 13 (81%) |
| 2008 | 1 (1%) | 0 | 0 |
| Missing | 0 | 0 | 0 |
| Mean BLL#(range) | 50.3 (7.3–74.0) | 41.2 (13.0–74.0) | 25.6 (4.3–74.0) |
| Chelation completed## | |||
| Yes | 17 (17%) | 1 (3%) | 0 |
| No | 81 (83%) | 30 (97%) | 16 (100%) |
| Characteristic . | Group 1 (born and tested December 1999 to July 2004 (n = 98) . | Group 2 (born and tested August 2004 to February 2006) (n = 31) . | Group 3 (born and tested March 2006 to July 2007) (n = 16) . |
|---|---|---|---|
| Mean age in years (range) | 3.4 (0.3–6.6) | 1.5 (0–2.5) | 0.6 (0–1.2) |
| Gender | |||
| Male | 55 (56%) | 14 (45%) | 7 (44%) |
| Female | 43 (44%) | 17 (55%) | 8 (50%) |
| Missing | 0 | 0 | 1 (6%) |
| Birthplace | |||
| Mitrovica/ë | 89 (91%) | 21 (68%) | 10 (63%) |
| Other | 9 (9%) | 10 (32%) | 6 (37%) |
| Suspected smelter | |||
| Yes | 16 (16%) | 4 (13%) | 3 (19%) |
| No | 80 (82%) | 26 (84%) | 12 (75%) |
| Missing | 2 (2%) | 1 (3%) | 1 (6%) |
| Camp | |||
| Cesmin Lug | 51 (52%) | 13 (42%) | 4 (25%) |
| Kablar | 20 (20%) | 4 (13%) | 2 (13%) |
| Zitkovic | 23 (24%) | 6 (19%) | 4 (25%) |
| Missing/other | 4 (4%) | 8 (26%) | 6 (37%) |
| Year of BLL9 | |||
| 2004 | 40 (41%) | 0 | 0 |
| 2005 | 11 (11%) | 4 (13%) | 0 |
| 2006 | 37 (38%) | 13 (42%) | 3 (19%) |
| 2007 | 9 (9%) | 14 (45%) | 13 (81%) |
| 2008 | 1 (1%) | 0 | 0 |
| Missing | 0 | 0 | 0 |
| Mean BLL#(range) | 50.3 (7.3–74.0) | 41.2 (13.0–74.0) | 25.6 (4.3–74.0) |
| Chelation completed## | |||
| Yes | 17 (17%) | 1 (3%) | 0 |
| No | 81 (83%) | 30 (97%) | 16 (100%) |
Note: Smaller sample sizes exist in cells due to missing test date
#P < 0.0001 (analysis of variance [ANOVA])
##P < 0.05 (chi-square test)
Comparison of characteristics of internally displaced children in Kosovo by group (N = 145)
| Characteristic . | Group 1 (born and tested December 1999 to July 2004 (n = 98) . | Group 2 (born and tested August 2004 to February 2006) (n = 31) . | Group 3 (born and tested March 2006 to July 2007) (n = 16) . |
|---|---|---|---|
| Mean age in years (range) | 3.4 (0.3–6.6) | 1.5 (0–2.5) | 0.6 (0–1.2) |
| Gender | |||
| Male | 55 (56%) | 14 (45%) | 7 (44%) |
| Female | 43 (44%) | 17 (55%) | 8 (50%) |
| Missing | 0 | 0 | 1 (6%) |
| Birthplace | |||
| Mitrovica/ë | 89 (91%) | 21 (68%) | 10 (63%) |
| Other | 9 (9%) | 10 (32%) | 6 (37%) |
| Suspected smelter | |||
| Yes | 16 (16%) | 4 (13%) | 3 (19%) |
| No | 80 (82%) | 26 (84%) | 12 (75%) |
| Missing | 2 (2%) | 1 (3%) | 1 (6%) |
| Camp | |||
| Cesmin Lug | 51 (52%) | 13 (42%) | 4 (25%) |
| Kablar | 20 (20%) | 4 (13%) | 2 (13%) |
| Zitkovic | 23 (24%) | 6 (19%) | 4 (25%) |
| Missing/other | 4 (4%) | 8 (26%) | 6 (37%) |
| Year of BLL9 | |||
| 2004 | 40 (41%) | 0 | 0 |
| 2005 | 11 (11%) | 4 (13%) | 0 |
| 2006 | 37 (38%) | 13 (42%) | 3 (19%) |
| 2007 | 9 (9%) | 14 (45%) | 13 (81%) |
| 2008 | 1 (1%) | 0 | 0 |
| Missing | 0 | 0 | 0 |
| Mean BLL#(range) | 50.3 (7.3–74.0) | 41.2 (13.0–74.0) | 25.6 (4.3–74.0) |
| Chelation completed## | |||
| Yes | 17 (17%) | 1 (3%) | 0 |
| No | 81 (83%) | 30 (97%) | 16 (100%) |
| Characteristic . | Group 1 (born and tested December 1999 to July 2004 (n = 98) . | Group 2 (born and tested August 2004 to February 2006) (n = 31) . | Group 3 (born and tested March 2006 to July 2007) (n = 16) . |
|---|---|---|---|
| Mean age in years (range) | 3.4 (0.3–6.6) | 1.5 (0–2.5) | 0.6 (0–1.2) |
| Gender | |||
| Male | 55 (56%) | 14 (45%) | 7 (44%) |
| Female | 43 (44%) | 17 (55%) | 8 (50%) |
| Missing | 0 | 0 | 1 (6%) |
| Birthplace | |||
| Mitrovica/ë | 89 (91%) | 21 (68%) | 10 (63%) |
| Other | 9 (9%) | 10 (32%) | 6 (37%) |
| Suspected smelter | |||
| Yes | 16 (16%) | 4 (13%) | 3 (19%) |
| No | 80 (82%) | 26 (84%) | 12 (75%) |
| Missing | 2 (2%) | 1 (3%) | 1 (6%) |
| Camp | |||
| Cesmin Lug | 51 (52%) | 13 (42%) | 4 (25%) |
| Kablar | 20 (20%) | 4 (13%) | 2 (13%) |
| Zitkovic | 23 (24%) | 6 (19%) | 4 (25%) |
| Missing/other | 4 (4%) | 8 (26%) | 6 (37%) |
| Year of BLL9 | |||
| 2004 | 40 (41%) | 0 | 0 |
| 2005 | 11 (11%) | 4 (13%) | 0 |
| 2006 | 37 (38%) | 13 (42%) | 3 (19%) |
| 2007 | 9 (9%) | 14 (45%) | 13 (81%) |
| 2008 | 1 (1%) | 0 | 0 |
| Missing | 0 | 0 | 0 |
| Mean BLL#(range) | 50.3 (7.3–74.0) | 41.2 (13.0–74.0) | 25.6 (4.3–74.0) |
| Chelation completed## | |||
| Yes | 17 (17%) | 1 (3%) | 0 |
| No | 81 (83%) | 30 (97%) | 16 (100%) |
Note: Smaller sample sizes exist in cells due to missing test date
#P < 0.0001 (analysis of variance [ANOVA])
##P < 0.05 (chi-square test)
Factors associated with mean initial BLL and group among 145 children from Kosovo tested for blood lead
| Characteristic . | Mean initial BLL (µg/dl) . | Standard deviation (µg/dl) . | Adjusted mean initial BLL (µg/dl) . | Relative change (%)a . | P-value . |
|---|---|---|---|---|---|
| Group | |||||
| One | 50.3 | 20.5 | 41.8 | 168.6 | <0.01 |
| Two | 41.2 | 18.3 | 35.0 | 124.8 | <0.01 |
| Three | 25.6 | 20.1 | 15.6 | Reference | |
| Age | |||||
| <2.9 years | 43.6 | 22.0 | NS | ||
| 3–4.9 years | 53.6 | 53.6 | |||
| 5–6 years | 42.1 | 21.8 | |||
| Gender | |||||
| Male | 48.2 | 20.7 | NS | ||
| Female | 43.2 | 22.0 | |||
| Family member suspected of smelting | |||||
| Yes | 64.0 | 15.7 | 26.8 | 71.9 | <0.01 |
| No | 42.6 | 20.8 | 15.6 | Reference | |
| Camp | |||||
| Cesmin Lug | 46.1 | 19.8 | NS | ||
| Kablar | 40.5 | 18.0 | |||
| Zitkovic | 59.2 | 20.7 | |||
| Chelation completed | |||||
| Yes | 68.1 | 10.2 | NS | ||
| No | 42.5 | 20.7 | |||
| Characteristic . | Mean initial BLL (µg/dl) . | Standard deviation (µg/dl) . | Adjusted mean initial BLL (µg/dl) . | Relative change (%)a . | P-value . |
|---|---|---|---|---|---|
| Group | |||||
| One | 50.3 | 20.5 | 41.8 | 168.6 | <0.01 |
| Two | 41.2 | 18.3 | 35.0 | 124.8 | <0.01 |
| Three | 25.6 | 20.1 | 15.6 | Reference | |
| Age | |||||
| <2.9 years | 43.6 | 22.0 | NS | ||
| 3–4.9 years | 53.6 | 53.6 | |||
| 5–6 years | 42.1 | 21.8 | |||
| Gender | |||||
| Male | 48.2 | 20.7 | NS | ||
| Female | 43.2 | 22.0 | |||
| Family member suspected of smelting | |||||
| Yes | 64.0 | 15.7 | 26.8 | 71.9 | <0.01 |
| No | 42.6 | 20.8 | 15.6 | Reference | |
| Camp | |||||
| Cesmin Lug | 46.1 | 19.8 | NS | ||
| Kablar | 40.5 | 18.0 | |||
| Zitkovic | 59.2 | 20.7 | |||
| Chelation completed | |||||
| Yes | 68.1 | 10.2 | NS | ||
| No | 42.5 | 20.7 | |||
a: Relative change in geometric mean BLL is calculated by using the formula 100 × (e[β] − 1), where β is the parameter estimate
Factors associated with mean initial BLL and group among 145 children from Kosovo tested for blood lead
| Characteristic . | Mean initial BLL (µg/dl) . | Standard deviation (µg/dl) . | Adjusted mean initial BLL (µg/dl) . | Relative change (%)a . | P-value . |
|---|---|---|---|---|---|
| Group | |||||
| One | 50.3 | 20.5 | 41.8 | 168.6 | <0.01 |
| Two | 41.2 | 18.3 | 35.0 | 124.8 | <0.01 |
| Three | 25.6 | 20.1 | 15.6 | Reference | |
| Age | |||||
| <2.9 years | 43.6 | 22.0 | NS | ||
| 3–4.9 years | 53.6 | 53.6 | |||
| 5–6 years | 42.1 | 21.8 | |||
| Gender | |||||
| Male | 48.2 | 20.7 | NS | ||
| Female | 43.2 | 22.0 | |||
| Family member suspected of smelting | |||||
| Yes | 64.0 | 15.7 | 26.8 | 71.9 | <0.01 |
| No | 42.6 | 20.8 | 15.6 | Reference | |
| Camp | |||||
| Cesmin Lug | 46.1 | 19.8 | NS | ||
| Kablar | 40.5 | 18.0 | |||
| Zitkovic | 59.2 | 20.7 | |||
| Chelation completed | |||||
| Yes | 68.1 | 10.2 | NS | ||
| No | 42.5 | 20.7 | |||
| Characteristic . | Mean initial BLL (µg/dl) . | Standard deviation (µg/dl) . | Adjusted mean initial BLL (µg/dl) . | Relative change (%)a . | P-value . |
|---|---|---|---|---|---|
| Group | |||||
| One | 50.3 | 20.5 | 41.8 | 168.6 | <0.01 |
| Two | 41.2 | 18.3 | 35.0 | 124.8 | <0.01 |
| Three | 25.6 | 20.1 | 15.6 | Reference | |
| Age | |||||
| <2.9 years | 43.6 | 22.0 | NS | ||
| 3–4.9 years | 53.6 | 53.6 | |||
| 5–6 years | 42.1 | 21.8 | |||
| Gender | |||||
| Male | 48.2 | 20.7 | NS | ||
| Female | 43.2 | 22.0 | |||
| Family member suspected of smelting | |||||
| Yes | 64.0 | 15.7 | 26.8 | 71.9 | <0.01 |
| No | 42.6 | 20.8 | 15.6 | Reference | |
| Camp | |||||
| Cesmin Lug | 46.1 | 19.8 | NS | ||
| Kablar | 40.5 | 18.0 | |||
| Zitkovic | 59.2 | 20.7 | |||
| Chelation completed | |||||
| Yes | 68.1 | 10.2 | NS | ||
| No | 42.5 | 20.7 | |||
a: Relative change in geometric mean BLL is calculated by using the formula 100 × (e[β] − 1), where β is the parameter estimate
In a subanalysis of the 92 children younger than 3 years old, the mean BLLs also varied significantly by group, with children in Group 1 having the highest geometric mean initial BLL of 51.7 µg/dl (P < 0.001). In addition, in the only group with children 3 years old and older, the geometric mean BLLs of children >3 years old was not statistically different from children <3 years old [49.1 and 51.7 µg/dl for the older versus the younger children, respectively (P > 0.2)]. As indicated in Figure 1, there was no correlation between age and BLL in these highly exposed children (r2 < 0.03).8
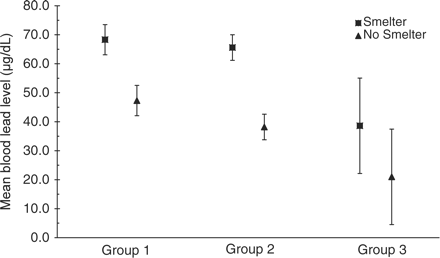
Comparison of mean blood lead levels of RAE children by cohort and whether family members may be involved in smelting lead.
Discussion
In this evaluation of population-based interventions among three groups of RAE children born before or after specific lead poisoning interventions were implemented in their community, we found that the mean BLL of children was lower after the interventions were implemented. For children living in families suspected of engaging in informal smelting activities, the mean BLL for children in Groups 1 and 2 were not significantly different; however, the mean BLL for children in Group 3 who lived in families suspected of informal smelting was significantly lower than that for children in either Group 1 or Group 2. In addition, we found that BLLs among displaced RAE children were unacceptably high.
Limitations
The association between geometric mean BLL and intervention strategy may be biased due to the cross-sectional and ecological nature of this study. Thus, we considered ecological, selection and misclassification bias.
Once children's BLLs are elevated it can take months to years for the levels to decrease despite efforts to reduce both exposure by controlling environmental sources of lead and BLLs by chelation therapy. This limits the usefulness of study designs that compare individuals pre-intervention BLLs with their post-interventions BLLs.10 To account for this limitation, we designed our groups based on date of birth and the date that specific interventions were provided to the population; we then compared children born into a cohort during the time that specific interventions were introduced, rather than comparing BLLs of individual children before and after an intervention. Thus, we cannot quantify the programme uptake on an individual basis. However, this ecological analysis is appropriate for evaluating population-based interventions over time. In addition, as many of the interventions were population-based, the risk of selection bias was decreased because individuals did not choose whether to participate in those interventions.
Except for gender, classifying all variables included in this evaluation proved difficult. For example, the team found assigning age problematic for some of the RAE IDPs because some adults were not sure of their own or their children's exact ages. In such cases the team assigned 1 January to the reported year of birth in cases where birth or health certificates were not available. This assignation probably did not produce a remarkable bias because the percentage of children whose birth date was assigned as 1 January did not vary by group.
We also considered whether the difference between mean initial BLL by group was simply a function of age because children in Group 1, who were significantly older than children in Groups 2 or 3, might have higher BLLs due to living in the contaminated area longer. Alternatively, younger children might have higher mean BLLs because of their increased hand-to-mouth activity. However, in a subanalysis restricted to children <3 years old, the initial mean BLL was still highest for children in Group 1.
In addition, assigning the camp of origin and relocation status was problematic. Some of the RAE IDPs moved between and outside of the camps as movement is a strong element of the RAE culture. However, the baseline data that were used to assign camp of origin and relocation status were correct at the time of the study. Note that the Cesmin Lug camp deserves special attention because both the Kablar and Zitkovac camps were completely relocated to Osterode. Cesmin Lug had only partial success with relocation, and new families have now recently moved into the vacant accommodation in the camp. The team could not identify with any certainty the relocation status of persons originally acknowledged as members of the Cesmin Lug camp during the baseline BLL period. Numerous attempts to verify relocation/residence status have resulted in conflicting information. The situation is further complicated by the new residents of Cesmin Lug. This camp population is transient, moving among Osterode, Cesmin Lug and Roma Mahalla, where members of their immediate and extended families live. In addition, because the blood lead testing of the various groups were conducted as circumstances allowed, we are unable in our analyses to control for the effect of seasonality on BLLs.4
Conflicts of interest: None declared.
The population-based interventions provided to RAE persons living in IDP camps in the UN Administered Province of Kosovo are associated with lower mean BLLs in children born following the implementation of the interventions as compared with children born before the interventions.
This decrease in mean BLL was attenuated in children born into families where some members were suspected of informal lead smelting.
All RAE children BLLs remain unacceptably high, with the geometric mean BLL in Group 3 > 20 µg/dl.
Further efforts are urgently needed to control or eliminate lead exposure in this population.
Continued blood lead surveillance of the population is also warranted.
References
Author notes
*The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC or WHO.




Comments