-
PDF
- Split View
-
Views
-
Cite
Cite
Anna K. Lugnér, Maarten J. Postma, Investment decisions in influenza pandemic contingency planning: cost-effectiveness of stockpiling antiviral drugs, European Journal of Public Health, Volume 19, Issue 5, October 2009, Pages 516–520, https://doi.org/10.1093/eurpub/ckp119
Close - Share Icon Share
Abstract
Background: The threat of an influenza pandemic has led to stockpiling of antiviral drugs in order to mitigate a plausible outbreak. If the stockpile would be used in relation to the recent pandemic alert, an investment decision about renewing the stock for a possible subsequent pandemic is essential. The decision should include cost-effectiveness considerations. Methods: We constructed a cost-effectiveness analysis in the Dutch context, explicitly including risk of an outbreak. Outcomes from a dynamic transmission model, comparing an intervention with a non-intervention scenario, were input in our health economic calculations. Results: Stockpiling was cost-effective from the health-care perspective if the actual risk is 37% for 30 years. If less than 60% of the population would take the antiviral drugs or the attack rate is about 50%, the investment would not be cost-effective from this perspective. Conclusion: Risk perception, realistic coverage among population and size of a pandemic are crucial parameters and highly decisive for the investment decision.
Introduction
In 1918–19, an influenza pandemic hit the world in three waves. Mainly, the second wave led to exceptionally elevated loss of lives, with the unique pattern involving the highest case fatality among the otherwise healthy young population (20–40 years of age).1,2 Without all details about the origin of the virus yet (or ever) being known, the A(H1N1) virus from the 1918 pandemic has been shown to be a distantly related avian virus. The viruses causing two later pandemics (the Asian flu in 1957–58 and the Hong Kong flu in 1968–69) were both related to the 1918-pandemic virus but with substantially lower case fatality rates.1
The cross-species transmission to humans of the avian influenza A(H5N1), circulating among wild and domesticated birds for a few years now has already raised concern about the possible occurrence of another influenza pandemic, should this virus acquire human-to-human transmission abilities. Another virus has recently raised additional concern about such a pandemic. In particular, by the end of April 2009 the World Health Organization (WHO) raised the level of influenza pandemic alert to phase five (out of six) due to the recent spread of the new influenza virus A(H1N1). Daily details of the outbreak can be found on the website of WHO.3
One strategy to minimize the consequences of a pandemic for public health and the economy is to mitigate the spread and the health consequences by therapeutic treatment with anti-viral (AV) drugs, advocated amongst others by the Dutch authorities.4 Stockpiling is necessary to be able to provide the population with these drugs in sufficient quantities and within time.5 Yet, such an investment raises the question whether such large-scale stockpiling is cost-effective, i.e. whether society's scarce resources are efficiently spent. There are only a few studies published yet that have investigated the cost-effectiveness of stockpiling AV drugs.6–8 All of these calculations assume a clinical attack rate (CAR) of ∼25–30% and predict that there would be a pandemic within 30 years. These assumptions may be conceived as realistic, as indeed three pandemics were seen during the last 100 years, all with CARs in the same order of magnitude.9 Yet, of course exact occurrences in the future remain inherently uncertain.
One aspect of AV therapy is the impact on the transmission of influenza. This can only be adequately modelled with a dynamic model, taking the force of infection explicitly into account. With such a model, the effect of AV therapy and other containment methods on the CAR can be estimated in detail and changing over time.10–16 One of these included costs for stockpiling, showing that this strategy would be cost-effective for the United States at a relatively high predicted CAR of 50%.16
Influenza pandemics are a continuous threat to public health. As long as there is no strain-specific, or universal vaccine against pandemic influenza, the question whether to stockpile AV drugs remains relevant. This certainly includes the cost-effectiveness aspect which requires regular updating, especially when new information about the seriousness of an infection becomes available.
The aim of this study is to estimate whether stockpiling of AV drugs is cost-effective for therapeutic use in the Netherlands, explicitly taking both a perceived risk of an influenza pandemic and multiple stock turnovers into account. The calculations of the effect of the therapy are based on a dynamic transmission model enabling sensitivity analyses on the effects of alternative CARs and percentages of the population receiving AV therapy on transmission and cost-effectiveness.
Methods
Time perspective of the analysis and risk of pandemic
We choose a time horizon of 30 years for our model design. A 30-year horizon reflects an inter-pandemic period based on the three influenza pandemics that occurred during the 20th century. This implies that on average a stock would have to be kept for 30 years. Also, the earlier analyses of stockpiling are mostly based on the assumption of an average yearly risk of a pandemic as 3% (3 pandemics in 100 years). Assuming that the risk of a pandemic is independent of the previous year, the probability of it occurring during a specified time interval can be approximated by a Poisson distribution; we refer to this as the observed risk. According to the assumption of an annual risk of 3%, the estimated risk that there would be at least one pandemic during the 30-year period that was modelled would be 59%, with a predicted risk of one, two and three outbreaks at 37, 16 and 5%, respectively.
The stock turnover depends on stock composition, the shelf life of the drugs stored and on the time horizon of the analysis. The exact shelf life of the stock-piled AV drugs depends on how they are stored. One option is to use bulk powder oseltamivir, a neuraminidase inhibitor with an expected shelf life of ten years. Another option is to stockpile ready-to-use oseltamivir (Tamiflu®) tablets, with a more limited shelf life of five years. In particular, two stockpiling options are investigated in this analysis: (i) as bulk powder (oseltamivir alone) and (ii) as a combination of two-third of bulk powder and one-third of Tamiflu® (combination oseltamivir and Tamiflu®). The combination option is the one previously chosen in the Netherlands, and has already been stockpiled. Currently, the stock consists of five million doses. Over a 30-year horizon, the stock of Tamiflu® would have to be renewed four times, whereas the oseltamivir has to be renewed two times after the first investment.
Costs of treatment and stockpiling
Health-care utilization, sick leave and deaths were estimated for both the non-intervention and the intervention scenario and subsequently compared in an incremental cost-effectiveness analysis, based on a dynamic transmission model.17 In the intervention scenario, individuals with influenza symptoms were therapeutically treated with AV drugs. Treatment was assumed to start within 48 h of the onset of symptoms, leading to a 50% reduction of health-care resource use due to complications, and a 50% reduction in deaths.18,19 Outpatient health-care utilization was based on opinions from an expert panel.20
Direct health-care costs that occur during a pandemic include telephone contact with an outpatient health-care centre or general practitioner (GP) for a prescription of the AV drug. Cost savings were expected in treatment with AV drugs, concerning less GP visits, antibiotics treatments of complications, costs for over-the-counter (OTC) medications and costs for hospitalizations due to severe complications of influenza. To reflect a societal perspective, productivity losses were included. These were estimated according to the Dutch guidelines using the friction-costing method, an alternative to the human capital method21,22 (table 1).
Data for use in cost-effectiveness analysis of stockpiling of AV drugs, base case analysis (30-year perspective), 80% of population receive AV therapy
| . | Non-intervention . | Intervention . | Incremental costs or LYG . |
|---|---|---|---|
| Infected individuals | 10 369 872 | 8 594 056 | |
| Symptomatic individuals | 6 221 923 | 5 156 433 | |
| Hospitalizations | 22 941 | 13 851 | |
| Deaths | 9012 | 5362 | |
| Life-years lost (discounted) | 96 795 | 57 912 | 38 883 |
| Costs health care | |||
| Outpatient GP visits | €33 240 996 | €16 529 133 | €16 711 863 |
| OTC drugs and antibiotics due to complications | €37 730 543 | €29 235 472 | €8 495 071 |
| Hospitalizations | €108 334 422 | €65 406 635 | €42 927 786 |
| Production losses | |||
| Production losses | €2 521 537 242 | €636 309 973 | €1 885 227 269 |
| Costs during pandemic | |||
| Telephone calls to GP | €44 077 556 | −€44 077 556 | |
| Pharmacy fee for AV prescriptions | €25 867 972 | −€25 867 972 | |
| Stockpiling costsa (5 million doses) | |||
| One time purchase, oseltamivir | €45 736 179 | ||
| One time purchase, combination Tamiflu® and oseltamivir | €56 185 269 | ||
| Yearly storing costs | €51 389 | ||
| 30 years stockpiling, PV | |||
| Purchase and storing, oseltamivir | €143 658 011 | ||
| Purchase and storing, combination | €176 881 136 | ||
| Average PV: costs during pandemic | −€1 085 347 | ||
| Average PV: savings including productivity losses during pandemic | €1 130 112 974 | ||
| Average life-years gained | 31 594 | ||
| Average quality adjusted life-years gained | 48 540 |
| . | Non-intervention . | Intervention . | Incremental costs or LYG . |
|---|---|---|---|
| Infected individuals | 10 369 872 | 8 594 056 | |
| Symptomatic individuals | 6 221 923 | 5 156 433 | |
| Hospitalizations | 22 941 | 13 851 | |
| Deaths | 9012 | 5362 | |
| Life-years lost (discounted) | 96 795 | 57 912 | 38 883 |
| Costs health care | |||
| Outpatient GP visits | €33 240 996 | €16 529 133 | €16 711 863 |
| OTC drugs and antibiotics due to complications | €37 730 543 | €29 235 472 | €8 495 071 |
| Hospitalizations | €108 334 422 | €65 406 635 | €42 927 786 |
| Production losses | |||
| Production losses | €2 521 537 242 | €636 309 973 | €1 885 227 269 |
| Costs during pandemic | |||
| Telephone calls to GP | €44 077 556 | −€44 077 556 | |
| Pharmacy fee for AV prescriptions | €25 867 972 | −€25 867 972 | |
| Stockpiling costsa (5 million doses) | |||
| One time purchase, oseltamivir | €45 736 179 | ||
| One time purchase, combination Tamiflu® and oseltamivir | €56 185 269 | ||
| Yearly storing costs | €51 389 | ||
| 30 years stockpiling, PV | |||
| Purchase and storing, oseltamivir | €143 658 011 | ||
| Purchase and storing, combination | €176 881 136 | ||
| Average PV: costs during pandemic | −€1 085 347 | ||
| Average PV: savings including productivity losses during pandemic | €1 130 112 974 | ||
| Average life-years gained | 31 594 | ||
| Average quality adjusted life-years gained | 48 540 |
a: Assumption based on estimates from national experts involved in pandemic preparedness
Data for use in cost-effectiveness analysis of stockpiling of AV drugs, base case analysis (30-year perspective), 80% of population receive AV therapy
| . | Non-intervention . | Intervention . | Incremental costs or LYG . |
|---|---|---|---|
| Infected individuals | 10 369 872 | 8 594 056 | |
| Symptomatic individuals | 6 221 923 | 5 156 433 | |
| Hospitalizations | 22 941 | 13 851 | |
| Deaths | 9012 | 5362 | |
| Life-years lost (discounted) | 96 795 | 57 912 | 38 883 |
| Costs health care | |||
| Outpatient GP visits | €33 240 996 | €16 529 133 | €16 711 863 |
| OTC drugs and antibiotics due to complications | €37 730 543 | €29 235 472 | €8 495 071 |
| Hospitalizations | €108 334 422 | €65 406 635 | €42 927 786 |
| Production losses | |||
| Production losses | €2 521 537 242 | €636 309 973 | €1 885 227 269 |
| Costs during pandemic | |||
| Telephone calls to GP | €44 077 556 | −€44 077 556 | |
| Pharmacy fee for AV prescriptions | €25 867 972 | −€25 867 972 | |
| Stockpiling costsa (5 million doses) | |||
| One time purchase, oseltamivir | €45 736 179 | ||
| One time purchase, combination Tamiflu® and oseltamivir | €56 185 269 | ||
| Yearly storing costs | €51 389 | ||
| 30 years stockpiling, PV | |||
| Purchase and storing, oseltamivir | €143 658 011 | ||
| Purchase and storing, combination | €176 881 136 | ||
| Average PV: costs during pandemic | −€1 085 347 | ||
| Average PV: savings including productivity losses during pandemic | €1 130 112 974 | ||
| Average life-years gained | 31 594 | ||
| Average quality adjusted life-years gained | 48 540 |
| . | Non-intervention . | Intervention . | Incremental costs or LYG . |
|---|---|---|---|
| Infected individuals | 10 369 872 | 8 594 056 | |
| Symptomatic individuals | 6 221 923 | 5 156 433 | |
| Hospitalizations | 22 941 | 13 851 | |
| Deaths | 9012 | 5362 | |
| Life-years lost (discounted) | 96 795 | 57 912 | 38 883 |
| Costs health care | |||
| Outpatient GP visits | €33 240 996 | €16 529 133 | €16 711 863 |
| OTC drugs and antibiotics due to complications | €37 730 543 | €29 235 472 | €8 495 071 |
| Hospitalizations | €108 334 422 | €65 406 635 | €42 927 786 |
| Production losses | |||
| Production losses | €2 521 537 242 | €636 309 973 | €1 885 227 269 |
| Costs during pandemic | |||
| Telephone calls to GP | €44 077 556 | −€44 077 556 | |
| Pharmacy fee for AV prescriptions | €25 867 972 | −€25 867 972 | |
| Stockpiling costsa (5 million doses) | |||
| One time purchase, oseltamivir | €45 736 179 | ||
| One time purchase, combination Tamiflu® and oseltamivir | €56 185 269 | ||
| Yearly storing costs | €51 389 | ||
| 30 years stockpiling, PV | |||
| Purchase and storing, oseltamivir | €143 658 011 | ||
| Purchase and storing, combination | €176 881 136 | ||
| Average PV: costs during pandemic | −€1 085 347 | ||
| Average PV: savings including productivity losses during pandemic | €1 130 112 974 | ||
| Average life-years gained | 31 594 | ||
| Average quality adjusted life-years gained | 48 540 |
a: Assumption based on estimates from national experts involved in pandemic preparedness
Next to the purchase costs, the stockpiling costs and opportunity costs were included. Costs for storing included heating/cooling of the storage room, electricity, regular inspection and control of the active substance and security arrangements. These storing costs were modelled as equally large annual payments. The stockpiling costs were calculated over the full-time horizon of 30 years. By investing in a stock, resources that could have been used for other purposes are tied up. To reflect this opportunity cost, 4% of the investment cost was added (purchase and storing costs). Unused, out-of-date stock is wasted at no cost assumed.
Some of the costs had to be neglected due to lack of information (e.g. costs for distribution of drugs to pharmacies from the central storing location and costs of dispensing the bulk powder into consumption doses). Consequently, the costs for the AV drugs could be slightly underestimated. These costs were investigated in a targeted sensitivity analysis. All costs were expressed in euro rates of the year 2007, costs were discounted with 4% and life-years gained with 1.5%;21 ergo, the analysis was made from a societal perspective including all relevant costs when available.
Transmission model
The virus transmission during an influenza pandemic was estimated with a dynamic model. A detailed description of the model and its parameters can be found in ref.17 In the model shown in figure 1, individuals start as being susceptible and upon infection they progress through a succession of stages, including being infected but not infectious (latent), being infected and infectious, recovered and immune (removed). The model includes key epidemiological parameters, such as contact rates among and within age groups, the length of the infectious period and the probability of transmission of the virus during a contact. The use of AV drugs affected the recovery rate of patients and the mean infectious time was assumed to be halved. At the start of the first pandemic wave, the whole population was assumed to be susceptible to infection with the virus. It was assumed in the calculations that the pandemic virus behaves as a seasonal influenza virus in the sense that risks of symptoms, illness and death upon infection are similar to those risks observed for seasonal influenza.
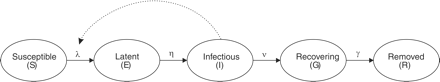
Schematic illustration of the dynamic model. λ is the rate of becoming infected (force of infection), η the rate of becoming infectious, ν the rate of losing infectiousness, γ rate of recovery or death. Source: Lugnér et al.17
The basic reproduction number, or reproductive ratio R0, reflects a key epidemiological variable that describes how many secondary cases of infections are caused by one primary case in a fully susceptible population (see e.g. Keeling and Rohani23). The estimate of R0 used in the model at 1.73, was based on data from the Asian Flu in 1957.24 Of all infected individuals, 60% were assumed to develop clinical symptoms.25,26 In the base-case non-intervention scenario the CAR predicted by the dynamic model equals 38%.
Cost-effectiveness ratio
Costs for renewal of the stock were included for the total period irrespective of the occurrence of an outbreak. The costs for the AV drugs and the savings due to the intervention (less health care costs due to less complications and less production losses due to less illness) as well as the health gains in terms of life-years gained (LYG), occur only when the pandemic occurs.
The annual and stock renewal costs (including alternative costs) were discounted and summed up to a present value (PV). Likewise, the costs of the AV drugs, the cost savings due to the intervention and the LYGs were discounted. Since we do not know in which year after the stockpiling investment a pandemic would exactly occur, costs, savings and LYGs were calculated as an average of the yearly discounted value during the 30-year time perspective. The net costs and LYG during an outbreak due to intervention are dependent on the perceived risk of an outbreak. The expected cost-effectiveness ratio is calculated as (assuming a risk of a pandemic larger than zero):
Sensitivity analysis
Epidemiological and distributional uncertainties were elaborated in several one-way sensitivity analyses estimating the cost-effectiveness for (i) size of pandemic with CAR of 25 and 50% (corresponding to R0 1.37 and 2.44, respectively17); (ii) lower percentage of the population receiving AV therapy, 60% instead of the policy goal of 80%. In these cases, the stock size is adjusted to equal expected number of symptomatic individuals; (iii) keeping the stock fixed (sufficient for five million cases) but with a CAR of 25%; (iv) using quality adjusted life-years (QALY) gained. The health-related quality of life (HRQoL) of patients with influenza treated with oseltamivir (HRQoL weight 0.65) compared with patients receiving placebo (HRQoL weight 0.61) were used.27 All clinical cases in the non-intervention scenario were assigned a weight of untreated illness (0.61). Non-treated individuals in the intervention scenario were assumed to have less severe illness and assigned the same HRQoL weight as for treated individuals (0.65); (v) possibly, there could be two pandemic outbreaks during a time perspective of 30 years. The observed risk of this would be 16% according to the Poisson distribution. Adding an extra refill of the stock (an average of the PV of the purchase cost) in combination with two times higher costs and savings due to a pandemic and a two times extra gain in life-years illustrates this; (vi) the cost of distribution and dispense of the drugs was investigated.
Results
Based on the Dutch population data (16.6 million people in 2007), there would be 10.4 million infected individuals if a pandemic was left uncontrolled. The policy goal in the Netherlands is that 80% of individuals with symptoms will use AV drugs, implying that there would be 8.6 million infected cases of which 60% would have symptoms, resulting in about 5 million individuals receiving AV drugs.
The stockpiling of oseltamivir alone is cost-effective if the risk of a pandemic influenza outbreak is perceived to be larger than 23% in the next 30 years. If the stock is a combination of oseltamivir and Tamiflu®, the perceived risk cut-off point would have to be a little larger (29%). Including production losses shows that the risk would have to be larger than 9% for the oseltamivir alone and 11% for the combination alternative (figure 2). The observed risk that there will be one pandemic during the 30 years is indicated with the horizontal lines in figure 2. This observed risk is higher than the risk cut-off point for the perceived risk. The interpretation is that along with the observed risk, the stock-piling would be cost-effective. The cost-effectiveness ratio for the combination alternative at the observed risk would be around €15 000 per LYG (including productivity losses is cost-saving).
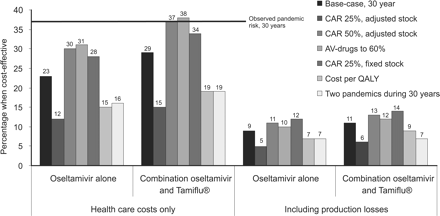
Cost-effectiveness ratio ≤€20 000 of stockpiling AV drugs depending on perceived risk of a pandemic outbreak, base case and sensitivity analyses. Observed risk cut-off point indicated with horizontal line
In the health-care perspective there are a couple of cases where stockpiling would not necessarily be cost-effective, as shown in the sensitivity analyses (figure 2). For two cases the cut-off cost-effectiveness point was equal to or higher than the observed risk. If the CAR is as high as 50% (in this case we assumed that the stock would cover to treat all symptomatic cases), and if AV drugs would only be delivered to 60% of the symptomatic individuals (instead of 80%), the stockpiling is not cost-effective. Finally, calculations revealed that costs due to distribution and dispensation could be up to €8 per dose at a cost-effectiveness ratio of €20 000 at a risk of 37%.
Discussion
We show how the cost-effectiveness of stockpiling AV drugs depends on the risk that there will be an outbreak of a pandemic influenza. At one extreme, if there will be no pandemic it would cost about 177 million to stockpile a combination of AV drugs for 30 years. On the other, if for sure there would be a pandemic outbreak the stockpiling would be very cost-effective, with a cost-effectiveness ratio below €6000 including production losses. We further show that the results are sensitive to the size of a pandemic and the proportion of the population that receives the AV drugs. Is it realistic that 80% of the population will use the drug on time? Is the spread of the pandemic similar to earlier influenza epidemics, or could we expect a higher R0? Recent estimations about the reproduction ratio based on data from the new influenza A(H1N1) gives various estimates of 2.0–3.228 and 1.4–1.629 showing the large uncertainty about the actual reproductive ratio in a new outbreak.
The model on which these calculations are based assumes that the virus would continue to be sensitive to the AV therapy. If resistance develops on a large scale and no alternative, equally effective AV drug at a similar cost is available to replace the current drug, this analysis would have to be reconsidered. This strengthens the argument that economic evaluations need to be reassessed when new information is available. Especially, this applies to new or better information about the characteristics of a pandemic virus and effects of a possible vaccine. Furthermore, a recent mathematical model showed that combining different AV drugs could significantly reduce the resistance of a virus against treatment and the attack rates of the pandemic.30 The recommendation to countries already holding a stock of oseltamivir was to include a small stockpile (enough for 1% of population) of a second AV drug. The costs for this extra stock are suggested to be small.30 Using the official price31 to estimate, the purchase cost of a zanamivir stock (another neuraminidase inhibitor) for the Netherlands would cost about €21 million for a one-time purchase. Stockpiling these drugs for 30 years, assuming a shelf life of 5 years, would cost about €90 million (discounted) including alternative costs. These rough estimates show that the costs are not likely to be negligible.
As pointed out by Beutels et al.,32 a health economic analysis is a partial equilibrium model that does not take into account all opportunity costs of treating symptomatic individuals during a pandemic. In case there is a pandemic outbreak of influenza, other health care procedures, mainly elective surgery and non-acute treatments, should have to be postponed to free up resources. For a more extensive economic evaluation, the disutility of postponing surgery should also be incorporated, measured as number of QALY lost.32 If current contingency plans include postponing of elective surgery the question arises if these plans would be changed during a pandemic if resource would become available. The chance that AV therapy would lead to less QALY lost among patients other than symptomatic ones compared with patients with no AV treatment is, in our opinion, likely to be very small, possibly even negligible. The effects of postponed elective surgery would have little influence on the incremental cost-effectiveness ratio and thus on our specific analysis.
The uncertainties about when and if a pandemic would manifest does not mean that an economic analysis of stockpiling either AV drugs or a vaccine is pointless: this analysis anticipate that the cost-effectiveness ratio of stockpiling a combination of AV drugs falls below the cost-effectiveness cut-off point (including avoided production losses due to treatment), if the risk of a pandemic during a 30-year period is about 11%. Currently, the new influenza A(H1N1) is causing outbreaks around the world, possibly adding another outbreak to the history of documented influenza pandemics. Including this outbreak into the calculus would raise the average annual risk to around 4% (4/100). The resulting observed risk for at least one pandemic for next 30 years would be higher (70%).
The beliefs about the risk and spread of a new influenza virus causing a pandemic are very important in the decision whether or not to invest in a stock of AV drugs or whenever available, a vaccine.
Conflicts of interest: None declared.
The beliefs about the risk of a new influenza virus causing a pandemic are very important in the decision whether or not to invest in a stock of AV drugs.
The cost-effectiveness of stockpiling is affected by the coverage of AV therapy in the population since the coverage influences the spread of the infection.
Stockpiling is cost-effective also in cases of lower coverage and higher attack rate when including indirect costs in the calculations, but not necessarily cost-saving.




Comments