-
PDF
- Split View
-
Views
-
Cite
Cite
Jacqueline Spayne, Ida Ackerman, Michael Milosevic, Allan Seidenfeld, Alan Covens, Lawrence Paszat, Invasive cervical cancer: a failure of screening, European Journal of Public Health, Volume 18, Issue 2, April 2008, Pages 162–165, https://doi.org/10.1093/eurpub/ckm043
Close - Share Icon Share
Abstract
Background: Cervical screening is an effective prevention measure. It is unclear whether cervical cancer results from non-participation in screening or from failures in detection by screening. Analysis of the screening history of patients with cervix cancer may contribute to understanding failures in prevention. Methods: A cohort of patients presenting during 1 year was identified. Dates and results of cervical smears in the 4 years prior to presentation were extracted from a screening database. Patients were grouped as follows: ‘No screening’—no Pap records; ‘Pre-diagnostic’—one or more Pap tests within 6 months of presentation; ‘Sporadic screening’—one Pap test between 6 and 48 months prior to presentation; and ‘Regular screening’—at least two Pap tests 6–48 months before presentation. Results: 225 patients were identified (median age: 48 years, range 25–107). Eighty- eight had no records of screening; a further 66 were categorized as pre-diagnostic. These two groups (68% of incident cases) were considered not to have participated in routine screening. A further 15% had sporadic screening tests, but only 37 patients (16%) had evidence of regular screening. Clinically, 53, 41 and 6% presented with early, locally advanced and metastatic disease, respectively. Older patients (>50 years) were more likely to present with advanced disease (61 vs 37% at least Stage II). Conclusions: These results suggest that the failure to prevent invasive cervix cancer in this population can largely be attributed to failures in recruitment for screening.
The introduction of cervical screening programs in developed countries has been associated with a decline in the incidence of invasive cervix cancer1–4 over several decades. In the future, it is possible that, with the development HPV vaccines,5,,6 the prevalence of the primary etiologic agent responsible for cervical cancer will be reduced to a level where continued large-scale cervical screening becomes unnecessary. However, for current and imminent generations of women, effective screening programs continue to be essential to prevent the significant morbidity and mortality associated with a diagnosis of invasive cervix cancer.
In Canada, in 2004, there were an estimated 1350 new cases of cervix cancer and ∼410 deaths attributed to this disease.7 While the absence of routine cervical screening is known to be a risk factor for invasive cervical cancer,8–15 it is unclear to what degree the failure of preventive measures, and development of invasive disease is attributable to non-participation in screening as opposed to the development of interval cancers in appropriately screened women. In two retrospective Canadian studies 30–37% of women with cervical cancer were reported as previously never having a cervical smear, with a further 10–15% with no screening for at least 3 years.16,,17 These studies identified the opportunistic nature of screening programs to be a major limitation and recommended the introduction of systematic approaches to screening.
One of the essential components of a systematic cervical screening program is a centralized database of screening test results. With appropriate security measures to protect confidentiality, such databases can also provide invaluable population-based data on the effectiveness of screening programs. In 1996, the Cytobase system was initiated in Ontario to provide a centralized electronic database of Pap test results. It captures ∼80% of Pap smears done in Ontario.
The purpose of this study was to analyze the screening history of patients who presented with invasive cervix cancer utilizing the Cytobase database, and to identify potential contributors to failures in cervical screening programs.
Methods
This is a descriptive study of sequential patients presenting to two cancer centers over a 1-year period. Research Ethics Board approval for the study was obtained at both institutions. Institutional records at each center were searched to identify all patients presenting with invasive cervix cancer between 1 April 2001 and 31 March 2002. As patients were referred to both Gynecological Oncology and Radiation Oncology services, clinic records for both services were searched. To ensure completeness of patient identification, institutional cancer registry files, pathology department records, radiation treatment records and operative lists were also searched. Each identified case was entered into a database identified by hospital file number and was assigned a study number. The hospital chart for each patient was reviewed to obtain the Ontario Health Insurance Plan (OHIP) number, date of birth and clinical data regarding tumor stage. It is a standard practice at both institutions for the pathology of all referred cases of cervix cancer to be reviewed by a gynecological pathologist. The pathology reports for all patients were reviewed to ensure that only cases of invasive uterine cervix cancer were included. Pathological diagnoses related to biopsy, conization or hysterectomy procedures were recorded. The date and result of any Pap smears performed for study patients at the participating hospitals were also recorded.
All aspects of health care are provided through the OHIP for all permanent Ontario residents, each of whom has a unique insurance number. These OHIP numbers were used to identify the cases; the date and result of all screening tests completed up to 48 months prior to presentation at the cancer center were extracted from Cytobase records.
Descriptive statistics were used to analyse the patient population. Proportional differences were compared using chi-square tests for categorical variables and Student's t-tests for mean differences of continuous variables. Variables included patient age, disease stage, histological diagnosis and screening history.
The time interval from date of each smear to presentation at the cancer center was computed and patients were grouped as follows:
| No screening: | no Pap screening record. |
| Pre-diagnostic test: | one or more Pap tests, all performed within 6 months of presentation at the cancer center. |
| Regular screening: | at least two Pap tests between 6 and 48 months prior to presentation, with or without additional tests done within 6 months of presentation. |
| Sporadic screening: | the remainder, namely patients with only one Pap test completed between 6 and 48 months prior to presentation, with or without additional tests done within 6 months of presentation. |
| No screening: | no Pap screening record. |
| Pre-diagnostic test: | one or more Pap tests, all performed within 6 months of presentation at the cancer center. |
| Regular screening: | at least two Pap tests between 6 and 48 months prior to presentation, with or without additional tests done within 6 months of presentation. |
| Sporadic screening: | the remainder, namely patients with only one Pap test completed between 6 and 48 months prior to presentation, with or without additional tests done within 6 months of presentation. |
| No screening: | no Pap screening record. |
| Pre-diagnostic test: | one or more Pap tests, all performed within 6 months of presentation at the cancer center. |
| Regular screening: | at least two Pap tests between 6 and 48 months prior to presentation, with or without additional tests done within 6 months of presentation. |
| Sporadic screening: | the remainder, namely patients with only one Pap test completed between 6 and 48 months prior to presentation, with or without additional tests done within 6 months of presentation. |
| No screening: | no Pap screening record. |
| Pre-diagnostic test: | one or more Pap tests, all performed within 6 months of presentation at the cancer center. |
| Regular screening: | at least two Pap tests between 6 and 48 months prior to presentation, with or without additional tests done within 6 months of presentation. |
| Sporadic screening: | the remainder, namely patients with only one Pap test completed between 6 and 48 months prior to presentation, with or without additional tests done within 6 months of presentation. |
Results
In the 1-year period of this study, 237 patients presented with a histologically confirmed diagnosis of invasive cervix cancer, approximately half at each of the participating cancer centres. Of these, 12 patients were non-resident in Ontario, without an OHIP number, and were, therefore, excluded from the study. The 225 patients included ranged in age from 25 to 107, with a median age of 48 years. Figure 1 shows a distribution of patients by age, emphasizing the incidence of this disease across all decades of female adulthood. Fifty-three percent of patients presented with disease confined to the cervix (FIGO Stage IA or IB). The remaining 47% had disease extending beyond the cervix including 12 patients with distant metastases at diagnosis. The most common histological diagnosis was squamous cell carcinoma, reported in 67% of patients. In 31% of the patients, a diagnosis of adenocarcinoma or adenosquamous carcinoma was reported. Other diagnoses (2% of the patients) included two cases of small cell carcinoma, one papillary serous carcinoma, one adenoid basal carcinoma and one lymphoepithelioma.
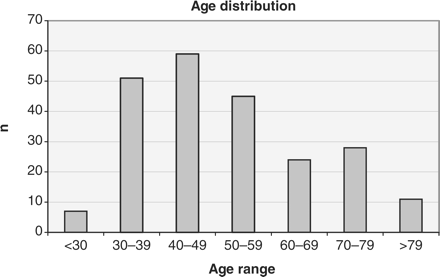
When stratified by age, for those patients 50 years or younger compared with those over 50, significant differences emerged in both the stage at presentation and histological diagnosis (table 1). Sixty-one percent of older patients had disease extending beyond the cervix compared with 37% of younger patients (P < 0.005). Diagnoses of adeno- or adenosquamous carcinoma were more prevalent among the younger than the older age group (40% compared with 22% of patients, P < 0.005).
Figure 2 provides a summary of the screening history of study patients. Results are categorized as detailed earlier. Thirty-one percent of patients appeared to have participated to some degree in cervical screening programs. Approximately half of these met the defined criteria for ‘regular’ screening with at least two tests between 6 and 48 months prior to presentation, and for the remainder (classified as ‘sporadic’ screening), records identified only one Pap test in this time period. Thirty-nine percent of patients had no record of screening tests within 4 years of presentation. A further 29% of patients had one or more Pap tests done within 6 months of presentation with none prior to this. The latter group was categorized as ‘pre-diagnostic’.
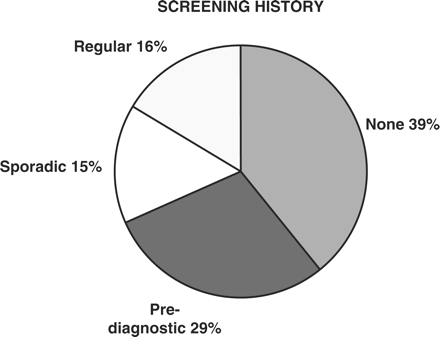
Cervical screening history of patients with cervix cancer (Categories defined in “Methods'” above)
Amongst the 37 ‘regularly’ screened women, 94 Pap smears were collected between 7 and 48 months prior to presentation. For 21 of these women all smears were benign. In 9 women, atypical squamous or glandular cells of unknown significance were seen, with occasional subsequent smears that were benign. In the remaining 7 women, the most recent smear showed a high-grade intra-epithelial lesion with prior benign or atypical results.
Table 2 demonstrates an analysis of patient characteristics grouped according to screening history, showing a correlation between screening participation and age. The median age of regularly screened patients at 41 years was significantly lower than that of all other groups, particularly those with no screening or pre-diagnostic tests only (P <0.000005). Furthermore, there was an inverse correlation between exposure to screening tests and stage at presentation with a significantly lower proportion of regularly screened patients presenting with disease beyond the cervix when compared with those patients with no screening tests (24 vs 65%, P <0.00005). Of the 12 patients presenting with distant metastases, 11 were in either the group with no screening or the ‘pre-diagnostic’ group. Forty-three percent of patients with a regular screening history had a histological diagnosis of adeno- or adenosquamous carcinoma compared with 30% of patients who were not screened, but this difference was not statistically significant.
| . | Screening history . | |||
|---|---|---|---|---|
| . | Regular . | Sporadic . | Pre-diagnostic . | None . |
| N (%) | 37 (16%) | 34 (15%) | 66 (29%) | 88 (39%) |
| Mean age | 41* | 49* | 54* | 54* |
| Stage I | 76%** | 62% | 58% | 35%** |
| Stage II–IV | 24%** | 38% | 42% | 65%** |
| Squamous cell | 54% | 59% | 74% | 68% |
| Adeno/adenosquamous | 43% | 41% | 23% | 30% |
| . | Screening history . | |||
|---|---|---|---|---|
| . | Regular . | Sporadic . | Pre-diagnostic . | None . |
| N (%) | 37 (16%) | 34 (15%) | 66 (29%) | 88 (39%) |
| Mean age | 41* | 49* | 54* | 54* |
| Stage I | 76%** | 62% | 58% | 35%** |
| Stage II–IV | 24%** | 38% | 42% | 65%** |
| Squamous cell | 54% | 59% | 74% | 68% |
| Adeno/adenosquamous | 43% | 41% | 23% | 30% |
*Mean age of the regular screening group is significantly different than all other groups (Student's t-test P < 0.01)
**χ2-test P < 0.00005
| . | Screening history . | |||
|---|---|---|---|---|
| . | Regular . | Sporadic . | Pre-diagnostic . | None . |
| N (%) | 37 (16%) | 34 (15%) | 66 (29%) | 88 (39%) |
| Mean age | 41* | 49* | 54* | 54* |
| Stage I | 76%** | 62% | 58% | 35%** |
| Stage II–IV | 24%** | 38% | 42% | 65%** |
| Squamous cell | 54% | 59% | 74% | 68% |
| Adeno/adenosquamous | 43% | 41% | 23% | 30% |
| . | Screening history . | |||
|---|---|---|---|---|
| . | Regular . | Sporadic . | Pre-diagnostic . | None . |
| N (%) | 37 (16%) | 34 (15%) | 66 (29%) | 88 (39%) |
| Mean age | 41* | 49* | 54* | 54* |
| Stage I | 76%** | 62% | 58% | 35%** |
| Stage II–IV | 24%** | 38% | 42% | 65%** |
| Squamous cell | 54% | 59% | 74% | 68% |
| Adeno/adenosquamous | 43% | 41% | 23% | 30% |
*Mean age of the regular screening group is significantly different than all other groups (Student's t-test P < 0.01)
**χ2-test P < 0.00005
Of the 225 patients with invasive cervix cancer included in this study, 85 had one Pap test and a further 15 had two or more Pap tests in the 6 months prior to presentation. These tests, including only the most recent for the 15 patients with multiple tests, reported the presence of frank malignancy in only 21%; pre-malignant changes were seen in 34%, atypia of unknown significance in 20% and benign changes or normal cells in the remaining 25%.
Discussion
Several population-based studies have shown a higher incidence of invasive cervix cancer in unscreened populations.8–12,,14,15 The present study supports the hypothesis that the failure to prevent invasive disease in this population is due to non-participation in regular cervical screening. In the preliminary analysis of data abstracted from patient charts, it was apparent that a majority of patients described a symptomatic period leading up to diagnosis, typically lasting several months, most often with complaints of dysfunctional vaginal bleeding (data not shown). This gives rise to conjecture that many Pap smears conducted in the months prior to presentation at a cancer center may have been associated with symptom investigation rather than true screening procedures. Therefore, we chose to categorize those patients whose only recorded Pap tests were done in the 6 months immediately prior to presentation at the cancer center as ‘pre-diagnostic’. This is intended to reflect the likelihood that, for the majority of these women who were not regularly screened, these tests were probably related to investigation of symptoms although a few may have been directly instrumental in reaching a diagnosis in asymptomatic women. If this assumption is correct, the 29% of patients with ‘pre-diagnostic’ tests can be added to the 39% of study patients with no record of prior screening Pap tests for an estimate of 68% of patients who did not participate in screening prior to a diagnosis of invasive cancer. This is somewhat higher than the 40–50% reported in other studies of patients with cervix cancer.16,,17 Several factors may contribute to these differences including different demographic profiles of study populations, or different methods of data collection, as patient self-reporting is known to overestimate participation in screening programs17–19 and the possibility that our estimates are slightly higher due to the assumptions made. Nonetheless, all these studies including ours found a screening participation rate amongst women with cervix cancer well below that reported in the general adult female population in Canada,20–22 confirming the hypothesis that absence of screening may have been a contributory risk factor for the development of invasive cervix cancer. However, to scientifically demonstrate that lack of screening contributes to the development of invasive cervix cancer, the incidence of invasive disease would have to be compared in a randomized trial of screened and unscreened women and such a study is not feasible or ethical.
This study does not examine reasons for non-participation in screening by this population, although contributory factors may include barriers to accessing healthcare. The correlation seen in this study between non-participation in screening and presentation with advanced disease suggests there may be a high-risk population of women who have limited contact with healthcare providers; consequently these women are not offered screening and also may experience delays in diagnosis. The barriers to accessing care in the context of a publicly funded health system remain unclear.
One potential limitation of this data is uncertainty over the completeness of the screening histories obtained. During the study period, it is estimated that the results of over 80% of Pap tests completed in Ontario were available through Cytobase. Notable exceptions were the results of tests done at teaching hospitals. For this reason, pathology records at both institutions participating in this study were also searched and additional Pap test results for two patients were identified and included in our analysis.
Although the incidence of invasive cervix cancer has fallen in recent decades,7 this study confirms that incident cases continue to occur throughout female adulthood. Unlike many cancers that occur predominantly in older age groups, over half this study population was under 50 years of age, suggesting this disease is responsible for a disproportionately greater loss of life-years and social cost. Nonetheless, older women, including those whose age is beyond the usual recommendations for discontinuation of screening, remain at risk for cervix cancer and tend to present with more advanced disease. These data reinforce recommendations23 that screening can only be discontinued in women aged 70 or older, if there is a documented history of consecutive prior normal tests. Otherwise efforts to complete additional opportunistic testing should continue.
The primary conclusions of this study are based only on patient participation in screening programs. We did not undertake any central audit of cytology results and, therefore, can draw only limited conclusions based on these. However, one useful aspect of this study is that it reinforces the known lack of sensitivity and specificity of the Pap smear in the setting of symptoms. The Pap smear is designed as a screening test for asymptomatic women. It is not intended as a diagnostic tool in symptomatic women and a negative Pap smear in symptomatic patients can be dangerously misleading. The high proportion of negative smears (45%) reported within 6 months of a diagnosis of cervix cancer in this study population confirms the unreliability of Pap tests in the presence of malignancy24,,25 and reinforces recommendations that symptomatic women must be investigated and evaluated by colposcopy and/or biopsy.
In the minority of women in this study (16%) who did develop cervix cancer despite reasonably regular screening tests, the available data offers little explanation for the failure to prevent this diagnosis. The potential explanations include poor test quality (including failures in collecting, processing and analysing samples) or rapidly progressive disease that developed without a sufficiently long pre-invasive stage to allow detection and intervention. Most of these women had a history of normal Pap smears and there is no evidence of a failure to follow-up the small number with abnormal results. Previous data has suggested that precursors for adenocarcinoma or adenosquamous carcinoma arising in the endocervical canal are less accessible to detection.26 While this is one possible explanation, our data did not show a significantly higher proportion of these diagnoses in those patients who had been regularly screened. However, the number of such patients in this study cohort may have been too small to detect such a difference. As new screening methodologies are adopted, including routine HPV testing, the sensitivity of screening tests may improve sufficiently to resolve this issue. However, given the evidence presented here that most screening failures are due to non-participation in screening programs, major preventive efforts should continue to be directed at optimizing participation rather than in maximizing test sensitivity. Futhermore, if HPV vaccination lives up to it's promise in protecting future generations of women, vigilance will still be required to ensure that older generations and unvaccinated women are still recruited in screening programs.
Acknowledgement
The authors would like to thank Incyte Corporation for access to the Cytobase data.
Conflicts of interest: None declared.
The majority of failures in prevention of invasive cervix cancer can be attributed to failures in screening participation.
The potential barriers to screening need to be evaluated and strategies are required to improve recruitment for regular screening.
A negative Pap test does not exclude invasive malignancy and must not be used to investigate symptoms.




Comments