-
PDF
- Split View
-
Views
-
Cite
Cite
Denise Felber Dietrich, Christian Schindler, Joel Schwartz, Jean-Claude Barthélémy, Jean-Marie Tschopp, Frédéric Roche, Arnold von Eckardstein, Otto Brändli, Philipppe Leuenberger, Diane R. Gold, Jean-Michel Gaspoz, Ursula Ackermann-Liebrich, SAPALDIA Team, Heart rate variability in an ageing population and its association with lifestyle and cardiovascular risk factors: results of the SAPALDIA study, EP Europace, Volume 8, Issue 7, July 2006, Pages 521–529, https://doi.org/10.1093/europace/eul063
Close - Share Icon Share
Abstract
Aims (i) To report associations between cardiovascular risk factors and heart rate variability (HRV) in a general population and (ii) to provide normal values for various HRV measurements in a healthy European general population sample aged ≥50.
Methods and results Twenty-four-hour electrocardiograms were recorded in 1742 randomly selected SAPALDIA (Swiss cohort study on Air Pollution and Lung Diseases in Adults) participants aged ≥50. In multivariate regression analyses, women (n=895) had a 6.1% lower standard deviation of all normal RR (NN) intervals (SDNN), a 11.4% lower total power (TP), and a 27.2% lower low-frequency (LF) power than men (n=847). Per unit increase in BMI, SDNN decreased by 0.7% and TP decreased by 1.2%. Persons with high blood pressure had a 9.2% lower LF than normotensive persons and current smokers a 15.5% lower LF than never smokers. Each hour of heavy physical exercise was associated with a 2.0% increase in SDNN, a 3.6% increase in the high frequency (HF) range power and a 4.2% increase in LF power. Higher levels of uric acid, high-sensitive C-reactive protein and non-HDL-cholesterol were associated with lower TP, HF and LF. Percentiles of TP and LF/HF as a function of age were calculated for an asymptomatic subsample of participants (n=499) free of cardioactive medications.
Conclusion Heart rate variability in a general population sample shows expected associations with all known cardiovascular risk factors, although not identically for all HRV domains. Together with our percentile estimates for HRV as a function of age, these findings could assist scientists in interpreting 24 h HRV values and factors influencing them in an ageing population.
Introduction
Heart rate variability (HRV), a measure of cardiac autonomic control (see Table 1 for physiological correlations of different HRV components), has been established as a strong predictor of death and non-fatal cardiovascular events both in survivors of a myocardial infarction1–3 and in an asymptomatic population. The Framingham Study has found that reduced HRV in short-term recordings (2 h) predicted new cardiac events, hypertension and hyperglycaemia, in a middle-aged general population,4–6 and the Whitehall Study showed associations between social position, behavioural factors, components of the metabolic syndrome, and HRV.7 However, little is known about 24 h HRV in a normal population.8 The Task Force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology have identified the need for large prospective population studies to establish normal HRV standards for various age and sex categories.9
| HRV component . | Spectrum . | Physiological correlation . |
|---|---|---|
| SDNN | — | Circadian rhythms |
| TP | ≤0.40 Hz | — |
| HF | 0.15–0.40 Hz | Vagal activity |
| LF | 0.04–0.15 Hz | Sympathetic and vagal activity, baroreflex sensitivity |
| LF/HF ratio | — | Proposed as balance between sympathetic and parasympathetic activities |
| VLF | 0.0033–0.04 Hz | Sympathetic activity+parasympathetic activity+thermoregulation+renin–angiotensin system |
| ULF | <0.0033 Hz | Circadian and neuroendocrine rhythms |
| HRV component . | Spectrum . | Physiological correlation . |
|---|---|---|
| SDNN | — | Circadian rhythms |
| TP | ≤0.40 Hz | — |
| HF | 0.15–0.40 Hz | Vagal activity |
| LF | 0.04–0.15 Hz | Sympathetic and vagal activity, baroreflex sensitivity |
| LF/HF ratio | — | Proposed as balance between sympathetic and parasympathetic activities |
| VLF | 0.0033–0.04 Hz | Sympathetic activity+parasympathetic activity+thermoregulation+renin–angiotensin system |
| ULF | <0.0033 Hz | Circadian and neuroendocrine rhythms |
| HRV component . | Spectrum . | Physiological correlation . |
|---|---|---|
| SDNN | — | Circadian rhythms |
| TP | ≤0.40 Hz | — |
| HF | 0.15–0.40 Hz | Vagal activity |
| LF | 0.04–0.15 Hz | Sympathetic and vagal activity, baroreflex sensitivity |
| LF/HF ratio | — | Proposed as balance between sympathetic and parasympathetic activities |
| VLF | 0.0033–0.04 Hz | Sympathetic activity+parasympathetic activity+thermoregulation+renin–angiotensin system |
| ULF | <0.0033 Hz | Circadian and neuroendocrine rhythms |
| HRV component . | Spectrum . | Physiological correlation . |
|---|---|---|
| SDNN | — | Circadian rhythms |
| TP | ≤0.40 Hz | — |
| HF | 0.15–0.40 Hz | Vagal activity |
| LF | 0.04–0.15 Hz | Sympathetic and vagal activity, baroreflex sensitivity |
| LF/HF ratio | — | Proposed as balance between sympathetic and parasympathetic activities |
| VLF | 0.0033–0.04 Hz | Sympathetic activity+parasympathetic activity+thermoregulation+renin–angiotensin system |
| ULF | <0.0033 Hz | Circadian and neuroendocrine rhythms |
The SAPALDIA (Swiss Cohort Study on Air Pollution and Lung Diseases in Adults) cohort was designed to measure the health effects from long-term exposure to air pollutants in the Swiss adult population.10,11 In 1991, a random sample of the Swiss population was recruited from eight areas featuring distinct geographical and environmental conditions. In a follow-up examination in 2002, 24 h measurements of HRV were included in those aged ≥50.
In this paper we (i) report the cross-sectional associations of cardiovascular risk factors and cardiovascular disease with HRV in the full sample and (ii) provide normative values for total power and the low to high frequency ratio in a healthy subsample of a large European population aged ≥50. Normal curves for other HRV parameters are available from the authors on request.
Methods
Participants
The design and objectives of the SAPALDIA cohort study have been reported in detail elsewhere.12 In brief, 9651 participants received intensive health examinations and a detailed health interview in 1991 and have been followed since then. In 2002, we were able to re-examine 8047 of the original participants. A random selection of the 4417 participants aged ≥50 were invited to participate in the HRV assessment and a total of 1846 subjects (955 women, 891 men) participated.
Exclusion criteria were general or spinal anaesthesia in 8 days prior to the ambulatory ECG recording (n=5), having had a myocardial infarction in 3 months prior to the exam (n=2), and taking digitalis (n=6); nobody had an artificial pacemaker. After exclusion of recordings showing atrial fibrillation (n=12), recordings of <18 h (recommendations of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology9) (n=73), and 6 recordings of insufficient quality, 1742 subjects were included in this analysis assessing the cross-sectional associations of cardiovascular risk factors or cardiovascular diseases with different parameters of HRV.
HRV measurements and analyses
For the Holter recording, digital devices (Aria, Del Mar Medical Systems, Irvine, CA, USA) with a frequency response of 0.05–40 Hz and a resolution of 128 samples/s were used. Three leads (V1, altered V3 with the electrode on the left midclavicular line on the lowest rib, and altered V5 with the electrode on the left anterior axillary line on the lowest rib) were recorded over 24 h. The mean duration of the Holter recordings was 22.3 (SD 2.1) h.
All recordings were scanned through a StrataScan 563 (Del Mar) and interpreted using the interactive method, with a final visual check on the full disclosure. The length of each RR interval was manually validated during this step. Resampling was made at 4 Hz. Spectral analysis was performed by the fast Fourier transform method using sliding 256 polynomial time approximation scheme (PTAs) windows for day and night periods. For 24 h periods, calculations of RR intervals were made without a sliding window, to allow measurement of ultra-low frequency (ULF) and very low frequency (VLF). Only normal-to-normal intervals were used, with intervals excluded due to ectopy or artefacts being replaced by holding the previous coupling interval level throughout the time interval to the next valid coupling interval. The standard deviation of all normal RR (NN) intervals (SDNN) and the following frequency domain variables have been calculated: Total power (TP) (≤0.40 Hz), ULF power (≤0.0033 Hz), VLF power (0.0033–0.04 Hz), LF power (0.04–0.15 Hz), high frequency (HF) power (0.15–0.40 Hz), and the ratio between LF and HF (LF/HF).
In order to avoid a biased result due to methacholine challenge, which was part of the SAPALDIA lung function testing and which, for practical reasons, was performed before the Holter recording, we excluded the first 2 h of all recordings.
The Holter recordings were made between August 2001 and March 2003. The recorders were placed on participants after a detailed health interview to those giving consent. Participants were asked to follow their regular daily routine and to complete a time-activity diary during the recording period.
Interview
During the interview, information about smoking habits (Questions: ‘Have you ever smoked for as long as a year?’ ‘Do you now smoke, as of 1 month ago?’ ‘Have you stopped or cut down smoking?’), recent myocardial infarction (‘Did you have a myocardial infarction in the past 3 months?’), comorbidities (‘Do you have any of the following conditions: …, hypertension,…, diabetes,…?’), drinking habits (‘How often do you normally drink alcoholic beverages like beer, wine, liqueurs, aperitifs, and strong drinks?’ ‘How often do you normally drink a glass of red wine?’), and amount of physical exercise (‘We are talking about physical exercise where you get out of breath at least a little bit, like fast walking, hiking, dancing, gardening, or a variety of sports. On how many days of the week do you do such exercises? On average, how many minutes per day do you do such exercises?’ ‘How many hours a week do you usually exercise such that you get out of breath or sweat?’) had been obtained. Participants were asked to bring all their drugs to the study centre in order to complete a medication list.
Other measurements
Serum creatinine, uric acid, total cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, and high sensitivity (hs) C-reactive protein (CRP) were measured in the blood as known cardiovascular risk factors. High density lipoprotein values could only be obtained if the participants had a triglyceride level of ≤9.4 mmol/L. Normal values for uric acid were 210–420 µmol/L for men and 150–350 µmol/L for women, for hs-CRP protein RP (should say: hs-CRP) <5.0 mg/L, for total cholesterol <5.0 mmol/L, and for HDL >1.0 mmol/L in both men and women8. Low density lipoprotein levels were calculated using the formula of Friedewald for subjects who had fasted for at least 6 h and had triglyceride levels of ≤4.7 mmol/L. As a further atherogenic marker, the difference between total cholesterol and HDL (non-HDL-cholesterol) was calculated, as well as the quotient between total cholesterol and HDL.
Blood pressure was measured twice with participants at rest and sitting by an automatic device (705CP, OMRON, Tokyo, Japan) positioned on the left upper arm according to WHO recommendations.13 Blood pressure values used in the regression models were the arithmetic mean of the two measurements.
High blood pressure was defined as either having a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg and/or having answered yes to the question ‘Do you have hypertension?’
Body height and weight were measured with the participant not wearing any shoes or coat. End-expiratory carbon monoxide (CO) was measured using an EC50 Micro-Smokerlyzer (BEDFONT, Rochester, UK).
This protocol was approved by the Ethics Committee of the Swiss Academy of Medical Sciences and the responsible Cantonal Ethics Committees.
Statistical analysis
Statistical analysis was performed using the software packages STATA 8.0 (Stata corporation, College Station, TX, USA) and SAS Vers. 8.2 (The SAS System, Cary, NC, USA).
To assess sex differences, in Tables 2–4, χ2 tests (for proportions) and t-tests (for means) were used. For comparison of the mean values across different age groups, one-way ANOVA was performed.
| . | Men . | Women . | All . |
|---|---|---|---|
| Sex | 847 (48.6%) | 895 (52.4%) | 1742 |
| Age (years) | 60.3 (SD 6.1) | 60.4 (SD 6.4) | 60.4 (SD 6.3) |
| Lifestyle factors | |||
| Never smokers | 277*** (32.7%) | 522 (58.5%) | 799 (45.9%) |
| Former smokers | 376*** (44.4%) | 227 (25.4%) | 603 (34.7%) |
| Current smokers | 193** (22.8%) | 144 (16.1%) | 337 (19.4%) |
| CO (ppm) | 5.6** (SD 9.6) | 4.3 (SD 7.6) | 4.9 (SD 8.7) |
| Alcohola,b | |||
| <1 glass/day | 499*** (59.3%) | 728 (81.7%) | 1227 (70.8%) |
| ≥1 glass/day | 343*** (40.7%) | 163 (18.3%) | 506 (29.2%) |
| Red winea | |||
| <1 glass/week | 318*** (37.8%) | 589 (66.1%) | 907 (52.3%) |
| ≥1 glass/week | 524*** (62.2%) | 302 (33.9%) | 826 (47.7%) |
| Light physical activitya | |||
| ≤−2 days/week | 443 (52.7%) | 438 (49.2%) | 881 (50.9%) |
| >2 days/week | 397 (47.3%) | 452 (50.8%) | 849 (49.1%) |
| Heavy physical acitivitya | |||
| ≤1 h/week | 575*** (68.8%) | 713 (80.5%) | 1288 (74.8%) |
| >1 h/week | 261*** (31.2%) | 173 (19.5%) | 434 (25.2%) |
| Cardiovascular health | |||
| SBP (mmHg) | 137.2*** (SD 19.1) | 127.2 (SD 18.6) | 132.1 (SD 19.5) |
| DBP (mmHg) | 84.3*** (SD 10.5) | 79.0 (SD 10.2) | 81.6 (SD 10.7) |
| Self-reported diabetes | 44 (5.2%) | 30 (3.4%) | 74 (4.3%) |
| BMIc (kg/m2) | 27.1*** (SD 3.5) | 26.3 (SD 4.9) | 26.7 (SD 4.3) |
| Medication | |||
| ACE-inhibitor | 53 (6.3%) | 57 (6.4%) | 110 (6.3%) |
| Antiarrhythmic drugs class I and III | 5 (0.6%) | 2 (0.2%) | 7 (0.4%) |
| Beta-blockers | 99 (11.7%) | 93 (10.4%) | 192 (11.0%) |
| Calcium channel blockers | 43 (5.1%) | 38 (4.2%) | 81 4.6% |
| Diuretics | 20** (2.4%) | 42 (4.7%) | 62 3.6% |
| Sympathomimetics | 31 (3.7%) | 26 (2.9%) | 57 3.3% |
| Laboratory parameters | |||
| Uric acid (µmol/L) | 367.5*** (SD 78.8) | 284.6 (SD 70.1) | 325.1 (SD 85.2) |
| hs-C-reactive protein (mg/L) | 2.6 (SD 6.3) | 2.5 (SD 3.7) | 2.6 (SD 5.2) |
| Non-HDL-cholesterold (mmol/L) | 4.8* (SD 1.1) | 4.7 (SD 1.1) | 4.8 (SD 1.1) |
| . | Men . | Women . | All . |
|---|---|---|---|
| Sex | 847 (48.6%) | 895 (52.4%) | 1742 |
| Age (years) | 60.3 (SD 6.1) | 60.4 (SD 6.4) | 60.4 (SD 6.3) |
| Lifestyle factors | |||
| Never smokers | 277*** (32.7%) | 522 (58.5%) | 799 (45.9%) |
| Former smokers | 376*** (44.4%) | 227 (25.4%) | 603 (34.7%) |
| Current smokers | 193** (22.8%) | 144 (16.1%) | 337 (19.4%) |
| CO (ppm) | 5.6** (SD 9.6) | 4.3 (SD 7.6) | 4.9 (SD 8.7) |
| Alcohola,b | |||
| <1 glass/day | 499*** (59.3%) | 728 (81.7%) | 1227 (70.8%) |
| ≥1 glass/day | 343*** (40.7%) | 163 (18.3%) | 506 (29.2%) |
| Red winea | |||
| <1 glass/week | 318*** (37.8%) | 589 (66.1%) | 907 (52.3%) |
| ≥1 glass/week | 524*** (62.2%) | 302 (33.9%) | 826 (47.7%) |
| Light physical activitya | |||
| ≤−2 days/week | 443 (52.7%) | 438 (49.2%) | 881 (50.9%) |
| >2 days/week | 397 (47.3%) | 452 (50.8%) | 849 (49.1%) |
| Heavy physical acitivitya | |||
| ≤1 h/week | 575*** (68.8%) | 713 (80.5%) | 1288 (74.8%) |
| >1 h/week | 261*** (31.2%) | 173 (19.5%) | 434 (25.2%) |
| Cardiovascular health | |||
| SBP (mmHg) | 137.2*** (SD 19.1) | 127.2 (SD 18.6) | 132.1 (SD 19.5) |
| DBP (mmHg) | 84.3*** (SD 10.5) | 79.0 (SD 10.2) | 81.6 (SD 10.7) |
| Self-reported diabetes | 44 (5.2%) | 30 (3.4%) | 74 (4.3%) |
| BMIc (kg/m2) | 27.1*** (SD 3.5) | 26.3 (SD 4.9) | 26.7 (SD 4.3) |
| Medication | |||
| ACE-inhibitor | 53 (6.3%) | 57 (6.4%) | 110 (6.3%) |
| Antiarrhythmic drugs class I and III | 5 (0.6%) | 2 (0.2%) | 7 (0.4%) |
| Beta-blockers | 99 (11.7%) | 93 (10.4%) | 192 (11.0%) |
| Calcium channel blockers | 43 (5.1%) | 38 (4.2%) | 81 4.6% |
| Diuretics | 20** (2.4%) | 42 (4.7%) | 62 3.6% |
| Sympathomimetics | 31 (3.7%) | 26 (2.9%) | 57 3.3% |
| Laboratory parameters | |||
| Uric acid (µmol/L) | 367.5*** (SD 78.8) | 284.6 (SD 70.1) | 325.1 (SD 85.2) |
| hs-C-reactive protein (mg/L) | 2.6 (SD 6.3) | 2.5 (SD 3.7) | 2.6 (SD 5.2) |
| Non-HDL-cholesterold (mmol/L) | 4.8* (SD 1.1) | 4.7 (SD 1.1) | 4.8 (SD 1.1) |
SBP, systolic blood pressure; DBP, diastolic blood pressure.
aMore categories have been used in the regression analysis.
bAlcoholic beverages including red wine.
cBMI=body weight/body height2.
dTotal cholesterol−HDL-cholesterol.
*P<0.05 (differences between sexes).
**P<0.01 (differences between sexes).
***P<0.001 (differences between sexes).
| . | Men . | Women . | All . |
|---|---|---|---|
| Sex | 847 (48.6%) | 895 (52.4%) | 1742 |
| Age (years) | 60.3 (SD 6.1) | 60.4 (SD 6.4) | 60.4 (SD 6.3) |
| Lifestyle factors | |||
| Never smokers | 277*** (32.7%) | 522 (58.5%) | 799 (45.9%) |
| Former smokers | 376*** (44.4%) | 227 (25.4%) | 603 (34.7%) |
| Current smokers | 193** (22.8%) | 144 (16.1%) | 337 (19.4%) |
| CO (ppm) | 5.6** (SD 9.6) | 4.3 (SD 7.6) | 4.9 (SD 8.7) |
| Alcohola,b | |||
| <1 glass/day | 499*** (59.3%) | 728 (81.7%) | 1227 (70.8%) |
| ≥1 glass/day | 343*** (40.7%) | 163 (18.3%) | 506 (29.2%) |
| Red winea | |||
| <1 glass/week | 318*** (37.8%) | 589 (66.1%) | 907 (52.3%) |
| ≥1 glass/week | 524*** (62.2%) | 302 (33.9%) | 826 (47.7%) |
| Light physical activitya | |||
| ≤−2 days/week | 443 (52.7%) | 438 (49.2%) | 881 (50.9%) |
| >2 days/week | 397 (47.3%) | 452 (50.8%) | 849 (49.1%) |
| Heavy physical acitivitya | |||
| ≤1 h/week | 575*** (68.8%) | 713 (80.5%) | 1288 (74.8%) |
| >1 h/week | 261*** (31.2%) | 173 (19.5%) | 434 (25.2%) |
| Cardiovascular health | |||
| SBP (mmHg) | 137.2*** (SD 19.1) | 127.2 (SD 18.6) | 132.1 (SD 19.5) |
| DBP (mmHg) | 84.3*** (SD 10.5) | 79.0 (SD 10.2) | 81.6 (SD 10.7) |
| Self-reported diabetes | 44 (5.2%) | 30 (3.4%) | 74 (4.3%) |
| BMIc (kg/m2) | 27.1*** (SD 3.5) | 26.3 (SD 4.9) | 26.7 (SD 4.3) |
| Medication | |||
| ACE-inhibitor | 53 (6.3%) | 57 (6.4%) | 110 (6.3%) |
| Antiarrhythmic drugs class I and III | 5 (0.6%) | 2 (0.2%) | 7 (0.4%) |
| Beta-blockers | 99 (11.7%) | 93 (10.4%) | 192 (11.0%) |
| Calcium channel blockers | 43 (5.1%) | 38 (4.2%) | 81 4.6% |
| Diuretics | 20** (2.4%) | 42 (4.7%) | 62 3.6% |
| Sympathomimetics | 31 (3.7%) | 26 (2.9%) | 57 3.3% |
| Laboratory parameters | |||
| Uric acid (µmol/L) | 367.5*** (SD 78.8) | 284.6 (SD 70.1) | 325.1 (SD 85.2) |
| hs-C-reactive protein (mg/L) | 2.6 (SD 6.3) | 2.5 (SD 3.7) | 2.6 (SD 5.2) |
| Non-HDL-cholesterold (mmol/L) | 4.8* (SD 1.1) | 4.7 (SD 1.1) | 4.8 (SD 1.1) |
| . | Men . | Women . | All . |
|---|---|---|---|
| Sex | 847 (48.6%) | 895 (52.4%) | 1742 |
| Age (years) | 60.3 (SD 6.1) | 60.4 (SD 6.4) | 60.4 (SD 6.3) |
| Lifestyle factors | |||
| Never smokers | 277*** (32.7%) | 522 (58.5%) | 799 (45.9%) |
| Former smokers | 376*** (44.4%) | 227 (25.4%) | 603 (34.7%) |
| Current smokers | 193** (22.8%) | 144 (16.1%) | 337 (19.4%) |
| CO (ppm) | 5.6** (SD 9.6) | 4.3 (SD 7.6) | 4.9 (SD 8.7) |
| Alcohola,b | |||
| <1 glass/day | 499*** (59.3%) | 728 (81.7%) | 1227 (70.8%) |
| ≥1 glass/day | 343*** (40.7%) | 163 (18.3%) | 506 (29.2%) |
| Red winea | |||
| <1 glass/week | 318*** (37.8%) | 589 (66.1%) | 907 (52.3%) |
| ≥1 glass/week | 524*** (62.2%) | 302 (33.9%) | 826 (47.7%) |
| Light physical activitya | |||
| ≤−2 days/week | 443 (52.7%) | 438 (49.2%) | 881 (50.9%) |
| >2 days/week | 397 (47.3%) | 452 (50.8%) | 849 (49.1%) |
| Heavy physical acitivitya | |||
| ≤1 h/week | 575*** (68.8%) | 713 (80.5%) | 1288 (74.8%) |
| >1 h/week | 261*** (31.2%) | 173 (19.5%) | 434 (25.2%) |
| Cardiovascular health | |||
| SBP (mmHg) | 137.2*** (SD 19.1) | 127.2 (SD 18.6) | 132.1 (SD 19.5) |
| DBP (mmHg) | 84.3*** (SD 10.5) | 79.0 (SD 10.2) | 81.6 (SD 10.7) |
| Self-reported diabetes | 44 (5.2%) | 30 (3.4%) | 74 (4.3%) |
| BMIc (kg/m2) | 27.1*** (SD 3.5) | 26.3 (SD 4.9) | 26.7 (SD 4.3) |
| Medication | |||
| ACE-inhibitor | 53 (6.3%) | 57 (6.4%) | 110 (6.3%) |
| Antiarrhythmic drugs class I and III | 5 (0.6%) | 2 (0.2%) | 7 (0.4%) |
| Beta-blockers | 99 (11.7%) | 93 (10.4%) | 192 (11.0%) |
| Calcium channel blockers | 43 (5.1%) | 38 (4.2%) | 81 4.6% |
| Diuretics | 20** (2.4%) | 42 (4.7%) | 62 3.6% |
| Sympathomimetics | 31 (3.7%) | 26 (2.9%) | 57 3.3% |
| Laboratory parameters | |||
| Uric acid (µmol/L) | 367.5*** (SD 78.8) | 284.6 (SD 70.1) | 325.1 (SD 85.2) |
| hs-C-reactive protein (mg/L) | 2.6 (SD 6.3) | 2.5 (SD 3.7) | 2.6 (SD 5.2) |
| Non-HDL-cholesterold (mmol/L) | 4.8* (SD 1.1) | 4.7 (SD 1.1) | 4.8 (SD 1.1) |
SBP, systolic blood pressure; DBP, diastolic blood pressure.
aMore categories have been used in the regression analysis.
bAlcoholic beverages including red wine.
cBMI=body weight/body height2.
dTotal cholesterol−HDL-cholesterol.
*P<0.05 (differences between sexes).
**P<0.01 (differences between sexes).
***P<0.001 (differences between sexes).
Unadjusted meana HRV values for men and women of different age groups of the total study population
| . | Age (years) . | ||||
|---|---|---|---|---|---|
| . | Sex . | 50–54 (nm=181; nf=219) . | 55–59 (nm=238; nf=217) . | 60–64 (nm=186; nf=189) . | 65–73 (nm=242; nf=270) . |
| SDNN (ms) | Men | 138.2 | 129.2 | 130.8 | 131.9* |
| (95% CI) | (132.5–144.1) | (124.8–133.8) | (126.0–135.7) | (127.0–137.1) | |
| Women | 135.8 | 134.7 | 130.6 | 123.6 | |
| (95% CI) | (131.0–140.7) | (130.4–139.2) | (126.2–135.3) | (119.5–127.9) | |
| TP (ms2) | Men | 3925.2 | 3616.1 | 3597.3 | 3585.8* |
| (95% CI) | (3568.6–4317.4) | (3340.5–3914.5) | (3295.8–3926.5) | (3288.4–3910.1) | |
| Women | 4134.0 | 3876.5 | 3514.6 | 3200.5 | |
| (95% CI) | (3818.3–4475.8) | (3600.2–4174.1) | (3223.4–3832.1) | (2973.4–3445.0) | |
| HF (ms2) | Men | 63.6*** | 59.7*** | 61.0 | 68.0 |
| (95% CI) | (55.4–72.9) | (53.0–67.2) | (53.4–69.7) | (59.4–77.8) | |
| Women | 87.8 | 81.0 | 66.1 | 65.3 | |
| (95% CI) | (79.1–97.4) | (72.6–90.4) | (57.6–75.7) | (58.6–72.8) | |
| LF (ms2) | Men | 318.1** | 260.7 | 228.4** | 196.0*** |
| (95% CI) | (284.4–355.8) | (237.9–285.7) | (205.9–253.4) | (175.7–218.5) | |
| Women | 258.2 | 234.9 | 179.0 | 147.7 | |
| (95% CI) | (238.0–280.0) | (214.5–257.3) | (161.0–199.0) | (135.1–161.4) | |
| LF/HF | Men | 5.0*** | 4.4*** | 3.7*** | 2.9*** |
| (95% CI) | (4.6–5.4) | (4.1–4.7) | (3.4–4.1) | (2.6–3.2) | |
| Women | 2.9 | 2.9 | 2.7 | 2.3 | |
| (95% CI) | (2.7–3.2) | (2.7–3.1) | (2.5–3.0) | (2.1–2.4) | |
| VLF (ms2) | Men | 719.1 | 641.7 | 637.9*** | 563.0*** |
| (95% CI) | (650.3–795.3) | (589.6–698.3) | (581.0–700.5) | (511.2–620.1) | |
| Women | 668.5 | 623.7 | 504.3 | 450.0 | |
| (95% CI) | (621.6–719.0) | (575.6–675.8) | (461.9–550.6) | (418.7–483.6) | |
| ULF (ms2) | Men | 2653.7 | 2479.2* | 2463.4 | 2515.2 |
| (95% CI) | (2390.7–2945.5) | (2273.1–2704.0) | (2232.5–2718.1) | (2293.2–2758.7) | |
| Women | 2928.7 | 2790.5 | 2605.8 | 2384.8 | |
| (95% CI) | (2674.4–3207.1) | (2578.7–3019.8) | (2375.1–2858.8) | (2200.5–2584.6) | |
| . | Age (years) . | ||||
|---|---|---|---|---|---|
| . | Sex . | 50–54 (nm=181; nf=219) . | 55–59 (nm=238; nf=217) . | 60–64 (nm=186; nf=189) . | 65–73 (nm=242; nf=270) . |
| SDNN (ms) | Men | 138.2 | 129.2 | 130.8 | 131.9* |
| (95% CI) | (132.5–144.1) | (124.8–133.8) | (126.0–135.7) | (127.0–137.1) | |
| Women | 135.8 | 134.7 | 130.6 | 123.6 | |
| (95% CI) | (131.0–140.7) | (130.4–139.2) | (126.2–135.3) | (119.5–127.9) | |
| TP (ms2) | Men | 3925.2 | 3616.1 | 3597.3 | 3585.8* |
| (95% CI) | (3568.6–4317.4) | (3340.5–3914.5) | (3295.8–3926.5) | (3288.4–3910.1) | |
| Women | 4134.0 | 3876.5 | 3514.6 | 3200.5 | |
| (95% CI) | (3818.3–4475.8) | (3600.2–4174.1) | (3223.4–3832.1) | (2973.4–3445.0) | |
| HF (ms2) | Men | 63.6*** | 59.7*** | 61.0 | 68.0 |
| (95% CI) | (55.4–72.9) | (53.0–67.2) | (53.4–69.7) | (59.4–77.8) | |
| Women | 87.8 | 81.0 | 66.1 | 65.3 | |
| (95% CI) | (79.1–97.4) | (72.6–90.4) | (57.6–75.7) | (58.6–72.8) | |
| LF (ms2) | Men | 318.1** | 260.7 | 228.4** | 196.0*** |
| (95% CI) | (284.4–355.8) | (237.9–285.7) | (205.9–253.4) | (175.7–218.5) | |
| Women | 258.2 | 234.9 | 179.0 | 147.7 | |
| (95% CI) | (238.0–280.0) | (214.5–257.3) | (161.0–199.0) | (135.1–161.4) | |
| LF/HF | Men | 5.0*** | 4.4*** | 3.7*** | 2.9*** |
| (95% CI) | (4.6–5.4) | (4.1–4.7) | (3.4–4.1) | (2.6–3.2) | |
| Women | 2.9 | 2.9 | 2.7 | 2.3 | |
| (95% CI) | (2.7–3.2) | (2.7–3.1) | (2.5–3.0) | (2.1–2.4) | |
| VLF (ms2) | Men | 719.1 | 641.7 | 637.9*** | 563.0*** |
| (95% CI) | (650.3–795.3) | (589.6–698.3) | (581.0–700.5) | (511.2–620.1) | |
| Women | 668.5 | 623.7 | 504.3 | 450.0 | |
| (95% CI) | (621.6–719.0) | (575.6–675.8) | (461.9–550.6) | (418.7–483.6) | |
| ULF (ms2) | Men | 2653.7 | 2479.2* | 2463.4 | 2515.2 |
| (95% CI) | (2390.7–2945.5) | (2273.1–2704.0) | (2232.5–2718.1) | (2293.2–2758.7) | |
| Women | 2928.7 | 2790.5 | 2605.8 | 2384.8 | |
| (95% CI) | (2674.4–3207.1) | (2578.7–3019.8) | (2375.1–2858.8) | (2200.5–2584.6) | |
nm, number of male participants; nf, number of female participants.
aGeometric means are shown because values were skewed.
*P<0.05 (differences between sexes).
**P<0.01 (differences between sexes).
***P<0.001 (differences between sexes).
Unadjusted meana HRV values for men and women of different age groups of the total study population
| . | Age (years) . | ||||
|---|---|---|---|---|---|
| . | Sex . | 50–54 (nm=181; nf=219) . | 55–59 (nm=238; nf=217) . | 60–64 (nm=186; nf=189) . | 65–73 (nm=242; nf=270) . |
| SDNN (ms) | Men | 138.2 | 129.2 | 130.8 | 131.9* |
| (95% CI) | (132.5–144.1) | (124.8–133.8) | (126.0–135.7) | (127.0–137.1) | |
| Women | 135.8 | 134.7 | 130.6 | 123.6 | |
| (95% CI) | (131.0–140.7) | (130.4–139.2) | (126.2–135.3) | (119.5–127.9) | |
| TP (ms2) | Men | 3925.2 | 3616.1 | 3597.3 | 3585.8* |
| (95% CI) | (3568.6–4317.4) | (3340.5–3914.5) | (3295.8–3926.5) | (3288.4–3910.1) | |
| Women | 4134.0 | 3876.5 | 3514.6 | 3200.5 | |
| (95% CI) | (3818.3–4475.8) | (3600.2–4174.1) | (3223.4–3832.1) | (2973.4–3445.0) | |
| HF (ms2) | Men | 63.6*** | 59.7*** | 61.0 | 68.0 |
| (95% CI) | (55.4–72.9) | (53.0–67.2) | (53.4–69.7) | (59.4–77.8) | |
| Women | 87.8 | 81.0 | 66.1 | 65.3 | |
| (95% CI) | (79.1–97.4) | (72.6–90.4) | (57.6–75.7) | (58.6–72.8) | |
| LF (ms2) | Men | 318.1** | 260.7 | 228.4** | 196.0*** |
| (95% CI) | (284.4–355.8) | (237.9–285.7) | (205.9–253.4) | (175.7–218.5) | |
| Women | 258.2 | 234.9 | 179.0 | 147.7 | |
| (95% CI) | (238.0–280.0) | (214.5–257.3) | (161.0–199.0) | (135.1–161.4) | |
| LF/HF | Men | 5.0*** | 4.4*** | 3.7*** | 2.9*** |
| (95% CI) | (4.6–5.4) | (4.1–4.7) | (3.4–4.1) | (2.6–3.2) | |
| Women | 2.9 | 2.9 | 2.7 | 2.3 | |
| (95% CI) | (2.7–3.2) | (2.7–3.1) | (2.5–3.0) | (2.1–2.4) | |
| VLF (ms2) | Men | 719.1 | 641.7 | 637.9*** | 563.0*** |
| (95% CI) | (650.3–795.3) | (589.6–698.3) | (581.0–700.5) | (511.2–620.1) | |
| Women | 668.5 | 623.7 | 504.3 | 450.0 | |
| (95% CI) | (621.6–719.0) | (575.6–675.8) | (461.9–550.6) | (418.7–483.6) | |
| ULF (ms2) | Men | 2653.7 | 2479.2* | 2463.4 | 2515.2 |
| (95% CI) | (2390.7–2945.5) | (2273.1–2704.0) | (2232.5–2718.1) | (2293.2–2758.7) | |
| Women | 2928.7 | 2790.5 | 2605.8 | 2384.8 | |
| (95% CI) | (2674.4–3207.1) | (2578.7–3019.8) | (2375.1–2858.8) | (2200.5–2584.6) | |
| . | Age (years) . | ||||
|---|---|---|---|---|---|
| . | Sex . | 50–54 (nm=181; nf=219) . | 55–59 (nm=238; nf=217) . | 60–64 (nm=186; nf=189) . | 65–73 (nm=242; nf=270) . |
| SDNN (ms) | Men | 138.2 | 129.2 | 130.8 | 131.9* |
| (95% CI) | (132.5–144.1) | (124.8–133.8) | (126.0–135.7) | (127.0–137.1) | |
| Women | 135.8 | 134.7 | 130.6 | 123.6 | |
| (95% CI) | (131.0–140.7) | (130.4–139.2) | (126.2–135.3) | (119.5–127.9) | |
| TP (ms2) | Men | 3925.2 | 3616.1 | 3597.3 | 3585.8* |
| (95% CI) | (3568.6–4317.4) | (3340.5–3914.5) | (3295.8–3926.5) | (3288.4–3910.1) | |
| Women | 4134.0 | 3876.5 | 3514.6 | 3200.5 | |
| (95% CI) | (3818.3–4475.8) | (3600.2–4174.1) | (3223.4–3832.1) | (2973.4–3445.0) | |
| HF (ms2) | Men | 63.6*** | 59.7*** | 61.0 | 68.0 |
| (95% CI) | (55.4–72.9) | (53.0–67.2) | (53.4–69.7) | (59.4–77.8) | |
| Women | 87.8 | 81.0 | 66.1 | 65.3 | |
| (95% CI) | (79.1–97.4) | (72.6–90.4) | (57.6–75.7) | (58.6–72.8) | |
| LF (ms2) | Men | 318.1** | 260.7 | 228.4** | 196.0*** |
| (95% CI) | (284.4–355.8) | (237.9–285.7) | (205.9–253.4) | (175.7–218.5) | |
| Women | 258.2 | 234.9 | 179.0 | 147.7 | |
| (95% CI) | (238.0–280.0) | (214.5–257.3) | (161.0–199.0) | (135.1–161.4) | |
| LF/HF | Men | 5.0*** | 4.4*** | 3.7*** | 2.9*** |
| (95% CI) | (4.6–5.4) | (4.1–4.7) | (3.4–4.1) | (2.6–3.2) | |
| Women | 2.9 | 2.9 | 2.7 | 2.3 | |
| (95% CI) | (2.7–3.2) | (2.7–3.1) | (2.5–3.0) | (2.1–2.4) | |
| VLF (ms2) | Men | 719.1 | 641.7 | 637.9*** | 563.0*** |
| (95% CI) | (650.3–795.3) | (589.6–698.3) | (581.0–700.5) | (511.2–620.1) | |
| Women | 668.5 | 623.7 | 504.3 | 450.0 | |
| (95% CI) | (621.6–719.0) | (575.6–675.8) | (461.9–550.6) | (418.7–483.6) | |
| ULF (ms2) | Men | 2653.7 | 2479.2* | 2463.4 | 2515.2 |
| (95% CI) | (2390.7–2945.5) | (2273.1–2704.0) | (2232.5–2718.1) | (2293.2–2758.7) | |
| Women | 2928.7 | 2790.5 | 2605.8 | 2384.8 | |
| (95% CI) | (2674.4–3207.1) | (2578.7–3019.8) | (2375.1–2858.8) | (2200.5–2584.6) | |
nm, number of male participants; nf, number of female participants.
aGeometric means are shown because values were skewed.
*P<0.05 (differences between sexes).
**P<0.01 (differences between sexes).
***P<0.001 (differences between sexes).
Adjusted estimatesa of mean per cent changes of HRV associated with different risk factorsb
| . | SDNN (%) . | TP (%) . | HF (%) . | LF (%) . | LF/HF (%) . | VLF (%) . | ULF (%) . |
|---|---|---|---|---|---|---|---|
| Female sex | −6.1*** | −11.4*** | 6.0 | −27.2*** | −31.3 *** | −23.1*** | −6.3 |
| Age (year) | −0.3* | −0.9*** | −1.4*** | −3.2*** | −1.9*** | −1.9*** | −0.5 |
| Lifestyle factors | |||||||
| Former vs. never smoking | −1.8 | −1.7 | 6.6 | 4.7 | −1.8 | −0.1 | −3.3 |
| Current vs. never smoking | −4.5* | −15.0** | −10.5 | −15.5** | −5.6 | −16.6*** | −14.9** |
| CO (ppm) | −0.3** | −0.3 | 0.0 | −0.6* | −0.6** | −0.6* | −0.3 |
| <1 glass alcohol/day vs. 0 | −1.0 | −6.0 | −5.7 | −8.8 | −3.3 | −8.1 | −5.8 |
| 1 glass alcohol/day vs. 0 | −3.0 | −15.7* | −19.2 | −19.0* | 0.3 | −17.7* | −13.7 |
| >1 glass alcohol/day vs. 0 | −2.0 | −13.5 | −11.0 | −14.6 | −4.0 | −11.6 | −14.6 |
| Exercise heavy vs. never | 2.0*** | 3.4** | 3.6*** | 4.2** | 0.6 | 4.1*** | 3.2** |
| Cardiovascular health | |||||||
| High BPc | −1.3 | −7.1* | −5.4 | −9.2* | −4.0 | −6.9* | −7.5* |
| Self-reported diabetes | −6.7 | −14.2 | −5.4 | −16.1 | −11.3 | −14.8 | −16.5* |
| BMI (kg/m2)d | −0.7*** | −1.2** | 0.5 | −1.0* | −1.5*** | −1.2** | −1.1** |
| Medications | |||||||
| ACE-inhibitors | 4.1 | 17.3* | 19.1 | 13.7 | −4.5 | 14.0* | 16.5* |
| Antiarrhythmics | 13.2 | 20.3 | 206.7** | 13.4 | −63.0*** | −7.6 | 2.5 |
| Beta-blockers | −6.9*** | 12.3* | 21.5** | 7.1 | −11.9* | 24.2*** | 10.5 |
| Ca blockers | −0.6 | −1.6 | 20.7 | −2.2 | −19.0** | −4.0 | −1.8 |
| Diuretics | −3.3 | −19.9* | −16.0 | −25.2** | −11.0 | −27.1*** | −21.4* |
| Sympathomimetics | −1.6 | −11.9 | −12.6 | −12.7 | −0.1 | −10.0 | −10.8 |
| Laboratory parameters | |||||||
| Uric acid (µmol/L) | 0.0** | −0.1** | −0.1* | −0.1** | 0.0 | −0.1** | −0.1* |
| hs-C-reactive protein (mg/L) | −0.4** | −1.0*** | −1.1** | −1.7*** | −0.6* | −1.5*** | −0.8** |
| Non-HDL-cholesterol (mmol/L)e | −0.2 | −2.8* | −7.5*** | −3.7* | 4.0** | −3.1* | −2.2 |
| . | SDNN (%) . | TP (%) . | HF (%) . | LF (%) . | LF/HF (%) . | VLF (%) . | ULF (%) . |
|---|---|---|---|---|---|---|---|
| Female sex | −6.1*** | −11.4*** | 6.0 | −27.2*** | −31.3 *** | −23.1*** | −6.3 |
| Age (year) | −0.3* | −0.9*** | −1.4*** | −3.2*** | −1.9*** | −1.9*** | −0.5 |
| Lifestyle factors | |||||||
| Former vs. never smoking | −1.8 | −1.7 | 6.6 | 4.7 | −1.8 | −0.1 | −3.3 |
| Current vs. never smoking | −4.5* | −15.0** | −10.5 | −15.5** | −5.6 | −16.6*** | −14.9** |
| CO (ppm) | −0.3** | −0.3 | 0.0 | −0.6* | −0.6** | −0.6* | −0.3 |
| <1 glass alcohol/day vs. 0 | −1.0 | −6.0 | −5.7 | −8.8 | −3.3 | −8.1 | −5.8 |
| 1 glass alcohol/day vs. 0 | −3.0 | −15.7* | −19.2 | −19.0* | 0.3 | −17.7* | −13.7 |
| >1 glass alcohol/day vs. 0 | −2.0 | −13.5 | −11.0 | −14.6 | −4.0 | −11.6 | −14.6 |
| Exercise heavy vs. never | 2.0*** | 3.4** | 3.6*** | 4.2** | 0.6 | 4.1*** | 3.2** |
| Cardiovascular health | |||||||
| High BPc | −1.3 | −7.1* | −5.4 | −9.2* | −4.0 | −6.9* | −7.5* |
| Self-reported diabetes | −6.7 | −14.2 | −5.4 | −16.1 | −11.3 | −14.8 | −16.5* |
| BMI (kg/m2)d | −0.7*** | −1.2** | 0.5 | −1.0* | −1.5*** | −1.2** | −1.1** |
| Medications | |||||||
| ACE-inhibitors | 4.1 | 17.3* | 19.1 | 13.7 | −4.5 | 14.0* | 16.5* |
| Antiarrhythmics | 13.2 | 20.3 | 206.7** | 13.4 | −63.0*** | −7.6 | 2.5 |
| Beta-blockers | −6.9*** | 12.3* | 21.5** | 7.1 | −11.9* | 24.2*** | 10.5 |
| Ca blockers | −0.6 | −1.6 | 20.7 | −2.2 | −19.0** | −4.0 | −1.8 |
| Diuretics | −3.3 | −19.9* | −16.0 | −25.2** | −11.0 | −27.1*** | −21.4* |
| Sympathomimetics | −1.6 | −11.9 | −12.6 | −12.7 | −0.1 | −10.0 | −10.8 |
| Laboratory parameters | |||||||
| Uric acid (µmol/L) | 0.0** | −0.1** | −0.1* | −0.1** | 0.0 | −0.1** | −0.1* |
| hs-C-reactive protein (mg/L) | −0.4** | −1.0*** | −1.1** | −1.7*** | −0.6* | −1.5*** | −0.8** |
| Non-HDL-cholesterol (mmol/L)e | −0.2 | −2.8* | −7.5*** | −3.7* | 4.0** | −3.1* | −2.2 |
aDerived from regression model including all factors in Table 2 and study site.
bEffect estimates for quantitative covariates refer to one unit increments in these variables. The respective units are given in parentheses.
cHigh blood pressure=systolic blood pressure ≥140 mmHg or diastolic blood pressure >90 mmHg or self-reported high blood pressure.
dBMI=body weight/body height2.
eTotal cholesterol−HDL-cholesterol.
*P<0.05.
**P<0.01.
***P<0.001.
Adjusted estimatesa of mean per cent changes of HRV associated with different risk factorsb
| . | SDNN (%) . | TP (%) . | HF (%) . | LF (%) . | LF/HF (%) . | VLF (%) . | ULF (%) . |
|---|---|---|---|---|---|---|---|
| Female sex | −6.1*** | −11.4*** | 6.0 | −27.2*** | −31.3 *** | −23.1*** | −6.3 |
| Age (year) | −0.3* | −0.9*** | −1.4*** | −3.2*** | −1.9*** | −1.9*** | −0.5 |
| Lifestyle factors | |||||||
| Former vs. never smoking | −1.8 | −1.7 | 6.6 | 4.7 | −1.8 | −0.1 | −3.3 |
| Current vs. never smoking | −4.5* | −15.0** | −10.5 | −15.5** | −5.6 | −16.6*** | −14.9** |
| CO (ppm) | −0.3** | −0.3 | 0.0 | −0.6* | −0.6** | −0.6* | −0.3 |
| <1 glass alcohol/day vs. 0 | −1.0 | −6.0 | −5.7 | −8.8 | −3.3 | −8.1 | −5.8 |
| 1 glass alcohol/day vs. 0 | −3.0 | −15.7* | −19.2 | −19.0* | 0.3 | −17.7* | −13.7 |
| >1 glass alcohol/day vs. 0 | −2.0 | −13.5 | −11.0 | −14.6 | −4.0 | −11.6 | −14.6 |
| Exercise heavy vs. never | 2.0*** | 3.4** | 3.6*** | 4.2** | 0.6 | 4.1*** | 3.2** |
| Cardiovascular health | |||||||
| High BPc | −1.3 | −7.1* | −5.4 | −9.2* | −4.0 | −6.9* | −7.5* |
| Self-reported diabetes | −6.7 | −14.2 | −5.4 | −16.1 | −11.3 | −14.8 | −16.5* |
| BMI (kg/m2)d | −0.7*** | −1.2** | 0.5 | −1.0* | −1.5*** | −1.2** | −1.1** |
| Medications | |||||||
| ACE-inhibitors | 4.1 | 17.3* | 19.1 | 13.7 | −4.5 | 14.0* | 16.5* |
| Antiarrhythmics | 13.2 | 20.3 | 206.7** | 13.4 | −63.0*** | −7.6 | 2.5 |
| Beta-blockers | −6.9*** | 12.3* | 21.5** | 7.1 | −11.9* | 24.2*** | 10.5 |
| Ca blockers | −0.6 | −1.6 | 20.7 | −2.2 | −19.0** | −4.0 | −1.8 |
| Diuretics | −3.3 | −19.9* | −16.0 | −25.2** | −11.0 | −27.1*** | −21.4* |
| Sympathomimetics | −1.6 | −11.9 | −12.6 | −12.7 | −0.1 | −10.0 | −10.8 |
| Laboratory parameters | |||||||
| Uric acid (µmol/L) | 0.0** | −0.1** | −0.1* | −0.1** | 0.0 | −0.1** | −0.1* |
| hs-C-reactive protein (mg/L) | −0.4** | −1.0*** | −1.1** | −1.7*** | −0.6* | −1.5*** | −0.8** |
| Non-HDL-cholesterol (mmol/L)e | −0.2 | −2.8* | −7.5*** | −3.7* | 4.0** | −3.1* | −2.2 |
| . | SDNN (%) . | TP (%) . | HF (%) . | LF (%) . | LF/HF (%) . | VLF (%) . | ULF (%) . |
|---|---|---|---|---|---|---|---|
| Female sex | −6.1*** | −11.4*** | 6.0 | −27.2*** | −31.3 *** | −23.1*** | −6.3 |
| Age (year) | −0.3* | −0.9*** | −1.4*** | −3.2*** | −1.9*** | −1.9*** | −0.5 |
| Lifestyle factors | |||||||
| Former vs. never smoking | −1.8 | −1.7 | 6.6 | 4.7 | −1.8 | −0.1 | −3.3 |
| Current vs. never smoking | −4.5* | −15.0** | −10.5 | −15.5** | −5.6 | −16.6*** | −14.9** |
| CO (ppm) | −0.3** | −0.3 | 0.0 | −0.6* | −0.6** | −0.6* | −0.3 |
| <1 glass alcohol/day vs. 0 | −1.0 | −6.0 | −5.7 | −8.8 | −3.3 | −8.1 | −5.8 |
| 1 glass alcohol/day vs. 0 | −3.0 | −15.7* | −19.2 | −19.0* | 0.3 | −17.7* | −13.7 |
| >1 glass alcohol/day vs. 0 | −2.0 | −13.5 | −11.0 | −14.6 | −4.0 | −11.6 | −14.6 |
| Exercise heavy vs. never | 2.0*** | 3.4** | 3.6*** | 4.2** | 0.6 | 4.1*** | 3.2** |
| Cardiovascular health | |||||||
| High BPc | −1.3 | −7.1* | −5.4 | −9.2* | −4.0 | −6.9* | −7.5* |
| Self-reported diabetes | −6.7 | −14.2 | −5.4 | −16.1 | −11.3 | −14.8 | −16.5* |
| BMI (kg/m2)d | −0.7*** | −1.2** | 0.5 | −1.0* | −1.5*** | −1.2** | −1.1** |
| Medications | |||||||
| ACE-inhibitors | 4.1 | 17.3* | 19.1 | 13.7 | −4.5 | 14.0* | 16.5* |
| Antiarrhythmics | 13.2 | 20.3 | 206.7** | 13.4 | −63.0*** | −7.6 | 2.5 |
| Beta-blockers | −6.9*** | 12.3* | 21.5** | 7.1 | −11.9* | 24.2*** | 10.5 |
| Ca blockers | −0.6 | −1.6 | 20.7 | −2.2 | −19.0** | −4.0 | −1.8 |
| Diuretics | −3.3 | −19.9* | −16.0 | −25.2** | −11.0 | −27.1*** | −21.4* |
| Sympathomimetics | −1.6 | −11.9 | −12.6 | −12.7 | −0.1 | −10.0 | −10.8 |
| Laboratory parameters | |||||||
| Uric acid (µmol/L) | 0.0** | −0.1** | −0.1* | −0.1** | 0.0 | −0.1** | −0.1* |
| hs-C-reactive protein (mg/L) | −0.4** | −1.0*** | −1.1** | −1.7*** | −0.6* | −1.5*** | −0.8** |
| Non-HDL-cholesterol (mmol/L)e | −0.2 | −2.8* | −7.5*** | −3.7* | 4.0** | −3.1* | −2.2 |
aDerived from regression model including all factors in Table 2 and study site.
bEffect estimates for quantitative covariates refer to one unit increments in these variables. The respective units are given in parentheses.
cHigh blood pressure=systolic blood pressure ≥140 mmHg or diastolic blood pressure >90 mmHg or self-reported high blood pressure.
dBMI=body weight/body height2.
eTotal cholesterol−HDL-cholesterol.
*P<0.05.
**P<0.01.
***P<0.001.
Because the distribution of the HRV values was skewed, values were log-transformed before analysis.
To assess associations of different risk factors with HRV measurements, a structured multivariable linear regression was performed. Because of the known association of HRV with age and sex, these variables were a priori included in the model.14 Variables were then examined by categories (cardiovascular health, alcohol intake, smoking, medication, exercise, laboratory results). Within each category, multiple variables were considered simultaneously, and backwards elimination was used to choose the best predictors of HRV from candidate variables. After the most important representatives of each category of covariates were combined in a full model, backward selection was used in order to identify covariates that might have lost their importance in the multivariable setting. In the final model, all baseline characteristics reported in Table 2, as well as dummy variables for the study areas, were included.
Calculation of percentiles
For calculating percentiles of HRV, a ‘healthy’ subsample of the sample described so far was defined by requiring the absence of each of the following conditions: high blood pressure (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg), current smoking, alcohol consumption of more than one glass per day, self-reported diabetes, intake of ACE-inhibitors, antiarrhythmic drugs classes I and III, calcium channel blockers, diuretics, or sympathomimetics in the preceding 30 days. Three hundred and twenty-nine women and 170 men fulfilled these criteria and could be included in the definition of ‘healthy individuals’.
Percentiles of power as a function of age in a healthy population have been estimated in the following way: for each of the probabilities P=0.05, 0.25, 0.5, 0.75, 0.95, the p-quantile of ln(TP) for a given age x was assumed to be of the form yp(x)=b0+b1zp+b2x+b3zpx+b4x2+b5zpx2, where zp denotes the p-quantile of the standard normal distribution.
This implies that, for each age x, the distribution of ln(TP) is normal with mean b0+b2x+b4x2 and standard deviation b1+b3x+b5x2. Exploration of the residuals of ln(TP) after regression against age and square of the age indicated that this assumption was tenable. The six parameters of this equation were estimated using weighted L1-regression. Details of this method are given in Braendli et al.15 and Koenker and Basset.16
Results
Table 2 gives an overview of the study population's baseline characteristics. Although there were slightly more women than men, their mean age was identical. Men more frequently had an unhealthy lifestyle (smoking, alcohol drinking), more often were overweight and had on average a higher blood pressure, but more frequently reported heavy physical exercise. Medication intake was similar for both sexes, except for diuretics which were taken twice as often by women. A surprisingly high proportion (11%) of participants were taking beta-blockers; 18.6% of the subjects were on at least one and 5.0% on more than one of the drugs listed in Table 2.
Table 3 shows the unadjusted geometric means of HRV values for the 1742 men and women in different age groups. Men of all combined age groups had significantly lower HF than women, but higher LF, LF/HF, and VLF. Looking at each age group separately, women of the oldest age group (65–73 years) had significantly lower SDNN, TP, LF, LF/HF, and VLF than men of the same age group. Across all age groups, women had significantly lower average LF/HF ratios than men, but women between 50 and 59 years had higher HF than men of their age.
Table 4 shows covariate-adjusted estimates of the per cent changes of frequency domain variables of HRV associated with different lifestyle or risk factors.
Even after adjustment for all other factors, women had significantly lower values for all HRV parameters, except for HF and ULF. TP, for example, was 11.4% lower in women than in men. All HRV parameters except ULF decreased with age in both sexes. HF decreased 1.4% per year and LF 3.2% (Table 4, first two rows).
Lifestyle factors
Although former smoking showed no influence, we found current smoking to be the strongest predictor for HRV with a decrease mainly in sympathetic activity (SDNN −4.5%, TP −15.0%, LF −15.5%, VLF −16.6%, and ULF −14.9%). Independent of being a current smoker, CO in exhaled air, as a measure of recent tobacco smoke exposure, showed a significant association with decreased SDNN (−0.3%), LF (−0.6%), LF/HF (−0.6%), and VLF (−0.6%). The consumption of one glass of alcoholic beverage per day showed a significant association with decreased TP (−15.7%), LF (−19.0%), and VLF (−17.7%). Heavy physical exercise was generally associated with higher HRV: SDNN, TP, HF, LF, VLF, and ULF increased significantly by ≥2.0% per weekly hour of heavy exercise.
Cardiovascular health
Having high blood pressure as defined above primarily affected sympathetic tone, with decreases in TP, LF, VLF, and ULF, whereas a higher body mass index (BMI) was associated with a decrease in all HRV parameters except HF. On average, TP decreased by 1.2% for each additional kg/m2 of BMI. Self-reported diabetes decreased ULF significantly by 16.5%.
Medications
Among the drugs examined, antiarrhythmics, as expected, showed the strongest association with HRV, followed by diuretics and beta-blockers. Persons taking diuretics showed significantly diminished TP, LF, VLF, and ULF. Subjects on beta-blockers had an increased vagal tone, with increases in TP of 13%, HF of 22%, and VLF of 25%, but with a 12% decrease in LF/HF. Subjects on ACE-inhibitors had a 18% increase in TP.
Laboratory parameters
All laboratory parameters of the regression model (uric acid, hs-CRP, and non-HDL-cholesterol) consistently showed negative associations with most HRV parameters. The exceptions were the LF/HF ratio for uric acid and SDNN as well as ULF for non-HDL-cholesterol. Both HF and LF decreased by 0.1%/µmol/L increase in uric acid. Per unit increase in hs-CRP, HF decreased by 1.1% and LF by 1.7%. Non-HDL-cholesterol showed a highly significant association with decreased vagal and sympathetic tone (with decreases in HF and LF by 7.5 and 3.7%, respectively).
Normal values
It is often useful to compare subjects or patients' HRV with that found in age- and sex-matched healthy populations. To construct such normal ageing curves for men and women between 50 and 70 years of age, we chose all non-smoking individuals without history of cardiovascular disease, high blood pressure or diabetes, and free of medications, as described in Methods. We then calculated normal ageing curves, which are shown in Figures 1 and 2.
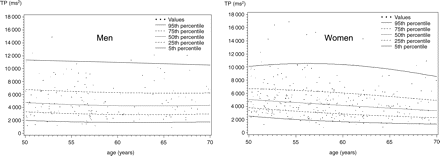
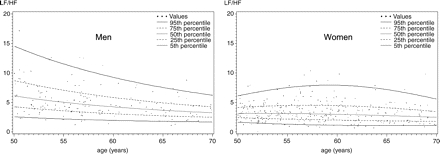
In men, LF/HF values clearly decrease between 50 and 70 years of age. In women, such a decrease is only observed after age 60.
Discussion
In this study, we have analysed different factors influencing HRV in a population-based sample aged 50–72. We are not aware of many studies about the respective importance of different predictors of HRV, although HRV is known to be influenced by a great variety of factors.8 Because of our relatively large sample size, we were also able to estimate age-dependent percentile curves for normotensive non-smoking subjects aged ≥50 without cardioactive drug intake or moderate to high alcohol consumption. As a result, we are able to show different ageing patterns for HRV in men and women. We found TP to decline clearly with age in men, whereas HF showed this pattern in women. This results in an earlier and more marked age-related decline of LF/HF ratio in men than in women, consistent with the higher life expectancy and the lower risk for cardiovascular diseases of women. This difference might explain why not all previous studies found an age-related decrease in HRV. Age has been described to be negatively associated with frequency domain measures,17–21 which represent both vagal and sympathetic activity. Some authors19 found this association only in men. Other authors found a negative association with frequency domain variables in the female sex,17,18,22 whereas Stein et al.19 describe this association only in subgroups. As stated by Parati and Di Rienzo,14 age and gender should always be taken into account when quantifying HRV.
Inconsistent findings concerning the effect of smoking on HRV have been reported in the literature.23,24 In our study, we found both a strong effect of smoking and an independent association with end expiratory CO levels. Although nicotine is a stimulant of the sympathetic nervous system, we found a significant reduction of LF in current smokers. This suggests that either the toxic effect of other components of tobacco smoke is stronger than the increase in the sympathetic tone expected from nicotine alone, leading to an accelerated ageing of the heart, or the continuous stimulation of the sympathetic nervous system eventually leads to the weakening of the response. In the context of all the risk factors considered, we also found an independent negative effect of alcohol consumption on HRV, which has been shown in other studies.25,26 In contrast, regular exercise has a positive effect on HRV. This has been described by others for both sympathetic and vagal nerve activity.27–30
The influence of BMI on HRV is also controversially discussed in the literature.20,22 We found a small effect of BMI on SDNN, TP, LF, LF/HF ratio, VLF, and ULF. Self-reported diabetes and high blood pressure show weak associations in our study (high blood pressure with TP, LF, VLF, and ULF, diabetes with ULF). Both of these cardiovascular risk factors have also been described as being associated with impaired autonomic function in the literature.23,31
We found that persons taking ACE-inhibitors had a higher TP and those taking antiarrhythmics a higher HF, whereas LF/HF was significantly decreased in the latter group. Diuretics had a statistically significant negative effect on TP, LF, VLF, and ULF. Obviously, there is a risk of the confounding factors of between medication commonly taken by the elderly, specifically ACE-inhibitors, antiarrhythmics, and beta-blockers, and the underlying condition requiring these chronic medications influencing the results. In these cases, it can be difficult to separate the effect of a disease from the effect of the drug used to treat it. However, in our study, we could show that the effect of drug intake was opposite to the one of the underlying disease, suggesting that we looked at the true effect and not at an effect confounded by diagnosis. Yet, in our results, the time domain measure SDNN is decreased by beta-blockers. It is possible that this result captures the effect of beta-blockers on sympathetic drive, whereas frequency domain variables, such as TP, may be more sensitive to subtle and fast changes such as enhancement of parasympathetic activity by beta-blockers. Effects from ACE-inhibitors have been demonstrated in smaller clinical studies: increases in HF32 and LF32,33 and decreases in LF/HF34,35 have been described. It is interesting to note that these effects were confirmed in our relatively healthy cohort. Adrenergic beta-antagonists36–38 and sympathomimetics39,40 have been widely studied, whereas little has been published about the effects of classes I and III antiarrhythmic agents,41 calcium channel blockers,42 or diuretics on frequency domain HRV.39 We can confirm most of these effects in our population-based sample, even after taking all other cardiovascular risk factors into account.
In our study, the laboratory parameters, uric acid and hs-CRP, were consistently and negatively associated with all HRV parameters but not the LF/HF ratio. In the literature, similar effects of hs-CRP have been described in elderly subjects with no apparent heart disease.43 However, the causal pathway of this association is not clear. Inflammation may influence autonomic balance or autonomic imbalance may activate inflammation. No information exists in the literature about the association between uric acid and HRV, but it is known that chronic renal failure is associated with impaired autonomic function and reduced HRV.44,45 Of all the laboratory parameters studied, we found non-HDL cholesterol to have the strongest effect on both HF and LF. We found no evidence for such an association in the literature, whereas studies examining the relation between hypercholesterolaemia and HRV in men without ischaemic heart disease showed non-uniform results.46,47
Thus, our findings confirm many of the results of individual smaller studies on the single or combined effects of drugs, lifestyle, or risk factors of cardiovascular disease on HRV.
From a clinical point of view, a shortcoming of this study is the fact that we examined a random sample of the general population not comparable with cardiac patients. Thus, the meaning of our results in clinical settings still needs to be explored.
In this cross-sectional study, we exclusively looked at people aged ≥50. In the future, additional age groups need to be examined and longitudinal assessments need to be performed, as stated in the report of the Task Force.9
We chose to report SDNN and frequency domain variables. Although the physiological basis for ULF and VLF power are far less clear than that for HF and LF power, they have been shown to be more powerful risk predictors in cardiovascular disease.48
To the best of our knowledge, this is the first study simultaneously able to assess the contribution of a wide range of risk factors on HRV and to provide percentile curves for 24 h HRV for an elderly population. We hope that these data will help the interpretation of HRV data in the general population and the clarification of the role of various factors influencing HRV.
Acknowledgements
The study could not have been done without the help of the study participants, technical and administrative support, and the medical teams and field workers at the local study sites. Local fieldworkers: Aarau: M. Broglie, M. Bünter, D. Gashi; Basel: R. Armbruster, T. Damm, M. Gut, L. Maier, A. Vögelin, L. Walter, Davos: D. Jud: Geneva: M. Ares, M. Bennour, B. Galobardes, E. Namer; Lugano: B. Baumberger, S. Boccia Soldati, E. Gehrig-Van Essen, S. Ronchetto; Montana: C. Bonvin, Payerne: S. Blanc, A.V. Ebinger, M.L. Fragnière, J. Jordan; Wald: N. Kourkoulos, U. Schafroth. Software technicians: S. Baur, P. Frankenbach, D. Burkhardt. Holter system technicians: M. Victoire, D. Maudoux. Mathematical HRV engineering: V. Pichot. Administrative assistants: D. Baehler, N. Bauer, R. Nilly.
Research support was provided by the National Science Foundation of Switzerland (grants no. 32-65896.01 and 32-47BO-104284); the Federal Office for Forest, Environment, and Landscape; the Federal Office of Public Health; the Federal Office of Roads and Transport; the Cantons Basel-Stadt, Basel-Land, Geneva, Zurich, Ticino, Aargau, Luzern; the Swiss Lung League; the Lung League of Ticino, Zurich, and Basel Stadt/Basel Landschaft; and the National Institute of Environmental Health Sciences (US) (grant no. 2 P01 ES009825, DR.G.).
Appendix
SAPALDIA Team
Senior scientific team
Ph. Leuenberger (p) co-dir and U. Ackermann-Liebrich (e) co-dir. J.C. Barthélémy (c), W. Berger (g), R. Bettschart (p), A. Bircher (a), K. Blaser (a), G. Bolognini (p), O. Brändli (p), M. Brutsche (p), L. Burdet (p), S. Downs (e/s), M. Frey (p), J.M. Gaspoz (c), M. Gerbase (p), D. Gold (e/c/p), W. Karrer (p), R. Keller (p), B. Knöpfli (p), N. Künzli (e/exp), A. Morabia (e), U. Neu (exp), L. Nicod (p), A.P. Perruchoud (p), M. Pons (p), N. Probst Hensch (g), Th. Rochat (p), E. Russi (p), C. Schindler (s), P. Schmid-Grendelmeyer (a), J. Schwartz (e), F. Schwarz (p), P. Straehl (exp), J.M. Tschopp (p), A. von Eckardstein (cc), J.P. Zellweger (p), E. Zemp Stutz (e).
Scientific team at coordinating centres
L. Bayer-Oglesby (exp), D. Felber Dietrich (c), M. Imboden (g), D. Keidel (s), S. Downs (e), P. Städele-Kessler (s), M. Gerbase (p).
(a) medicine of allergy, (c) cardiology, (cc) clinical chemistry, (e) epidemiology, (exp) exposure, (g) genetic and molecular biology, (m) meteorology, (p) pneumology, (s) statistics.
Scientific team at local study sites
C. Burrus, D. Felber Dietrich, U. Egermann, M. Gerbase, R. Gimmi, A. Kick, N. Lutz.



