-
PDF
- Split View
-
Views
-
Cite
Cite
Martin Stockburger, Suzanne Fateh-Moghadam, Aischa Nitardy, Holger Langreck, Wilhelm Haverkamp, Rainer Dietz, Optimization of cardiac resynchronization guided by Doppler echocardiography: haemodynamic improvement and intraindividual variability with different pacing configurations and atrioventricular delays, EP Europace, Volume 8, Issue 10, October 2006, Pages 881–886, https://doi.org/10.1093/europace/eul088
Close - Share Icon Share
Abstract
Aims Cardiac resynchronization therapy (CRT) improves symptoms in heart failure patients with intraventricular conduction delay (IVCD). Different pacing modalities produce variable activation patterns and are likely to result in different haemodynamic changes. The objective of this study was to demonstrate acute haemodynamic changes with different CRT configurations.
Methods and results In 26 patients (left ventricular ejection fraction 22.7±6.1%, QRS 176±29 ms, New York Heart Association III/IV 18/8), a CRT device was implanted. An optimization procedure was performed including left (LVPEI) and right ventricular pre-ejection intervals, interventricular mechanical delay (IVD), left ventricular filling fraction (FTc), and myocardial performance index (MPI) during left and biventricular pacing with three different atrioventricular (AV) delays. An optimal mode and AV delay were defined. LVPEI changed from 166±27 to 139±25 ms, IVD from 49±19 to 6±18 ms, MPI from 0.98±0.25 to 0.62±0.22, and FTc from 0.42±0.08 to 0.51±0.08 (P<0.001 for all comparisons). The variability was 39±20 ms for LVPEI, 55±24 ms for IVD, 0.11±0.07 for FTc, and 0.35±0.18 for MPI.
Conclusion Optimized resynchronization in heart failure patients with IVCD produces marked acute improvement of the altered cardiac cycle timing. The variability of Doppler parameters with different CRT modalities underlines the necessity of individualized settings and suggests that the patients' benefit may be jeopardized without optimization.
Introduction
Cardiac resynchronization therapy (CRT) has been shown to improve haemodynamics1,2 and the functional status3–5 in most patients with depressed left ventricular ejection fraction (LVEF), intraventricular conduction delay (IVCD), and symptomatic heart failure. The treatment effect has been demonstrated for biventricular pacing as well as for left ventricular pacing.6 But a minority does not benefit from CRT.5 Possible reasons include the absence of ventricular asynchrony despite prolonged QRS duration7 and the subsequent inability to improve intraventricular synchronicity, inadequate left ventricular lead placement,8 but possibly also suboptimal setting of the CRT device.
The timing of the cardiac cycle is altered in left bundle branch block (LBBB). Isovolumic intervals are prolonged and the left ventricular diastolic filling period is shortened. A discordance of right and left ventricular ejection is also present.9 These alterations are reflected in Doppler echocardiographic parameters10 such as left ventricular pre-ejection interval (LVPEI), the interventricular mechanical delay (IVD), the left ventricular filling fraction of the cycle length (FTc), and the myocardial performance index (MPI).
The tissue Doppler technique appears to be the most promising method of asynchrony detection and pre-operative patient selection.11,12 But conventional Doppler echocardiography can be expected to serve as a useful tool for the definition of the best individual CRT setting with regard to atrioventricular (AV) delay and pacing mode (left vs. biventricular pacing). It reflects the partial restoration of cardiac cycle timing, and thus can provide a relatively simple and readily applicable method of haemodynamic optimization.
This study aimed to show the magnitude of the achievable CRT treatment effect with respect to Doppler echocardiography and also the variability of the parameters mentioned earlier with different CRT modalities, i.e. possibly missed benefit.
Methods
Study participants
The study population consisted of 26 consecutive patients with severe systolic left ventricular dysfunction of any aetiology and an angiographic LVEF below 35%, symptomatic heart failure in at least New York Heart Association (NYHA) Class III and LBBB with a QRS duration of above 150 ms.
CRT device implantation
The indication for a CRT pacemaker vs. CRT device with additional cardioverter/defibrillator capabilities (CRT-D) was according to the current pacemaker/ICD guidelines.13 After an initial coronary venogram, lateral veins were selected for lead placement. The implantation was attempted transvenously with an over-the-wire technique in all patients, and the procedure was performed in the catheterization laboratory under local anaesthesia, unless a lateral thoracotomy and epicardial lead placement was necessary.
Doppler echocardiographic follow-up
During the first 3 postoperative days, the Doppler echocardiographic optimization procedure was performed by two observers (MS, SF-M) with the patient at rest in the left lateral supine position. A General Electric Medical Systems™ (Chalfont St. Giles, UK) Vingmed System 5 or Vivid 7 Pro echocardiography machine with a 2.5 MHz sector transducer was used for all examinations. The pulsed wave Doppler spectra from the mitral, aortic, and pulmonary valve were analysed at a horizontal speed of 100 mm/s. In each modality, three values for all of the following parameters were obtained and averaged. The left ventricular ejection time (LVET) was measured from the transaortic flow signal and the LVPEI was assessed between the QRS onset and the beginning of the transaortic ejection signal. The right ventricular pre-ejection interval (RVPEI) was measured from the QRS onset to the beginning of the transpulmonary ejection signal. The difference of LVPEI and RVPEI is referred to as the IVD. Positive IVD values indicate delayed left ventricular ejection and negative values delayed right ventricular ejection. FTc as the left ventricular filling fraction of the cycle length and the interval between an A wave and the subsequent E wave (AET) were obtained from the transmitral left ventricular inflow signal. The absence of truncated A waves with short AV delays was verified. The cycle length correction for the filling time was chosen, because a close linear relationship of heart rate and filling time is well known and was also reproduced in our patients (r=−0.78, r2=0.60, P<0.001). The MPI was obtained from the transmitral inflow spectrum and the transaortic left ventricular ejection time (LVET) following the formula MPI=(AET−LVET)/LVET. Figure 1 explains the performed Doppler measurements and Figure 2 shows an example of the measurements without CRT and during optimized CRT. All measurements were made without resynchronization and with left and biventricular pacing. In 24 patients, ventricular pacing could be triggered on the intrinsic sinus rhythm (VDD mode). In these patients, three AV delays were tested (long, 150 ms; intermediate, 120 ms; short, 90 ms). The intermediate AV delay (120 ms after a sensed atrial event) and biventricular pacing corresponded to the devices' standard setting. The two patients with sinus bradycardia were paced in the atria and ventricle(s) (DDD mode). In these patients, 20 and 40 ms, respectively, were added to the three AV delays after initial analysis of the transmitral Doppler spectrum. The devices' standard AV delay after a paced atrial event was 150 ms. Ventricular pacing was ensured with all AV delays. The pacing mode (i.e. left or biventricular pacing) and AV delay with most of the four parameters at an optimal level was considered the optimal CRT configuration. The variability of the parameters mentioned before with all different CRT modes and AV delays was assessed and expressed as absolute values and percentage of the baseline values.
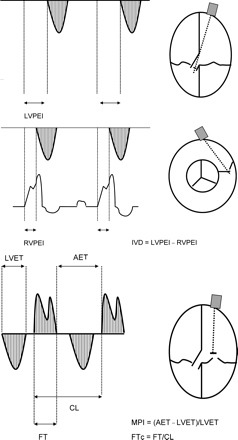
Assessment of LVPEI, RVPEI, IVD, FTc, and MPI from the ECG, the transaortic, the transpulmonary, and transmitral pulsed wave Doppler signals in the apical four-chamber view and the parasternal short axis view, respectively. CL, cycle length; ECG, electrocardiogram; AET, time between two diastolic filling periods.
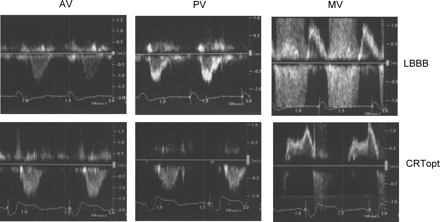
Pulsed wave Doppler tracings of transaortic (AV), transpulmonary (PV), and transmitral (MV) flow from one patient during intrinsic LBBB and during optimized CRT. Optimal mode in this case: biventricular pacing with 90 ms sensed AV delay. Reduction in LVPEI (195–153 ms), IVD (73 ms to −11 ms), and MPI (0.53–0.48), and prolongation of FTc (0.48–0.51), and RVPEI (122–164 ms). Note also the reduced mitral regurgitation signal with CRTopt. CRTopt, cardiac resynchronization therapy with optimal settings.
Statistical analysis
Categorical variables are reported as absolute numbers and percentages. Continuous variables are presented as means±SD. Paired and unpaired Student's t-tests were used for comparisons between groups and between baseline values and the optimal CRT mode as appropriate. The relationship between MPI and baseline LVEF was assessed by univariate linear regression analysis. Multivariate analysis was performed with the changes in Doppler parameters as dependent variables. A P-value <0.05 was considered statistically significant.
Results
Study population characteristics
Demographic data of the study population are outlined in Table 1. The patients represent a severely compromised heart failure cohort. The majority had coronary artery disease as the aetiology for LV dysfunction. The average ejection fraction was severely depressed, marked IVCD was present and almost one-third of the patients were in NYHA class IV.
| Age (years) | 64±10 |
| Men (%) | 21 (81) |
| Prior myocardial infarction (%) | 14 (54) |
| Non-ischaemic cardiomyopathy (%) | 9 (34) |
| Prior aortic valve replacement for stenosis (%) | 3 (12) |
| Diabetes mellitus (%) | 9 (34) |
| Systemic hypertension (%) | 12 (46) |
| LVEF (%) | 23±6 |
| QRS width (ms) | 176±29 |
| NYHA Class III/IV (%) | 18 (69)/8 (31) |
| Age (years) | 64±10 |
| Men (%) | 21 (81) |
| Prior myocardial infarction (%) | 14 (54) |
| Non-ischaemic cardiomyopathy (%) | 9 (34) |
| Prior aortic valve replacement for stenosis (%) | 3 (12) |
| Diabetes mellitus (%) | 9 (34) |
| Systemic hypertension (%) | 12 (46) |
| LVEF (%) | 23±6 |
| QRS width (ms) | 176±29 |
| NYHA Class III/IV (%) | 18 (69)/8 (31) |
| Age (years) | 64±10 |
| Men (%) | 21 (81) |
| Prior myocardial infarction (%) | 14 (54) |
| Non-ischaemic cardiomyopathy (%) | 9 (34) |
| Prior aortic valve replacement for stenosis (%) | 3 (12) |
| Diabetes mellitus (%) | 9 (34) |
| Systemic hypertension (%) | 12 (46) |
| LVEF (%) | 23±6 |
| QRS width (ms) | 176±29 |
| NYHA Class III/IV (%) | 18 (69)/8 (31) |
| Age (years) | 64±10 |
| Men (%) | 21 (81) |
| Prior myocardial infarction (%) | 14 (54) |
| Non-ischaemic cardiomyopathy (%) | 9 (34) |
| Prior aortic valve replacement for stenosis (%) | 3 (12) |
| Diabetes mellitus (%) | 9 (34) |
| Systemic hypertension (%) | 12 (46) |
| LVEF (%) | 23±6 |
| QRS width (ms) | 176±29 |
| NYHA Class III/IV (%) | 18 (69)/8 (31) |
CRT device implantation
All patients successfully received a CRT device. An atriobiventricular pacemaker was used in three patients and the remainder were treated with a CRT-D device. The primary preventive defibrillator option was not preferred by two patients with coronary artery disease. In 25 patients, left ventricular lead placement was achieved via lateral coronary sinus tributaries, whereas in one patient a rigid ostial coronary venous valve made the transluminal approach impossible and an epicardial lead was placed at the left ventricular lateral wall after minithoracotomy.
Doppler echocardiographic optimization
The MPI at baseline was modestly but significantly correlated with the initial angiographic LVEF (r=−0.46, r2=0.21, P=0.019). Values for LVPEI, IVD, FT, FTc, and MPI at baseline and with optimized CRT and the respective variability with different CRT modalities are presented in Table 2 and Figure 3. All variables referring to left ventricular haemodynamics were clearly and significantly improved. RVPEI was significantly but slightly prolonged. The variability with different CRT modes and AV delays exceeded the treatment effect for LVPEI, IVD, and FT and approached the achievable improvement for the MPI. CRT with standard settings resulted also in significantly more favourable Doppler parameters in comparison with baseline values. But the improvement of the parameters was larger with optimized CRT. The optimization produced significantly lower MPI (0.62 vs. 0.80, P<0.01) and longer FTc (0.51 vs. 0.47, P<0.01) compared with standard settings. The additional shortenings of LVPEI (139 vs. 141 ms) and IVD (3 vs. 6 ms) were less pronounced and did not reach statistical significance. The resulting Doppler measurements for standard CRT settings are included in Table 2 and Figure 3.
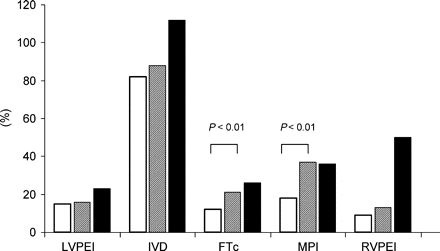
Relative improvement in the Doppler parameters vs. baseline values with standard settings (open bars) and with optimized CRT (hatched bars). The solid bars indicate the relative variability of the parameters during optimization.
| Variable . | Baseline . | Standard settings . | Optimized CRT . | Difference baseline vs. optimized CRT (%) . | Variability (%) . |
|---|---|---|---|---|---|
| LVPEI (ms) | 166±27 | 141±24* | 139±25* | 27 (16) | 39±20 (23) |
| IVD (ms) | 49±19 | 9±32* | 6±18* | 43 (88) | 55±24 (112) |
| FTc | 0.42±0.08 | 0.47±0.07* | 0.51±0.08** | 0.09 (21) | 0.11±0.07 (26) |
| MPI | 0.98±0.25 | 0.80±0.35*** | 0.62±0.22** | 0.36 (37) | 0.35±0.18 (36) |
| RVPEI (ms) | 117±29 | 128±31 | 132±26*** | 15 (13) | 59±31 (50) |
| Variable . | Baseline . | Standard settings . | Optimized CRT . | Difference baseline vs. optimized CRT (%) . | Variability (%) . |
|---|---|---|---|---|---|
| LVPEI (ms) | 166±27 | 141±24* | 139±25* | 27 (16) | 39±20 (23) |
| IVD (ms) | 49±19 | 9±32* | 6±18* | 43 (88) | 55±24 (112) |
| FTc | 0.42±0.08 | 0.47±0.07* | 0.51±0.08** | 0.09 (21) | 0.11±0.07 (26) |
| MPI | 0.98±0.25 | 0.80±0.35*** | 0.62±0.22** | 0.36 (37) | 0.35±0.18 (36) |
| RVPEI (ms) | 117±29 | 128±31 | 132±26*** | 15 (13) | 59±31 (50) |
*P<0.001 vs. baseline.
**P<0.001 vs. baseline and <0.01 vs. standard settings.
***P<0.05 vs. baseline.
| Variable . | Baseline . | Standard settings . | Optimized CRT . | Difference baseline vs. optimized CRT (%) . | Variability (%) . |
|---|---|---|---|---|---|
| LVPEI (ms) | 166±27 | 141±24* | 139±25* | 27 (16) | 39±20 (23) |
| IVD (ms) | 49±19 | 9±32* | 6±18* | 43 (88) | 55±24 (112) |
| FTc | 0.42±0.08 | 0.47±0.07* | 0.51±0.08** | 0.09 (21) | 0.11±0.07 (26) |
| MPI | 0.98±0.25 | 0.80±0.35*** | 0.62±0.22** | 0.36 (37) | 0.35±0.18 (36) |
| RVPEI (ms) | 117±29 | 128±31 | 132±26*** | 15 (13) | 59±31 (50) |
| Variable . | Baseline . | Standard settings . | Optimized CRT . | Difference baseline vs. optimized CRT (%) . | Variability (%) . |
|---|---|---|---|---|---|
| LVPEI (ms) | 166±27 | 141±24* | 139±25* | 27 (16) | 39±20 (23) |
| IVD (ms) | 49±19 | 9±32* | 6±18* | 43 (88) | 55±24 (112) |
| FTc | 0.42±0.08 | 0.47±0.07* | 0.51±0.08** | 0.09 (21) | 0.11±0.07 (26) |
| MPI | 0.98±0.25 | 0.80±0.35*** | 0.62±0.22** | 0.36 (37) | 0.35±0.18 (36) |
| RVPEI (ms) | 117±29 | 128±31 | 132±26*** | 15 (13) | 59±31 (50) |
*P<0.001 vs. baseline.
**P<0.001 vs. baseline and <0.01 vs. standard settings.
***P<0.05 vs. baseline.
The intraobserver and interobserver variability during conventional Doppler measurements have been reported to be 6–9%,10 which is far below the variability with different pacing modalities.
By multivariate analysis including underlying heart disease, QRS duration, LVEF, NYHA class, and baseline values of the Doppler variables, NYHA class was predictive for the achievable MPI improvement with larger acute improvement for the class IV (class IV-0.60 vs. class III-0.25; P=0.024) patients. LVPEI at baseline was linked with MPI (P=0.021) and LVPEI (P=0.020) improvement with enhanced benefit for higher baseline values, a higher IVD at baseline was linked with larger IVD improvement (P<0.001) and shorter FTc baseline values were linked with larger obtained FTc prolongation (P=0.04). Worse MPI values at baseline were linked with a larger MPI reduction in optimized CRT (P=0.017). These findings indicate that larger acute improvements can be achieved in patients whose cardiac cycle is severely impaired and in patients with more pronounced heart failure symptoms. No influence of the underlying heart disease, ejection fraction, or QRS width on the acute CRT benefit could be identified.
Long-term echocardiographic follow-up was not pre-specified in the study design, but in 18/26 patients long-term data on the left ventricular end diastolic diameter (LVEDD) are available. After a mean follow-up duration of 21 months on the optimized pacing configuration, the average LVEDD decreased significantly from 68±8 to 60±9 mm (P<0.001).
Distribution of optimal CRT modes and AV delays
The distribution of optimal CRT modes and AV delays is given in Table 3, showing a clear tendency towards biventricular pacing and intermediate or short AV delays as an optimal configuration in most patients. But some patients appear to benefit more from LV pacing or from longer AV delays. The five patients who benefited most from LV only pacing improved also with biventricular pacing with respect to LVPEI, FTc, and IVD, but to a lesser extent. With respect to the MPI, no acute improvement could be demonstrated with biventricular pacing (MPI without CRT 0.94; with biventricular pacing 0.93; with LV only pacing 0.67). In five patients (19%), the optimal setting corresponded to the standard device setting.
| Biventricular pacing, n (%) | 21 (81) |
| Left ventricular pacing, n (%) | 5 (19) |
| Long AV delay, n (%) | 3 (12) |
| Intermediate AV delay, n (%) | 10 (38) |
| Short AV delay, n (%) | 13 (50) |
| Biventricular pacing, n (%) | 21 (81) |
| Left ventricular pacing, n (%) | 5 (19) |
| Long AV delay, n (%) | 3 (12) |
| Intermediate AV delay, n (%) | 10 (38) |
| Short AV delay, n (%) | 13 (50) |
| Biventricular pacing, n (%) | 21 (81) |
| Left ventricular pacing, n (%) | 5 (19) |
| Long AV delay, n (%) | 3 (12) |
| Intermediate AV delay, n (%) | 10 (38) |
| Short AV delay, n (%) | 13 (50) |
| Biventricular pacing, n (%) | 21 (81) |
| Left ventricular pacing, n (%) | 5 (19) |
| Long AV delay, n (%) | 3 (12) |
| Intermediate AV delay, n (%) | 10 (38) |
| Short AV delay, n (%) | 13 (50) |
Discussion
Given the wide variety of ventricular activation patterns with different resynchronization modes (i.e. left ventricular and biventricular pacing) and AV delays leading to variable degrees of fusion between pacing sites and intrinsic activation, considerable haemodynamic changes are to be expected with different device settings. A simple method for optimization based on the pathophysiology of heart failure and ventricular asynchrony is required. Invasive methods have been suggested using left ventricular dP/dt and arterial pulse pressure as target parameters.8,14 The disadvantages of invasive optimization following dP/dt and pulse pressure include time and resource consumption and lack of repeatability.
Doppler parameters as optimization tools
Doppler echocardiography has earlier been proposed for initial CRT optimization,4 but without giving further details on how to apply it. Breithard et al.10 performed a Doppler-guided analysis of CRT effects with a similar approach, but only 4 weeks after implantation, so that an influence of early remodelling processes could not be ruled out with certainty.
The MPI reflects systole as well as diastole and is well known to be linked with the clinical severity of heart failure and with haemodynamic parameters such as positive and negative peak left ventricular dP/dt.15,16 It does not depend on heart rate.17 The significant, although, modest negative linear correlation of MPI and LVEF was reproduced in our patient cohort confirming the clinical usefulness of this parameter. The pre-ejection intervals reflect the electromechanical delay and isovolumic contraction time of the ventricles, which is inversely correlated with dP/dt.16 The LVPEI has previously been described as a predictor of the CRT treatment effect in a preliminary report from the MIRACLE study.18 The IVD reflects the mismatch between right and left ventricular contraction, which is known to be present in many LBBB patients. The IVD has also been identified as a predictor of the CRT treatment effect by some investigators19 and was included in patient's selection in the CARE-HF trial.20 QRS morphology and ventricular activation time change with different activation patterns during different ventricular pacing configurations and AV delays. Haemodynamic improvement is not necessarily linked with a shorter QRS. Thus, the changing QRS duration may have a confounding influence on the changes of LVPEI with CRT vs. baseline. The calculation of IVD as the difference of LVPEI and RVPEI eliminates the influence of the variation in QRS duration with baseline values.
Impaired and shortened left ventricular filling is a major detrimental consequence of LBBB9 and LV asynchrony. Hence, prolongation of FTc by CRT appears to be a reasonable goal during haemodynamic optimization.
Acute improvement by CRT
The present study showed that a clear and significant acute improvement in the altered cardiac cycle can be achieved by optimized cardiac resynchronization. The magnitude of the effect was almost equal to that described by Breithard et al.10 The best setting for each individual patient was expectedly non-uniform, although the distribution of pacing modes being identified as optimal by the suggested algorithm revealed intermediate or short AV delays and biventricular pacing as the best configuration for the majority of patients. The result regarding the AV delays is also in line with previous data, but our findings favouring biventricular over LV pacing in >80% of patients differ from earlier statements, where the two pacing configurations were found to be equivalent.10 In the present study, we still identified a considerable subgroup of patients in whom LV pacing combined with intrinsic activation was superior to biventricular pacing. In addition, despite the general statement that intermediate or short AV delays are optimal in the majority, in some patients long AV delays are appropriate. Only in about one-fifth of the patients did the optimal configuration correspond to standard device settings. CRT with unchanged standard settings in all patients would have produced significantly improved ventricular filling and ejection, but the optimization procedure resulted in significant further reduction in MPI and prolongation of FTc.
The also significant, but modest prolongation of the RVPEI by left and biventricular pacing should stimulate further long-term characterization of right ventricular function in CRT patients in order not to overlook possible adverse effects of right ventricular asynchrony.
Variability of Doppler parameters
The variability of Doppler parameters with different configurations exceeded or at least approached the optimal treatment effect. With some settings haemodynamics were even worsened. The findings have important clinical implications. They underline the advantage of an individually adapted setting and indicate that the CRT treatment benefit might be jeopardized, if an optimization process is not performed.
Predictors of the acute benefit from CRT
NYHA Class IV and a higher extent of initial impairment of LV filling and ejection were linked with a larger acute benefit from optimized CRT. Despite this finding, we would not advocate to base patient selection solely on the presence of a prolonged LVPEI, wide IVD, shortened FTc, or increased MPI. Impaired filling and ejection are to be considered a consequence of ventricular asynchrony,21,22 but ventricular asynchrony is not the unalterable condition for impaired filling and ejection. The increasing knowledge of patient selection11,12 rather points to tissue Doppler analysis as a more direct means for the assessment of asynchrony. On the other hand, a relevant symptomatic benefit can hardly be expected in a patient with LV asynchrony without an altered cardiac cycle. In this light, Doppler parameters can play a helpful additional role for patient selection. Doppler analysis can and should serve as the first line optimization tool that is easily applicable, reflects impaired filling and ejection of the asynchronous ventricle, and helps to achieve the optimal benefit for the patient.
Study limitations
The study only examined acute changes in the Doppler parameters, which can be obtained in the context of post-operative optimization. The acute functional changes do not necessarily correlate with chronic improvement of the patients' functional status. Different AV delays for the left and right ventricles have not been examined in order to limit the complexity of the procedure. In patients who lack CRT response, despite conventional optimization, right–left ventricular delays should be assessed as adjustment may provide additional benefit.23
References
- left ventricular ejection fraction
- hemodynamics
- doppler echocardiography
- heart failure
- left ventricle
- follicular thyroid carcinoma
- heart ventricle
- united states federal trade commission
- incomplete bundle branch block
- heart
- cardiac resynchronization therapy
- emtricitabine
- biventricular pacing devices
- cardiac cycle
- medical devices
- myocardial performance index



