-
PDF
- Split View
-
Views
-
Cite
Cite
José Luis Merino, Aiming for the STARs: radiotherapy for ventricular tachycardia—bright future or cosmic gamble?, EP Europace, Volume 27, Issue 1, January 2025, euae306, https://doi.org/10.1093/europace/euae306
Close - Share Icon Share
This editorial refers to ‘Stereotactic cardiac radiotherapy for refractory ventricular tachycardia in structural heart disease patients: a systematic review’ by A. Gupta et al., https://doi.org/10.1093/europace/euae305.
Ventricular tachycardia (VT) is a life-threatening arrhythmia that can lead to haemodynamic compromise, heart failure, and sudden death. While implantable cardioverter defibrillators (ICDs) effectively terminate VT episodes, frequent ICD shocks are painful and may adversely affect long-term outcomes. Antiarrhythmic drugs help reduce VT burden but have limited efficacy and considerable side effects. Catheter ablation can offer a more definitive approach, yet the VT substrate often lies in mid-myocardial or epicardial regions, making it technically challenging and sometimes requiring epicardial access. Prior cardiac surgeries may result in pericardial adhesions that further complicate the procedure. Additionally, VT ablation carries a significant procedural risk, with major complications reported in up to 6.3%.1
A non-invasive therapy that can reduce VT burden without the risks of invasive procedures would be highly attractive. In 2015, Loo et al.2 introduced the concept of stereotactic arrhythmia radioablation (STAR), and Cuculich et al.3 popularized it in 2017. Yet, despite promising early results, STAR has not gained widespread adoption. Published data largely consist of case reports and small series.
In this issue of the journal, Gupta et al.4 present a meta-analysis summarizing much of the existing STAR experience. Given that VT remains difficult to treat and that reducing ICD shocks is always desirable, this contribution merits attention. However, before embracing a new therapy, it is crucial to critically examine the evidence regarding its efficacy, safety, and applicability (Figure 1).
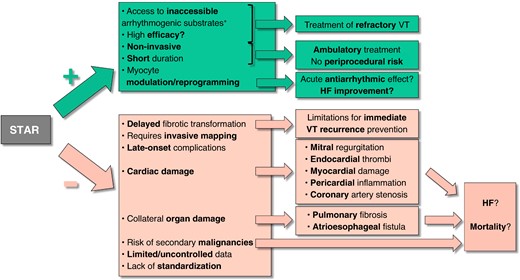
Positive (+) and negative (−) characteristics, potential effects, and consequences of stereotactic arrhythmia radioablation (STAR). HF, heart failure; VT, ventricular tachycardia. *Includes intramural VT substrates, contraindications for endocardial ablation (e.g. LV thrombi), or challenges to endocardial or epicardial ablation, such as valvular prostheses or pericardial adhesions.
Efficacy considerations
The authors report a 10-fold reduction in VT episodes, a seemingly robust finding. However, several caveats need mentioning. VT often occurs in clusters, with periods of frequent activity followed by long quiescent intervals. Previous data show that after an initial VT storm, only about half of patients have a second storm, and 20% of these occur more than a year after the first event.5 This natural fluctuation can create the illusion of therapeutic success if follow-up coincides with a natural ‘cooling off’ period.
In this meta-analysis, a three-month blanking period was employed—VT recurrences during this window were not counted, potentially skewing results. Such an approach may introduce regression to the mean, comparing a peak VT episode period (before therapy) to a naturally quieter phase (after therapy), and thus overestimating efficacy. The three-month blanking also shortened effective follow-up from 13.3 to 10.3 months, complicating comparisons to catheter ablation studies, which rarely use blanking periods. Moreover, 18 patients died during the blanking period, potentially removing patients with a high propensity for VT from later analysis. While the rationale behind a blanking period is the presumed need for STAR-induced fibrotic changes to mature, recent studies suggesting acute changes6 and a consensus statement from the European Heart Rhythm Association (EHRA) and the Heart Rhythm Society (HRS) discourage the routine use of a blanking period for STAR.7
Safety concerns
Safety is paramount, especially for a therapy that may offer incremental benefits. Several red flags emerge.
High mortality and heart failure
The meta-analysis reports a 43% mortality rate at two years. Although patients had advanced heart disease, this figure is substantially higher than those seen in other VT populations, typically under 10% at two years.8 This raises the possibility that radiation may contribute to progressive myocardial or collateral damage. The targeted areas averaged ∼3.5 of 17 left ventricle segments—around 20% of the left ventricle mass—potentially compromising a substantial portion of myocardium. Some studies suggest no significant myocardial damage as left ventricular ejection fraction (LVEF) was slightly improved by 1.5%, and others propose a cardiomyocyte ‘conditioning’ phenomenon.6,9 However, transient reduction in LVEF due to VT episodes or ICD shocks may recover once these episodes abate, potentially masking subtle radiation damage. Longer follow-up is needed to clarify STAR’s net effect on cardiac function and mortality.
Atrioesophageal fistula
Two cases of atrioesophageal or pericardioesophageal fistula were reported. Such a complication is essentially unknown in VT catheter ablation and is extremely rare (0.016–0.1%) in atrial fibrillation ablation.10 The latency of these complications—9 months to 2 years post-STAR—is particularly concerning. Radioablation may cause late-appearing tissue injury not captured by short-term studies.
Mitral regurgitation and other complications
Mitral regurgitation progression occurred in 4.4% of patients—rarely heard of in VT ablation and far more frequent than in AF ablation. Such changes likely reflect radiation-induced fibrotic remodelling of the mitral apparatus, potentially worsening heart failure. Other acute injuries (pneumonitis, pericarditis) and late complications (fibrosis, vascular damage, potential malignancies) may also emerge over time. With a mean follow-up of around one year, the current data may underestimate long-term risks. The future incidence of secondary malignancies, while less critical in patients with poor prognosis, should not be dismissed if STAR use expands to broader populations.
Applicability and adoption challenges
Despite early enthusiasm, fewer than 300 STAR cases have been reported worldwide. Reasons may include as follows.
Mapping limitations
Stereotactic arrhythmia radioablation ideally requires precise arrhythmia localization to target the critical VT isthmus without harming surrounding tissue. While non-invasive mapping technology has improved, it still cannot consistently match the precision of invasive mapping.11 Often, failure of catheter ablation stems from incomplete substrate characterization rather than technical inaccessibility.12 This limitation reduces STAR’s utility to a ‘bailout’ after ablation failure due to the latter.
Procedural complexity
Although non-invasive in delivery, STAR often requires at least some form of mapping—be it non-invasive body surface mapping or data from previous invasive procedures—to identify the target. Thus, the theoretical advantage of a completely non-invasive therapy is limited in practice.
Future directions
Given the current evidence, STAR remains experimental and best reserved for carefully selected cases where other therapies have failed or are not feasible.13 Controlled studies with longer follow-up, such as the ongoing RADIATE-VT trial, are needed to better understand the long-term safety profile and to determine which patients may benefit most.
While STAR brings exciting possibilities as a novel, non-invasive modality for treating VT, we must acknowledge the limitations of the current evidence. The potential for significant and sometimes delayed complications requires prolonged surveillance, and the true efficacy remains uncertain due to methodological factors like blanking periods and regression to the mean. As our understanding evolves, careful, evidence-based patient selection and long-term follow-up will be essential before STAR can earn its place alongside established therapies.
Funding
This work was funded in part by a research grant from the Instituto de Salud Carlos III (Clinical Research Project PI22/00278, 2022).
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of Europace or of the European Society of Cardiology.
Conflict of interest: J.L.M. has received fees and honoraria for lectures, education, and scientific advice from Abbott, Biosense-Webster, Biotronik, iRhythm Technologies, Microport, and Zoll.



