-
PDF
- Split View
-
Views
-
Cite
Cite
Jinhee Ahn, Dong Geum Shin, Sang-Jin Han, Hong Euy Lim, Safety and efficacy of intracardiac echocardiography–guided zero-fluoroscopic cryoballoon ablation for atrial fibrillation: a prospective randomized controlled trial, EP Europace, Volume 25, Issue 5, May 2023, euad086, https://doi.org/10.1093/europace/euad086
Close - Share Icon Share
Abstract
The development of intracardiac echocardiography (ICE) has enabled fluoroless atrial fibrillation (AF) ablation using three-dimensional electroanatomical mapping systems. However, fluoroless cryoballoon ablation (CBA) remains challenging, mainly because of the lack of a visual mapping system. Hence, this study aimed to investigate the safety and efficacy of fluoroless CBA for AF under ICE guidance.
Patients (n = 100) who underwent CBA for paroxysmal AF were randomly assigned to zero-fluoroscopic (Zero-X) and conventional groups. Intracardiac echocardiography was used to guide the transseptal puncture and catheter and balloon manipulation in all enrolled patients. The patients were prospectively followed for 12 months after CBA. The mean age was 60.4 years, and the left atrial (LA) size was 39.4 mm. Pulmonary vein isolation (PVI) was achieved in all patients. In the Zero-X group, fluoroscopy was used in only one patient because of unstable phrenic nerve capture during right-sided PVI. The procedure time and LA indwelling time in the Zero-X group were not statistically different compared with that in the conventional group. Fluoroscopic time (9.0 vs. 0.008 min) and radiation exposure (29.4 vs. 0.02 mGy) were significantly shorter in the Zero-X group than in the conventional group (P < 0.001). The complication rate did not differ between the two groups. During a mean follow-up of 663.3 ± 172.3 days, the recurrence rate was similar (16.0 vs. 18.0%; P = 0.841) between the groups. Multivariate analysis revealed that LA size was the only independent predictor of clinical recurrence.
Intracardiac echocardiography–guided fluoroless CBA for AF was a feasible strategy without compromising acute and long-term success or complication rates.
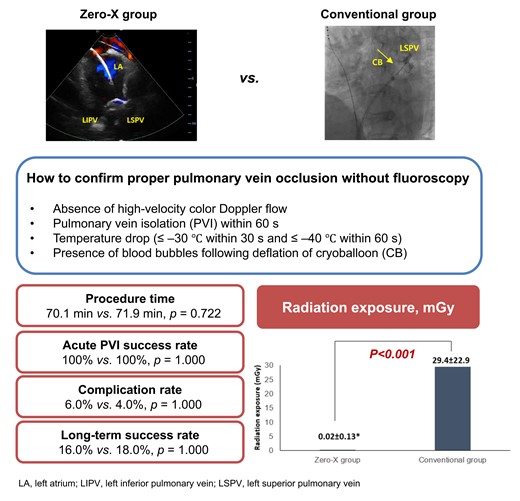
This randomized controlled trial presents the safety and efficacy of intracardiac echocardiography (ICE)–guided zero-fluoroscopic cryoballoon ablation (CBA) in patients with paroxysmal atrial fibrillation.
Fluoroless CBA using ICE alone is a feasible strategy with similar acute and long-term success and complication rates compared with conventional CBA.
This strategy is particularly beneficial for patients with impaired renal function because it does not use contrast agents to confirm adequate pulmonary vein occlusion.
Introduction
Catheter ablation (CA) is a well-established procedure for managing symptomatic atrial fibrillation (AF).1 Since the procedure is traditionally conducted under fluoroscopy guidance, radiation exposure is always a concern for operators and patients.2 Efforts have been made to reduce fluoroscopic time to ‘as low as reasonably achievable’, as recommended by the American College of Cardiology with the ALARA statement.3 Consequently, fluoroless or near-fluoroless radiofrequency (RF) CA for AF has been enabled using a three-dimensional (3D) electroanatomical mapping system, transoesophageal echocardiography (TEE), or intracardiac echocardiography (ICE).4–6
Cryoballoon ablation (CBA) is a single-shot ablation technique that has replaced RFCA with similar anticipated outcomes, especially for paroxysmal AF targeting pulmonary vein (PV) isolation.7–9 During CBA, proper occlusion of each PV is traditionally confirmed by fluoroscopic imaging with contrast injection. Therefore, a considerable limitation of CBA compared with RFCA might be larger radiation exposure. Although recent studies have reported a reduction in radiation with TEE, ICE, or pressure-guided cryoablation,10–12 fluoroless CBA remains challenging because of the lack of visual 3D virtual cardiac chambers.
Hence, this study aims to (i) investigate the feasibility of fluoroless CBA under the guidance of ICE in patients with symptomatic paroxysmal AF and (ii) compare the safety and efficacy of fluoroless CBA with conventional CBA using fluoroscopy.
Methods
Study participants
This prospective randomized controlled trial (RCT) recruited 100 consecutive patients undergoing de novo CBA for symptomatic paroxysmal AF between January 2020 and July 2021. The exclusion criteria were (i) age <18 years, (ii) history of AF ablation or cardiac surgery, (iii) contraindication to oral anticoagulants (OACs), (iv) presence of intracardiac thrombus, or (v) unstable coronary artery disease.
All participants received uninterrupted OAC therapy for at least 3 weeks before and 8 weeks after the procedure. Central randomization at a 1:1 ratio using computer-generated random permutation sequences assigned the patients to either the zero-fluoroscopic (Zero-X) or conventional group on the day of the procedure. Written informed consent was obtained from all the enrolled participants. The study protocol was approved by the institutional review board of the participating institutions and complied with the Declaration of Helsinki. The procedure was conducted in accordance with the ethical standards of our research committee. The study was registered at ClinicalTrials.gov (unique identifier: NCT05711589).
Zero-fluoroscopic cryoballoon ablation
The workflow for CBA has been previously described.13 In brief, under deep sedation with a continuous infusion of propofol and intermittent bolus of fentanyl,14 the right femoral vein was percutaneously accessed using the Seldinger technique. A single transseptal puncture was performed under the guidance of ICE (ViewFlex™; Abbott, Inc., USA). Then, a 28 mm cryoballoon (CB) catheter (Arctic Front Advance™; Medtronic, Inc., USA) assembled with an intraluminal mapping catheter (Achieve™; Medtronic, Inc., USA) was gently advanced into the left atrial (LA) cavity through a 15-Fr steerable sheath (FlexCath™; Medtronic, Inc., USA). The best-fit occlusion of the inflated CB in each PV antrum was confirmed by colour Doppler ICE imaging (Figure 1A–D and see Supplementary material online, Video S1A–D). Then, cryoenergy was delivered depending on time-to-isolation (TTI) of PV for 180 s if TTI was shorter than 30 s; otherwise, for 240 s. Diaphragmatic stimulation with continuous pacing of the ipsilateral phrenic nerve was performed during the right-sided CBA to avoid phrenic nerve paralysis. Four indicators defined proper PV occlusion in the Zero-X group (see Supplementary material online, Video S2): (i) TTI within 60 s after freezing if PV potentials were observed, (ii) temperature drop of ≤−30°C within 30 s and ≤−40°C within 60 s after freezing, (iii) absence of high-velocity colour Doppler flow around the CB after freezing, and (iv) presence of blood bubbles following deflation of CB after thawing (Figure 1E–H and see Supplementary material online, Video S1E–H). If TTI was not achieved within 60 s or the temperature did not reach −40°C within 60 s, freezing was stopped, and the CB catheter was repositioned. If any remaining PV potentials and any atrial capture by a high-output pacing inside the PVs were observed, additional attempt with the same strategy was performed to achieve complete PV isolation (PVI). After PVI, additional freezing was applied for 150 s at a more proximal antrum than the previous CBA site, using the segmental non-occlusive technique, to create a wide antral circumferential ablation.
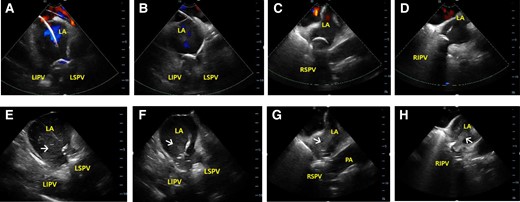
Intracardiac echocardiographic images. Colour Doppler images during pulmonary vein (PV) occlusion and blood bubbles (arrows) from PV after deflation of cryoballoon at the LSPV (A and E), LIPV (B and F), RSPV (C and G), and RIPV (D and H). LA, left atrial; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PA, pulmonary artery; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Linear ablation for the cavotricuspid isthmus (CTI) was performed in cases with previously documented or induced atrial flutter (AFL). Fluoroscopy was used during CTI ablation in the Zero-X group because a 3D electroanatomical mapping system was not used. If AF was sustained after completing the CBA lesion set, direct-current cardioversion was performed to restore sinus rhythm.
The procedure in the conventional group followed the same protocol as that in the Zero-X group, except for the use of fluoroscopy during PV occlusion with an inflated CB. A frame rate of 3.5 frame/s and low-dose energy levels per frame were applied during the procedure.
Femoral venous haemostasis was achieved using a fisherman’s knot suture closure after sheath removal without protamine injection. Complete haemostasis was confirmed after 8 h of immobilization, and then OAC was administered at the scheduled time on the day of the procedure.
Study follow-up
Antiarrhythmic drug therapy was maintained for a 3-month blanking period following CBA unless any adverse effects were observed. Clinical follow-up was scheduled at 1, 3, 6, 9, and 12 months after the procedure, and every 6 months after that. Twelve-lead electrocardiography (ECG) at each visit or whenever a patient presented with palpitations, and 72 h Holter monitoring at 3, 6, and 12 months were performed. Any atrial tachyarrhythmias lasting at least 30 s documented on ECG after the initial 3-month blanking period was defined as clinical recurrence.
Statistical analysis
Continuous variables were assessed for normality and expressed as mean ± standard deviation or median (interquartile interval), as appropriate. Categorical variables were presented as numbers with proportions. The Student’s t-test or Mann–Whitney U test for continuous variables and Pearson’s χ2 or Fisher’s exact test for categorical variables were applied as appropriate for intergroup comparisons. Kaplan–Meier (K-M) curves were used to analyse the recurrence-free survival rate during the follow-up period. Both univariate and multivariate Cox regression analyses were used to identify the independent predictors of clinical recurrence. All statistical analyses were performed using SPSS (version 23.0; SPSS Inc., Chicago, IL, USA), and P-values <0.05 were considered statistically significant.
Results
Patient characteristics
Table 1 presents the baseline characteristics of the enrolled participants. The mean age was 60.4 ± 10.1 years, and the LA diameter was 39.4 ± 6.2 mm. The median duration from the first AF detection to CBA was 18.5 months. Except for a history of vascular disease, variables were well-balanced between the Zero-X and conventional groups.
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Age, year | 58.9 ± 11.2 | 61.8 ± 9.8 | 0.174 |
| Female sex, n (%) | 12 (24.0%) | 11 (22.0%) | 0.812 |
| Body mass index, kg/m2 | 24.8 ± 3.2 | 24.6 ± 2.9 | 0.753 |
| Paroxysmal AF | 50 (100%) | 50 (100%) | 1.000 |
| AF duration, months | 16.5 (9.0–25.3) | 22.0 (9.8–38.0) | 0.314 |
| Heart failure, n (%) | 8 (16.0%) | 7 (14.0%) | 0.779 |
| Hypertension, n (%) | 31 (62.0%) | 27 (54.0%) | 0.418 |
| Diabetes mellitus, n (%) | 12 (24.0%) | 10 (20.0%) | 0.629 |
| CVA, n (%) | 6 (12.0%) | 7 (14.0%) | 0.766 |
| Vascular disease, n (%) | 2 (4.0%) | 12 (24.0%) | 0.008 |
| CHA2DS2-VASc score | 1.9 ± 1.4 | 2.1 ± 1.7 | 0.520 |
| LVEF, % | 61.4 ± 5.5 | 62.5 ± 4.3 | 0.233 |
| LA volume index | 39.9 ± 8.7 | 42.2 ± 13.8 | 0.361 |
| LA diameter, mm | 38.8 ± 5.5 | 39.9 ± 6.9 | 0.416 |
| Creatinine | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.308 |
| Creatinine clearance | 89.1 ± 21.4 | 87.7 ± 18.8 | 0.736 |
| Follow-up duration, day | 645.7 ± 177.8 | 680.9 ± 166.9 | 0.311 |
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Age, year | 58.9 ± 11.2 | 61.8 ± 9.8 | 0.174 |
| Female sex, n (%) | 12 (24.0%) | 11 (22.0%) | 0.812 |
| Body mass index, kg/m2 | 24.8 ± 3.2 | 24.6 ± 2.9 | 0.753 |
| Paroxysmal AF | 50 (100%) | 50 (100%) | 1.000 |
| AF duration, months | 16.5 (9.0–25.3) | 22.0 (9.8–38.0) | 0.314 |
| Heart failure, n (%) | 8 (16.0%) | 7 (14.0%) | 0.779 |
| Hypertension, n (%) | 31 (62.0%) | 27 (54.0%) | 0.418 |
| Diabetes mellitus, n (%) | 12 (24.0%) | 10 (20.0%) | 0.629 |
| CVA, n (%) | 6 (12.0%) | 7 (14.0%) | 0.766 |
| Vascular disease, n (%) | 2 (4.0%) | 12 (24.0%) | 0.008 |
| CHA2DS2-VASc score | 1.9 ± 1.4 | 2.1 ± 1.7 | 0.520 |
| LVEF, % | 61.4 ± 5.5 | 62.5 ± 4.3 | 0.233 |
| LA volume index | 39.9 ± 8.7 | 42.2 ± 13.8 | 0.361 |
| LA diameter, mm | 38.8 ± 5.5 | 39.9 ± 6.9 | 0.416 |
| Creatinine | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.308 |
| Creatinine clearance | 89.1 ± 21.4 | 87.7 ± 18.8 | 0.736 |
| Follow-up duration, day | 645.7 ± 177.8 | 680.9 ± 166.9 | 0.311 |
AF, atrial fibrillation; CVA, cerebrovascular accident; LA, left atrium; LVEF, left ventricular ejection fraction.
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Age, year | 58.9 ± 11.2 | 61.8 ± 9.8 | 0.174 |
| Female sex, n (%) | 12 (24.0%) | 11 (22.0%) | 0.812 |
| Body mass index, kg/m2 | 24.8 ± 3.2 | 24.6 ± 2.9 | 0.753 |
| Paroxysmal AF | 50 (100%) | 50 (100%) | 1.000 |
| AF duration, months | 16.5 (9.0–25.3) | 22.0 (9.8–38.0) | 0.314 |
| Heart failure, n (%) | 8 (16.0%) | 7 (14.0%) | 0.779 |
| Hypertension, n (%) | 31 (62.0%) | 27 (54.0%) | 0.418 |
| Diabetes mellitus, n (%) | 12 (24.0%) | 10 (20.0%) | 0.629 |
| CVA, n (%) | 6 (12.0%) | 7 (14.0%) | 0.766 |
| Vascular disease, n (%) | 2 (4.0%) | 12 (24.0%) | 0.008 |
| CHA2DS2-VASc score | 1.9 ± 1.4 | 2.1 ± 1.7 | 0.520 |
| LVEF, % | 61.4 ± 5.5 | 62.5 ± 4.3 | 0.233 |
| LA volume index | 39.9 ± 8.7 | 42.2 ± 13.8 | 0.361 |
| LA diameter, mm | 38.8 ± 5.5 | 39.9 ± 6.9 | 0.416 |
| Creatinine | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.308 |
| Creatinine clearance | 89.1 ± 21.4 | 87.7 ± 18.8 | 0.736 |
| Follow-up duration, day | 645.7 ± 177.8 | 680.9 ± 166.9 | 0.311 |
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Age, year | 58.9 ± 11.2 | 61.8 ± 9.8 | 0.174 |
| Female sex, n (%) | 12 (24.0%) | 11 (22.0%) | 0.812 |
| Body mass index, kg/m2 | 24.8 ± 3.2 | 24.6 ± 2.9 | 0.753 |
| Paroxysmal AF | 50 (100%) | 50 (100%) | 1.000 |
| AF duration, months | 16.5 (9.0–25.3) | 22.0 (9.8–38.0) | 0.314 |
| Heart failure, n (%) | 8 (16.0%) | 7 (14.0%) | 0.779 |
| Hypertension, n (%) | 31 (62.0%) | 27 (54.0%) | 0.418 |
| Diabetes mellitus, n (%) | 12 (24.0%) | 10 (20.0%) | 0.629 |
| CVA, n (%) | 6 (12.0%) | 7 (14.0%) | 0.766 |
| Vascular disease, n (%) | 2 (4.0%) | 12 (24.0%) | 0.008 |
| CHA2DS2-VASc score | 1.9 ± 1.4 | 2.1 ± 1.7 | 0.520 |
| LVEF, % | 61.4 ± 5.5 | 62.5 ± 4.3 | 0.233 |
| LA volume index | 39.9 ± 8.7 | 42.2 ± 13.8 | 0.361 |
| LA diameter, mm | 38.8 ± 5.5 | 39.9 ± 6.9 | 0.416 |
| Creatinine | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.308 |
| Creatinine clearance | 89.1 ± 21.4 | 87.7 ± 18.8 | 0.736 |
| Follow-up duration, day | 645.7 ± 177.8 | 680.9 ± 166.9 | 0.311 |
AF, atrial fibrillation; CVA, cerebrovascular accident; LA, left atrium; LVEF, left ventricular ejection fraction.
Procedural details
As shown in Table 2, successful PVI was achieved in all patients. Linear ablation for CTI was conducted in 20 cases: 9 and 11 in the Zero-X and conventional groups, respectively (P = 0.617). Total procedure time, defined as the duration from groin puncture to fisherman’s knot suture, was 70.1 min in the Zero-X and 71.9 min in the conventional group (P = 0.722). The LA indwelling or total ablation times were also similar between the two groups. However, the mean fluoroscopy time was significantly shorter in the Zero-X group than in the conventional group (0.008 vs. 9.0 min, P < 0.001; Figure 2A). Fluoroscopy was used in only one patient assigned to the Zero-X group because of unstable phrenic nerve capture during right-sided PVI. Radiation exposure was also significantly less in the Zero-X group (0.02 vs. 29.4 mGy, P < 0.001; Figure 2B).
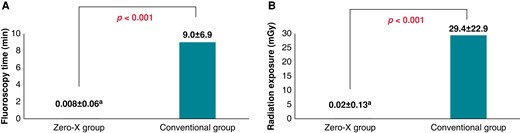
Fluoroscopy time and radiation exposure during cryoballoon ablation between the Zero-X and conventional groups. Data are presented as mean ± standard deviation. aRadiation exposure during catheter manipulation for phrenic nerve capture in one patient assigned to the Zero-X group.
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Ablation lesion | |||
| PVI, n (%) | 50 (100.0%) | 50 (100.0%) | 1.000 |
| Cavotricuspid isthmus, n (%) | 9 (18.0%) | 11 (22.0%) | 0.617 |
| Procedure-related time, min | |||
| Procedure time, min | 70.1 ± 11.2 | 71.9 ± 15.4 | 0.722 |
| Ablation time, min | 25.5 ± 4.8 | 25.4 ± 5.6 | 0.926 |
| LA indwelling time, min | 53.9 ± 7.9 | 55.9 ± 11.6 | 0.324 |
| Fluoroscopic time, min | 0.008 ± 0.06 | 9.0 ± 6.9 | <0.001 |
| Fluoroscopic exposure, mGy | 0.02 ± 0.13 | 29.4 ± 22.9 | <0.001 |
| LA pressure | |||
| Systolic pressure, mmHg | 15.9 ± 6.4 | 13.9 ± 5.3 | 0.115 |
| Diastolic pressure, mmHg | 3.5 ± 3.4 | 2.6 ± 2.0 | 0.109 |
| Mean pressure, mmHg | 8.8 ± 4.1 | 7.4 ± 3.2 | 0.062 |
| Any complication, n (%) | 3 (6.0%) | 2 (4.0%) | 1.000 |
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Ablation lesion | |||
| PVI, n (%) | 50 (100.0%) | 50 (100.0%) | 1.000 |
| Cavotricuspid isthmus, n (%) | 9 (18.0%) | 11 (22.0%) | 0.617 |
| Procedure-related time, min | |||
| Procedure time, min | 70.1 ± 11.2 | 71.9 ± 15.4 | 0.722 |
| Ablation time, min | 25.5 ± 4.8 | 25.4 ± 5.6 | 0.926 |
| LA indwelling time, min | 53.9 ± 7.9 | 55.9 ± 11.6 | 0.324 |
| Fluoroscopic time, min | 0.008 ± 0.06 | 9.0 ± 6.9 | <0.001 |
| Fluoroscopic exposure, mGy | 0.02 ± 0.13 | 29.4 ± 22.9 | <0.001 |
| LA pressure | |||
| Systolic pressure, mmHg | 15.9 ± 6.4 | 13.9 ± 5.3 | 0.115 |
| Diastolic pressure, mmHg | 3.5 ± 3.4 | 2.6 ± 2.0 | 0.109 |
| Mean pressure, mmHg | 8.8 ± 4.1 | 7.4 ± 3.2 | 0.062 |
| Any complication, n (%) | 3 (6.0%) | 2 (4.0%) | 1.000 |
LA, left atrium; PVI, pulmonary vein isolation.
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Ablation lesion | |||
| PVI, n (%) | 50 (100.0%) | 50 (100.0%) | 1.000 |
| Cavotricuspid isthmus, n (%) | 9 (18.0%) | 11 (22.0%) | 0.617 |
| Procedure-related time, min | |||
| Procedure time, min | 70.1 ± 11.2 | 71.9 ± 15.4 | 0.722 |
| Ablation time, min | 25.5 ± 4.8 | 25.4 ± 5.6 | 0.926 |
| LA indwelling time, min | 53.9 ± 7.9 | 55.9 ± 11.6 | 0.324 |
| Fluoroscopic time, min | 0.008 ± 0.06 | 9.0 ± 6.9 | <0.001 |
| Fluoroscopic exposure, mGy | 0.02 ± 0.13 | 29.4 ± 22.9 | <0.001 |
| LA pressure | |||
| Systolic pressure, mmHg | 15.9 ± 6.4 | 13.9 ± 5.3 | 0.115 |
| Diastolic pressure, mmHg | 3.5 ± 3.4 | 2.6 ± 2.0 | 0.109 |
| Mean pressure, mmHg | 8.8 ± 4.1 | 7.4 ± 3.2 | 0.062 |
| Any complication, n (%) | 3 (6.0%) | 2 (4.0%) | 1.000 |
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Ablation lesion | |||
| PVI, n (%) | 50 (100.0%) | 50 (100.0%) | 1.000 |
| Cavotricuspid isthmus, n (%) | 9 (18.0%) | 11 (22.0%) | 0.617 |
| Procedure-related time, min | |||
| Procedure time, min | 70.1 ± 11.2 | 71.9 ± 15.4 | 0.722 |
| Ablation time, min | 25.5 ± 4.8 | 25.4 ± 5.6 | 0.926 |
| LA indwelling time, min | 53.9 ± 7.9 | 55.9 ± 11.6 | 0.324 |
| Fluoroscopic time, min | 0.008 ± 0.06 | 9.0 ± 6.9 | <0.001 |
| Fluoroscopic exposure, mGy | 0.02 ± 0.13 | 29.4 ± 22.9 | <0.001 |
| LA pressure | |||
| Systolic pressure, mmHg | 15.9 ± 6.4 | 13.9 ± 5.3 | 0.115 |
| Diastolic pressure, mmHg | 3.5 ± 3.4 | 2.6 ± 2.0 | 0.109 |
| Mean pressure, mmHg | 8.8 ± 4.1 | 7.4 ± 3.2 | 0.062 |
| Any complication, n (%) | 3 (6.0%) | 2 (4.0%) | 1.000 |
LA, left atrium; PVI, pulmonary vein isolation.
Apart from transient diaphragmatic paralysis, no procedure-related complications developed in any enrolled patients. Transient diaphragmatic paralysis was observed in three and two cases assigned to the Zero-X and conventional groups, respectively, and there was no statistically significant difference between the two groups (P = 1.000).
Clinical outcomes
During a mean follow-up of 663.3 ± 172.3 days, recurrence of atrial tachyarrhythmia occurred in eight (16%) and nine (18%) participants in the Zero-X and conventional groups, respectively (P = 1.000), as listed in Table 3. A mean of 295 days was recorded until recurrent arrhythmia was detected. The type of recurrence (AF or AFL) was not significantly different between the two groups. Figure 3 shows the K-M curve for the atrial tachyarrhythmia-free survival rate, which was similar between the two groups (log-rank P = 0.841). After adjusting for confounding factors, the only independent predictor for recurrence was the LA dimension (hazard ratio, 1.263; 95% confidence interval, 1.127–1.146; P < 0.001). The fluoroless procedure itself did not affect clinical outcomes (Table 4).
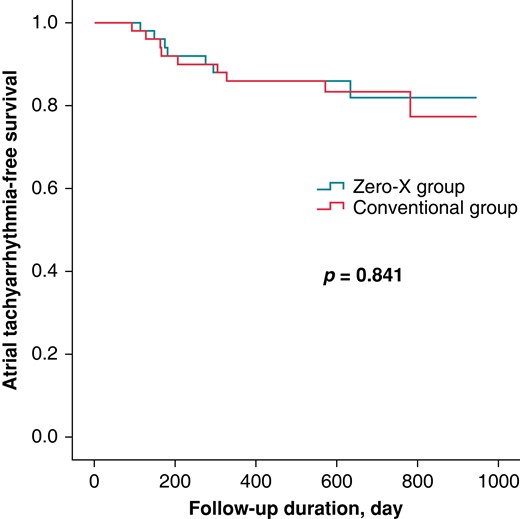
Kaplan–Meier curve presenting atrial tachyarrhythmia-free survival in the Zero-X and conventional groups.
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Recurrence of atrial tachyarrhythmia, n (%) | 8 (16.0%) | 9 (18.0%) | 1.000 |
| Duration until detection, day | 268.6 ± 166.1 | 304.3 ± 230.4 | 0.717 |
| Type of recurrence | |||
| Atrial fibrillation, n (%) | 5 (62.5%) | 6 (66.7%) | 1.000 |
| Atrial flutter, n (%) | 3 (37.5%) | 3 (33.3%) | 1.000 |
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Recurrence of atrial tachyarrhythmia, n (%) | 8 (16.0%) | 9 (18.0%) | 1.000 |
| Duration until detection, day | 268.6 ± 166.1 | 304.3 ± 230.4 | 0.717 |
| Type of recurrence | |||
| Atrial fibrillation, n (%) | 5 (62.5%) | 6 (66.7%) | 1.000 |
| Atrial flutter, n (%) | 3 (37.5%) | 3 (33.3%) | 1.000 |
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Recurrence of atrial tachyarrhythmia, n (%) | 8 (16.0%) | 9 (18.0%) | 1.000 |
| Duration until detection, day | 268.6 ± 166.1 | 304.3 ± 230.4 | 0.717 |
| Type of recurrence | |||
| Atrial fibrillation, n (%) | 5 (62.5%) | 6 (66.7%) | 1.000 |
| Atrial flutter, n (%) | 3 (37.5%) | 3 (33.3%) | 1.000 |
| . | Zero-X group (n = 50) . | Conventional group (n = 50) . | P-value . |
|---|---|---|---|
| Recurrence of atrial tachyarrhythmia, n (%) | 8 (16.0%) | 9 (18.0%) | 1.000 |
| Duration until detection, day | 268.6 ± 166.1 | 304.3 ± 230.4 | 0.717 |
| Type of recurrence | |||
| Atrial fibrillation, n (%) | 5 (62.5%) | 6 (66.7%) | 1.000 |
| Atrial flutter, n (%) | 3 (37.5%) | 3 (33.3%) | 1.000 |
| Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|
| Risk factor . | HR (95% CI) . | P-value . | Risk factor . | HR (95% CI) . | P-value . |
| Age, year | 1.003 (0.959–1.049) | 0.899 | |||
| Female sex | 1.449 (0.510–4.119) | 0.486 | |||
| Body mass index, kg/m2 | 0.976 (0.827–1.151) | 0.772 | |||
| Heart failure | 1.160 (0.333–4.038) | 0.816 | |||
| Hypertension | 1.351 (0.500–3.665) | 0.55 | |||
| Diabetes mellitus | 1.461 (0.515–4.148) | 0.476 | |||
| Cerebrovascular accident | 3.178 (1.117–9.042) | 0.03 | Cerebrovascular accident | 1.008 (0.265–3.839) | 0.99 |
| Vascular disease | 0.800 (0.183–3.505) | 0.767 | |||
| CHA2DS2-VASC score | 1.278 (0.961–1.699) | 0.092 | |||
| AF duration, mon | 0.972 (0.939–1.005) | 0.1 | AF duration, mon | 0.981 (0.935–1.029) | 0.429 |
| LA diameter, mm | 1.287 (1.163–1.423) | <0.001 | LA diameter, mm | 1.263 (1.127–1.416) | <0.001 |
| LV ejection fraction, % | 0.993 (0.902–1.092) | 0.879 | |||
| Procedure time, min | 1.017 (0.983–1.052) | 0.329 | |||
| LA indwelling time, min | 1.031 (0.989–1.075) | 0.145 | |||
| Ablation time, min | 1.105 (1.014–1.204) | 0.022 | Ablation time, min | 1.013 (0.930–1.104) | 0.761 |
| Fluoroscopic time, min | 1.006 (0.942–1.074) | 0.869 | |||
| Radiation exposure, mGy | 1.001 (0.981–1.021) | 0.937 | |||
| Mean LA pressure | 0.950 (0.821–1.099) | 0.491 | |||
| Zero-X (vs. conventional group) | 1.103 (0.425–2.861) | 0.841 | |||
| Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|
| Risk factor . | HR (95% CI) . | P-value . | Risk factor . | HR (95% CI) . | P-value . |
| Age, year | 1.003 (0.959–1.049) | 0.899 | |||
| Female sex | 1.449 (0.510–4.119) | 0.486 | |||
| Body mass index, kg/m2 | 0.976 (0.827–1.151) | 0.772 | |||
| Heart failure | 1.160 (0.333–4.038) | 0.816 | |||
| Hypertension | 1.351 (0.500–3.665) | 0.55 | |||
| Diabetes mellitus | 1.461 (0.515–4.148) | 0.476 | |||
| Cerebrovascular accident | 3.178 (1.117–9.042) | 0.03 | Cerebrovascular accident | 1.008 (0.265–3.839) | 0.99 |
| Vascular disease | 0.800 (0.183–3.505) | 0.767 | |||
| CHA2DS2-VASC score | 1.278 (0.961–1.699) | 0.092 | |||
| AF duration, mon | 0.972 (0.939–1.005) | 0.1 | AF duration, mon | 0.981 (0.935–1.029) | 0.429 |
| LA diameter, mm | 1.287 (1.163–1.423) | <0.001 | LA diameter, mm | 1.263 (1.127–1.416) | <0.001 |
| LV ejection fraction, % | 0.993 (0.902–1.092) | 0.879 | |||
| Procedure time, min | 1.017 (0.983–1.052) | 0.329 | |||
| LA indwelling time, min | 1.031 (0.989–1.075) | 0.145 | |||
| Ablation time, min | 1.105 (1.014–1.204) | 0.022 | Ablation time, min | 1.013 (0.930–1.104) | 0.761 |
| Fluoroscopic time, min | 1.006 (0.942–1.074) | 0.869 | |||
| Radiation exposure, mGy | 1.001 (0.981–1.021) | 0.937 | |||
| Mean LA pressure | 0.950 (0.821–1.099) | 0.491 | |||
| Zero-X (vs. conventional group) | 1.103 (0.425–2.861) | 0.841 | |||
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; LA, left atrium; LV, left ventricle.
| Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|
| Risk factor . | HR (95% CI) . | P-value . | Risk factor . | HR (95% CI) . | P-value . |
| Age, year | 1.003 (0.959–1.049) | 0.899 | |||
| Female sex | 1.449 (0.510–4.119) | 0.486 | |||
| Body mass index, kg/m2 | 0.976 (0.827–1.151) | 0.772 | |||
| Heart failure | 1.160 (0.333–4.038) | 0.816 | |||
| Hypertension | 1.351 (0.500–3.665) | 0.55 | |||
| Diabetes mellitus | 1.461 (0.515–4.148) | 0.476 | |||
| Cerebrovascular accident | 3.178 (1.117–9.042) | 0.03 | Cerebrovascular accident | 1.008 (0.265–3.839) | 0.99 |
| Vascular disease | 0.800 (0.183–3.505) | 0.767 | |||
| CHA2DS2-VASC score | 1.278 (0.961–1.699) | 0.092 | |||
| AF duration, mon | 0.972 (0.939–1.005) | 0.1 | AF duration, mon | 0.981 (0.935–1.029) | 0.429 |
| LA diameter, mm | 1.287 (1.163–1.423) | <0.001 | LA diameter, mm | 1.263 (1.127–1.416) | <0.001 |
| LV ejection fraction, % | 0.993 (0.902–1.092) | 0.879 | |||
| Procedure time, min | 1.017 (0.983–1.052) | 0.329 | |||
| LA indwelling time, min | 1.031 (0.989–1.075) | 0.145 | |||
| Ablation time, min | 1.105 (1.014–1.204) | 0.022 | Ablation time, min | 1.013 (0.930–1.104) | 0.761 |
| Fluoroscopic time, min | 1.006 (0.942–1.074) | 0.869 | |||
| Radiation exposure, mGy | 1.001 (0.981–1.021) | 0.937 | |||
| Mean LA pressure | 0.950 (0.821–1.099) | 0.491 | |||
| Zero-X (vs. conventional group) | 1.103 (0.425–2.861) | 0.841 | |||
| Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|
| Risk factor . | HR (95% CI) . | P-value . | Risk factor . | HR (95% CI) . | P-value . |
| Age, year | 1.003 (0.959–1.049) | 0.899 | |||
| Female sex | 1.449 (0.510–4.119) | 0.486 | |||
| Body mass index, kg/m2 | 0.976 (0.827–1.151) | 0.772 | |||
| Heart failure | 1.160 (0.333–4.038) | 0.816 | |||
| Hypertension | 1.351 (0.500–3.665) | 0.55 | |||
| Diabetes mellitus | 1.461 (0.515–4.148) | 0.476 | |||
| Cerebrovascular accident | 3.178 (1.117–9.042) | 0.03 | Cerebrovascular accident | 1.008 (0.265–3.839) | 0.99 |
| Vascular disease | 0.800 (0.183–3.505) | 0.767 | |||
| CHA2DS2-VASC score | 1.278 (0.961–1.699) | 0.092 | |||
| AF duration, mon | 0.972 (0.939–1.005) | 0.1 | AF duration, mon | 0.981 (0.935–1.029) | 0.429 |
| LA diameter, mm | 1.287 (1.163–1.423) | <0.001 | LA diameter, mm | 1.263 (1.127–1.416) | <0.001 |
| LV ejection fraction, % | 0.993 (0.902–1.092) | 0.879 | |||
| Procedure time, min | 1.017 (0.983–1.052) | 0.329 | |||
| LA indwelling time, min | 1.031 (0.989–1.075) | 0.145 | |||
| Ablation time, min | 1.105 (1.014–1.204) | 0.022 | Ablation time, min | 1.013 (0.930–1.104) | 0.761 |
| Fluoroscopic time, min | 1.006 (0.942–1.074) | 0.869 | |||
| Radiation exposure, mGy | 1.001 (0.981–1.021) | 0.937 | |||
| Mean LA pressure | 0.950 (0.821–1.099) | 0.491 | |||
| Zero-X (vs. conventional group) | 1.103 (0.425–2.861) | 0.841 | |||
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; LA, left atrium; LV, left ventricle.
Discussion
This study is the first prospective RCT to compare ICE-guided zero-fluoroscopic CBA with conventional fluoroscopy-guided CBA in patients with paroxysmal AF. In the present study, several noteworthy findings were derived: (i) fluoroscopy could be of remarkably limited use when performing ICE-guided CBA; (ii) PVI was successfully achieved in all patients who underwent ICE-guided CBA using the four indicators of good occlusion; and (iii) long-term recurrence-free survival rate, procedure time, and procedure-related complication rates were similar between ICE-guided CBA and traditional CBA using fluoroscopy with contrast injection.
The American College of Cardiology with ‘the ALARA statement’ recommends keeping the radiation ‘as low as reasonably achievable’ because there is no threshold for completely safe ionized radiation exposure.3 Previous studies have demonstrated that interventional cardiologists or cardiac electrophysiologists are at 2.8-, 3-, 6.3-, and 2-fold higher risk of skin lesions, cancer, cataracts, and chromosomal damage in lymphocytes as well as orthopaedic strain, respectively, compared with general population.15,16 In addition, excess radiation exposure is also inevitable for patients undergoing complex or repeat procedures. For AF ablation, patients are exposed to an average of 16.6 mSv which is equivalent to 830 chest X-rays and a 6.9-fold higher dose than natural worldwide background exposure per year.17 Many efforts to reduce radiation doses have been made so far, and most electrophysiology laboratories are adjusting fluoroscopy systems, such as optimization of collimation and reduction in the use of cine, frame rate, and energy per frame.
Above all, zero or near-zero fluoroscopy AF ablation has become possible with the development of echocardiography and non-fluoroscopy imaging systems.17,18 Sommer et al.19 reported a fluoroscopic time of 0.9 min and dose of 345.1 cGy·cm2 in their cohort of 1000 patients undergoing RFCA for AF using non-fluoroscopic catheter visualization technology in combination with a 3D electroanatomical mapping system. Falasconi et al.6 demonstrated 96.7% of risk reduction in fluoroscopic dose compared with the conventional approach in 111 cases of RFCA for AF based on TEE. Recently, ICE-guided fluoroless or near-zero-fluoroscopic RFCA for AF has been performed in many experienced centres. Ferguson et al.20 reported in their small observational study that a complete fluoroless procedure using ICE with 3D electroanatomic mapping navigation was conducted in 19 out of 21 cases. Minciuna et al.21 also demonstrated that adopting ICE significantly reduced radiation exposure by 34.8%. In these studies, fluoroless or near-fluoroless procedures showed similar acute and long-term success rates and similar or even shorter procedure times compared with conventional strategies. In addition, the radiation exposure dose was gradually decreased along the learning curve using a echocardiography and non-fluoroscopic imaging system.5,19,21 These findings are consistent with our results, but derived from studies using RFCA, not CBA.
Fluoroless CBA for AF remains challenging mainly because of the lack of a visual 3D mapping system. Traditionally, CBA is conducted based on fluoroscopic imaging with contrast agent injection.7 This strategy has been considered routine during CBA to confirm proper PV occlusion with CB.22 However, recently, several studies have demonstrated that contrast agent-free and/or fluoroscopy-free approaches are also feasible for PVI during CBA. Avitall et al.23 reported in their animal study that good PV occlusion could be discriminated by a marked temperature drop and slow rewarming after injecting 5 cc cold saline at 4°C into the PV, which was assessed based on pre-specified images created by a 3D navigation system. Kühne et al.24 reported a comparable no-contrast protocol, that is positioning the CB using tactile feedback and ensuring CB catheter and sheath alignment with the orientation of the PV based on pre-procedural cardiac magnetic resonance imaging, to the conventional protocol.
These protocols benefit patients with renal impairment because contrast media can deteriorate renal function. In addition, these protocols greatly reduce radiation exposure by omitting the contrast confirmation step. However, they still depend on fluoroscopic imaging for CB positioning and movement.
Alyesh et al.12 conducted a first-of-its-kind, proof-of-concept case–control study comparing fluoroless CBA with the traditional fluoroscopic technique. The procedure was exclusively performed under ICE guidance and employed ICE imaging combined with haemodynamic measures with continuous-wave pressure monitoring (CWPM). The indicators of PV occlusion used in their study included absence of high-velocity colour Doppler flow around the CB, a change in the mean CWPM of 5 mmHg or greater, increase in the V-wave, and decrease in the A-wave in case of sinus rhythm. They concluded that their new strategy showed similar acute safety and efficacy outcomes for PVI compared with the conventional CBA approach. Their strategy is similar to our protocol with regard to the adoption of ICE as a visual imaging modality. In contrast, our protocol used ICE alone to observe for indicators of good PV occlusion, that is, the presence of abundant blood bubbles from the occluded PV following deflation of CB after thawing as well as absence of colour flow signals around the inflated CB. Furthermore, a temperature drop of ≤−30°C within 30 s and ≤−40°C within 60 s after freezing was applied in the present study as one of the four indicators of good PV occlusion, which have been suggested as predictors of durable PVI.25 Consequently, the acute and long-term success rate were comparable between ICE-guided fluoroless CBA and conventional CBA in the present study. The overall success rate (83%) was also similar with that from the largest cohort including >1000 procedures by Bordignon et al.22
Limitations
This study could not assess durable PVI because the redo procedure was not performed during the study period. Furthermore, the efficacy may be overestimated given that continuous ECG monitoring was not applied during the follow-up period. However, the long-term recurrence-free rate during the mean follow-up time of 663.3 ± 172.3 days was not statistically different between the Zero-X and the conventional groups. All procedures were performed at a highly reputed centre. In addition, the number of enrolled patients may have been too small to assess the safety of ICE-guided fluoroless CBA fully. Therefore, large-scale, multicentre RCTs are needed to generalize this study’s findings.
Conclusions
Fluoroless CBA was performed safely and effectively under ICE guidance. Pulmonary vein isolation was achieved in all enrolled patients with AF using our CBA protocol based on the four indicators of good PV occlusion. This strategy showed comparable long-term clinical outcomes after CBA, without increasing the procedure time and procedure-related complications. Conventional CBA using fluoroscopy with contrast agent injection can be safely and effectively replaced by the ICE-guided fluoroless technique, especially in patients with impaired renal function.
Supplementary material
Supplementary material is available at Europace online.
Funding
Clinical research grants were obtained from Pusan National University Hospital in 2022 and Hallym University Medical Center (H20190710) in 2019.
Data availability
The data in the study will be available on reasonable request.
References
Author notes
Conflict of interest: None declared.



