-
PDF
- Split View
-
Views
-
Cite
Cite
Vanessa Rubesch-Kütemeyer, Stephan Molatta, Jürgen Vogt, Klaus-Jürgen Gutleben, Dieter Horstkotte, Georg Nölker, Reduction of radiation exposure in cryoballoon ablation procedures: a single-centre study applying intracardiac echocardiography and other radioprotective measures, EP Europace, Volume 19, Issue 6, June 2017, Pages 947–953, https://doi.org/10.1093/europace/euw139
Close - Share Icon Share
Abstract
The population treated with cryoballoon (CB) ablation is relatively young, and radiation protection is of major importance. We aimed to demonstrate that radiation exposure can be markedly reduced by intracardiac echocardiography (ICE) and optimized settings of the X-ray system.
We analysed 100 patients undergoing CB pulmonary vein isolation (PVI) for treatment of paroxysmal atrial fibrillation. In 50 consecutive patients (25 male, 59 ± 13 years; Group 1), we used ICE, skipped PV angiography prior to CB inflation, and avoided fluoroscopy whenever possible. Furthermore, we reduced the frame rate, minimized distance between patient and detector, and consequently applied collimation. These patients were compared with 50 similar preceding patients in Group 2 (29 male, 61 ± 12 years). Total fluoroscopy time was reduced from 18 ± 6 min in Group 2 to 12 ± 5 min in Group 1 (P < 0.001). Moreover, the dose area product was significantly lower (1555 ± 1219 vs. 4935 ± 2094 cGycm2, P < 0.001), total freezing time was significantly shortened (1855 ± 399 vs. 2121 ± 756 s, P = 0.031), and contrast media use was significantly reduced (66 ± 25 vs. 109 ± 27 mL, P < 0.001). At the same time, total procedure duration and complication rates did not differ significantly between both groups. After a 12 months follow-up, a similar percentage of patients was free from recurrences (74% in Group 1 vs. 78% in Group 2, P = 0.640).
Radiation exposure in CB PVI can be markedly reduced without prolonging procedure times, affecting the outcome or complication rates. Moreover, ICE seems to shorten total freezing time.
Cryoballoon (CB) ablation for paroxysmal atrial fibrillation is an alternative to radiofrequency (RF) ablation. Whereas non-fluoroscopic navigation systems can be used with RF current to reduce radiation exposure, alternative approaches are necessary in CB ablation.
This single-centre study provides evidence that intracardiac echocardiography in combination with minor modifications of the X-ray system settings and application of basic radioprotective measures can be effectively used to significantly reduce radiation exposure, contrast media use, and freezing time.
Procedure time, as well as outcome and complication rates, is unaffected by our modified protocol for CB ablation.
Introduction
Isolation of the pulmonary veins (PVs) for patients suffering from paroxysmal atrial fibrillation (pxAF) is a Class I recommendation after drug failure and nowadays considered reasonable as first-line treatment.1 Radiofrequency (RF) current is still the standard source of energy.2 However, studies revealed comparable success and complication rates for using the cryoballoon (CB) instead of RF for ablation of pxAF but show controversial results with respect to procedure duration, as well as ablation and fluoroscopy time.3,4 Luik et al.5 even showed a non-inferiority for CB ablation compared with RF ablation. Only recently, CB ablation has been proved as effective as RF for persistent atrial fibrillation (perAF).6 Considering the rising number of procedures, the increasing risk to develop malignancies caused by radiation exposure during electrophysiology studies is a major concern.7 In addition, occupational exposure to the electrophysiologist and catheter laboratory staff is another important matter of discussion.8,9 This exposure is mainly caused by scatter radiation from the patient so that reducing patient's radiation dose will result in decline of occupational exposure.8 It has been shown that every 10 mSv of radiation exposure increases the malignancy risk by 0.05% added to the lifetime risk of 20% for developing a fatal cancer.9,10 Common measures to reduce radiation doses for patients and staff include application of the ALARA principle, meaning ‘As Low As Reasonably Achievable’, and use of personal radioprotection as well as optimal adjustment of the catheter laboratory equipment.7 Moreover, non-fluoroscopic imaging technologies and catheter guidance by intracardiac echocardiography (ICE) have been developed and are used in clinical practice. In particular, it has been shown that ICE is a feasible and safe technique for CB ablation of AF and has the potential to reduce radiation exposure.11,12
The aim of our study was to evaluate the possible amount of reduction in radiation exposure achievable by implementation of several radioprotective measures and the use of ICE. Furthermore, the impact of applying these methods on mid-term outcome was studied.
Materials and methods
Patients
A total of 100 consecutive patients who underwent CB ablation for pxAF between March 2012 and July 2013 at our centre and met the inclusion criteria were included. The patients had to have an indication for catheter ablation of AF in accordance with the guidelines1 and a 12-lead ECG documentation of an AF episode. Further inclusion criteria were age over 18 years, no prior interventional treatment for AF, and the completion of at least 6 months follow-up. The study was designed as a retrospective cohort study comparing patients who were treated before and after implementation of additional radioprotective measures. All patients signed informed consent prior to the ablation. The local ethics committee approved this study (Reg. No. 35/2014).
Periprocedural management
Transthoracic echocardiography was performed to measure left atrial (LA) diameter in a parasternal long-axis view in M-mode, and Simpson's method was applied for assessment of the left ventricular ejection fraction (LVEF). To rule out LA thrombi, patients received a transoesophageal ultrasound within 24 h prior to ablation.
Antiarrhythmic drugs (AADs) except amiodarone were discontinued at least three half-lives before the ablation procedure. Anticoagulation with phenprocoumon was continued aiming for an International Normalized Ratio (INR) between 2.0 and 3.0. The direct anticoagulants (DOAC) were stopped one half-life before the ablation. After the procedure, all patients received a transthoracic echo within 2 h to rule out pericardial effusion (PE). Anticoagulation was continued within 2 h after the procedure either with phenprocoumon, DOAC, or, if necessary, intermittent use of heparin. Antiarrhythmic drugs were prescribed to the operator's discretion for a period of 3 months after the ablation.
Ablation procedure
Group 2 received the CB ablation procedure similar to the approach as described before by Nölker et al.11 and Vogt et al.13 without use of ICE. In brief, for vascular access, usually the right femoral vein was used. The ablation was performed under conscious sedation and analgesia with fentanyl and propofol as required. A quadripolar catheter (Dynamic XT™ Boston Scientific, Marlborough, MA, USA) was introduced into the coronary sinus. Transseptal puncture was performed with a steerable sheath (Flexcath®, Medtronic, Minneapolis, MN, USA) under fluoroscopic guidance. A 28 mm CB (Arctic Front® Advance, Medtronic, Minneapolis, MN, USA) was almost always the first choice in all applications regardless of PV diameters. A multipolar mapping catheter (Achieve™ Mapping Catheter, Medtronic, Minneapolis, MN, USA) was introduced for mapping of the PV before and after the ablation, followed by PV angiography (NIH closed-end four side holes, Cordis Corporation, Miami Lakes, FL, USA). The degree of the PV occlusion was measured by contrast injection after balloon inflation and then verified by PV angiography in the initial freezing period. Freezing time was 2 × 180 s. To guarantee complete occlusion, the previously described14 ‘hockey stick’ and ‘pull-down’ techniques were applied if necessary. Freezing time after ‘pull-down’ manoeuvre after 120 s was prolonged for further 180 s. During ablation of the right superior PV, the phrenic nerve function was monitored in both groups by constant palpation of the abdomen for diaphragmatic contractions without any use of fluoroscopy. To confirm persistence of pulmonary vein isolation (PVI), the PVs were re-evaluated 20 min after ablation in the order of freezing.
Group 1 received a modified approach to minimize radiation exposure to both patient and operator. The technique of applying ICE for confirmation of PV occlusion, guiding of wires, and the CB was previously described.11 Briefly, a 10 F 64 element phased array ultrasound imaging catheter (ACUSON AcuNav™, Siemens AG, Erlangen, Germany) connected to the ACUSON Cypress™ platform (Siemens AG, Erlangen, Germany) was inserted into the right atrium via the left femoral vein. Transseptal puncture was guided by ICE and performed at a site directed towards the left-sided PVs (see Figure 1). Subsequent steps like placement of guidewires, circular mapping catheters, and the CB as well as evaluation of total PV occlusion after pull-down manoeuvres were controlled by ICE. The same angiocardiography system was used for both groups (AXIOM Artis dBC, Siemens AG, Erlangen, Germany). All ablations were conducted as single-operator procedures without a technician or second electrophysiologist for the application of ICE. Compared with the procedure described above, the following modifications were made:
– Transseptal access to the LA was guided towards the left-sided PVs by ICE.
– Initial placement of the quadripolar catheter in the coronary sinus for orientation during transseptal puncture was skipped, and the catheter directly advanced into the superior vena cava for phrenic nerve stimulation during freezing of the right superior PV.
– Positioning of the guidewire and the Achieve™ catheter was also guided by ICE.
– The CB was placed at the antrum of the PVs guided by fluoroscopy and ICE.
– Pulmonary vein angiography prior to balloon placement for orientation was skipped and replaced by ICE.
– Pulmonary vein occlusion after pull-downs during the freezing period was evaluated by colour flow Doppler in ICE.
– When total occlusion could be demonstrated after CB placement by contrast media injection and opacification remained in the initial freezing period, additional PV angiography was skipped.
– Collimation was applied consequently whenever possible.
– The distance between patient and detector was kept minimal.
– The frame rate during fluoroscopy was reduced from 7.5 to 3/s.
– Fluoroscopy during table movements was replaced by the CAREposition feature (Siemens, Erlangen, Germany).
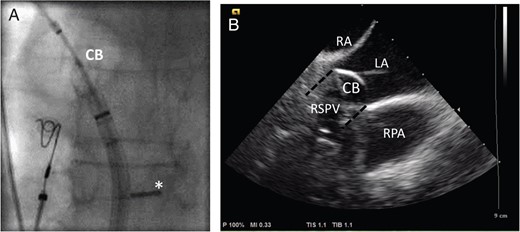
(A) The fluoroscopic image of the inflated CB in the antrum of the right superior pulmonary vein (RSPV) in a posterior–anterior view. The ICE probe has been bent backwards and pushed against the interatrial septum (*) allowing for a long-axis view. (B) The corresponding ICE view with the CB placed in the RSPV (symbolized by a black dashed line), which is not visible when the balloon is inflated due to acoustic shadowing of the balloon. Other structures useful for orientation are the right pulmonary artery (RPA), the left (LA), and the right atrium (RA).
Data collection
Patient data, medication at baseline and discharge, and complications were gathered from patient records and discharge letters. Procedural parameters such as duration, fluoroscopy time, radiation exposure, and contrast media use were documented during the ablation process. As an easy accessible and comparable measure of radiation exposure, we used the dose area product (DAP) in cGycm².
Follow-up
Routinely, patients were scheduled for follow-up including a clinical visit and ambulatory 7-day Holter monitors at 3, 6, and 12 months after the ablation. Data from an implantable device (loop recorder, ICD, or pacemaker) were available for follow-up in 14 patients of Group 1 and 6 patients of Group 2. Atrial fibrillation episodes lasting longer than 30 s were counted as recurrence. Some patients did not present for follow-up in our institution, and information about these patients was obtained by retrieving results of the follow-up with their cardiologist or physician.
Statistical analysis
Nominal scale variables were expressed as frequency and percentage, and comparison between the two groups was then conducted with Pearson's χ2 test or if applicable, Fisher's exact test. Continuous variables such as age, LVEF, LA size, procedure time, DAP, fluoroscopy time, contrast media use, and total freezing time were expressed as mean ± standard deviation. Levene's test was used for comparison of homogeneity of variances. Equal and unequal variances were analysed with Student's t-test for independent samples and Welch's t-test, respectively. Outcome was calculated using a Kaplan–Meier analysis, and comparison between the groups was calculated with the log-rank test. A P-value of <0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 100 patients with 50 patients in each group were analysed. There were no significant differences between both groups regarding baseline characteristics including antiarrhythmic medication. For two patients in Group 1, data on LA size and LVEF were missing. Details of the patient characteristics are outlined in Table 1.
| . | Group 1 (n= 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Sex (male/female) | 25/25 | 29/21 | 0.422 |
| Age (years) | 59.1 ± 13.4 | 60.7 ± 11.9 | 0.529 |
| LVEF (%) | 54.9 ± 0.7 | 54.0 ± 4.5 | 0.172 |
| LA (mm) | 38.1 ± 5.9 | 40.42 ± 5.8 | 0.051 |
| BMI (kg/m²) | 26.5 ± 4.0 | 26.3 ± 3.5 | 0.746 |
| Devices (loop recorder/ICD or pacemaker) | 14 (13/1) | 6 (2/4) | 0.078 |
| CHA2DS2-VASc score | 1.8 ± 1.5 | 1.8 ± 1.2 | 0.943 |
| AADs at baseline | |||
| None | 7 (14%) | 5 (10%) | 0.095 |
| Amiodarone | 4 (8%) | 4 (8%) | 1.00 |
| Flecainide | 10 (20%) | 15 (30%) | 0.356 |
| Beta-blockers | 21 (42%) | 24 (48%) | 0.687 |
| Others | 5 (10%) | 2 (4%) | 0.395 |
| . | Group 1 (n= 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Sex (male/female) | 25/25 | 29/21 | 0.422 |
| Age (years) | 59.1 ± 13.4 | 60.7 ± 11.9 | 0.529 |
| LVEF (%) | 54.9 ± 0.7 | 54.0 ± 4.5 | 0.172 |
| LA (mm) | 38.1 ± 5.9 | 40.42 ± 5.8 | 0.051 |
| BMI (kg/m²) | 26.5 ± 4.0 | 26.3 ± 3.5 | 0.746 |
| Devices (loop recorder/ICD or pacemaker) | 14 (13/1) | 6 (2/4) | 0.078 |
| CHA2DS2-VASc score | 1.8 ± 1.5 | 1.8 ± 1.2 | 0.943 |
| AADs at baseline | |||
| None | 7 (14%) | 5 (10%) | 0.095 |
| Amiodarone | 4 (8%) | 4 (8%) | 1.00 |
| Flecainide | 10 (20%) | 15 (30%) | 0.356 |
| Beta-blockers | 21 (42%) | 24 (48%) | 0.687 |
| Others | 5 (10%) | 2 (4%) | 0.395 |
BMI, body mass index; ICD, implantable cardioverter defibrillator; kg, kilograms; LA, left atrial diameter; LVEF, left ventricular ejection fraction; mm, millimetres.
| . | Group 1 (n= 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Sex (male/female) | 25/25 | 29/21 | 0.422 |
| Age (years) | 59.1 ± 13.4 | 60.7 ± 11.9 | 0.529 |
| LVEF (%) | 54.9 ± 0.7 | 54.0 ± 4.5 | 0.172 |
| LA (mm) | 38.1 ± 5.9 | 40.42 ± 5.8 | 0.051 |
| BMI (kg/m²) | 26.5 ± 4.0 | 26.3 ± 3.5 | 0.746 |
| Devices (loop recorder/ICD or pacemaker) | 14 (13/1) | 6 (2/4) | 0.078 |
| CHA2DS2-VASc score | 1.8 ± 1.5 | 1.8 ± 1.2 | 0.943 |
| AADs at baseline | |||
| None | 7 (14%) | 5 (10%) | 0.095 |
| Amiodarone | 4 (8%) | 4 (8%) | 1.00 |
| Flecainide | 10 (20%) | 15 (30%) | 0.356 |
| Beta-blockers | 21 (42%) | 24 (48%) | 0.687 |
| Others | 5 (10%) | 2 (4%) | 0.395 |
| . | Group 1 (n= 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Sex (male/female) | 25/25 | 29/21 | 0.422 |
| Age (years) | 59.1 ± 13.4 | 60.7 ± 11.9 | 0.529 |
| LVEF (%) | 54.9 ± 0.7 | 54.0 ± 4.5 | 0.172 |
| LA (mm) | 38.1 ± 5.9 | 40.42 ± 5.8 | 0.051 |
| BMI (kg/m²) | 26.5 ± 4.0 | 26.3 ± 3.5 | 0.746 |
| Devices (loop recorder/ICD or pacemaker) | 14 (13/1) | 6 (2/4) | 0.078 |
| CHA2DS2-VASc score | 1.8 ± 1.5 | 1.8 ± 1.2 | 0.943 |
| AADs at baseline | |||
| None | 7 (14%) | 5 (10%) | 0.095 |
| Amiodarone | 4 (8%) | 4 (8%) | 1.00 |
| Flecainide | 10 (20%) | 15 (30%) | 0.356 |
| Beta-blockers | 21 (42%) | 24 (48%) | 0.687 |
| Others | 5 (10%) | 2 (4%) | 0.395 |
BMI, body mass index; ICD, implantable cardioverter defibrillator; kg, kilograms; LA, left atrial diameter; LVEF, left ventricular ejection fraction; mm, millimetres.
Procedural parameters
Procedure times were similar in both groups, but radiation exposure, fluoroscopy time, and contrast media use showed highly significant differences favouring Group 1 (all P < 0.001, see Figures 23–4). The total freezing time was also significantly shorter in Group 1 (P = 0.031).
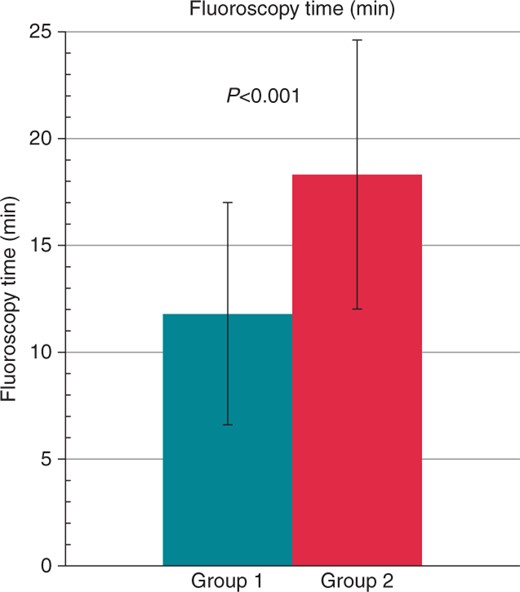
Comparison of fluoroscopy time in minutes between Group 1 (turquoise) and Group 2 (red). The values are given as mean ± standard deviation.
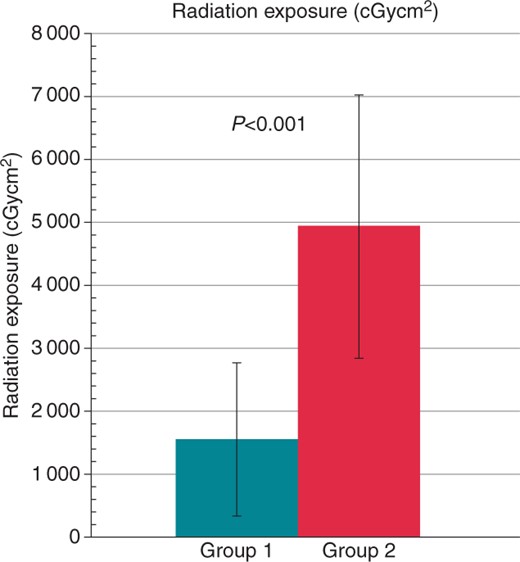
Comparison of radiation exposure between Group 1 (turquoise) and Group 2 (red). The values are given as mean ± standard deviation.
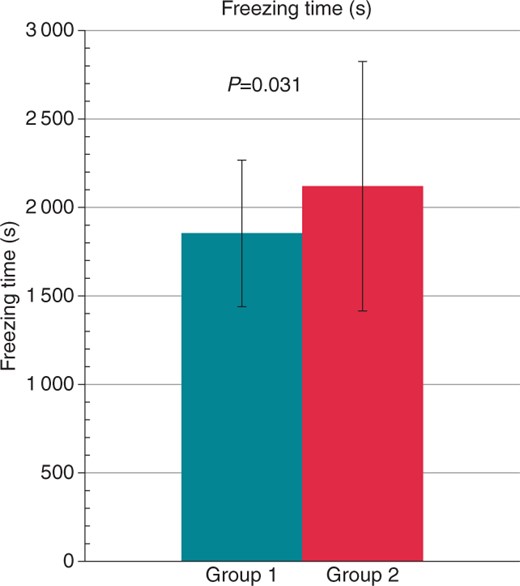
Comparison of freezing time in seconds between Group 1 (turquoise) and Group 2 (red). The values are given as mean ± standard deviation.
A switch to the smaller, 23 mm balloon was considered necessary for ablation of one vein in Group 1 and for 12 veins in Group 2 (P = 0.003). Data of 2 patients for radiation exposure and fluoroscopy time in Group 2 and for contrast media use in 3 patients (1 patient in Group 1) were missing. Details of the procedural parameters are shown in Table 2.
| . | Group 1 (n = 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Procedure time (min) | 108 ± 23 | 105 ± 23 | 0.500 |
| Radiation exposure (cGycm²) | 1555 ± 1219 | 4935 ± 2094 | <0.001 |
| Fluoroscopy time (min) | 12 ± 5 | 18 ± 6 | <0.001 |
| Contrast media (mL) | 66 ± 25 | 109 ± 27 | <0.001 |
| Total freezing time (s) | 1855 ± 399 | 2121 ± 756 | 0.031 |
| Major bleeding from puncture site | 3 (6%) | 0 | 0.246 |
| PE | 1 (2%) | 0 | 1.00 |
| Transient PNP | 0 | 1 (2%) | 1.00 |
| Transitory ischaemic attack | 1 (2%) | 0 | 1.00 |
| Haemoptysis | 0 | 2 (4%) | 0.495 |
| . | Group 1 (n = 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Procedure time (min) | 108 ± 23 | 105 ± 23 | 0.500 |
| Radiation exposure (cGycm²) | 1555 ± 1219 | 4935 ± 2094 | <0.001 |
| Fluoroscopy time (min) | 12 ± 5 | 18 ± 6 | <0.001 |
| Contrast media (mL) | 66 ± 25 | 109 ± 27 | <0.001 |
| Total freezing time (s) | 1855 ± 399 | 2121 ± 756 | 0.031 |
| Major bleeding from puncture site | 3 (6%) | 0 | 0.246 |
| PE | 1 (2%) | 0 | 1.00 |
| Transient PNP | 0 | 1 (2%) | 1.00 |
| Transitory ischaemic attack | 1 (2%) | 0 | 1.00 |
| Haemoptysis | 0 | 2 (4%) | 0.495 |
min, minutes; mL, millilitres; s, seconds.
| . | Group 1 (n = 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Procedure time (min) | 108 ± 23 | 105 ± 23 | 0.500 |
| Radiation exposure (cGycm²) | 1555 ± 1219 | 4935 ± 2094 | <0.001 |
| Fluoroscopy time (min) | 12 ± 5 | 18 ± 6 | <0.001 |
| Contrast media (mL) | 66 ± 25 | 109 ± 27 | <0.001 |
| Total freezing time (s) | 1855 ± 399 | 2121 ± 756 | 0.031 |
| Major bleeding from puncture site | 3 (6%) | 0 | 0.246 |
| PE | 1 (2%) | 0 | 1.00 |
| Transient PNP | 0 | 1 (2%) | 1.00 |
| Transitory ischaemic attack | 1 (2%) | 0 | 1.00 |
| Haemoptysis | 0 | 2 (4%) | 0.495 |
| . | Group 1 (n = 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Procedure time (min) | 108 ± 23 | 105 ± 23 | 0.500 |
| Radiation exposure (cGycm²) | 1555 ± 1219 | 4935 ± 2094 | <0.001 |
| Fluoroscopy time (min) | 12 ± 5 | 18 ± 6 | <0.001 |
| Contrast media (mL) | 66 ± 25 | 109 ± 27 | <0.001 |
| Total freezing time (s) | 1855 ± 399 | 2121 ± 756 | 0.031 |
| Major bleeding from puncture site | 3 (6%) | 0 | 0.246 |
| PE | 1 (2%) | 0 | 1.00 |
| Transient PNP | 0 | 1 (2%) | 1.00 |
| Transitory ischaemic attack | 1 (2%) | 0 | 1.00 |
| Haemoptysis | 0 | 2 (4%) | 0.495 |
min, minutes; mL, millilitres; s, seconds.
Complications
Procedure-related complications occurred in 4 patients in Group 1 and 3 patients in Group 2. Complication rates did not differ significantly between the groups. However, severe bleeding events only occurred in Group 1 with a rate of 6%.
While PE occurred only in 1 patient of Group 1, haemoptysis and phrenic nerve palsy (PNP) only occurred in Group 2. Further details on complications are presented in Table 2.
Clinical outcome
In 13 patients of Group 1 and 11 patients of Group 2, a recurrence of AF or atrial tachyarrhythmias (ATs) was documented within the follow-up time after the 3 months blanking period. This means 74% in Group 1 vs. 78% in Group 2 were free of AF/AT at follow-up and after only one procedure with no significant difference in the log-rank test (P = 0.39, see Figure 5). From the patients with a recurrence, 4 in Group 1 and 3 in Group 2 received a second procedure within the follow-up period (P = 1.0). In Group 1, all procedures were RF ablations with CARTO. In Group 2, one patient received RF ablation with Ensite, and the other two had another CB ablation. Considering these second procedures does not significantly change the differences in DAP and fluoroscopy time in favour of Group 1 (12 ± 6 min, 1690 ± 1352 cGycm2 vs. 19 ± 6 min, 4962 ± 2114 cGycm2, both P < 0.001). Significantly more recurrences were detected by an implantable device in Group 1. Whereas 38% in Group 1 and 42% in Group 2 were treated with Class I or III AAD at baseline, only 14% of the patients in both groups received such drugs at the time of follow-up. This difference was significant for both groups (P = 0.02 and P = 0.006, respectively). For 2 patients in Group 1 and 4 patients in Group 2, medication at follow-up could not be clarified. Further details are shown in Table 3.
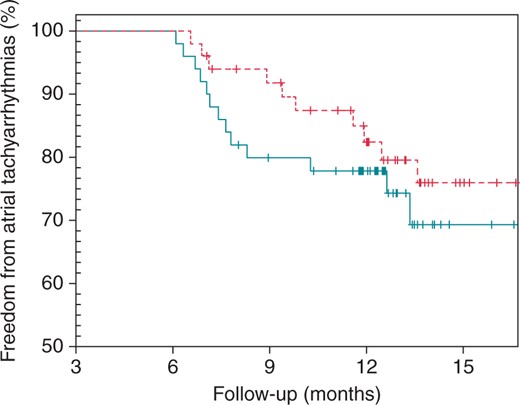
The Kaplan–Meyer plot for comparison of freedom from ATs between Group 1 (turquoise line) and Group 2 (dashed red line). The log-rank test revealed no significant differences between the groups (P = 0.39).
| . | Group 1 (n= 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Recurrences | 13 (26%) | 11 (22%) | 0.640 |
| Overall follow-up (months) | 11.8 ± 3.1 | 12.8 ± 3.2 | 0.142 |
| Recurrences detected by implanted device | 5 (38.5%) | 2 (18.2%) | 0.046 |
| Antiarrhythmic medication at follow-up | |||
| None | 12 (24%) | 5 (10%) | 0.108 |
| Amiodarone | 1 (2%) | 2 (4%) | 1.000 |
| Flecainide | 5 (10%) | 4 (8%) | 1.000 |
| Beta-blocker | 29 (58%) | 34 (68%) | 0.407 |
| Others | 1 (2%) | 1 (2%) | 1.000 |
| Missing | 2 (4%) | 4 (8%) | 0.667 |
| . | Group 1 (n= 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Recurrences | 13 (26%) | 11 (22%) | 0.640 |
| Overall follow-up (months) | 11.8 ± 3.1 | 12.8 ± 3.2 | 0.142 |
| Recurrences detected by implanted device | 5 (38.5%) | 2 (18.2%) | 0.046 |
| Antiarrhythmic medication at follow-up | |||
| None | 12 (24%) | 5 (10%) | 0.108 |
| Amiodarone | 1 (2%) | 2 (4%) | 1.000 |
| Flecainide | 5 (10%) | 4 (8%) | 1.000 |
| Beta-blocker | 29 (58%) | 34 (68%) | 0.407 |
| Others | 1 (2%) | 1 (2%) | 1.000 |
| Missing | 2 (4%) | 4 (8%) | 0.667 |
| . | Group 1 (n= 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Recurrences | 13 (26%) | 11 (22%) | 0.640 |
| Overall follow-up (months) | 11.8 ± 3.1 | 12.8 ± 3.2 | 0.142 |
| Recurrences detected by implanted device | 5 (38.5%) | 2 (18.2%) | 0.046 |
| Antiarrhythmic medication at follow-up | |||
| None | 12 (24%) | 5 (10%) | 0.108 |
| Amiodarone | 1 (2%) | 2 (4%) | 1.000 |
| Flecainide | 5 (10%) | 4 (8%) | 1.000 |
| Beta-blocker | 29 (58%) | 34 (68%) | 0.407 |
| Others | 1 (2%) | 1 (2%) | 1.000 |
| Missing | 2 (4%) | 4 (8%) | 0.667 |
| . | Group 1 (n= 50) . | Group 2 (n = 50) . | P-value . |
|---|---|---|---|
| Recurrences | 13 (26%) | 11 (22%) | 0.640 |
| Overall follow-up (months) | 11.8 ± 3.1 | 12.8 ± 3.2 | 0.142 |
| Recurrences detected by implanted device | 5 (38.5%) | 2 (18.2%) | 0.046 |
| Antiarrhythmic medication at follow-up | |||
| None | 12 (24%) | 5 (10%) | 0.108 |
| Amiodarone | 1 (2%) | 2 (4%) | 1.000 |
| Flecainide | 5 (10%) | 4 (8%) | 1.000 |
| Beta-blocker | 29 (58%) | 34 (68%) | 0.407 |
| Others | 1 (2%) | 1 (2%) | 1.000 |
| Missing | 2 (4%) | 4 (8%) | 0.667 |
Discussion
Main findings
To the best of our knowledge, this is the first study focusing on radiation reduction in CB PVI without additional use of a 3D mapping system. It shows that a significant reduction of radiation exposure is possible applying ICE and other radioprotective measures without increasing complication rates or affecting the clinical outcome.
As Heidbuchel et al.9 stated earlier, the resulting radiation exposure during an intervention does not only depend on duration of exposure during fluoroscopy or cineangiography but also, among others, on the X-ray system settings, collimation, and awareness of the team for radioprotection. Besides basic radioprotective measures, which should be implemented in every catheter laboratory,8,9 we could demonstrate that ICE seems to be an effective way to avoid radiation by replacing angiography with ultrasound imaging. We were able to reduce the mean DAP to 1555 cGycm², which is almost one-third of the original dose. This value is markedly smaller than in recent studies for CB ablation with or without ICE, ranging from 2633 to 5176 cGycm2,11,12,15,16 which supports the effectiveness of the measures taken to reduce radiation exposure. In order to convert the DAP to an estimation of the effective dose, Heidbuchel et al.9 recommend applying a factor of 0.20 for cardiac ablation procedures. Consequently, the estimated effective dose was 9.8 mSv in Group 2 and 3.2 mSv in Group 1. As a value of 10 mSv corresponds to an increased risk of 0.05% for developing a fatal malignancy,10 our changes in the DAP indicate a long-term positive effect on patients safety.
The decrease of the DAP in our study was also a result of the decline of fluoroscopy time. We were able to shorten it significantly from 18 to 12 min. This is less than in previous studies applying CB and CB with ICE with reported fluoroscopy times between 16 and 34 min.3,4,11–13,15,16 One factor contributing to our low fluoroscopy time in both groups might be the expertise of the operators. Despite the fact that fluoroscopy time reduces with operator experience,13 we do not assume that a learning curve might have caused the lower fluoroscopy time in Group 1 due to our long-term experience in CB ablation. Although the overall reduction of 6 min does not seem impressive at first glance, it contributes to an important decline of the DAP and thus radiation exposure, which is in compliance with the ALARA principle to avoid radiation whenever possible. Moreover, Lickfett et al.7 showed that every 60 min of fluoroscopy accounts for an increased risk of development of a fatal malignancy of 0.07% in women and 0.1% in men. This fact is probably of even higher importance for the involved staff than for the patients as the staff is usually involved in several procedures every day.
By omitting angiography, we could reduce contrast media use by almost 40% to a mean of 66 mL, which is below the values reported in previous studies of CB with ICE (88–108 mL)11,12,17 and less than one-third of what was reported for CB ablation without ICE.12 This is especially important for patients with known sensitivity to contrast media, for example diabetic patients or patients with already impaired renal function who might be prone to the adverse effects of contrast media. In future clinical practice, we suggest to further reduce contrast media use and angiography time by replacing the angiography used to confirm the first balloon placement with colour flow Doppler imaging.
Besides the reduction in fluoroscopy time, we could also significantly shorten the total freezing time in Group 1 compared with Group 2. This is probably due to the enhanced visibility with ICE during the freezing process allowing for better balloon placement and enabling the operator to discontinue ineffective freezes detected by remaining leak flow in colour flow Doppler when PV occlusion or ‘pull-down’ manoeuvres are insufficient.11 Overall procedure time of 108 vs. 105 min did not differ significantly between the groups, which is well in line with procedure durations previously reported for the used 28 mm Arctic Front® Advance CB ranging from 67 to 107 min.18–20 This leads to the conclusion that the application of our radioprotective measures did not prolong the procedure.
Complications
Between the groups, there were no statistical differences regarding complication rates. This leads to the conclusion that our radioprotective measures including use of ICE did not negatively influence patient safety. However, the observed bleeding rate of 6% appears a little higher compared with the literature with reported values of 0.63 and 3.2%.17 Possibly, our higher bleeding rate can be attributed to continuation of effective oral anticoagulation during the ablation procedure. Regarding the possibility of ICE catheter-induced groin haematoma, this might have been the case in one patient with bilateral haematoma, while the other two patients were bleeding from the opposite groin. Stroke and TIA are major but fortunately rare complications in CB PVI. Reported numbers lie between 0 and 1.3%,3,4,13,17 which is comparable with the rate of 2% in our study. The incidence of PE in our study (2%) is in good agreement with previously reported rates of PE or combined PE and cardiac tamponade between 0.2 and 7.3%.3,4,13,17 Haemoptysis was reported in 4% of the patients in Group 2, comparable with the data of a large series of patients.13 Of note, this complication did not occur in Group 1. Haemoptysis most likely results from lung tissue damage during the freezing process when freezing more distally in at least one PV. Therefore, the lower rate in Group 1 can be at least partially attributed to the use of ICE, which provides optimal visibility of the CB during the whole freezing process ensuring a more antral position of the CB. In one of the cases in Group 2, the use of the probably too small 23 mm balloon may have resulted in a more distal lesion contributing to freezing of pulmonary tissue.
A single transient PNP (2%) occurred in Group 2. It was detected during isolation of the right upper PV in Group 2. After cessation of freezing, the nerve function immediately recovered. This seems to be relatively low compared with the rate of PNP reported in the literature. For the 28 mm Arctic Front® Advance, Martins et al.18 reported a rate of 24.4%, but others found rates from 920 to 19%.19
Previous studies by Schmidt et al.3 and Mugnai et al.4 have shown no significant differences regarding complication rates between RF and CB ablation, except for PNP, which occurs significantly more often after CB ablation. This indicates that our novel approach using CB ablation with ICE provides at least a similar safety level as RF ablation.
Outcome
No differences were found between the groups regarding freedom from AT after one procedure. More recurrences in Group 1 were detected by an implanted device than in Group 2. This might indicate that over time more, shorter, and subclinical episodes were registered in this group, which are probably missed when following patients with a 7-day Holter monitor instead. Although we did not record Holter monitors on patients with a device, research suggests that monitoring and follow-up by implanted devices is more accurate than intermittent Holter monitoring.21
Overall, the outcome in both groups of our study (74 vs. 78%) is comparable with the current literature regarding the Arctic Front® Advance CB. Authors report rates of 71 up to 91% with different durations and methods of follow-up.15,16,19,20
Clinical implications
As growing numbers of at least partly young patients are treated, the suggested measures have the potential to significantly reduce lifetime risk for development of malignant diseases caused by radiation exposure. This seems to be true not only for the patients but possibly also for the catheter laboratory staff.
Limitations
One major limitation of this study is its retrospective design and the relatively small number of patients. However, a prospective study exposing one group of patients to more radiation than possibly necessary does not seem reasonable from an ethical point of view. Further investigation is required to verify the reliability of our findings in daily routine.
In addition, the use of ICE might increase costs of the procedure and not be adequately reimbursed, thus making ICE financially less attractive. Due to the different reimbursement systems around the world, it is beyond the scope of this study to conduct any cost simulation.
Conclusions
This single-centre cohort study demonstrates that radiation exposure in CB ablation can be markedly reduced by awareness for and consequent application of radioprotective measures. Intracardiac echocardiography may play an important role in this scenario.
Conflicts of interest: J.V. has a proctorship in CB ablation and is course director of new to left course of Medtronic. G.N. has received honoraria for lectures from Medtronic and Biosense Webster and serves as advisory board member for Siemens.



