-
PDF
- Split View
-
Views
-
Cite
Cite
Axel Buob, Stephanos Siaplaouras, Dietmar Tscholl, Hans-Joachim Schäfers, Michael Böhm, Jens Jung, Clinical value of routine predischarge testing after ICD-implantation, EP Europace, Volume 6, Issue 2, January 2004, Pages 159–164, https://doi.org/10.1016/j.eupc.2003.11.007
Close - Share Icon Share
Abstract
After implantation of a cardioverter/defibrillator (ICD) predischarge testing is often performed to ensure appropriate therapy function. Nevertheless there is no proven evidence for the necessity of this examination. In this retrospective single-centre analysis we investigated the clinical value of routine predischarge testing.
Predischarge testing was performed in 161 patients 6±2 days after primary implantation of an ICD. There were no complications related to ICD-testing. In 17 of 161 patients (11%) there was at least one pathological finding. In 4 of 17 patients we observed a defibrillation energy requirement (DER) with a safety margin of less than 10 J. In two of these patients an early lead repositioning was undertaken and in two patients reversal of the shock polarity was used to achieve an adequate DER. In 13 of 17 patients we detected a distinct deviation of pacing thresholds or R-wave sensing amplitudes. In two of these patients an early electrode repositioning was performed because of lead displacement. In the remaining 11 patients we found an adequate DER at first, whereas in two patients a further lead repositioning was still necessary during follow-up. In 144 of 161 patients (89%) predischarge testing was without pathological findings. None of these patients needed revision of the ICD-lead during a mean follow-up of 24±13 months.
Abnormal measurements during predischarge testing are not rare findings in ICD-recipients. Noninvasive methods cannot rule out inadequate defibrillation function. A normal predischarge test seems to be a reliable predictor for a stable electrode function during the first years of follow-up.
Introduction
After implantation of a cardioverter/defibrillator (ICD) retesting of the device is often performed to ensure appropriate electrode positioning, to confirm adequacy of the defibrillation function and to evaluate sensing parameters before discharge from the hospital [1]. Nevertheless there is no clear evidence for this so-called predischarge testing and the value of its routine use has been questioned. Detection of a life-threatening device failure (mainly rise of the defibrillation threshold due to dislocation of the ICD-lead) is a possible benefit of this examination. But there is also a potential risk associated with the induction of ventricular arrhythmias and the use of anaesthetic or sedative agents. Furthermore, economical aspects are not negligible in the light of the expanding indications for ICD-therapy and the increasing number of implantations, because predischarge testing adds additional costs to ICD-therapy [2]. Currently, clinical practice differs between the implanting centres and there are no mandatory guidelines to perform routine predischarge testing [1].
The focus of our retrospective single-centre study was on the results and consequences of routine predischarge testing to assess the clinical value and the diagnostic significance of this examination in an unselected population after primary ICD-implantation.
Methods
All patients underwent implantation of a transvenous ICD with an active can configuration and the capacity for biphasic shock waveforms. All systems were implanted subpectorally. Operation was performed under general anaesthesia and intraoperative testing was performed in a standard fashion. Successful implantation was defined as demonstration of adequate sensing (R-wave amplitude > 5.0 mV), adequate pacing threshold (<2.0 V/0.5 ms) and a safety margin of at least 10 J between the maximum shock energy of the implanted device and the defibrillation energy requirement (tested twice).
One day before discharge from the hospital retesting was undertaken in every patient. This evaluation included noninvasive measurements of R-wave sensing, pacing threshold, impedance of the pacing lead and high voltage pathway impedance. After fluoroscopic examination for correct electrode position, testing of appropriate sensing and termination of induced ventricular fibrillation with a safety margin of 10 J was performed under deep sedation.
Pathological findings were defined as follows: decrease of R-wave sensing ≥ 50%, increase in stimulation amplitude greater than threefold (with a constant impulse duration of 0.5 ms), fluoroscopically obvious dislocation of the ventricular electrode or a safety margin of the defibrillation energy requirement of less than 10 J.
Data are presented as mean ± standard deviation. For statistical analysis, Mann–Whitney rank sum test or Chi-square test were used. A p-value of <0.05 was considered statistically significant.
Results
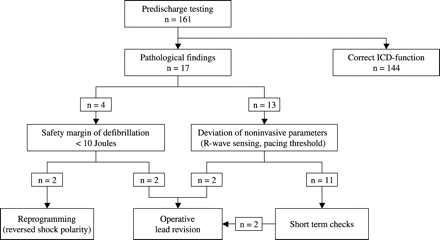
Predischarge testing was performed in 161 patients between January 1998 and December 2001 (48 months). Clinical data of the 161 patients are summarized in Table 1. No patient underwent change of antiarrhythmic medication between implantation and predischarge testing. Complications occurred in none of the patients. In 17 of 161 patients (11%) there was at least one pathological finding. Clinical variables and measured parameters during implantation were similar in patients with normal and pathological findings during predischarge testing (Table 2), only time delay between implantation and ICD-retesting was statistically different (6 vs. 7 days). Clinical consequences of pathological predischarge testing are illustrated in Fig. 1. Measured parameters in patients with immediate therapeutically relevant results are listed in detail (Table 3).
| Patients . | n=161 . |
|---|---|
| Gender | |
| Men | 127 (79%) |
| Women | 34 (21%) |
| Mean age | 63 ± 11 years |
| LVEF | 0.41 ± 0.14 |
| Cardiac disease | |
| CHD | 112 (70%) |
| Dilated cardiomyopathy | 30 (19%) |
| Valvular disease | 6 (4%) |
| HOCM | 1 |
| LQTS | 1 |
| None | 11 (7%) |
| Clinical arrhythmia | |
| VF/SCD | 64 (40%) |
| VT | 68 (42%) |
| nsVT | 14 (9%) |
| Syncope | 15 (9%) |
| Antiarrhythmic drugs | |
| Amiodarone | 89 (55%) |
| Sotalol | 17 (11%) |
| None | 55 (34%) |
| Device | |
| Single chamber | 123 (76%) |
| Dual chamber | 37 (24%) |
| PDT delay | 6 ± 2 days |
| Patients . | n=161 . |
|---|---|
| Gender | |
| Men | 127 (79%) |
| Women | 34 (21%) |
| Mean age | 63 ± 11 years |
| LVEF | 0.41 ± 0.14 |
| Cardiac disease | |
| CHD | 112 (70%) |
| Dilated cardiomyopathy | 30 (19%) |
| Valvular disease | 6 (4%) |
| HOCM | 1 |
| LQTS | 1 |
| None | 11 (7%) |
| Clinical arrhythmia | |
| VF/SCD | 64 (40%) |
| VT | 68 (42%) |
| nsVT | 14 (9%) |
| Syncope | 15 (9%) |
| Antiarrhythmic drugs | |
| Amiodarone | 89 (55%) |
| Sotalol | 17 (11%) |
| None | 55 (34%) |
| Device | |
| Single chamber | 123 (76%) |
| Dual chamber | 37 (24%) |
| PDT delay | 6 ± 2 days |
LVEF = left ventricular ejection fraction, CHD = coronary heart disease, HOCM = hypertrophic obstructive cardiomyopathy, LQTS = long QT-syndrome, VF = ventricular fibrillation, SCD = sudden cardiac death, VT = ventricular tachycardia, nsVT = non-sustained ventricular tachycardia, PDT = predischarge testing.
| Patients . | n=161 . |
|---|---|
| Gender | |
| Men | 127 (79%) |
| Women | 34 (21%) |
| Mean age | 63 ± 11 years |
| LVEF | 0.41 ± 0.14 |
| Cardiac disease | |
| CHD | 112 (70%) |
| Dilated cardiomyopathy | 30 (19%) |
| Valvular disease | 6 (4%) |
| HOCM | 1 |
| LQTS | 1 |
| None | 11 (7%) |
| Clinical arrhythmia | |
| VF/SCD | 64 (40%) |
| VT | 68 (42%) |
| nsVT | 14 (9%) |
| Syncope | 15 (9%) |
| Antiarrhythmic drugs | |
| Amiodarone | 89 (55%) |
| Sotalol | 17 (11%) |
| None | 55 (34%) |
| Device | |
| Single chamber | 123 (76%) |
| Dual chamber | 37 (24%) |
| PDT delay | 6 ± 2 days |
| Patients . | n=161 . |
|---|---|
| Gender | |
| Men | 127 (79%) |
| Women | 34 (21%) |
| Mean age | 63 ± 11 years |
| LVEF | 0.41 ± 0.14 |
| Cardiac disease | |
| CHD | 112 (70%) |
| Dilated cardiomyopathy | 30 (19%) |
| Valvular disease | 6 (4%) |
| HOCM | 1 |
| LQTS | 1 |
| None | 11 (7%) |
| Clinical arrhythmia | |
| VF/SCD | 64 (40%) |
| VT | 68 (42%) |
| nsVT | 14 (9%) |
| Syncope | 15 (9%) |
| Antiarrhythmic drugs | |
| Amiodarone | 89 (55%) |
| Sotalol | 17 (11%) |
| None | 55 (34%) |
| Device | |
| Single chamber | 123 (76%) |
| Dual chamber | 37 (24%) |
| PDT delay | 6 ± 2 days |
LVEF = left ventricular ejection fraction, CHD = coronary heart disease, HOCM = hypertrophic obstructive cardiomyopathy, LQTS = long QT-syndrome, VF = ventricular fibrillation, SCD = sudden cardiac death, VT = ventricular tachycardia, nsVT = non-sustained ventricular tachycardia, PDT = predischarge testing.
Comparison between patients with normal and pathological findings at predischarge testing (PDT)
| Normal PDT | Pathological PDT | p-Value | |
| Patients (n) | 144 | 17 | |
| Clinical variables | |||
| Mean age (years) | 64 ± 11 | 61 ± 13 | n.s. |
| Men/women | 113/31 (78%/22%) | 14/3 (82%/18%) | n.s. |
| CHD | 101 (78%) | 11 (65%) | n.s. |
| LVEF | 0.41 ± 0.14 | 0.41 ± 0.18 | n.s. |
| Amiodarone therapy | 78 (54%) | 11 (65%) | n.s. |
| Single chamber device | 113 (78%) | 12 (71%) | n.s. |
| PDT delay (days) | 6 ± 2 | 7 ± 1 | <0.001 |
| Measurements during implantation | |||
| R-wave sensing (mV) | 13.4 ± 5.0 | 13.1 ± 4.7 | n.s. |
| Pacing threshold ([email protected] ms) | 0.6 ± 0.2 | 0.7 ± 0.4 | n.s. |
| HV lead impedance (Ω) | 42 ± 11 | 42 ± 6 | n.s. |
| Normal PDT | Pathological PDT | p-Value | |
| Patients (n) | 144 | 17 | |
| Clinical variables | |||
| Mean age (years) | 64 ± 11 | 61 ± 13 | n.s. |
| Men/women | 113/31 (78%/22%) | 14/3 (82%/18%) | n.s. |
| CHD | 101 (78%) | 11 (65%) | n.s. |
| LVEF | 0.41 ± 0.14 | 0.41 ± 0.18 | n.s. |
| Amiodarone therapy | 78 (54%) | 11 (65%) | n.s. |
| Single chamber device | 113 (78%) | 12 (71%) | n.s. |
| PDT delay (days) | 6 ± 2 | 7 ± 1 | <0.001 |
| Measurements during implantation | |||
| R-wave sensing (mV) | 13.4 ± 5.0 | 13.1 ± 4.7 | n.s. |
| Pacing threshold ([email protected] ms) | 0.6 ± 0.2 | 0.7 ± 0.4 | n.s. |
| HV lead impedance (Ω) | 42 ± 11 | 42 ± 6 | n.s. |
CHD = coronary heart disease, LVEF = left ventricular ejection fraction, HV = high voltage.
Comparison between patients with normal and pathological findings at predischarge testing (PDT)
| Normal PDT | Pathological PDT | p-Value | |
| Patients (n) | 144 | 17 | |
| Clinical variables | |||
| Mean age (years) | 64 ± 11 | 61 ± 13 | n.s. |
| Men/women | 113/31 (78%/22%) | 14/3 (82%/18%) | n.s. |
| CHD | 101 (78%) | 11 (65%) | n.s. |
| LVEF | 0.41 ± 0.14 | 0.41 ± 0.18 | n.s. |
| Amiodarone therapy | 78 (54%) | 11 (65%) | n.s. |
| Single chamber device | 113 (78%) | 12 (71%) | n.s. |
| PDT delay (days) | 6 ± 2 | 7 ± 1 | <0.001 |
| Measurements during implantation | |||
| R-wave sensing (mV) | 13.4 ± 5.0 | 13.1 ± 4.7 | n.s. |
| Pacing threshold ([email protected] ms) | 0.6 ± 0.2 | 0.7 ± 0.4 | n.s. |
| HV lead impedance (Ω) | 42 ± 11 | 42 ± 6 | n.s. |
| Normal PDT | Pathological PDT | p-Value | |
| Patients (n) | 144 | 17 | |
| Clinical variables | |||
| Mean age (years) | 64 ± 11 | 61 ± 13 | n.s. |
| Men/women | 113/31 (78%/22%) | 14/3 (82%/18%) | n.s. |
| CHD | 101 (78%) | 11 (65%) | n.s. |
| LVEF | 0.41 ± 0.14 | 0.41 ± 0.18 | n.s. |
| Amiodarone therapy | 78 (54%) | 11 (65%) | n.s. |
| Single chamber device | 113 (78%) | 12 (71%) | n.s. |
| PDT delay (days) | 6 ± 2 | 7 ± 1 | <0.001 |
| Measurements during implantation | |||
| R-wave sensing (mV) | 13.4 ± 5.0 | 13.1 ± 4.7 | n.s. |
| Pacing threshold ([email protected] ms) | 0.6 ± 0.2 | 0.7 ± 0.4 | n.s. |
| HV lead impedance (Ω) | 42 ± 11 | 42 ± 6 | n.s. |
CHD = coronary heart disease, LVEF = left ventricular ejection fraction, HV = high voltage.
| Patient | R-wave sensing (mV) | Pacing threshold ([email protected] ms) | Pacing impedance (Ω) | HV lead impedance (Ω) | Fluoroscopy (lead position) | DER (J) | Intervention | |||||
| Impl. | PDT | Impl. | PDT | Impl. | PDT | Impl. | PDT | PDT | Impl. | PDT | ||
| 1 | 19.8 | 24.1 | 0.6 | 0.6 | 699 | 979 | 40 | 35 | Unchanged | 17 | >31a | Reprogramming |
| 2 | 10.3 | 5.3 | 0.4 | 0.8 | 1093 | 789 | 46 | 48 | Unchanged | 17 | 31b | Reprogramming |
| 3 | 11.9 | 2.6 | 0.6 | 1.0 | 949 | 721 | 49 | n.d. | Dislodgement | 17 | n.d. | Lead revision |
| 4 | 16.4 | 3.6 | 1.0 | 5.5 | 650 | 518 | 35 | 33 | Unchanged | 17 | n.d. | Lead revision |
| 5 | 4.2 | 8.1 | 1.0 | 1.4 | 1100 | 883 | 40 | 37 | Unchanged | 21 | 31b | Lead revision |
| 6 | 8.8 | 4.6 | 1.0 | 1.2 | 767 | 783 | 45 | 31 | Unchanged | 21 | 31b | Lead revision |
| Patient | R-wave sensing (mV) | Pacing threshold ([email protected] ms) | Pacing impedance (Ω) | HV lead impedance (Ω) | Fluoroscopy (lead position) | DER (J) | Intervention | |||||
| Impl. | PDT | Impl. | PDT | Impl. | PDT | Impl. | PDT | PDT | Impl. | PDT | ||
| 1 | 19.8 | 24.1 | 0.6 | 0.6 | 699 | 979 | 40 | 35 | Unchanged | 17 | >31a | Reprogramming |
| 2 | 10.3 | 5.3 | 0.4 | 0.8 | 1093 | 789 | 46 | 48 | Unchanged | 17 | 31b | Reprogramming |
| 3 | 11.9 | 2.6 | 0.6 | 1.0 | 949 | 721 | 49 | n.d. | Dislodgement | 17 | n.d. | Lead revision |
| 4 | 16.4 | 3.6 | 1.0 | 5.5 | 650 | 518 | 35 | 33 | Unchanged | 17 | n.d. | Lead revision |
| 5 | 4.2 | 8.1 | 1.0 | 1.4 | 1100 | 883 | 40 | 37 | Unchanged | 21 | 31b | Lead revision |
| 6 | 8.8 | 4.6 | 1.0 | 1.2 | 767 | 783 | 45 | 31 | Unchanged | 21 | 31b | Lead revision |
Impl. = implantation, PDT = predischarge testing, HV = high voltage, DER = defibrillation energy requirement, n.d. = not done. Maximum shock energy of the implanted devices was 31 J.
aVentricular fibrillation was terminated by an external applied monophasic 360J rescue shock.
bInternal 21J shock failed.
| Patient | R-wave sensing (mV) | Pacing threshold ([email protected] ms) | Pacing impedance (Ω) | HV lead impedance (Ω) | Fluoroscopy (lead position) | DER (J) | Intervention | |||||
| Impl. | PDT | Impl. | PDT | Impl. | PDT | Impl. | PDT | PDT | Impl. | PDT | ||
| 1 | 19.8 | 24.1 | 0.6 | 0.6 | 699 | 979 | 40 | 35 | Unchanged | 17 | >31a | Reprogramming |
| 2 | 10.3 | 5.3 | 0.4 | 0.8 | 1093 | 789 | 46 | 48 | Unchanged | 17 | 31b | Reprogramming |
| 3 | 11.9 | 2.6 | 0.6 | 1.0 | 949 | 721 | 49 | n.d. | Dislodgement | 17 | n.d. | Lead revision |
| 4 | 16.4 | 3.6 | 1.0 | 5.5 | 650 | 518 | 35 | 33 | Unchanged | 17 | n.d. | Lead revision |
| 5 | 4.2 | 8.1 | 1.0 | 1.4 | 1100 | 883 | 40 | 37 | Unchanged | 21 | 31b | Lead revision |
| 6 | 8.8 | 4.6 | 1.0 | 1.2 | 767 | 783 | 45 | 31 | Unchanged | 21 | 31b | Lead revision |
| Patient | R-wave sensing (mV) | Pacing threshold ([email protected] ms) | Pacing impedance (Ω) | HV lead impedance (Ω) | Fluoroscopy (lead position) | DER (J) | Intervention | |||||
| Impl. | PDT | Impl. | PDT | Impl. | PDT | Impl. | PDT | PDT | Impl. | PDT | ||
| 1 | 19.8 | 24.1 | 0.6 | 0.6 | 699 | 979 | 40 | 35 | Unchanged | 17 | >31a | Reprogramming |
| 2 | 10.3 | 5.3 | 0.4 | 0.8 | 1093 | 789 | 46 | 48 | Unchanged | 17 | 31b | Reprogramming |
| 3 | 11.9 | 2.6 | 0.6 | 1.0 | 949 | 721 | 49 | n.d. | Dislodgement | 17 | n.d. | Lead revision |
| 4 | 16.4 | 3.6 | 1.0 | 5.5 | 650 | 518 | 35 | 33 | Unchanged | 17 | n.d. | Lead revision |
| 5 | 4.2 | 8.1 | 1.0 | 1.4 | 1100 | 883 | 40 | 37 | Unchanged | 21 | 31b | Lead revision |
| 6 | 8.8 | 4.6 | 1.0 | 1.2 | 767 | 783 | 45 | 31 | Unchanged | 21 | 31b | Lead revision |
Impl. = implantation, PDT = predischarge testing, HV = high voltage, DER = defibrillation energy requirement, n.d. = not done. Maximum shock energy of the implanted devices was 31 J.
aVentricular fibrillation was terminated by an external applied monophasic 360J rescue shock.
bInternal 21J shock failed.
In 4 of 17 patients we found a DER with a safety margin of less than 10 J. No significant changes in sensing or pacing parameters or changes in lead position were observed in these patients, so noninvasive measurements and fluoroscopy failed to predict inadequate defibrillation function. In two patients (no. 1 and 2) reversion of the shock polarity was able to achieve adequate defibrillation function. In the other two patients (no. 5 and 6) early operative lead revision was necessary.
In 13 of 17 patients we found a distinct deviation of pacing thresholds or R-wave amplitudes according to the criteria defined above. In two of these patients (no. 3 and 4) an early operative lead revision was necessary because of an obvious lead dislodgement or an unacceptably high pacing threshold without prior testing of the defibrillation function. In the remaining 11 patients we found an adequate DER at first and at the short-term follow-up examination. In two of these patients a further lead repositioning was necessary after 4 weeks. In one patient recurrent episodes of oversensing had been documented, in the other patient a progressive rise in the stimulation threshold was observed and repeated fluoroscopy showed a modest lead tip dislocation.
In 144 of 161 patients (89%) predischarge testing was without pathological findings. Follow-up was available in 138 of these 144 patients. No patient needed revision of the ICD-lead during a mean follow-up of 24±13 months. Forty of 138 patients (29%) received adequate ICD-shocks during this period, 40 of 138 patients (29%) received adequate antitachycardia pacing therapy (ATP). Incidence of inappropriate shocks due to atrial fibrillation or sinus tachycardia was 5% (7 of 138 patients). Three patients received an inadequate shock therapy due to oversensing of myopotentials, but all problems could be managed by reprogramming of the implanted device. All shock therapies were programmed to the maximum shock energy and there was no occurrence of shocks failing to defibrillate the heart. Two patients needed to undergo change in the pulse generator because of dysfunction, and two patients required temporary explantation of the whole system because of an infection.
Regarding the patients with pathological findings at the time of predischarge testing, follow-up was available in 16 of 17 patients. Mean follow-up was 22±15 months. Incidence of appropriate shock therapy was 13% (2 of 16 patients) and that of appropriate ATP was 31% (5 of 16 patients). Three patients (19%) received inappropriate shocks due to atrial fibrillation.
Discussion
This retrospective analysis of predischarge testing in 161 patients after primary implantation of an ICD-system yielded immediate therapeutically relevant findings in six patients (4%). In four patients an operative lead revision was necessary because of an obvious “macro”-dislodgement of the ICD-lead, unacceptably high pacing thresholds or an inadequate safety margin for defibrillation. In two patients reversal of the shock polarity provided acceptable defibrillation function. Other studies of the utility of postoperative ICD-testing showed different and inconsistent results. In the most extensive retrospective analysis of 573 routine predischarge tests internal defibrillation failed in one patient only. This patient had a haematoma below the subcutaneous patch electrode. Other therapeutically relevant results were seen in two patients and consisted of inefficacy of the first shock or inappropriate shocks due to induction of an accelerated idioventricular rhythm [3]. In a study of Wieckhorst et al. [4] routine predischarge tests revealed severe lead problems prompting reoperation in 3 of 268 patients because of lead dislocation or lead fracture. The maximum increase in defibrillation threshold was from 10 to 20 J without any consequences. Glikson et al. [5] reported important findings in 8.8% of routine predischarge ventricular fibrillation induction, all in patients with an intraoperative defibrillation threshold of ≥15 J or concomitant pacemaker systems. These authors concluded, that routine predischarge testing of the defibrillation function does not result in additional information because most of the observed problems could be detected using noninvasive measurements.
Goldberger et al. [6] detected major abnormalities during predischarge testing in 3 of 97 patients. Despite adequate intraoperative defibrillation safety margins, in two patients a maximum output shock was not effective and in one patient an adequate safety margin was no longer present. The identified failures could not be predicted by noninvasive measurements or fluoroscopy. In another study critical findings were seen in 10 of 122 predischarge tests: six patients had a DER increase of ≥25 J, two patients had sensing abnormalities of the induced arrhythmias and two patients had pacemaker interactions [7]. These authors recommended routine postoperative ICD-testing because of the possible increase in the diagnostic yield of clinically silent critical system problems. Correction of these problems would likely result in clinical advantages including prevention of adverse events like sudden cardiac death.
In the present study we found an unexpectedly reduced safety margin of defibrillation in four patients, one patient had a DER greater than the maximum shock energy of the implanted device. No significant changes in the noninvasive measurements or an obvious lead dislodgement during fluoroscopy had been observed in these patients. At the time of implantation induced ventricular fibrillation had been terminated twice with a shock energy of 17 J in two patients corresponding to a large safety margin of 14 J. In the other two patients intraoperative defibrillation threshold was 21 J corresponding to a borderline safety margin of 10 J. It is reasonable to conclude that retesting of the defibrillation function would have been done anyway because of the borderline safety margin. On the other hand, in the first two patients the identified inadequate defibrillation function could not be predicted and it is reasonable to conclude that without routine predischarge testing further life-threatening device failures could have been missed.
Some authors stress the potential risks and discomfort for patients associated with the induction of ventricular arrhythmias. Brunn et al. [3] reported complications in 1.6% of 1007 ICD tests, mainly consisting of transient neurological disorders or induction of other arrhythmias with consecutive inappropriate ICD-shocks. No complications during routine predischarge testing occurred in our institution. Our present routine is to perform induction of ventricular arrhythmias under deep sedation with noninvasive monitoring of oxygen saturation and blood pressure in the presence of an electrophysiologist and two trained nurses which conforms closely with accepted clinical practice.
In our study, lead failure remained a significant problem in ICD-recipients. All relevant lead-related complications were observed at the time of predischarge testing or during short-term follow-up in patients with a pathological predischarge testing. On the other hand, no ICD-lead revision was necessary in patients with a normal predischarge test during a mean follow-up of 24 months. According to this observation, Schwacke et al. [8] detected lead dysfunctions in 33 of 340 patients during a period of 7 years after a median of only 2 months after implantation. It is possible to presume that normal predischarge test seems to be a predictor for proper electrode function at least during the first years after implantation. This is corresponding with the reported long-term stability of the defibrillation threshold in devices of the latest generation with transvenous lead systems and biphasic waveforms [9,,10]. Recent observations proved the stability of the defibrillation efficacy even with a safety margin of about 5 J but with rigorous implantation technique and use of testing algorithms [11].
Some authors suggest performing predischarge testing only in patients with a safety margin of less than 15 J between the intraoperative defibrillation threshold and the maximum shock energy of the device or less than 10 J when defibrillation threshold was tested twice [3]. In uncomplicated cases, we prefer an abbreviated implantation protocol without detailed determination of the defibrillation threshold but with double testing of an energy level with a safety margin greater than 10 J and performing a predischarge test to ensure a safety margin of at least 10 J in every patient. Data presented in our retrospective analysis confirmed the safety and feasibility of this clinical practice.
Limitations
As mentioned above, a limitation of the study is the fact that in most patients no systematic determination of the defibrillation threshold has been performed. Because of the increasing number of complications coupled with repeated reinductions of ventricular fibrillation and a longer duration of the procedure, double testing of a sufficient safety margin (at least 10 J between the maximum shock output and the tested energy) is common clinical practice. Focus of our study is on the clinical value of predischarge testing not after a detailed determination of the defibrillation threshold during implantation, but after performing a commonly used abbreviated implantation protocol.
A minor limitation of the study is the fact that data of a retrospective analysis are presented. In our opinion, this has no major influence on the results and the validity of the study. The predischarge testing was performed relatively late in this study which does not match the practice of many ICD implanting centres.
Conclusions
Abnormal findings during predischarge testing are not rare in ICD-recipients. Probably, failure of the defibrillation function is predictable in some but not all patients by noninvasive measurements or fluoroscopic examination of the electrode position. One major finding of this study is the fact that noninvasive methods cannot rule out inadequate defibrillation function. We believe that the absence or at least rareness of complications and the potential to detect clinically relevant device failures justifies performing routine predischarge testing in every patient. To provide better evidence for this strategy a prospective randomized study will be necessary.



