-
PDF
- Split View
-
Views
-
Cite
Cite
Johan Engdahl, Katrin Kemp Gudmundsdottir, Mårten Rosenqvist, Screening for atrial fibrillation: all invitees are equal, but some are more equal than others?, EP Europace, Volume 25, Issue 5, May 2023, euad133, https://doi.org/10.1093/europace/euad133
Close - Share Icon Share
Atrial fibrillation (AF) is established as the most prevalent clinical arrhythmia and a significant risk factor for stroke, death, heart failure, cognitive decline, and hospitalization.
Unfortunately, many patients are not aware of their AF diagnosis, due to the often asymptomatic and intermittent presentation. The conditions for early detection and treatment have, however, improved significantly over the last decade.
The advent of direct-acting oral anticoagulants (DOACs), backed up with a redundance of clinical data, has lowered the threshold for treatment initiation in AF patients with an increased risk for stroke, and a new generation of drugs is being studied. In Sweden, the prescription of oral anticoagulants (OACs) has doubled since the introduction of DOACs.
As for detection of AF, the development has been even more comprehensive. Investigation of the heart rhythm is available in every pocket and soon on every wrist, and the availability of heart rhythm detection devices for consumers is probably the largest revolution in this matter, although its effect on public health is far from established.
The landscape of medical-graded long-term electrocardiogram (ECG) recording devices is improving, where longer and continuous recordings are possible at a relatively low cost and with acceptable patient comfort.
With all these pieces in place, the conditions for AF screening have never been more favourable. Atrial fibrillation is a common disease, sometimes with asymptomatic presentation, with an increased risk of serious complications and a well-accepted treatment for risk reduction of stroke and death, most probably fulfiling the World Health Organization screening criteria.1 However, when motivating individuals in participating in screening programmes, there must be some perception of the disease as a serious threat to your health. Are we there yet with AF?
In this issue of Europace, Sandberg et al.2 report the results on a fully digital, siteless self-screening for AF pilot study, using a patch ECG device. Similar studies with similar ECG devices have been performed previously, the novelty being mainly the invitation procedure and the use of a low-cost device using the examinees’ smartphone for data transfer.
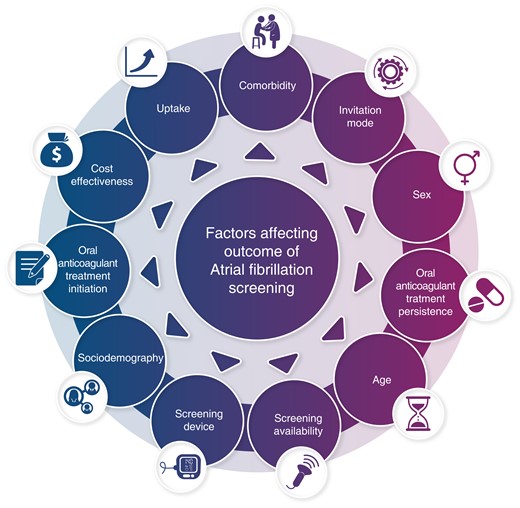
Invitation to participate was made through advertising in social media, newspapers, and radio. The study enroled 2118 individuals ≥65 years of age with a CHADS–VASc risk score of 2.6 and 74% being women. Of these, 87% completed the self-screening procedure. The mean ECG monitoring time was 153 h, and AF was detected in 2.2% (41 individuals).
The relatively large proportion of women is an interesting finding and merits further comment. Given the number of participants, one could assume that this result is statistically solid. Females dominating participation has been rare in previous AF screening studies, with the exception of the Fitbit Study.3 However, the advertising and invitation procedure used by Sandberg et al. differs from most previous AF screening trials. Most similar previous studies not linked to smartwatches and wearables, like the mSToPS4 and eBRAVE5 trials, have used targeted emails in different health care insurance populations. The mega-trials using smartwatches and wearables3,6,7 from Apple, Huawei, and Fitbit used invitations within their own systems.
If the result with regard to sex distribution from this Norwegian study will be reproduced in future AF screening studies using social media for advertising, it will be a trend to follow closely. First of all, it is a case of unequal sex distribution rarely seen in cardiovascular medicine with the risk of excluding elderly males with less digital literacy and/or interest in social media. In the future, digital literacy will probably be more widespread. Secondly, in the age span dominating this study, AF prevalence and incidence are lower in females, meaning that the lower proportion of men included lead to lower AF detection overall. As the reported from this Norwegian study, AF detection was three-fold higher in the participating men. No such sex difference was reported from the LOOP study8 but present in the STROKESTOP study9 and STROKESTOP 2 study10 (post hoc analysis). We need to learn much more about these mechanisms when continuing into the era of completely siteless and digital AF screening, and the results from Sandberg et al. suggest that different selection and exclusion mechanisms come into play with digital advertising. However, there are examples from screening programmes using traditional invitation and inviting both sexes in which uptake is higher among women.11
The AF yield reported from Sandberg et al. of 2.2% following a little more than 6 days of continuous ECG monitoring is within the expected range when comparing to similar studies like mSToPs4 and GUARD-AF,12 the two latter however using at least 14 days of ECG monitoring and hence reporting a higher total AF yield. The ECG device used in this study is capable of longer recordings than the mean of 6 days achieved here, and we are looking forward to learning if the feasibility and usability will be unchanged for longer recordings in future studies. Three days of ECG recording was the minimum recommendation for participants, which must be regarded as a conservative amount considering the performance of the device. The expected duration of the recording is not mentioned in the advertisement attached to the paper, and we would like to know for the future if there is an association between the expected monitoring duration and participation uptake?
Another clue for the AF yield is to be found in the baseline characteristics: this was a rather healthy population by selection. Another finding supporting this is the very few cases diagnosed with other relevant arrhythmias.
Sociodemographic factors have been reported to influence participation both in AF screening and in medical screening in general, where wealthy, healthy, married non-immigrants have significantly higher uptake. There were unfortunately no sociodemographic data collected in the Norwegian pilot study, and we hope that further studies could reveal if this mode of AF screening could reduce the sociodemographic gradient between participants and non-participants apparent in screening studies.
Electrocardiogram screening per se will not reduce the number of complications associated to AF, but oral anticoagulation treatment started in positive cases has the potential of doing that. It would have been of interest to learn the proportion of OAC treatment in the newly diagnosed cases, as the whole point of screening is to be able to act on a finding. The OAC treatment proportion is reported from several other AF screening trials and given the novel invitation mode from this study, will it affect treatment acceptance? We are convinced that further studies from this group will report on initiation on OAC treatment and clinical endpoints.
Sandberg et al. should be congratulated for moving the boundaries of AF screening, not at least in terms of combining digital advertising and recruitment with a device capable of delivering real-time ECG data in the first step of the screening procedure at a relatively low cost, ready for diagnosing arrhythmias without the use of additional devices and procedures. Concluding questions: Will this mode of AF screening have a net clinical benefit? Will this mode of recruitment make AF screening more equal? Because in the screening game, some are still more equal than others.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of Europace or of the European Society of Cardiology.
Conflict of interest: Johan Engdahl has received consultant or lecture fees from Roche Diagnostics, Pfizer, Bristol Myers Squibb, Boehringer Ingelheim, Piotrode, and Philips. Research grants from the Swedish Research Council, The Swedish Heart & Lung Foundation, The Swedish Innovation Agency, and The Stockholm Region. Katrin Kemp Gudmundsdottir has received speaker/lecture fees from Pfizer, Boehringer Ingelheim, and Roche Diagnostics. Research grants from Roche Diagnostics, The Stockholm Region, Carl Bennet AB, and The Swedish Heart & Lung Foundation. Mårten Rosenqvist has received consulting fees from BMS-Pfizer, Roche, Zenicor, Medtronic, and Janssen, payment or honoraria for lectures from Roche and BMS-Pfizer, support for attending meetings and/or travel from BMS-Pfizer, Medtronic, and Roche, and participation on advisory board for Medtronic SAE committee ICD, is a board member for Heart Runner Inc., and is the chairman for the Heart Foundation.



