-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas Metzner, Karl-Heinz Kuck, Julian K R Chun, What we have learned: is pulmonary vein isolation still the cornerstone of atrial fibrillation ablation?, EP Europace, Volume 24, Issue Supplement_2, June 2022, Pages ii8–ii13, https://doi.org/10.1093/europace/euab268
Close - Share Icon Share
Abstract
Ablation of atrial fibrillation (AF) is an established treatment option for symptomatic patients. The cornerstone of all ablation strategies is electrical isolation of the pulmonary veins (PVs). Ablation strategies going beyond PV isolation (PVI) might be considered in the setting of recurrent AF despite durably isolated PVs. The lack of persistent PVI, however, limits the opportunities to perceive the real impact of this endpoint on AF suppression and to fully understand the benefit of extended ablation strategies going beyond. To overcome this limitation, novel and innovative ablation systems have been developed to facilitate acute PVI and to increase its durability. These systems include balloon-based ablation devices incorporating different energy sources such as cryo energy, laser, or radiofrequency current, but also new energy sources such as pulsed field ablation as a non-thermal energy source. These technologies could advance catheter ablation of AF to an early stage of the disease and to the primary treatment tool. The current manuscript focuses on the past, the present, and the future value of PVI as the cornerstone for interventional treatment of AF and on how to achieve durable PVI during the first procedure and to further improve the clinical success rates of AF ablation. It also analyses extended ablation strategies going beyond PVI and their impact.
Introduction
Ablation of atrial fibrillation (AF) is an established treatment option for symptomatic patients as recommended in the current AF guidelines.1 The cornerstone of all AF ablation strategies is electrical isolation of the pulmonary veins (PVs) while ablation strategies going beyond PV isolation (PVI) might be considered in the setting of recurrent AF despite durably isolated PVs or in case of atrial tachycardia. Since the PVs have been identified as the major trigger for initiation and perpetuation of AF, different strategies and technologies were developed aiming at durable PVI.2 However, 23 years after the initial report, it still remains a challenge to achieve durable PVI and to prevent AF recurrences. The lack of persistent PVI, however, limits the opportunities to perceive the real impact of PVI on AF suppression and to fully understand the benefit of extended ablation strategies going beyond PVI in non-responders to PVI. The current manuscript focuses on the past, the present, and the future value of PVI as the cornerstone for interventional treatment of AF and on how to achieve durable PVI during the first procedure and to further improve the clinical success rates of AF ablation. It also analyses extended ablation strategies going beyond PVI and their impact.
The past
While initial ablation attempts for the treatment of AF were based on radiofrequency (RF) energy induced linear lesion sets within the right and/or left atrium mimicking surgical strategies3 with rather limited success, the PVs soon emerged as the main and specific ablation targets.4 Catheter ablation of arrhythmogenic foci inside the PVs was the initial approach, but was associated with only modest success rates and a substantial risk of PV stenosis. Therefore, circular lesion sets guided by three-dimensional (3D) electroanatomical mapping around the PV ostia were performed without aiming at isolation of the PVs.5 Antral lesions surrounding the ipsilateral PVs with a single wide area circumferential ablation line based on 3D mapping6 with the goal to electrically isolate the PVs from the left atrial myocardium became the mainstay of RF-based AF ablation.6–8 However, to create transmural, contiguous, and durable lesions remained a challenge. To prove that complete isolation of the PVs is superior to incomplete PV ablation, the Gap-AF–AFNET 1 trial was designed to investigate the impact of a complete vs. an intentionally incomplete circular line around the PVs in patients with paroxysmal AF.9 The study did not only demonstrate clinical superiority of complete PVI but also, during an invasive follow-up study, a high incidence of electrical reconnection of previously isolated PVs with the left atrium. This finding was reproducibly shown in multiple studies focusing on reablation procedures in patients with AF recurrence after previous complete electrical PVI.10–13 Therefore, the demonstration of PVI durability became an ongoing challenge. One way to improve durable PVI was the assessment of dormant electrical conduction after acute PVI by administration of adenosine14,15 and, in case of acute reconduction, further ablation at these sites. However, the results were contradictory and therefore routine administration of adenosine was not recommended.16 Also, non-excitability along previously performed ablation lines was suggested as an endpoint to further improve durability but this did not become an established endpoint.17 Not only ablation strategies but also ablation systems were further refined. Remote systems allowing for magnetic or robotic navigation and ablation (Stereotaxis, Hansen) were introduced and assessed but failed to prove superiority over manually guided ablation.18 Finally, the Hansen robotic system was taken from the market because of safety issues.
The addition of contact force (CF) to time, temperature, and power as another physical variable into RF ablation, improved lesion formation, and lesion quality significantly.19,20 However, randomized clinical trials demonstrating clinical superiority of CF-guided ablation over conventional ablation are still lacking and only non-inferiority trials have been performed.
To improve RF-based point-by-point ablation novel catheter technologies with novel catheter designs were introduced to simplify PVI and to increase efficacy, reproducibility, and safety. Cryoballoon-based PVI revolutionized catheter ablation of AF and became equivalent to RF-based ablation, particularly as a first-line treatment of PAF and short-lasting persistent AF.21–23 The randomized FIRE AND ICE trial demonstrated non-inferiority regarding efficacy and similar safety of cryoballoon-based compared with RF-based ablation in patients with paroxysmal AF. Therefore, the cryoballoon became an established treatment option and the gold standard next to RF ablation, as recommended in the current AF guidelines.1
However, even with all the advancements in ablation technologies and strategies aiming at higher durability of PVI, stand-alone PVI might be insufficient in more advanced stages of AF and certainly in so-called PVI non-responders. Since more advanced or chronic AF stages are often associated with different levels of atrial fibrosis, ablation strategies were extended beyond stand-alone PVI. Such strategies include ablation of complex fractionated atrial electrograms (CFAE), of linear lesions, a combination of both, or also identification and ablation of rotors, rotational activity or of focal sources.24–28 Initial single-centre studies described the superiority in clinical efficacy of extended ablation approaches over stand-alone PVI only in persistent forms of AF, but none of these strategies revealed clinical superiority in randomized controlled studies.24–29 In addition, those extended ablation strategies were oftentimes associated with prolonged procedure and fluoroscopy times as well as an increased incidence of peri- and postinterventional complications.1 However, as electrical reconduction into previously isolated PVs is a common finding, it remains unclear if AF recurrences in patients with PVI plus extended ablation strategies occur because of electrical reconduction into the PVs, because of ineffectiveness of the additional lesion sets, or because additional lesions are also not durable. Therefore, durable PVI is a prerequisite to further assess the real clinical impact of ablation strategies beyond PVI and all randomized studies comparing stand-alone PVI with PVI plus additional ablation are ultimately inconclusive. Due to the mentioned limitations PVI was the only established endpoint in ablation of AF in the past and all ablation strategies going beyond PVI were not recommended during index ablation procedures for the treatment of AF.
The present
While CF guidance has become a standard in RF ablation, novel ablation strategies emerged to further reduce the incidence of reconduction gaps. The ‘ablation index’ is a complex mathematical formula including power, ablation time, but also the CF in order to further improve RF lesion quality. Latest clinical studies could prove the high efficacy of ‘ablation index’-guided PVI by improved clinical outcomes as a surrogate for lesions quality and durability of PVI.30 In addition to the ablation index the interlesion distance assessment was introduced and a combination of both modalities further reduces the likelihood of lesion gaps enabling electrical reconduction.31 Moreover, traditional RF ablation is challenged by modified application duration and energy settings. High power or even very high power and short duration is increasingly used and induces broader and shallower lesions with the goal to shorten procedure time and to reduce collateral damage to surrounding tissue. First clinical outcome studies are promising.32,33
However, apart from conventional RF-based ablation, novel and innovative ablation tools have been emerging, aiming for more effective, safer, and easier PVI. Therefore, balloon (Laser, Cryo)-based anatomic PVI tools have been introduced and proved to facilitate acute PVI. Importantly, the introduction of the second-generation Cryoballoon (CB) marked the onset of a new ‘ice age’. The uniform cooling was linked to less operator dependent and improved procedural and favourable rhythm outcome for PAF and persistent AF.34 The POLARx Cryoballoon (Boston Scientific) was launched and features a 28 mm balloon catheter in combination with a steerable sheath and a modified spiral mapping catheter. Very similar to the established and widespread ArcticFront Advance (Medtronic), first studies demonstrated high acute efficacy, safety, and feasibility.35 Clinical outcome data are not yet available. Furthermore, encouraged by the benefits of balloon-based catheter technologies and incorporating the long and unique experience of RF energy (energy titration at different electrodes, segmental ablation, etc.), the Heliostar (Biosense Webster) catheter, a balloon catheter equipped with RF electrodes on its surface entered the field of AF ablation (Radiance, Shine). Also, novel cryotechnologies reaching ultra-low temperatures have been launched and are under clinical evaluation (Adagio, Coolloop). The non-RF single shot tool (Adagio) utilizes near-critical nitrogen technology which allows ultra-low cooling temperatures. Along with different catheter stylets, PVI and linear lesion ablation is enabled. First data are underway to better understand the future role of this strategy in the arena of AF ablation technologies.
A novel, potentially disruptive non-thermal energy source, namely, electroporation or pulsed field ablation (PFA) was recently introduced. Based on an old electrophysical principle it was rediscovered and incorporated into differently shaped catheter designs (Figure 1). In preclinical studies, high acute and chronic efficacy and especially high specificity for myocardial cells was demonstrated.36 At the same time almost no damage to non-myocardial cells or extracardiac structures such as the oesophagus or the phrenic nerve was shown.37 First clinical results of the only currently available system (Farapulse, California) appear to be encouraging. The incidence of first-pass PVI and durability of lesions sets along the PVs or the posterior wall are extremely high if a certain pattern of PFA pulses has been deployed using two catheter configurations (basket, flower).38 However, initial 12-month success rates indicate success rates similar to cryoballoon-based PVI.39 If 100% durable PVI can be reproduced in multicentre trials and in larger patient populations, important implications may arise with regard to the role of PVI and non-PV mechanisms.
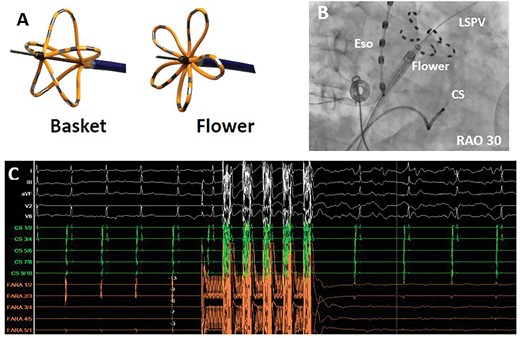
The new Farapulse-Farawave© pulsed field ablation catheter. (A) The Farawave (Farapulse©) catheter in its two application shapes (basket and flower). (B) Fluoroscopy in RAO 30° showing the Farawave catheter in the flower shape at the ostium of the left superior pulmonary vein. (C) Electrical isolation of a pulmonary vein induced by a pulsed field ablation application. Shown are surface electrocardiogram leads (white), coronary sinus leads (green), and Farawave catheter leads (orange). CS, coronary sinus catheter; Eso, oesophageal temperature probe; LSPV, left superior pulmonary vein; RAO, right anterior oblique.
Durable PVI at the initial ablation procedure would allow further assessment of the final impact of ablation targets and strategies outside the PVs. If previous studies comparing stand-alone PVI with PVI plus additional lesions would be repeated but would be based on durable PVI at the initial procedure, additional ablation strategies outside of the PVs might demonstrate their benefit, particularly in patients with persistent forms of AF. Such extended ablation strategies may also include the detection and ablation of atrial fibrosis, although the pathophysiological impact of atrial fibrosis or low-voltage areas (LVA) on the initiation and perpetuation of AF is not yet fully understood. As shown in the DECAAF I [Association of atrial fibrosis identified by delayed enhancement magnetic resonance imaging (MRI) and atrial catheter ablation] study, atrial fibrosis was independently associated with the likelihood of AF recurrence.40 A recent study did not demonstrate superiority of LVA ablation over non-LVA ablation in addition to PVI in patients with PAF.41 Whether ablation of fibrotic areas impacts the outcome of catheter ablation in persistent AF was investigated in the DECAAF II study.42 The recently presented but not yet published results did not prove superiority of ablation of LA fibrosis as assessed by cardiac MRI in comparison to sole PVI.42 Further technological advancements of currently applied 3D mapping systems in conjunction with multipolar mapping catheters allow for high-resolution characterization of atrial fibrosis. However, the pathophysiological impact and potential treatment options of these areas need further evaluation. Also the left atrial appendage (LAA) might gain further importance in the treatment of persistent AF. While first studies such as BELIEF could demonstrate superiority of LAA isolation in addition to PVI over stand-alone PVI in patients with persistent AF, the currently recruiting ASTRO-AF study assesses the clinical benefit of LAA isolation in patients with isolated PVs and will further enhance our understanding of extended ablation strategies43 (Chun clinical trials: NCT04056390).
The future
Durable PVI at the first procedure will be achieved in the future, either by new energy sources as addressed above or by improvement and real-time assessment of the lesion quality based on conventional energy sources. If so, the role of PVI as the cornerstone of AF ablation will be re-enforced and also re-assessed. The role of additional ablation beyond PVI could then be tested in the index procedure.
Novel ablation technologies such as PFA will play a key role. New mapping systems will not only provide anatomical and electrical information such as unipolar, bipolar, and activation analysis but also real-time information on lesion formation, lesion quality, and wall thickness. One platform is KODEX-EPD (EPD-Philips), a 3D imaging system that is not based on impedance or electromagnetism but on tissue dielectricity. Changes in the dielectric tissue properties can be used for real-time assessment and visualization of ablation lesions. First studies demonstrated the feasibility of KODEX-EPD in conjunction with RF ablation or the cryoballoon, while current and future evaluations will focus on real-time lesion formation assessment and measurement of wall thickness44–46 (Figure 2). If wall thickness could reliably be measured, energy settings at the respective anatomical location could be individualized and adapted. Future mapping systems might be evolutionized but also revolutionized. They might be based on current and further refined technologies or on completely different platforms and might include contact and/or non-contact mapping, intracavitary and/or body surface mapping, high resolution and/or modified point-by-point mapping, or single electrode and/or multielectrode mapping. But we may also experience a comeback of remote and robotic systems. Platforms such as a modified magnetic navigation and ablation system allowing for automated mapping, automated lesion design, and automated ablation just by pressing a button in the control room are conceivable.
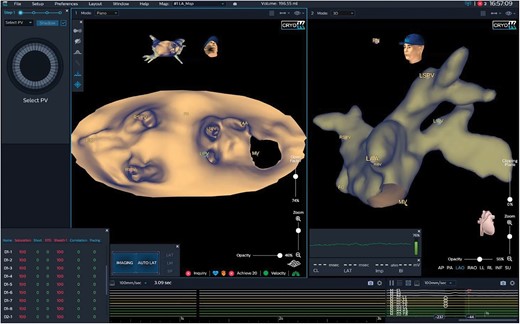
Three-dimensional imaging of the left atrium applying the KODEX-EPD system. Three-dimensional imaging of a left atrium applying the KODEX-EPD system. The left atrium is shown in a panoramic view and in an anterior–posterior orientation (left panel) and in a left anterior oblique view (right panel). LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MV, mitral valve; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Conclusions
Pulmonary vein isolation was, is, and will be the established cornerstone for catheter-based treatment of AF. However, today electrical reconduction from acutely isolated PVs is frequent and a common cause for AF recurrence. To overcome this limitation, novel and innovative ablation systems have been developed to facilitate acute PVI and to increase PVI durability. These systems include balloon-based ablation systems incorporating different energy sources such as cryo energy, laser, or RF current, but also new energy sources such as PFA as a non-thermal energy source. Currently, PFA seems to have the greatest potential to make catheter ablation of AF more effective but also rather safe at the initial procedure. More data are needed to support the promising initial experience, most likely incorporating better energy delivering tools than presently available. This could advance catheter ablation of AF to an early stage of the disease and to the primary treatment tool for AF. Ablation strategies beyond PVI such as linear lesions, ablation of CFAE, or isolation of the LAA were tested but the clinical results were inconsistent. However, the real impact of these ablation strategies can only be evaluated in the setting of persistently isolated PVs. As soon as durable PVI is provided at the initial procedure, ablation strategies for AF beyond PVI need to be re-assessed in randomized controlled trials in patients with persistent forms of AF.
Funding
This supplement was supported by an educational grant from Medtronic.
Conflict of interest: A.M.: travel and speaker’s honoraria: Medtronic, KODEX-EPD, Pfizer, and Biosense Webster; consulting fees: Medtronic. K.-H.K.: grant/research support from Medtronic, Biosense Webster, and K2; consulting fees/honoraria from CardioValve; equity: Nuvera; ownership/founder: MTEx, Cardiac Implants; intellectual property rights: MTEx, Cardiac Implants, and A-Rhythmik. J.K.R.C.: advisory board: Medtronic; research grant: Biosense Webster, Cardiofocus, Medtronic, and Biotronic; travel and speaker’s honoraria: Medtronic, Boston Scientific, Biosense Webster, Cardiofocus, Abbott, and Bayer.



