-
PDF
- Split View
-
Views
-
Cite
Cite
Jason G Andrade, Gian-Battista Chierchia, Malte Kuniss, Oussama M Wazni, New evidence: Cryoballoon ablation vs. antiarrhythmic drugs for first-line therapy of atrial fibrillation, EP Europace, Volume 24, Issue Supplement_2, June 2022, Pages ii14–ii21, https://doi.org/10.1093/europace/euab246
Close - Share Icon Share
Abstract
Atrial fibrillation (AF) is a commonly encountered chronic and progressive heart rhythm disorder, characterized by exacerbations and remissions. Contemporary clinical practice guidelines recommend a trial of antiarrhythmic drugs (AADs) as the initial therapy for sinus rhythm maintenance; however, these medications have modest efficacy and are associated with significant adverse effects. Recently, several trials have demonstrated that an initial treatment strategy of cryoballoon catheter ablation significantly improves arrhythmia outcomes (e.g. freedom atrial tachyarrhythmia and reduction in arrhythmia burden), produces clinically meaningful improvements in patient-reported outcomes (e.g. symptoms and quality of life), and significantly reduces subsequent healthcare resource utilization (e.g. hospitalization), without increasing the risk of serious or any adverse events. These findings are relevant to patients, providers, and healthcare systems, helping inform the decision regarding the initial choice of rhythm-control therapy in patients with treatment-naïve AF.
Introduction
Atrial fibrillation (AF) is the most common sustained dysrhythmia confronted in clinical practice.1 While rarely life-threatening, AF is associated with significant impairments in quality-of-life and an increased risk of heart failure, thromboembolism, and premature mortality.2–4 Given the combination of disease prevalence (e.g. AF is present in 2% of the general population), the magnitude of symptomatic impairment, as well as the chronic and progressive nature of AF the economic burden of AF is substantial. Recent estimates suggest that AF is responsible for up to 2.5% of total annual healthcare expenditures, which is largely driven by the provision of acute clinical care through arrhythmia-related emergency department visits and hospital admissions.5,6 As such, while the contemporary goals of AF management are centred on improvement of arrhythmia-related symptoms and reduction in the morbidity associated with AF, substantial societal benefits would be expected from management strategies that meaningfully reduce the direct costs (e.g., healthcare utilization) and the indirect costs (e.g., lost productivity).7
AF is a chronic progressive disorder. Without preventative therapy, AF relapses in up to 75% of patients within a year of the index episode.8–10 Recently, the Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) trial evaluated the utility of an early rhythm-control strategy in patients with newly diagnosed AF (enrolled median 36 days after AF diagnosis).11 This trial demonstrated that early rhythm control significantly reduced the composite primary outcome of cardiovascular death, stroke, and hospitalization for worsening heart failure and acute coronary syndrome by 21% (from 5.0%/year to 3.9%/year). Consistent with major North American and European guidelines,12–14 rhythm control in the EAST-AFNET 4 trial was predominantly attained with pharmacological antiarrhythmic drug (AAD) therapy.11 While AAD therapy has been definitively proven to be superior to placebo in the prevention of arrhythmia recurrence in randomized clinical trials [odds ratio (OR) 0.22–0.53 for arrhythmia recurrence vs. placebo], these agents have only modest efficacy at maintaining sinus rhythm.15,16 Moreover, AAD therapy has been associated with significant side-effects, leading to high rates of withdrawal (OR 1.63–2.91).11,15,16 In some cases, the use of AAD therapy has been associated with increased all-cause mortality [OR 2.73, 95% confidence interval (CI), 1.00–7.41; P = 0.049 for amiodarone and OR 4.32, 95% CI, 1.59–11.70; P = 0.013 for sotalol, vs. placebo].15
Over the past 25 years, there has been a significant evolution in percutaneous catheter ablation procedures for the management of AF. Up to the mid-late 1980s, the dominant mechanistic theory of AF pathophysiology was based on the ‘multiple wavelet hypothesis’, which postulated that AF results from the presence of multiple independent spontaneously occurring wavelets that coexist and propagate randomly throughout the atria. As this hypothesis postulated that AF will continue as long as the atrium had a sufficient area with adequately short refractory periods, interventions were designed to reduce the excitable mass of atrial tissue by compartmentalizing the atria into smaller regions incapable of sustaining the critical number of circulating wavelets. The interventional procedure was technically complicated and associated with moderate success and high rates of complication.
In the late 1990s, Haïssaguerre et al.17 demonstrated that AF was a triggered arrhythmia initiated by rapid repetitive discharges predominantly originating from the pulmonary veins (PVs), and perpetuated via micro re-entrant circuits around the pulmonary venous-left atrial junction. This knowledge spurred the development of percutaneous procedures designed to electrically isolate the PVs from the left atrium, evolving from focal ablation of triggers within the PV to circumferential ablation of the peri-venous left atrial myocardium with a goal of circumferential electrical pulmonary venous isolation (PVI). This contemporary approach effectively targeted both the initiating triggers of AF (e.g. the PVs) as well as the mass of electrically active left atrial tissue capable of perpetuating AF. Since then multiple randomized clinical trials have demonstrated catheter ablation to be superior to AAD therapy for sinus rhythm maintenance, symptomatic improvement, and enhancement in quality of life when AADs have been ineffective, are contraindicated, or are not tolerated.18,19
While it has been postulated that earlier intervention may provide significant benefits, we cannot extrapolate the evidence supporting the role of catheter ablation as a second-line therapy to a population who has never received AAD therapy. By design, the aforementioned clinical trials enrolled patients who had already failed AAD therapy, thus pre-selecting a population in whom AADs have proven to be ineffectual. As such, these trials significantly skewed the therapeutic benefit towards the ablation group (e.g. selection bias). While it has been postulated that early invasive intervention with catheter ablation offers an opportunity to halt the progressive pathoanatomical changes associated with AF,20,21 it remained unknown whether the benefits of catheter ablation would be maintained when delivered as a ‘first-line’ therapy (e.g. prior to AAD failure).
Atrial fibrillation ablation as a first-line therapy
The most recent practice guidelines recommended a trial of AADs prior to considering an invasive ablation procedure, providing only a conditional recommendation for catheter ablation as first-line therapy [Class IIa for Paroxysmal AF and Class IIb for persistent AF in the European Society of Cardiology (ESC) and the American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) guidelines; and a weak recommendation for both paroxysmal and persistent AF in the Canadian Cardiovascular Society (CCS) guidelines].12–14
However, while predominantly an isolated electrical disorder early in its course the intermittent AF episodes lead to progressive electrical, contractile, and structural atrial remodelling. As such it has been postulated that the early delivery of a tailored catheter ablation procedure designed to modify the pathogenic mechanism of AF initiation and perpetuation may alter the disease trajectory and impart clinical benefits. Specifically, several studies have attempted to identify whether a patient population exists where the effectiveness of a catheter ablation procedure would be sufficiently favourable, and the risks sufficiently low, that it would be appropriate to offer catheter ablation as first-line therapy.
Initially, this question was addressed in three studies employing point-by-point radiofrequency energy: the Radiofrequency Ablation vs Antiarrhythmic Drugs as First-line Treatment of Symptomatic Atrial Fibrillation (RAAFT-1) trial, the Medical ANtiarrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation (MANTRA-PAF) trial, and the Radiofrequency Ablation vs Antiarrhythmic Drugs as First-Line Treatment of Paroxysmal Atrial Fibrillation (RAAFT-2) trial.22–24 These three trials had relatively small sample size, and were limited by their use of intermittent non-invasive rhythm monitoring, as well as high rates of cross-over from AADs to ablation, which impacted their ability to detect significant differences between treatment groups.22–24 These studies observed low absolute success rates and limited relative benefit of first-line radiofrequency ablation [risk ratio (RR) 0.81 for any arrhythmia; 95% CI 0.68–0.96, P = 0.01, Figure 1; and RR 0.62 for symptomatic arrhythmia; 95% CI 0.38–1.01, P = 0.06, Figure 2].25 Given the combination of modest efficacy, lack of procedural standardization, and inconsistent procedural endpoints these studies had a limited impact on clinical practice.
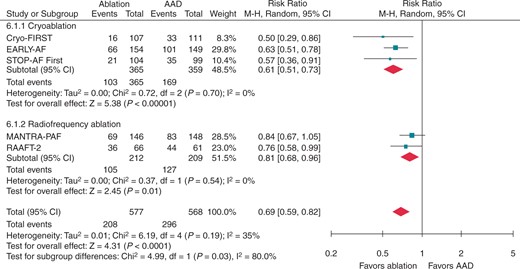
Recurrence of any atrial tachyarrhythmia, stratified by ablation energy. Andrade et al. 2021.25 Reproduced with permission from Elsevier. AAD, antiarrhythmic drug; CI, confidence interval.
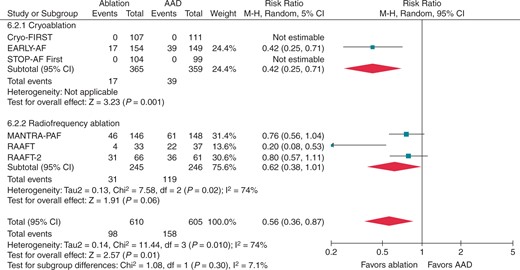
Recurrence of symptomatic atrial tachyarrhythmia, stratified by ablation energy. Andrade et al. 2021.25 Reproduced with permission from Elsevier. AAD, antiarrhythmic drug; CI, confidence interval.
Cryoballoon ablation as a first-line therapy
In recent years, there has been an emergence of technologies designed to reduce the technical complexity of AF ablation procedures. While focal point-by-point radiofrequency catheter ablation has shown success, the procedure is time-consuming and highly dependent on operator proficiency.26 In response, single-shot AF ablation toolsets, such as the Arctic Front Cryoballoon (Medtronic, USA), have been developed.27 These toolsets are less reliant on operator dexterity, enabling procedural standardization28 and ensuring consistent outcomes26 with low rates of complications.29,30 The balance of safety, efficacy, and generalizability has led to renewed interest in the role of first-line ablation for the initial management of treatment-naïve AF.16,31,32
This question was addressed in three multicentre parallel-group, single-blinded randomized clinical trials published in 2021: the Early Aggressive Invasive Intervention for Atrial Fibrillation (EARLY-AF), the Cryoballoon Catheter Ablation in an Antiarrhythmic Drug Naive Paroxysmal Atrial Fibrillation (STOP-AF First) trial, and the Catheter Cryoablation vs. Antiarrhythmic Drug as First-Line Therapy of Paroxysmal Atrial Fibrillation (Cryo-FIRST) trials.16,31,32 Together three trials included a total of 724 patients with treatment-naïve AF in their intention-to-treat16,31 or modified intention-to-treat populations.32
The patients included in these studies were relatively young (mean age 57.4 years) and predominantly male (67%). Characteristics of the studies and their populaitons are presented in Table 1. Included patients were relatively healthy and had normal left ventricular function and left atrial size, although differences between study populations were noted. Specifically, the CRYO-FIRST population was younger, had the lowest prevalence of hypertension, ischaemic heart disease, and heart failure, and had the lowest CHA2DS2-VASc score.
| . | Cryo-FIRST31 . | EARLY-AF16 . | STOP-AF First32 . | |||
|---|---|---|---|---|---|---|
| Setting (number of centres) . | Australia, Europe, Latin America (20) . | Canada (18) . | USA (24) . | |||
| . | Cryoablation . | AAD . | Cryoablation . | AAD . | Cryoablation . | AAD . |
| Randomized, n | 107 | 111 | 154 | 149 | 108 | 102 |
| Included in analysis, n (%) | 107 | 111 | 154 | 149 | 104 | 99 |
| Baseline demographics | ||||||
| Age, years | 50.5 | 54.1 | 57.7 | 59.5 | 60.4 | 61.6 |
| Male, n (%) | 76 (71) | 72 (65) | 112 (73) | 102 (68) | 63 (61) | 57 (58) |
| Paroxysmal AF | 107 (100) | 111 (100) | 147 (96) | 140 (94) | 104 (100) | 99 (100) |
| Time from AF diagnosis, years | Mean 0.7 | Mean 0.8 | Median 1 | Median 1 | Mean 1.3 | Mean 1.3 |
| AFEQT, mean | 62.0 | 59.9 | 61.4 | 57.4 | 58.5 | 62.9 |
| CHA2DS2-VASc score | ||||||
| 0/1 | 82 (77) | 78 (70) | 68 (44) | 65 (44) | 48 (46) | 44 (44) |
| 2 | 13 (12) | 15 (14) | 53 (34) | 48 (32) | 33 (32) | 19 (19) |
| ≥3 | 7 (7) | 12 (11) | 33 (21) | 36 (24) | 23 (22) | 26 (26) |
| Co-morbidities | ||||||
| Hypertension | 33 (31) | 40 (36) | 57 (37) | 55 (37) | 58 (54) | 57 (56) |
| Ischaemic heart disease | 2 (2) | 1 (1) | 12 (8) | 7 (5) | 13 (12) | 12 (12) |
| Heart failure, n (%) | 0 | 0 | 14 (9) | 14 (9) | 1 (1) | 3 (3) |
| Previous stroke/TIA, n (%) | 0 | 1 (1) | 4 (3) | 5 (3) | 2 (2) | 3 (3) |
| Left atrial diameter, mm | 37.0 | 38.0 | 39.5 | 38.1 | 38.7 | 38.2 |
| Follow-up duration | 12 months from day from cryoablation procedure or AAD initiation excluding a 90-day blanking period | |||||
| Primary outcome | Any recurrence of atrial tachyarrhythmia (AF, AT, AFL) lasting longer than 30 seconds | |||||
| Monitoring protocol | 7-day Holter every 3 months | Implantable cardiac monitor with daily transmissions | 24-h Holter every 6 months Weekly patient-activated trans-telephonic event recorder | |||
| Key secondary outcomes |
|
|
| |||
| . | Cryo-FIRST31 . | EARLY-AF16 . | STOP-AF First32 . | |||
|---|---|---|---|---|---|---|
| Setting (number of centres) . | Australia, Europe, Latin America (20) . | Canada (18) . | USA (24) . | |||
| . | Cryoablation . | AAD . | Cryoablation . | AAD . | Cryoablation . | AAD . |
| Randomized, n | 107 | 111 | 154 | 149 | 108 | 102 |
| Included in analysis, n (%) | 107 | 111 | 154 | 149 | 104 | 99 |
| Baseline demographics | ||||||
| Age, years | 50.5 | 54.1 | 57.7 | 59.5 | 60.4 | 61.6 |
| Male, n (%) | 76 (71) | 72 (65) | 112 (73) | 102 (68) | 63 (61) | 57 (58) |
| Paroxysmal AF | 107 (100) | 111 (100) | 147 (96) | 140 (94) | 104 (100) | 99 (100) |
| Time from AF diagnosis, years | Mean 0.7 | Mean 0.8 | Median 1 | Median 1 | Mean 1.3 | Mean 1.3 |
| AFEQT, mean | 62.0 | 59.9 | 61.4 | 57.4 | 58.5 | 62.9 |
| CHA2DS2-VASc score | ||||||
| 0/1 | 82 (77) | 78 (70) | 68 (44) | 65 (44) | 48 (46) | 44 (44) |
| 2 | 13 (12) | 15 (14) | 53 (34) | 48 (32) | 33 (32) | 19 (19) |
| ≥3 | 7 (7) | 12 (11) | 33 (21) | 36 (24) | 23 (22) | 26 (26) |
| Co-morbidities | ||||||
| Hypertension | 33 (31) | 40 (36) | 57 (37) | 55 (37) | 58 (54) | 57 (56) |
| Ischaemic heart disease | 2 (2) | 1 (1) | 12 (8) | 7 (5) | 13 (12) | 12 (12) |
| Heart failure, n (%) | 0 | 0 | 14 (9) | 14 (9) | 1 (1) | 3 (3) |
| Previous stroke/TIA, n (%) | 0 | 1 (1) | 4 (3) | 5 (3) | 2 (2) | 3 (3) |
| Left atrial diameter, mm | 37.0 | 38.0 | 39.5 | 38.1 | 38.7 | 38.2 |
| Follow-up duration | 12 months from day from cryoablation procedure or AAD initiation excluding a 90-day blanking period | |||||
| Primary outcome | Any recurrence of atrial tachyarrhythmia (AF, AT, AFL) lasting longer than 30 seconds | |||||
| Monitoring protocol | 7-day Holter every 3 months | Implantable cardiac monitor with daily transmissions | 24-h Holter every 6 months Weekly patient-activated trans-telephonic event recorder | |||
| Key secondary outcomes |
|
|
| |||
AAD, antiarrhythmic drug; AFEQT, Atrial Fibrillation Effect on QualiTy-of-life; LVEF, left ventricular ejection fraction; TIA, transient ischaemic attack.
| . | Cryo-FIRST31 . | EARLY-AF16 . | STOP-AF First32 . | |||
|---|---|---|---|---|---|---|
| Setting (number of centres) . | Australia, Europe, Latin America (20) . | Canada (18) . | USA (24) . | |||
| . | Cryoablation . | AAD . | Cryoablation . | AAD . | Cryoablation . | AAD . |
| Randomized, n | 107 | 111 | 154 | 149 | 108 | 102 |
| Included in analysis, n (%) | 107 | 111 | 154 | 149 | 104 | 99 |
| Baseline demographics | ||||||
| Age, years | 50.5 | 54.1 | 57.7 | 59.5 | 60.4 | 61.6 |
| Male, n (%) | 76 (71) | 72 (65) | 112 (73) | 102 (68) | 63 (61) | 57 (58) |
| Paroxysmal AF | 107 (100) | 111 (100) | 147 (96) | 140 (94) | 104 (100) | 99 (100) |
| Time from AF diagnosis, years | Mean 0.7 | Mean 0.8 | Median 1 | Median 1 | Mean 1.3 | Mean 1.3 |
| AFEQT, mean | 62.0 | 59.9 | 61.4 | 57.4 | 58.5 | 62.9 |
| CHA2DS2-VASc score | ||||||
| 0/1 | 82 (77) | 78 (70) | 68 (44) | 65 (44) | 48 (46) | 44 (44) |
| 2 | 13 (12) | 15 (14) | 53 (34) | 48 (32) | 33 (32) | 19 (19) |
| ≥3 | 7 (7) | 12 (11) | 33 (21) | 36 (24) | 23 (22) | 26 (26) |
| Co-morbidities | ||||||
| Hypertension | 33 (31) | 40 (36) | 57 (37) | 55 (37) | 58 (54) | 57 (56) |
| Ischaemic heart disease | 2 (2) | 1 (1) | 12 (8) | 7 (5) | 13 (12) | 12 (12) |
| Heart failure, n (%) | 0 | 0 | 14 (9) | 14 (9) | 1 (1) | 3 (3) |
| Previous stroke/TIA, n (%) | 0 | 1 (1) | 4 (3) | 5 (3) | 2 (2) | 3 (3) |
| Left atrial diameter, mm | 37.0 | 38.0 | 39.5 | 38.1 | 38.7 | 38.2 |
| Follow-up duration | 12 months from day from cryoablation procedure or AAD initiation excluding a 90-day blanking period | |||||
| Primary outcome | Any recurrence of atrial tachyarrhythmia (AF, AT, AFL) lasting longer than 30 seconds | |||||
| Monitoring protocol | 7-day Holter every 3 months | Implantable cardiac monitor with daily transmissions | 24-h Holter every 6 months Weekly patient-activated trans-telephonic event recorder | |||
| Key secondary outcomes |
|
|
| |||
| . | Cryo-FIRST31 . | EARLY-AF16 . | STOP-AF First32 . | |||
|---|---|---|---|---|---|---|
| Setting (number of centres) . | Australia, Europe, Latin America (20) . | Canada (18) . | USA (24) . | |||
| . | Cryoablation . | AAD . | Cryoablation . | AAD . | Cryoablation . | AAD . |
| Randomized, n | 107 | 111 | 154 | 149 | 108 | 102 |
| Included in analysis, n (%) | 107 | 111 | 154 | 149 | 104 | 99 |
| Baseline demographics | ||||||
| Age, years | 50.5 | 54.1 | 57.7 | 59.5 | 60.4 | 61.6 |
| Male, n (%) | 76 (71) | 72 (65) | 112 (73) | 102 (68) | 63 (61) | 57 (58) |
| Paroxysmal AF | 107 (100) | 111 (100) | 147 (96) | 140 (94) | 104 (100) | 99 (100) |
| Time from AF diagnosis, years | Mean 0.7 | Mean 0.8 | Median 1 | Median 1 | Mean 1.3 | Mean 1.3 |
| AFEQT, mean | 62.0 | 59.9 | 61.4 | 57.4 | 58.5 | 62.9 |
| CHA2DS2-VASc score | ||||||
| 0/1 | 82 (77) | 78 (70) | 68 (44) | 65 (44) | 48 (46) | 44 (44) |
| 2 | 13 (12) | 15 (14) | 53 (34) | 48 (32) | 33 (32) | 19 (19) |
| ≥3 | 7 (7) | 12 (11) | 33 (21) | 36 (24) | 23 (22) | 26 (26) |
| Co-morbidities | ||||||
| Hypertension | 33 (31) | 40 (36) | 57 (37) | 55 (37) | 58 (54) | 57 (56) |
| Ischaemic heart disease | 2 (2) | 1 (1) | 12 (8) | 7 (5) | 13 (12) | 12 (12) |
| Heart failure, n (%) | 0 | 0 | 14 (9) | 14 (9) | 1 (1) | 3 (3) |
| Previous stroke/TIA, n (%) | 0 | 1 (1) | 4 (3) | 5 (3) | 2 (2) | 3 (3) |
| Left atrial diameter, mm | 37.0 | 38.0 | 39.5 | 38.1 | 38.7 | 38.2 |
| Follow-up duration | 12 months from day from cryoablation procedure or AAD initiation excluding a 90-day blanking period | |||||
| Primary outcome | Any recurrence of atrial tachyarrhythmia (AF, AT, AFL) lasting longer than 30 seconds | |||||
| Monitoring protocol | 7-day Holter every 3 months | Implantable cardiac monitor with daily transmissions | 24-h Holter every 6 months Weekly patient-activated trans-telephonic event recorder | |||
| Key secondary outcomes |
|
|
| |||
AAD, antiarrhythmic drug; AFEQT, Atrial Fibrillation Effect on QualiTy-of-life; LVEF, left ventricular ejection fraction; TIA, transient ischaemic attack.
| . | CRYO-FIRST . | EARLY-AF . | STOP-AF FIRST . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Endpoint . | Ablation group (n = 107) . | AAD group (n = 111) . | Treatment effect (95% CI) . | Ablation group (n = 154) . | AAD group (n = 149) . | Treatment effect (95% CI) . | Ablation group (n = 104) . | AAD group (n = 99) . | Treatment effect (95% CI) . |
| Documented recurrence of any atrial tachyarrhythmiaa | 16 (17.8) | 33 (29.7) | 0.48 (0.26–0.86) | 66 (42.9) | 101 (67.8) | 0.48 (0.35–0.66) | 21 (20.2) | 35 (35.4) | 0.57 (0.36–0.91) |
| Absolute risk reduction | 14.6% | 24.9% | 15.2% | ||||||
| Atrial fibrillation burdenb | |||||||||
| Mean | NR | NR | 0.6 ± 3.3 | 3.9 ± 12.4 | −3.3 ± 1.0b | NR | NR | ||
| Symptoms | |||||||||
| Documented recurrence of symptomatic arrhythmia | NR | NR | 17 (11.0) | 39 (26.2) | 0.39 (0.22–0.68) | NR | NR | ||
| Asymptomatic at 12 months | 77 (86.5) | 69 (70.4) | 1.23 (1.06–1.43) | 131 (85.1) | 109 (73.2) | 1.16 (1.03–1.31) | NR | NR | |
| Quality of life | |||||||||
| AFEQT score 12 months following treatment initiationc | 88.9 ± 12.8 | 78.1 ± 19.8 | 9.9 (5.5,14.2) | 88.3 ± 19.1 | 80.3 ± 19.1 | 8.0 (3.69–12.31) | 91.9 ± 15.4 | 84.9 ± 15.4 | 7.0 (2.6–11.4) |
| Healthcare utilizationd | 25 | 39 | 0.66 (0.43–1.02) | 30 | 36 | 0.81 (0.53–1.24) | 31 | 43 | 0.69 (0.47–0.99) |
| Cardioversion | NR | NR | 10 | 14 | 0.69 (0.32–1.51) | 3 | 7 | 0.41 (0.11–1.53) | |
| Emergency department visit | NR | NR | 28 | 30 | 0.90 (0.57–1.43) | 10 | 17 | 0.56 (0.27–1.16) | |
| Hospitalization >24 h | NR | NR | 5 | 13 | 0.37 (0.14–1.02) | 13 | 32 | 0.39 (0.22–0.69) | |
| Adverse events | 26 (24.3) | 37 (33.3) | 0.73 (0.48–1.12) | 14 (9.1) | 24 (16.1) | 0.56 (0.30–1.05) | 34 | 45 | 0.72 (0.51–1.02) |
| 42 events | 56 events | 15 events | 27 events | 67 events | 82 events | ||||
| . | CRYO-FIRST . | EARLY-AF . | STOP-AF FIRST . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Endpoint . | Ablation group (n = 107) . | AAD group (n = 111) . | Treatment effect (95% CI) . | Ablation group (n = 154) . | AAD group (n = 149) . | Treatment effect (95% CI) . | Ablation group (n = 104) . | AAD group (n = 99) . | Treatment effect (95% CI) . |
| Documented recurrence of any atrial tachyarrhythmiaa | 16 (17.8) | 33 (29.7) | 0.48 (0.26–0.86) | 66 (42.9) | 101 (67.8) | 0.48 (0.35–0.66) | 21 (20.2) | 35 (35.4) | 0.57 (0.36–0.91) |
| Absolute risk reduction | 14.6% | 24.9% | 15.2% | ||||||
| Atrial fibrillation burdenb | |||||||||
| Mean | NR | NR | 0.6 ± 3.3 | 3.9 ± 12.4 | −3.3 ± 1.0b | NR | NR | ||
| Symptoms | |||||||||
| Documented recurrence of symptomatic arrhythmia | NR | NR | 17 (11.0) | 39 (26.2) | 0.39 (0.22–0.68) | NR | NR | ||
| Asymptomatic at 12 months | 77 (86.5) | 69 (70.4) | 1.23 (1.06–1.43) | 131 (85.1) | 109 (73.2) | 1.16 (1.03–1.31) | NR | NR | |
| Quality of life | |||||||||
| AFEQT score 12 months following treatment initiationc | 88.9 ± 12.8 | 78.1 ± 19.8 | 9.9 (5.5,14.2) | 88.3 ± 19.1 | 80.3 ± 19.1 | 8.0 (3.69–12.31) | 91.9 ± 15.4 | 84.9 ± 15.4 | 7.0 (2.6–11.4) |
| Healthcare utilizationd | 25 | 39 | 0.66 (0.43–1.02) | 30 | 36 | 0.81 (0.53–1.24) | 31 | 43 | 0.69 (0.47–0.99) |
| Cardioversion | NR | NR | 10 | 14 | 0.69 (0.32–1.51) | 3 | 7 | 0.41 (0.11–1.53) | |
| Emergency department visit | NR | NR | 28 | 30 | 0.90 (0.57–1.43) | 10 | 17 | 0.56 (0.27–1.16) | |
| Hospitalization >24 h | NR | NR | 5 | 13 | 0.37 (0.14–1.02) | 13 | 32 | 0.39 (0.22–0.69) | |
| Adverse events | 26 (24.3) | 37 (33.3) | 0.73 (0.48–1.12) | 14 (9.1) | 24 (16.1) | 0.56 (0.30–1.05) | 34 | 45 | 0.72 (0.51–1.02) |
| 42 events | 56 events | 15 events | 27 events | 67 events | 82 events | ||||
Plus–minus values are expressed as mean ± SE, except for atrial fibrillation burden, which is expressed as mean ± SD. Data in the 2nd and 3rd columns are observed data, and data in column 4 are model-based effect estimates.
AAD, antiarrhythmic drug; AFEQT, Atrial Fibrillation Effect on QualiTy-of-life; LVEF, left ventricular ejection fraction; NR, not reported; TIA, transient ischaemic attack.
The treatment effect is expressed as the hazard ratio and 95% confidence interval, which were calculated with the use of Cox regression or the risk ratio via Mantel-Haenszel random-effects model (STOP-AF First).
The between-group absolute difference in atrial fibrillation burden, expressed as the beta coefficient ±SE, was calculated with the use of linear regression analysis.
Changes in quality-of-life scores at 6 months and 12 months from baseline are expressed as least-squares means ±SE and were analysed with the use of a linear mixed-effects model for repeated measures, including group, visit, and interaction between group and visit.
The treatment effect is expressed as the relative risk and 95% confidence interval.
| . | CRYO-FIRST . | EARLY-AF . | STOP-AF FIRST . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Endpoint . | Ablation group (n = 107) . | AAD group (n = 111) . | Treatment effect (95% CI) . | Ablation group (n = 154) . | AAD group (n = 149) . | Treatment effect (95% CI) . | Ablation group (n = 104) . | AAD group (n = 99) . | Treatment effect (95% CI) . |
| Documented recurrence of any atrial tachyarrhythmiaa | 16 (17.8) | 33 (29.7) | 0.48 (0.26–0.86) | 66 (42.9) | 101 (67.8) | 0.48 (0.35–0.66) | 21 (20.2) | 35 (35.4) | 0.57 (0.36–0.91) |
| Absolute risk reduction | 14.6% | 24.9% | 15.2% | ||||||
| Atrial fibrillation burdenb | |||||||||
| Mean | NR | NR | 0.6 ± 3.3 | 3.9 ± 12.4 | −3.3 ± 1.0b | NR | NR | ||
| Symptoms | |||||||||
| Documented recurrence of symptomatic arrhythmia | NR | NR | 17 (11.0) | 39 (26.2) | 0.39 (0.22–0.68) | NR | NR | ||
| Asymptomatic at 12 months | 77 (86.5) | 69 (70.4) | 1.23 (1.06–1.43) | 131 (85.1) | 109 (73.2) | 1.16 (1.03–1.31) | NR | NR | |
| Quality of life | |||||||||
| AFEQT score 12 months following treatment initiationc | 88.9 ± 12.8 | 78.1 ± 19.8 | 9.9 (5.5,14.2) | 88.3 ± 19.1 | 80.3 ± 19.1 | 8.0 (3.69–12.31) | 91.9 ± 15.4 | 84.9 ± 15.4 | 7.0 (2.6–11.4) |
| Healthcare utilizationd | 25 | 39 | 0.66 (0.43–1.02) | 30 | 36 | 0.81 (0.53–1.24) | 31 | 43 | 0.69 (0.47–0.99) |
| Cardioversion | NR | NR | 10 | 14 | 0.69 (0.32–1.51) | 3 | 7 | 0.41 (0.11–1.53) | |
| Emergency department visit | NR | NR | 28 | 30 | 0.90 (0.57–1.43) | 10 | 17 | 0.56 (0.27–1.16) | |
| Hospitalization >24 h | NR | NR | 5 | 13 | 0.37 (0.14–1.02) | 13 | 32 | 0.39 (0.22–0.69) | |
| Adverse events | 26 (24.3) | 37 (33.3) | 0.73 (0.48–1.12) | 14 (9.1) | 24 (16.1) | 0.56 (0.30–1.05) | 34 | 45 | 0.72 (0.51–1.02) |
| 42 events | 56 events | 15 events | 27 events | 67 events | 82 events | ||||
| . | CRYO-FIRST . | EARLY-AF . | STOP-AF FIRST . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Endpoint . | Ablation group (n = 107) . | AAD group (n = 111) . | Treatment effect (95% CI) . | Ablation group (n = 154) . | AAD group (n = 149) . | Treatment effect (95% CI) . | Ablation group (n = 104) . | AAD group (n = 99) . | Treatment effect (95% CI) . |
| Documented recurrence of any atrial tachyarrhythmiaa | 16 (17.8) | 33 (29.7) | 0.48 (0.26–0.86) | 66 (42.9) | 101 (67.8) | 0.48 (0.35–0.66) | 21 (20.2) | 35 (35.4) | 0.57 (0.36–0.91) |
| Absolute risk reduction | 14.6% | 24.9% | 15.2% | ||||||
| Atrial fibrillation burdenb | |||||||||
| Mean | NR | NR | 0.6 ± 3.3 | 3.9 ± 12.4 | −3.3 ± 1.0b | NR | NR | ||
| Symptoms | |||||||||
| Documented recurrence of symptomatic arrhythmia | NR | NR | 17 (11.0) | 39 (26.2) | 0.39 (0.22–0.68) | NR | NR | ||
| Asymptomatic at 12 months | 77 (86.5) | 69 (70.4) | 1.23 (1.06–1.43) | 131 (85.1) | 109 (73.2) | 1.16 (1.03–1.31) | NR | NR | |
| Quality of life | |||||||||
| AFEQT score 12 months following treatment initiationc | 88.9 ± 12.8 | 78.1 ± 19.8 | 9.9 (5.5,14.2) | 88.3 ± 19.1 | 80.3 ± 19.1 | 8.0 (3.69–12.31) | 91.9 ± 15.4 | 84.9 ± 15.4 | 7.0 (2.6–11.4) |
| Healthcare utilizationd | 25 | 39 | 0.66 (0.43–1.02) | 30 | 36 | 0.81 (0.53–1.24) | 31 | 43 | 0.69 (0.47–0.99) |
| Cardioversion | NR | NR | 10 | 14 | 0.69 (0.32–1.51) | 3 | 7 | 0.41 (0.11–1.53) | |
| Emergency department visit | NR | NR | 28 | 30 | 0.90 (0.57–1.43) | 10 | 17 | 0.56 (0.27–1.16) | |
| Hospitalization >24 h | NR | NR | 5 | 13 | 0.37 (0.14–1.02) | 13 | 32 | 0.39 (0.22–0.69) | |
| Adverse events | 26 (24.3) | 37 (33.3) | 0.73 (0.48–1.12) | 14 (9.1) | 24 (16.1) | 0.56 (0.30–1.05) | 34 | 45 | 0.72 (0.51–1.02) |
| 42 events | 56 events | 15 events | 27 events | 67 events | 82 events | ||||
Plus–minus values are expressed as mean ± SE, except for atrial fibrillation burden, which is expressed as mean ± SD. Data in the 2nd and 3rd columns are observed data, and data in column 4 are model-based effect estimates.
AAD, antiarrhythmic drug; AFEQT, Atrial Fibrillation Effect on QualiTy-of-life; LVEF, left ventricular ejection fraction; NR, not reported; TIA, transient ischaemic attack.
The treatment effect is expressed as the hazard ratio and 95% confidence interval, which were calculated with the use of Cox regression or the risk ratio via Mantel-Haenszel random-effects model (STOP-AF First).
The between-group absolute difference in atrial fibrillation burden, expressed as the beta coefficient ±SE, was calculated with the use of linear regression analysis.
Changes in quality-of-life scores at 6 months and 12 months from baseline are expressed as least-squares means ±SE and were analysed with the use of a linear mixed-effects model for repeated measures, including group, visit, and interaction between group and visit.
The treatment effect is expressed as the relative risk and 95% confidence interval.
The patients included in these three studies had predominantly paroxysmal AF (98%), with only the EARLY-AF study including patients with persistent AF (5.3%). Despite being enrolled relatively early in their disease (median time from first AF diagnosis was 1 year), most patients had a significantly impaired quality of life [mean baseline (AFEQT) score of 57.4–62.9]. Those patients enrolled in EARLY-AF were experiencing a median of three symptomatic AF episodes per month[interquartile range (IQR) 1-10], and were relatively more symptomatic (55% having a moderate-severe symptoms vs. 26% in CRYO-FIRST).
Median time from randomization to catheter ablation ranged from 24 to 50 days. Three-minute freezes were recommended in STOP-AF First, protocolized in EARLY-AF, and deferred to the operator in Cryo-FIRST. Procedure duration was longest in STOP-AF First (139 ± 74 min; P < 0.0001), intermediate in EARLY-AF [106 min (IQR 89–131)], and shortest in Cryo-FIRST (84 ± 29 min). Despite these differences, the fluoroscopy time was similar between studies [18.2 ± 11.8 min in STOP-AF First, 18.9 min (IQR 12.6–27.0) in EARLY-AF, and 16 ± 14 min in Cryo-FIRST].
Consistent with EAST-AFNET 4, Class Ic AADs were the most frequently prescribed agent in the AAD group (first agent prescribed in 92% in Cryo-FIRST, 82% in EARLY-AF, and 79% in STOP-AF First). In EARLY-AF, as the study protocol mandated the dose of AADs be aggressively up-titrated using standardized protocols with a goal of complete suppression of AF on the implantable cardiac monitor, slightly more than 30% of patients in the AAD group required multiple AAD trials before exiting the blanking period. Subtherapeutic AAD dosing was observed in 7% in Cryo-FIRST, 0% in EARLY-AF, and 21% in STOP-AF First, with 18%, 0%, and 12% of patients permanently discontinuing AAD therapy, respectively. Cross-over from AAD to ablation occurred in 14% in Cryo-FIRST, 0% in EARLY-AF, and 15% in STOP-AF First.
Cryoballoon ablation as a first-line therapy: outcomes
In each of the three studies, the primary outcome INCLUDED the first recurrence of any atrial tachyarrhythmia (defined as AF, atrial flutter, or atrial tachycardia) lasting 30 s or longer between 91 and 365 days after treatment initiation (i.e. catheter ablation or AAD initiation). Secondary outcomes included the first recurrence of symptomatic atrial tachyarrhythmia (EARLY-AF), AF burden (EARLY-AF), symptom status (EARLY-AF, Cryo-FIRST), disease-specific and generic quality of life (all studies), healthcare utilization (all studies, though the components of cardioversion, emergency department visit, and hospitalization were reported only in EARLY-AF and STOP-AF First), and adverse events (all studies). Serious adverse events included events causing death or functional disability, warranted intervention, or resulted in or prolonged hospitalization for more than 24 h.
Documented recurrence of any atrial tachyarrhythmia occurred in 17.2–42.9% of patients randomized to cryoballoon ablation and 32.4–67.8% of patients randomized to AADs within 1 year following treatment initiation (Table 2). Of note, between study differences in the absolute rates of are due to differences in the arrhythmia monitoring protocols. Specifically, non-invasive intermittent rhythm monitoring (e.g. Holter monitors, as employed in Cryo-FIRST and STOP-AF First) lack sensitivity in detecting paroxysmal arrhythmia, providing higher estimates of arrhythmia-free survival compared to continuous rhythm monitoring (e.g. implantable cardiac monitors, as employed in EARLY-AF). Importantly, as the monitoring strategy was applied consistently within the studies, the between study differences in arrhythmia monitoring protocols are unlikely to affect the relative rates of recurrence between the randomized groups . In other words, while the absolute reduction in the rates of atrial tachyarrhythmia recurrence ranged from 15% (Cryo-FIRST and STOP-AF First) to 25% (EARLY-AF), the relative benefit of first-line cryoablation was remarkbly consistent [hazard ratio (HR) of 0.50 (0.29–0.86) in Cryo-FIRST, 0.57 (0.36–0.91) in STOP-AF First, and 0.63 (0.51–0.78) in EARLY-AF].
Freedom from symptomatic atrial tachyarrhythmia and AF burden (percentage time in AF) were only available in the EARLY-AF trial. For symptomatic atrial tachyarrhythmia, there was a significant 15.2% absolute reduction in the rate of recurrence (RR 0.42, 95% CI 0.25–0.71; number needed to treat 7). AF burden was significantly lower in the ablation group (mean difference between of 3.3 ± 1.0% between the ablation group and AAD group, effectively 1 day less AF per month for patients randomized to ablation).
The proportion of patients who were asymptomatic at 1 year was reported in Cryo-FIRST and EARLY-AF. In aggregate, 86% of patients randomized to ablation and 72% of patients randomized to AADs were free of symptoms at 1 year following enrolment (RR 1.19; 95% CI: 1.08–1.30).
In all three studies, the change in health-related quality of life at 1 year was significantly improved from baseline for both the ablation and the AAD groups. For the disease-specific AFEQT questionnaire, those randomized to ablation attained a 27–33-point improvement from baseline at 1 year, with those randomized to AADs achieving a 19–22-point improvement from baseline. In aggregate, at 1 year the mean treatment effect (i.e. the difference between the randomized groups) was 8.3 points (95% CI 5.8–10.8) in favour of ablation,25 which exceeded the minimally clinically relevant difference (e.g. 5 points) for the AFEQT score.33 Similar clinically relevant improvements were observed for the generic EQ-5D questionnaire (0.07 ± 0.03 points in EARLY-AF) but not for the SF-36 questionnaire employed in Cryo-FIRST.
Within each study the aggregate healthcare utilization did not differ between randomized groups; however, it is important to recall that these studies were not individually powered for this endpoint. When these three studies were combined, pooled analysis demonstrated that significantly fewer patients randomized to first-line cryoballoon ablation experienced the composite healthcare utilization outcome with an absolute reduction of 9% or a number need to treat of 11 (RR 0.71, 95% CI 0.56–0.90). This result was propelled by a compelling reduction in hospitalization (RR 0.38; 95% CI 0.23–0.63), although non-significant reductions in emergency department visits (RR 0.78, 95% CI 0.50–1.20), and cardioversions (RR 0.60, 95% CI 0.31–1.18) were also observed.25
With respect to safety outcomes, clinically significant serious adverse events (bradycardia requiring pacemaker implantation, pericardial effusion or tamponade, phrenic nerve injury, stroke or systemic thromboembolism, syncope, and ventricular pro-arrhythmia) were comparable between those randomized to first-line cryoballoon catheter ablation and AAD therapy (RR 0.74, 95% CI 0.35–1.56), with ablation being associated with a lower incidence of any adverse event (RR 0.70; 95% CI 0.54–0.89).25
First-line cryoablation: unanswered questions
Despite the evidence supporting first-line cryoablation (Figure 3) there remain several unanswered questions. Specifically, (i) can these results be extrapolated to other ablation energy sources?, (ii) can these results be extrapolated to patients with more advanced forms of AF (e.g. persistent AF)?, (iii) Does early ablation results in beneficial effects on the progression to more persistent forms of AF?, and (iv) are these results durable over long-term follow-up?
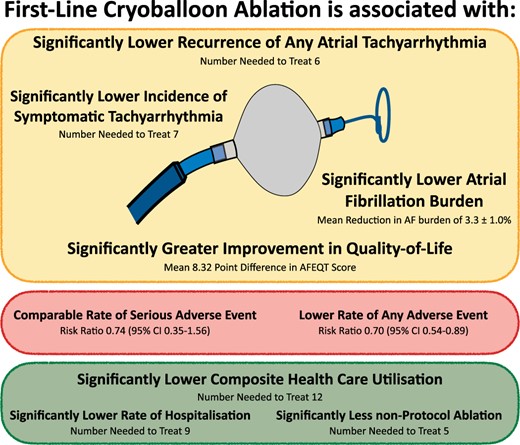
The effect of first-line cryoballoon ablation for treatment-naive AF. AF, atrial fibrillation; CI, confidence interval.
Regarding the first question, while recent randomized clinical trials have observed similar arrhythmia outcomes for patients with AAD-refractory AF treated with cryoballoon ablation and contact-force radiofrequency ablation,34 the previous studies of first-line radiofrequency ablation failed to demonstrate a clinically meaningful difference in arrhythmia outcomes, quality of life improvement, and healthcare utilization.22–24 It is possible that these differences may be related to operator and centre experience, as previous studies have demonstrated that outcomes with radiofrequency energy are more closely tied to procedural volume.26 Conversely, the outcomes with cryoballoon ablation appear to be more generalizable, with outcomes being fairly consistent between low- and high-volume centres.26 As such, while it may be reasonable to extrapolate the results of these first-line cryoballoon studies to high-volume radiofrequency centres, further study is required to determine if the results of first-line radiofrequency catheter ablation are comparable in lower volume or less experienced centres.
Regarding the second question, most patients included in the three randomized first-line cryoablation trials were experiencing paroxysmal AF. Although it is unknown whether the results of these first-line ablation studies can be extrapolated to patients with more persistent forms of AF, there is currently no evidence to suggest that the relative benefit of ablation (vs. AAD therapy) differs between those with paroxysmal and persistent AF. Specifically, while it is known that PV isolation alone is less successful in patients with persistent AF (relative to paroxysmal AF),35,36 the CABANA study demonstrated comparable relative reductions in non-invasive AF burden observed after ablation of both paroxysmal AF and persistent AF.37 This suggests that the benefit of ablation relative to AAD therapy is maintained with more advanced forms of AF.
Regarding the third question, it is postulated that the cumulative effect of the intermittent AF episodes is electrical and structural atrial remodelling, predisposing towards more sustained arrhythmia and driving the progression from paroxysmal to more persistent forms of AF.9,21 By extrapolation, it is hypothesized that early intervention with a tailored catheter ablation procedure targeting the underlying mechanism of AF could halt the progressive pathoanatomical changes associated with AF. While not definitely proven, the randomized ‘Catheter ablation or medical therapy to delay progression of atrial fibrillation’ (ATTEST) trial observed that catheter ablation was superior to guideline-directed AAD therapy in delaying the progression from paroxysmal to persistent AF at 3 years of follow-up (2.4% vs. 17.5%; P < 0.001).21
Regarding the last question, the follow-up reported in these three randomized clinical trials was limited to 12 months following randomization. Longer-term follow-up is required to determine the durability of the result in terms of arrhythmia recurrence, AF progression, and healthcare utilization. Longer-term follow-up from the EARLY-AF trial is underway and will provide information regarding the natural history of AF (e.g. disease progression), the relative long-term efficacy of the treatment approaches (first-line catheter ablation vs. arrhythmic drug therapy), and the downstream healthcare utilization associated with these treatment approaches, as well as the long-term impact on patient-reported outcomes.
Conclusions
An initial treatment strategy of first-line cryoballoon ablation has been shown to be superior to AAD therapy for the management of patients with treatment-naïve AF. Compared to AADs, initial cryoballoon catheter ablation resulted in greater freedom from arrhythmia recurrence, a greater improvement in quality of life, and significantly lower subsequent healthcare resource utilization. These findings are relevant to patients, providers, and healthcare systems, helping inform the decision regarding the initial choice of rhythm-control therapy in patients with treatment-naïve AF.
Acknowledgements
The authors wish to thank the patients participated in the trials, as well as the study sites and co-ordinators.
Funding
This work was not funded. The EARLY-AF Trial was funded by a peer-reviewed grant from the Cardiac Arrhythmia Network of Canada [grant number SRG-15-P15-001], with additional unrestricted support Medtronic and Baylis Medical. The STOP-AF First trial and the Cryo-FIRST trial were supported by Medtronic.
Conflict of interest: J.G.A. reports grants and personal fees from Medtronic, grants from Baylis, personal fees from Biosense-Webster. G.B.C. reports speaker fees for Medtronic, Biotronik, Biosense Webster, and Abbott. O.M.W. reports grants from Medtronic and personal fees from Biosense Webster and Boston Scientific. M.K. reports speaker fees from Abbott and Medtronic; proctoring, consultancy, and advisory board services for Medtronic; research grants from Medtronic and Biosense Webster.



