-
PDF
- Split View
-
Views
-
Cite
Cite
Li-Bin Shi, Ole Rossvoll, Pål Tande, Peter Schuster, Eivind Solheim, Jian Chen, Cryoballoon vs. radiofrequency catheter ablation: insights from NOrwegian randomized study of PERSistent Atrial Fibrillation (NO-PERSAF study), EP Europace, Volume 24, Issue 2, February 2022, Pages 226–233, https://doi.org/10.1093/europace/euab281
Close - Share Icon Share
Abstract
Pulmonary vein isolation (PVI) is still regarded as a cornerstone for treatment of persistent atrial fibrillation (AF). This study evaluated the effectiveness of PVI performed with cryoballoon ablation (CBA) in comparison with radiofrequency ablation (RFA) in patients with persistent AF.
A total of 101 patients with symptomatic persistent AF were enrolled and randomized (1:1) to CBA or RFA groups and followed up for 12 months. The primary endpoint was any documented recurrent atrial tachyarrhythmia (ATA) lasting longer than 30 s following a 3-month blanking period. Secondary endpoints were procedure-related complications, procedure and ablation duration, and fluoroscopy time. The ATA-free survival curves were estimated by Kaplan–Meier method and analysed by the log-rank test. According to intention-to-treat analysis, freedom from ATA was achieved in 36 out of 52 patients in the CBA group and 30 out of 49 patients in the RFA group (69.2% vs. 61.2%, P = 0.393). No difference in AF recurrence was found between the two groups (27.5% in CBA vs. 38.0% in RFA, P = 0.258), and less atrial flutter recurrence was documented in the CBA group compared with the RFA group (3.9% vs. 18.0%, P = 0.020). The procedure and ablation duration were significantly shorter in the CBA group (160 ± 31 vs. 197 ± 38 min, P < 0.0001; 36.7 ± 9.5 vs. 55.3 ± 16.7 min, P < 0.0001). There was no difference regarding fluoroscopy time (21.5 ± 7.8 vs. 23.4 ± 11.2 min, P > 0.05).
Compared with RFA, PVI performed by CBA led to shorter procedure and ablation duration, with less atrial flutter recurrence and similar freedom from ATA at 12-month follow-up.
This is so far the first randomized study comparing cryoballoon vs. radiofrequency catheter ablation in treatment of persistent atrial fibrillation (AF).
Pulmonary vein isolation achieved by cryoballoon is as effective as radiofrequency ablation for treatment of persistent AF in terms of atrial-tachyarrhythmia freedom at 12-month follow-up, with less atrial flutter recurrence and shorter procedure and ablation times.
Introduction
It has been demonstrated that catheter ablation is effective and safe for treatment of paroxysmal atrial fibrillation (AF), but the long-term outcome is poor for patients with persistent or long-standing persistent AF.1–3 Although a variety of additional ablation strategies have been attempted, electrical pulmonary vein isolation (PVI) is still regarded as a cornerstone of treatment for persistent AF.1,3,4 Radiofrequency ablation (RFA) by point-by-point mode is the most common method to achieve PVI, while cryoballoon ablation (CBA) has emerged as an alternative technique. Similar clinical outcomes and durability of PVI in ablation of paroxysmal AF have been demonstrated by randomized controlled trials.5–7 For persistent AF, the success rate of CBA ranged from 60% to 70% at 1-year follow-up,8–11 while a relatively low success rate of 35.6–41.0% for RFA was reported from earlier studies.1–3,12 Hoffmann et al.13 reported a similar recurrence rate of atrial tachyarrhythmia (ATA) for RFA and CBA in patients with persistent AF in a prospective multicentre and multinational observational cluster cohort study. Randomized controlled trials are called for to compare CBA and RFA for PVI in treatment of persistent AF.
We aimed to evaluate the effectiveness of PVI performed with CBA in comparison with contact-force-sensing RFA in patients with persistent or long-standing persistent AF.
Methods
Study design
NO-PERSAF study (ClinicalTrials.gov number, NCT03008811) is a prospective, randomized (1:1), open-label, multicentre clinical trial to compare clinical outcomes of PVI achieved using cryoballoon and contact-force-sensing RFA catheter in persistent or long-standing persistent AF. All patients were recruited in three Norwegian centres (two high volume and one middle volume). This study was conducted following the Declaration of Helsinki and approved by the Regional Ethical Committee of Western Norway.
Study participants and randomization
Between November 2016 and March 2020, this study enrolled patients who underwent PV isolation as the first ablation procedure for symptomatic persistent AF (lasting for >7 days, but <12 months) or long-standing persistent AF (lasting for >12 months) refractory to at least one antiarrhythmic drug. All patients had received at least one direct current cardioversion. Eligibility criteria were 18–75 years old and able and willing to give informed consent. Exclusion criteria were any previous left atrial (LA) ablation or surgery, presence of an intracavitary thrombus, uncontrolled severe heart failure, severe valvular disease, LA anteroposterior diameter >60 mm confirmed by echocardiography, AF lasting longer than 36 months, contraindications to systemic anticoagulation with heparin or Warfarin, severe renal dysfunction, and acute coronary syndrome. After the written informed consent had been signed, patients were randomly assigned in a 1:1 ratio to the CBA group or RFA group.
Ablation procedure
All patients had taken oral anticoagulants for at least 4 weeks. Transoesophageal echocardiography, but not cardiac computed tomography, was performed on all patients before the procedure. The patients underwent the ablation procedure under conscious sedation. Heparin was administered immediately after transseptal access to the LA. Activated clotting time was kept between 250 and 350 s throughout the procedure. Angiography of the pulmonary veins (PVs) was performed after the transseptal puncture. The procedural endpoint was defined as electrical isolation of the PV demonstrated by the elimination of PV potentials in the ostium.
Cryoballoon ablation
After the transseptal puncture, a steerable 12-Fr sheath (Flexcath®, Medtronic) was placed in the LA. All patients were treated with a 28-mm diameter cryoballoon (Arctic Front Advance®, Medtronic). A circular mapping catheter (Achieve™, Medtronic) was inserted through the lumen of the cryoballoon and was advanced more distally to stabilize the cryoballoon at the PV ostium. Occlusion of the PV by the balloon was confirmed by venography. The ablation regimen consisted of two freezing applications of 240 s in each PV, no matter the PV was isolated or not after the first CBA. If the PV was still not isolated after two attempts, extra freezing should be applied. To prevent damage of the phrenic nerve while ablating the right PVs, visual inspection of diaphragmatic contraction and monitoring of the diaphragmatic compound motor action potential were performed during phrenic nerve pacing with another diagnostic catheter at a high output (up to 20 mA and 2 ms duration).
Radiofrequency ablation
After the transseptal puncture, a long sheath (Swartz™, Abbott Medical) was placed in the LA. A circular mapping catheter (Advisor™ FL, Sensor Enabled™, Abbott Medical) was inserted in the PVs for monitoring the pulmonary potentials. All patients were treated with a contact-force-sensing irrigated ablation catheter (TactiCath™ Quartz, Abbott Medical) with support of a deflectable long sheath (Agilis™, Abbott Medical). An encircling ablation strategy was performed in all PVs, with targeting force-time-integral of 400 g·s for each lesion.
A three-dimensional mapping system (EnSite NavX, Abbott Medical) was employed to reconstruct the LA geometry in all patients, and bipolar voltage mapping was performed with the circular mapping catheter in AF before ablation and in sinus rhythm after PVI. If the procedure started with sinus rhythm, we induced AF with burst atrial pacing. If the patient was still in AF after PVI, direct current cardioversion was conducted to resume sinus rhythm.
Patients without complications were discharged from the hospital within 1–2 days of the procedure. Oral anticoagulation was continued for at least 3 months.
Follow-up and endpoints
Antiarrhythmic drugs were maintained for at least 3 months and then discontinued at the physicians’ discretion. The patients received direct current cardioversion if they suffered persistent AF during the first month after the procedure. All patients were followed up in an out-patient clinic with a 7-day ambulatory ECG at 3, 6, and 12 months after ablation. Post-procedural cardiac computed tomography was performed between 3 and 6 months. The primary endpoint was defined as any documented ATA, including AF, atrial flutter, and atrial tachycardia, lasting longer than 30 s in duration after a 3-month blanking period. Secondary endpoints were defined as procedure and ablation duration, fluoroscopy time, and procedure-related complications, including bleeding/haematoma, phrenic nerve palsy, stroke, pericardial effusion or tamponade, PV stenosis, coronary artery stenosis/occlusion, and atrio-oesophageal fistula.
Statistical analysis
The sample size was calculated based on previously published data covering a range of ablation modalities and methodologies. We estimated that the success rate in patients with persistent AF was around 40% for RFA and 65% for CBA. In order to statistically assess the difference in success rate between these two techniques, at least 94 patients needed to be randomized in the two groups (1:1) for 80% power at a 5% of two-sided significance level. Assuming a dropout rate of 5%, we needed a minimum of 50 patients in each group.
The Shapiro–Wilk test was used for testing normality. Continuous variables were presented as mean ± standard deviation if normally distributed, otherwise presented as median and interquartile ranges (IQRs). To compare means of continuous data, a two-sample t-test and Mann–Whitney U test were employed for normally and skewed distributed data, respectively. Categorical values were presented as percentages and analysed by the χ2 test or Fisher’s exact test as appropriate. The Kaplan–Meier method was used to estimate the survival curves for the time to first primary endpoint and was analysed by the log-rank test. Logistic regression analysis was performed to evaluate predictors for the recurrence of ATA. Variables with a P-value over 0.1 in the univariate analysis were removed from the model for subsequent multivariate analysis. A P-value of <0.05 was considered statistically significant.
Assessment of the primary endpoint was conducted by both intention-to-treat (ITT) and per-protocol analysis.
Results
Population characteristics
A total of 101 patients (79.2% men; mean age 63.2 ± 8.6 years) were randomly enrolled in the study: 52 patients were assigned to the CBA group and 49 to the RFA group (ITT). The patient flow diagram is shown in Figure 1. One patient in the CBA group did not receive the allocated treatment because of a technical problem and underwent RFA treatment instead (crossover). Thus, 51 patients underwent CBA and 50 patients received RFA treatment (per-protocol). One patient in the RFA group suffered cardiac tamponade during the transseptal puncture, after which the procedure was interrupted without ablation. This patient received a new RFA procedure 3 months later and was followed up for 12 months after the second procedure. The median duration of persistent AF before the procedure was 8.0 (0.3–12.0) months, and long-standing persistent AF was presented in 24 patients (14 in CBA and 10 in RFA). Seven patients had a history of earlier cavotricuspid isthmus ablation for typical atrial flutter (AFL). No significant differences in clinical characteristics were found between the two groups (Table 1).
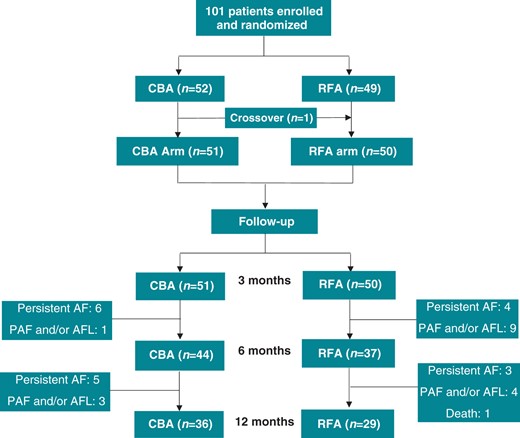
Randomization and patient flow for NO-PERSAF study. The number of patients with AF recurrence and recurrence-free during follow-up was based on per-protocol analysis. The crossover patient suffered AF recurrence. AF, atrial fibrillation; AFL, atrial flutter; CBA, cryoballoon ablation; PAF, paroxysmal atrial fibrillation; RFA, radiofrequency ablation.
| . | CBA group (n = 52) . | RFA group (n = 49) . | P value . |
|---|---|---|---|
| Age (years) | 62.4 ± 8.4 | 64.0 ± 8.7 | 0.363 |
| Male, n (%) | 45 (86.5%) | 35 (71.4%) | 0.061 |
| Body mass index, kg/m2 | 29.6 ± 4.7 | 28.8 ± 4.5 | 0.368 |
| Hypertension, n (%) | 29 (55.8%) | 28 (58.0%) | 0.889 |
| Diabetes mellitus, n (%) | 1 (1.9%) | 4 (8.2%) | 0.148 |
| Coronary heart disease, n (%) | 4 (7.7%) | 6 (12.2%) | 0.444 |
| Obstructive sleep apnoea, n (%) | 5 (9.6%) | 3 (6.1%) | 0.516 |
| CHA2DS2VASC score | 0.294 | ||
| 0 | 14 (26.9%) | 7 (14.3%) | |
| 1 | 11 (21.2%) | 12 (24.5%) | |
| ≥2 | 27 (51.9%) | 30 (61.2%) | |
| Serum creatinine (μmol/L) | 89.2 ± 15.8 | 87.0 ± 16.3 | 0.478 |
| History of AF, n (%) | 0.518 | ||
| <1 year | 6 (11.5%) | 10 (20.4%) | |
| 1–2 years | 20 (38.5%) | 17 (34.7%) | |
| >2 years | 26 (50.0%) | 22 (44.9%) | |
| Duration of persistent AF before procedure (months) | 8 (1–14) | 8 (0–12) | 0.689 |
| Sinus rhythm before procedure, n (%) | 7 (13.5%) | 10 (20.4%) | 0.351 |
| <6 months | 15 (28.8%) | 13 (26.5%) | 0.795 |
| 6–12 months | 16 (30.8%) | 16 (32.7%) | 0.839 |
| >12 months | 14 (26.9%) | 10 (20.4%) | 0.490 |
| History of previous cavotricuspid ablation, n (%) | 3 (5.8%) | 4 (8.2%) | 0.710 |
| Left atrial diameter (cm) | 4.6 ± 0.6 | 4.4 ± 0.7 | 0.110 |
| Left ventricular ejection fraction (%) | 56.0 ± 7.2 | 56.8 ± 8.1 | 0.679 |
| Basal medication | |||
| Beta-blocker | 36 (69.2%) | 30 (61.2%) | 0.398 |
| Amiodarone | 14 (26.9%) | 13 (26.5%) | 0.617 |
| Dronedarone | 28 (53.8%) | 17 (34.7%) | 0.053 |
| Flecainide | 3 (5.8%) | 3 (6.1%) | 1.000 |
| . | CBA group (n = 52) . | RFA group (n = 49) . | P value . |
|---|---|---|---|
| Age (years) | 62.4 ± 8.4 | 64.0 ± 8.7 | 0.363 |
| Male, n (%) | 45 (86.5%) | 35 (71.4%) | 0.061 |
| Body mass index, kg/m2 | 29.6 ± 4.7 | 28.8 ± 4.5 | 0.368 |
| Hypertension, n (%) | 29 (55.8%) | 28 (58.0%) | 0.889 |
| Diabetes mellitus, n (%) | 1 (1.9%) | 4 (8.2%) | 0.148 |
| Coronary heart disease, n (%) | 4 (7.7%) | 6 (12.2%) | 0.444 |
| Obstructive sleep apnoea, n (%) | 5 (9.6%) | 3 (6.1%) | 0.516 |
| CHA2DS2VASC score | 0.294 | ||
| 0 | 14 (26.9%) | 7 (14.3%) | |
| 1 | 11 (21.2%) | 12 (24.5%) | |
| ≥2 | 27 (51.9%) | 30 (61.2%) | |
| Serum creatinine (μmol/L) | 89.2 ± 15.8 | 87.0 ± 16.3 | 0.478 |
| History of AF, n (%) | 0.518 | ||
| <1 year | 6 (11.5%) | 10 (20.4%) | |
| 1–2 years | 20 (38.5%) | 17 (34.7%) | |
| >2 years | 26 (50.0%) | 22 (44.9%) | |
| Duration of persistent AF before procedure (months) | 8 (1–14) | 8 (0–12) | 0.689 |
| Sinus rhythm before procedure, n (%) | 7 (13.5%) | 10 (20.4%) | 0.351 |
| <6 months | 15 (28.8%) | 13 (26.5%) | 0.795 |
| 6–12 months | 16 (30.8%) | 16 (32.7%) | 0.839 |
| >12 months | 14 (26.9%) | 10 (20.4%) | 0.490 |
| History of previous cavotricuspid ablation, n (%) | 3 (5.8%) | 4 (8.2%) | 0.710 |
| Left atrial diameter (cm) | 4.6 ± 0.6 | 4.4 ± 0.7 | 0.110 |
| Left ventricular ejection fraction (%) | 56.0 ± 7.2 | 56.8 ± 8.1 | 0.679 |
| Basal medication | |||
| Beta-blocker | 36 (69.2%) | 30 (61.2%) | 0.398 |
| Amiodarone | 14 (26.9%) | 13 (26.5%) | 0.617 |
| Dronedarone | 28 (53.8%) | 17 (34.7%) | 0.053 |
| Flecainide | 3 (5.8%) | 3 (6.1%) | 1.000 |
AF, atrial fibrillation; CBA, cryoballoon ablation; RFA, radiofrequency ablation.
| . | CBA group (n = 52) . | RFA group (n = 49) . | P value . |
|---|---|---|---|
| Age (years) | 62.4 ± 8.4 | 64.0 ± 8.7 | 0.363 |
| Male, n (%) | 45 (86.5%) | 35 (71.4%) | 0.061 |
| Body mass index, kg/m2 | 29.6 ± 4.7 | 28.8 ± 4.5 | 0.368 |
| Hypertension, n (%) | 29 (55.8%) | 28 (58.0%) | 0.889 |
| Diabetes mellitus, n (%) | 1 (1.9%) | 4 (8.2%) | 0.148 |
| Coronary heart disease, n (%) | 4 (7.7%) | 6 (12.2%) | 0.444 |
| Obstructive sleep apnoea, n (%) | 5 (9.6%) | 3 (6.1%) | 0.516 |
| CHA2DS2VASC score | 0.294 | ||
| 0 | 14 (26.9%) | 7 (14.3%) | |
| 1 | 11 (21.2%) | 12 (24.5%) | |
| ≥2 | 27 (51.9%) | 30 (61.2%) | |
| Serum creatinine (μmol/L) | 89.2 ± 15.8 | 87.0 ± 16.3 | 0.478 |
| History of AF, n (%) | 0.518 | ||
| <1 year | 6 (11.5%) | 10 (20.4%) | |
| 1–2 years | 20 (38.5%) | 17 (34.7%) | |
| >2 years | 26 (50.0%) | 22 (44.9%) | |
| Duration of persistent AF before procedure (months) | 8 (1–14) | 8 (0–12) | 0.689 |
| Sinus rhythm before procedure, n (%) | 7 (13.5%) | 10 (20.4%) | 0.351 |
| <6 months | 15 (28.8%) | 13 (26.5%) | 0.795 |
| 6–12 months | 16 (30.8%) | 16 (32.7%) | 0.839 |
| >12 months | 14 (26.9%) | 10 (20.4%) | 0.490 |
| History of previous cavotricuspid ablation, n (%) | 3 (5.8%) | 4 (8.2%) | 0.710 |
| Left atrial diameter (cm) | 4.6 ± 0.6 | 4.4 ± 0.7 | 0.110 |
| Left ventricular ejection fraction (%) | 56.0 ± 7.2 | 56.8 ± 8.1 | 0.679 |
| Basal medication | |||
| Beta-blocker | 36 (69.2%) | 30 (61.2%) | 0.398 |
| Amiodarone | 14 (26.9%) | 13 (26.5%) | 0.617 |
| Dronedarone | 28 (53.8%) | 17 (34.7%) | 0.053 |
| Flecainide | 3 (5.8%) | 3 (6.1%) | 1.000 |
| . | CBA group (n = 52) . | RFA group (n = 49) . | P value . |
|---|---|---|---|
| Age (years) | 62.4 ± 8.4 | 64.0 ± 8.7 | 0.363 |
| Male, n (%) | 45 (86.5%) | 35 (71.4%) | 0.061 |
| Body mass index, kg/m2 | 29.6 ± 4.7 | 28.8 ± 4.5 | 0.368 |
| Hypertension, n (%) | 29 (55.8%) | 28 (58.0%) | 0.889 |
| Diabetes mellitus, n (%) | 1 (1.9%) | 4 (8.2%) | 0.148 |
| Coronary heart disease, n (%) | 4 (7.7%) | 6 (12.2%) | 0.444 |
| Obstructive sleep apnoea, n (%) | 5 (9.6%) | 3 (6.1%) | 0.516 |
| CHA2DS2VASC score | 0.294 | ||
| 0 | 14 (26.9%) | 7 (14.3%) | |
| 1 | 11 (21.2%) | 12 (24.5%) | |
| ≥2 | 27 (51.9%) | 30 (61.2%) | |
| Serum creatinine (μmol/L) | 89.2 ± 15.8 | 87.0 ± 16.3 | 0.478 |
| History of AF, n (%) | 0.518 | ||
| <1 year | 6 (11.5%) | 10 (20.4%) | |
| 1–2 years | 20 (38.5%) | 17 (34.7%) | |
| >2 years | 26 (50.0%) | 22 (44.9%) | |
| Duration of persistent AF before procedure (months) | 8 (1–14) | 8 (0–12) | 0.689 |
| Sinus rhythm before procedure, n (%) | 7 (13.5%) | 10 (20.4%) | 0.351 |
| <6 months | 15 (28.8%) | 13 (26.5%) | 0.795 |
| 6–12 months | 16 (30.8%) | 16 (32.7%) | 0.839 |
| >12 months | 14 (26.9%) | 10 (20.4%) | 0.490 |
| History of previous cavotricuspid ablation, n (%) | 3 (5.8%) | 4 (8.2%) | 0.710 |
| Left atrial diameter (cm) | 4.6 ± 0.6 | 4.4 ± 0.7 | 0.110 |
| Left ventricular ejection fraction (%) | 56.0 ± 7.2 | 56.8 ± 8.1 | 0.679 |
| Basal medication | |||
| Beta-blocker | 36 (69.2%) | 30 (61.2%) | 0.398 |
| Amiodarone | 14 (26.9%) | 13 (26.5%) | 0.617 |
| Dronedarone | 28 (53.8%) | 17 (34.7%) | 0.053 |
| Flecainide | 3 (5.8%) | 3 (6.1%) | 1.000 |
AF, atrial fibrillation; CBA, cryoballoon ablation; RFA, radiofrequency ablation.
Clinical results
A total of 397 PVs were targeted. Left common PV was found in 7 patients (2 in CBA and 5 in RFA). Four PVs (two left inferior and two right inferior) from three patients in the CBA group were not isolated by the cryoballoon so a cryo (Freezor™, Medtronic) or RFA catheter (TactiCath™ Quartz, Abbott Medical) had to be employed. All PVs were successfully isolated by the end of the procedure. After PVI, AF was converted to sinus rhythm in three patients (two in RFA, one in CBA). In two patients in the RFA group, AF changed to typical AFL and was terminated after cavotricuspid isthmus block. One patient in the CBA group was converted to AFL which was not further treated during the index procedure. Fluoroscopy times were similar between the CBA and RFA groups, while shorter procedure and ablation times were found in the CBA group (P < 0.001) (Table 2).
| . | Total (n = 101) . | Intention-to-treat analysis . | Per-protocol analysis . | ||||
|---|---|---|---|---|---|---|---|
| CBA group (n = 52) . | RFA group (n = 49) . | P value . | CBA group (n = 51) . | RFA group (n = 50) . | P value . | ||
| Procedure started in sinus rhythm | 17 (16.8%) | 7 (13.5%) | 10 (20.4%) | 0.351 | 7 (13.7%) | 10 (20.0%) | 0.399 |
| AF terminated during ablation | 5 (5.0%) | 1 (1.9%) | 4 (8.0%) | 0.205 | 1 (2.0%) | 4 (8.0%) | 0.162 |
| Ablation time (min) | 45.6 ± 16.3 | 36.7 ± 9.5 | 55.3 ± 16.7 | 0.000 | 35.8 ± 6.5 | 55.9 ± 16.7 | 0.000 |
| Fluoroscopy time (min) | 22.4 ± 9.6 | 21.5 ± 7.8 | 23.4 ± 11.2 | 0.317 | 21.2 ± 7.6 | 23.6 ± 11.2 | 0.208 |
| Procedure time (min) | 178.2 ± 39.1 | 160.4 ± 30.6 | 197.2 ± 38.4 | 0.000 | 158.9 ± 28.9 | 197.9 ± 38.4 | 0.000 |
| Recurrence before discharge | 9 (8.9%) | 5 (9.6%) | 4 (8.2%) | 0.704 | 4 (7.8%) | 5 (10.0%) | 0.704 |
| Recurrence in the blanking period | 34 (33.7%) | 18 (34.6%) | 16 (32.7%) | 0.835 | 17 (33.3%) | 17 (34.0%) | 0.943 |
| Recurrence during follow-up | 35 (34.7%) | 16 (30.8%) | 19 (38.8%) | 0.398 | 15 (29.4%) | 20 (40.0%) | 0.264 |
| Persistent AF | 18 | 11 | 7 | 0.049 | 11 | 7 | 0.023 |
| Paroxysmal AF alone | 6 | 2 | 4 | 2 | 4 | ||
| Paroxysmal AF with AFL | 9 | 2 | 7 | 1 | 8 | ||
| AFL alone | 2 | 1 | 1 | 1 | 1 | ||
| Recurrence of AFL | 11 | 3 (5.8%) | 8 (16.3%) | 0.063 | 2 (3.9%) | 9 (18.0%) | 0.020 |
| Typical AFL | 2 | 0 | 2 | 0 | 2 | ||
| Atypical AFL | 8 | 2 | 6 | 1 | 7 | ||
| Both | 1 | 1 | 0 | 1 | 0 | ||
| . | Total (n = 101) . | Intention-to-treat analysis . | Per-protocol analysis . | ||||
|---|---|---|---|---|---|---|---|
| CBA group (n = 52) . | RFA group (n = 49) . | P value . | CBA group (n = 51) . | RFA group (n = 50) . | P value . | ||
| Procedure started in sinus rhythm | 17 (16.8%) | 7 (13.5%) | 10 (20.4%) | 0.351 | 7 (13.7%) | 10 (20.0%) | 0.399 |
| AF terminated during ablation | 5 (5.0%) | 1 (1.9%) | 4 (8.0%) | 0.205 | 1 (2.0%) | 4 (8.0%) | 0.162 |
| Ablation time (min) | 45.6 ± 16.3 | 36.7 ± 9.5 | 55.3 ± 16.7 | 0.000 | 35.8 ± 6.5 | 55.9 ± 16.7 | 0.000 |
| Fluoroscopy time (min) | 22.4 ± 9.6 | 21.5 ± 7.8 | 23.4 ± 11.2 | 0.317 | 21.2 ± 7.6 | 23.6 ± 11.2 | 0.208 |
| Procedure time (min) | 178.2 ± 39.1 | 160.4 ± 30.6 | 197.2 ± 38.4 | 0.000 | 158.9 ± 28.9 | 197.9 ± 38.4 | 0.000 |
| Recurrence before discharge | 9 (8.9%) | 5 (9.6%) | 4 (8.2%) | 0.704 | 4 (7.8%) | 5 (10.0%) | 0.704 |
| Recurrence in the blanking period | 34 (33.7%) | 18 (34.6%) | 16 (32.7%) | 0.835 | 17 (33.3%) | 17 (34.0%) | 0.943 |
| Recurrence during follow-up | 35 (34.7%) | 16 (30.8%) | 19 (38.8%) | 0.398 | 15 (29.4%) | 20 (40.0%) | 0.264 |
| Persistent AF | 18 | 11 | 7 | 0.049 | 11 | 7 | 0.023 |
| Paroxysmal AF alone | 6 | 2 | 4 | 2 | 4 | ||
| Paroxysmal AF with AFL | 9 | 2 | 7 | 1 | 8 | ||
| AFL alone | 2 | 1 | 1 | 1 | 1 | ||
| Recurrence of AFL | 11 | 3 (5.8%) | 8 (16.3%) | 0.063 | 2 (3.9%) | 9 (18.0%) | 0.020 |
| Typical AFL | 2 | 0 | 2 | 0 | 2 | ||
| Atypical AFL | 8 | 2 | 6 | 1 | 7 | ||
| Both | 1 | 1 | 0 | 1 | 0 | ||
Values are presented as mean ± SD, or n (%).
AF, atrial fibrillation; AFL, atrial flutter; CBA, cryoballoon ablation; RFA, radiofrequency ablation.
| . | Total (n = 101) . | Intention-to-treat analysis . | Per-protocol analysis . | ||||
|---|---|---|---|---|---|---|---|
| CBA group (n = 52) . | RFA group (n = 49) . | P value . | CBA group (n = 51) . | RFA group (n = 50) . | P value . | ||
| Procedure started in sinus rhythm | 17 (16.8%) | 7 (13.5%) | 10 (20.4%) | 0.351 | 7 (13.7%) | 10 (20.0%) | 0.399 |
| AF terminated during ablation | 5 (5.0%) | 1 (1.9%) | 4 (8.0%) | 0.205 | 1 (2.0%) | 4 (8.0%) | 0.162 |
| Ablation time (min) | 45.6 ± 16.3 | 36.7 ± 9.5 | 55.3 ± 16.7 | 0.000 | 35.8 ± 6.5 | 55.9 ± 16.7 | 0.000 |
| Fluoroscopy time (min) | 22.4 ± 9.6 | 21.5 ± 7.8 | 23.4 ± 11.2 | 0.317 | 21.2 ± 7.6 | 23.6 ± 11.2 | 0.208 |
| Procedure time (min) | 178.2 ± 39.1 | 160.4 ± 30.6 | 197.2 ± 38.4 | 0.000 | 158.9 ± 28.9 | 197.9 ± 38.4 | 0.000 |
| Recurrence before discharge | 9 (8.9%) | 5 (9.6%) | 4 (8.2%) | 0.704 | 4 (7.8%) | 5 (10.0%) | 0.704 |
| Recurrence in the blanking period | 34 (33.7%) | 18 (34.6%) | 16 (32.7%) | 0.835 | 17 (33.3%) | 17 (34.0%) | 0.943 |
| Recurrence during follow-up | 35 (34.7%) | 16 (30.8%) | 19 (38.8%) | 0.398 | 15 (29.4%) | 20 (40.0%) | 0.264 |
| Persistent AF | 18 | 11 | 7 | 0.049 | 11 | 7 | 0.023 |
| Paroxysmal AF alone | 6 | 2 | 4 | 2 | 4 | ||
| Paroxysmal AF with AFL | 9 | 2 | 7 | 1 | 8 | ||
| AFL alone | 2 | 1 | 1 | 1 | 1 | ||
| Recurrence of AFL | 11 | 3 (5.8%) | 8 (16.3%) | 0.063 | 2 (3.9%) | 9 (18.0%) | 0.020 |
| Typical AFL | 2 | 0 | 2 | 0 | 2 | ||
| Atypical AFL | 8 | 2 | 6 | 1 | 7 | ||
| Both | 1 | 1 | 0 | 1 | 0 | ||
| . | Total (n = 101) . | Intention-to-treat analysis . | Per-protocol analysis . | ||||
|---|---|---|---|---|---|---|---|
| CBA group (n = 52) . | RFA group (n = 49) . | P value . | CBA group (n = 51) . | RFA group (n = 50) . | P value . | ||
| Procedure started in sinus rhythm | 17 (16.8%) | 7 (13.5%) | 10 (20.4%) | 0.351 | 7 (13.7%) | 10 (20.0%) | 0.399 |
| AF terminated during ablation | 5 (5.0%) | 1 (1.9%) | 4 (8.0%) | 0.205 | 1 (2.0%) | 4 (8.0%) | 0.162 |
| Ablation time (min) | 45.6 ± 16.3 | 36.7 ± 9.5 | 55.3 ± 16.7 | 0.000 | 35.8 ± 6.5 | 55.9 ± 16.7 | 0.000 |
| Fluoroscopy time (min) | 22.4 ± 9.6 | 21.5 ± 7.8 | 23.4 ± 11.2 | 0.317 | 21.2 ± 7.6 | 23.6 ± 11.2 | 0.208 |
| Procedure time (min) | 178.2 ± 39.1 | 160.4 ± 30.6 | 197.2 ± 38.4 | 0.000 | 158.9 ± 28.9 | 197.9 ± 38.4 | 0.000 |
| Recurrence before discharge | 9 (8.9%) | 5 (9.6%) | 4 (8.2%) | 0.704 | 4 (7.8%) | 5 (10.0%) | 0.704 |
| Recurrence in the blanking period | 34 (33.7%) | 18 (34.6%) | 16 (32.7%) | 0.835 | 17 (33.3%) | 17 (34.0%) | 0.943 |
| Recurrence during follow-up | 35 (34.7%) | 16 (30.8%) | 19 (38.8%) | 0.398 | 15 (29.4%) | 20 (40.0%) | 0.264 |
| Persistent AF | 18 | 11 | 7 | 0.049 | 11 | 7 | 0.023 |
| Paroxysmal AF alone | 6 | 2 | 4 | 2 | 4 | ||
| Paroxysmal AF with AFL | 9 | 2 | 7 | 1 | 8 | ||
| AFL alone | 2 | 1 | 1 | 1 | 1 | ||
| Recurrence of AFL | 11 | 3 (5.8%) | 8 (16.3%) | 0.063 | 2 (3.9%) | 9 (18.0%) | 0.020 |
| Typical AFL | 2 | 0 | 2 | 0 | 2 | ||
| Atypical AFL | 8 | 2 | 6 | 1 | 7 | ||
| Both | 1 | 1 | 0 | 1 | 0 | ||
Values are presented as mean ± SD, or n (%).
AF, atrial fibrillation; AFL, atrial flutter; CBA, cryoballoon ablation; RFA, radiofrequency ablation.
Primary endpoint during follow-up
Nine patients experienced AF recurrence before discharge and underwent direct current cardioversion. Thirty-four patients reported AF recurrence in the first 3 months. One hundred patients completed 12-month follow-up. One patient in RFA group died 11 months after the procedure because of a serious surgical disease unrelated to the procedure. He had not experienced any ATAs. One patient underwent atrioventricular junction ablation 4 months after CBA due to intolerable fast AF and heart failure associated with AF recurrence. After the 3-month blanking period, 36 patients in the CBA group and 30 patients in the RFA group maintained sinus rhythm without any episode of ATA over 30 s at 12-month follow-up. No difference in ATA-freedom was found between the groups (69.2% in CBA vs. 61.2% in RFA, P = 0.398) after ITT analysis. ATA-free survival curves are shown in Figure 2. Ten patients (five in CBA and five in RFA) who were free from ATAs continued with antiarrhythmic drugs. In a per-protocol analysis, 36 patients in the CBA group and 30 patients in the RFA group were free from ATAs (70.6% vs. 60.0%, P = 0.264). No difference in AF recurrence was found between the two groups (27.5% in CBA vs. 38.0% in RFA, P = 0.258). Less AFL recurrence was documented in the CBA group compared with RFA (3.9% in CBA vs.18.0% in RFA, P = 0.020; Table 2). Among those patients with recurrence, 17 patients suffered paroxysmal AF and/or AFL, and 18 were still in persistent AF. The proportion of persistent AF was significantly higher in the CBA group compared with RFA (11/15, 73.3% vs. 7/20, 35.0%, P = 0.023; Table 2).
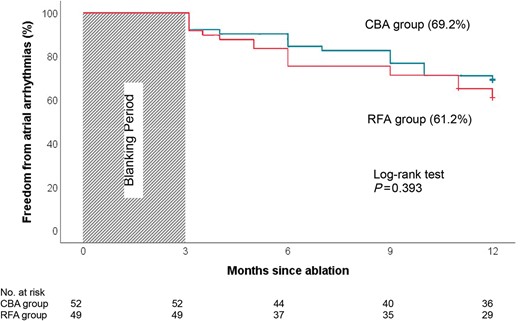
Kaplan–Meier survival curve of freedom from atrial arrhythmias. There is no difference of freedom from atrial tachyarrhythmias between CBA (blue) and RFA (red) groups during a 12-month follow-up. CBA, cryoballoon ablation; RFA, radiofrequency ablation.
Risk factors were analysed and compared between the ATA-free and recurrence groups (Table 3). Multivariable analysis showed that ATA recurrence was related to longer duration of persistent AF before the procedure [odds ratio (OR) 1.08, 95% confidence interval (CI) 1.02–1.15; P = 0.008], long-standing persistent AF (OR 3.24, 95% CI 1.11–9.47; P = 0.032), and early recurrence of AF in the blanking period (OR 6.43, 95% CI 2.35–17.59; P = 0.000). Among 34 patients who experienced early AF recurrence in the blanking period, ATAs were recorded in 22 patients (64.7%) during long-term follow-up, and no difference was observed between the two groups (64.7% in each group).
| . | Recurrence (n = 35) . | Atrial arrhythmia free (n = 66) . | P value . |
|---|---|---|---|
| Age (years) | 63.7 ± 8.4 | 63.0 ± 8.7 | 0.704 |
| Male, n (%) | 28 (80.0%) | 52 (78.8%) | 0.886 |
| Body mass index, kg/m2 | 29.1 ± 4.8 | 29.5 ± 4.3 | 0.699 |
| Hypertension, n (%) | 22 (62.9%) | 35 (53.0%) | 0.343 |
| Diabetes mellitus, n (%) | 2 (5.7%) | 3 (4.5%) | 0.797 |
| Coronary heart disease, n (%) | 6 (17.1%) | 4 (6.1%) | 0.076 |
| Obstructive sleep apnoea, n (%) | 2 (5.7%) | 6 (9.1%) | 0.550 |
| CHA2DS2VASC score | 0.139 | ||
| 0 | 8 (22.9%) | 13 (19.7%) | |
| 1 | 4 (11.4%) | 19 (28.8%) | |
| ≥2 | 23 (65.7%) | 34 (51.5%) | |
| Serum creatinine (μmol/L) | 88.2 ± 16.6 | 88.1 ± 15.8 | 0.974 |
| History of AF | 0.105 | ||
| <1 year | 2 (5.7%) | 14 (21.2%) | |
| 1–2 years | 13 (37.1%) | 24 (36.4%) | |
| >2 years | 20 (57.1%) | 28 (42.4%) | |
| Duration of persistent AF before procedure (months) | 12 (5–24) | 6 (0–12) | 0.004 |
| Long-standing persistent AF, n (%) | 13 (37.1%) | 11 (16.7%) | 0.021 |
| History of previous cavotricuspid isthmus block, n (%) | 1 (2.9%) | 6 (9.1%) | 0.240 |
| Left atrial diameter (cm) | 4.7 ± 0.6 | 4.5 ± 0.6 | 0.175 |
| Left ventricular ejection fraction (%) | 54.8 ± 7.4 | 57.1 ± 7.6 | 0.236 |
| Basal medication, n (%) | |||
| Beta-blocker | 25 (71.4%) | 41 (62.1%) | 0.350 |
| Amiodarone | 8 (22.9%) | 19 (28.8%) | 0.666 |
| Dronedarone | 14 (40.0%) | 31 (47.0%) | 0.502 |
| Flecainide | 2 (3.0%) | 4 (11.4%) | 0.089 |
| Procedure started in sinus rhythm, n (%) | 3 (8.6%) | 14 (21.2%) | 0.106 |
| AF terminated during procedure, n (%) | 2 (5.7%) | 3 (4.5%) | 0.797 |
| AF recurrence in the blanking period, n (%) | 22 (62.9%) | 12 (18.2%) | 0.000 |
| . | Recurrence (n = 35) . | Atrial arrhythmia free (n = 66) . | P value . |
|---|---|---|---|
| Age (years) | 63.7 ± 8.4 | 63.0 ± 8.7 | 0.704 |
| Male, n (%) | 28 (80.0%) | 52 (78.8%) | 0.886 |
| Body mass index, kg/m2 | 29.1 ± 4.8 | 29.5 ± 4.3 | 0.699 |
| Hypertension, n (%) | 22 (62.9%) | 35 (53.0%) | 0.343 |
| Diabetes mellitus, n (%) | 2 (5.7%) | 3 (4.5%) | 0.797 |
| Coronary heart disease, n (%) | 6 (17.1%) | 4 (6.1%) | 0.076 |
| Obstructive sleep apnoea, n (%) | 2 (5.7%) | 6 (9.1%) | 0.550 |
| CHA2DS2VASC score | 0.139 | ||
| 0 | 8 (22.9%) | 13 (19.7%) | |
| 1 | 4 (11.4%) | 19 (28.8%) | |
| ≥2 | 23 (65.7%) | 34 (51.5%) | |
| Serum creatinine (μmol/L) | 88.2 ± 16.6 | 88.1 ± 15.8 | 0.974 |
| History of AF | 0.105 | ||
| <1 year | 2 (5.7%) | 14 (21.2%) | |
| 1–2 years | 13 (37.1%) | 24 (36.4%) | |
| >2 years | 20 (57.1%) | 28 (42.4%) | |
| Duration of persistent AF before procedure (months) | 12 (5–24) | 6 (0–12) | 0.004 |
| Long-standing persistent AF, n (%) | 13 (37.1%) | 11 (16.7%) | 0.021 |
| History of previous cavotricuspid isthmus block, n (%) | 1 (2.9%) | 6 (9.1%) | 0.240 |
| Left atrial diameter (cm) | 4.7 ± 0.6 | 4.5 ± 0.6 | 0.175 |
| Left ventricular ejection fraction (%) | 54.8 ± 7.4 | 57.1 ± 7.6 | 0.236 |
| Basal medication, n (%) | |||
| Beta-blocker | 25 (71.4%) | 41 (62.1%) | 0.350 |
| Amiodarone | 8 (22.9%) | 19 (28.8%) | 0.666 |
| Dronedarone | 14 (40.0%) | 31 (47.0%) | 0.502 |
| Flecainide | 2 (3.0%) | 4 (11.4%) | 0.089 |
| Procedure started in sinus rhythm, n (%) | 3 (8.6%) | 14 (21.2%) | 0.106 |
| AF terminated during procedure, n (%) | 2 (5.7%) | 3 (4.5%) | 0.797 |
| AF recurrence in the blanking period, n (%) | 22 (62.9%) | 12 (18.2%) | 0.000 |
AF, atrial fibrillation.
| . | Recurrence (n = 35) . | Atrial arrhythmia free (n = 66) . | P value . |
|---|---|---|---|
| Age (years) | 63.7 ± 8.4 | 63.0 ± 8.7 | 0.704 |
| Male, n (%) | 28 (80.0%) | 52 (78.8%) | 0.886 |
| Body mass index, kg/m2 | 29.1 ± 4.8 | 29.5 ± 4.3 | 0.699 |
| Hypertension, n (%) | 22 (62.9%) | 35 (53.0%) | 0.343 |
| Diabetes mellitus, n (%) | 2 (5.7%) | 3 (4.5%) | 0.797 |
| Coronary heart disease, n (%) | 6 (17.1%) | 4 (6.1%) | 0.076 |
| Obstructive sleep apnoea, n (%) | 2 (5.7%) | 6 (9.1%) | 0.550 |
| CHA2DS2VASC score | 0.139 | ||
| 0 | 8 (22.9%) | 13 (19.7%) | |
| 1 | 4 (11.4%) | 19 (28.8%) | |
| ≥2 | 23 (65.7%) | 34 (51.5%) | |
| Serum creatinine (μmol/L) | 88.2 ± 16.6 | 88.1 ± 15.8 | 0.974 |
| History of AF | 0.105 | ||
| <1 year | 2 (5.7%) | 14 (21.2%) | |
| 1–2 years | 13 (37.1%) | 24 (36.4%) | |
| >2 years | 20 (57.1%) | 28 (42.4%) | |
| Duration of persistent AF before procedure (months) | 12 (5–24) | 6 (0–12) | 0.004 |
| Long-standing persistent AF, n (%) | 13 (37.1%) | 11 (16.7%) | 0.021 |
| History of previous cavotricuspid isthmus block, n (%) | 1 (2.9%) | 6 (9.1%) | 0.240 |
| Left atrial diameter (cm) | 4.7 ± 0.6 | 4.5 ± 0.6 | 0.175 |
| Left ventricular ejection fraction (%) | 54.8 ± 7.4 | 57.1 ± 7.6 | 0.236 |
| Basal medication, n (%) | |||
| Beta-blocker | 25 (71.4%) | 41 (62.1%) | 0.350 |
| Amiodarone | 8 (22.9%) | 19 (28.8%) | 0.666 |
| Dronedarone | 14 (40.0%) | 31 (47.0%) | 0.502 |
| Flecainide | 2 (3.0%) | 4 (11.4%) | 0.089 |
| Procedure started in sinus rhythm, n (%) | 3 (8.6%) | 14 (21.2%) | 0.106 |
| AF terminated during procedure, n (%) | 2 (5.7%) | 3 (4.5%) | 0.797 |
| AF recurrence in the blanking period, n (%) | 22 (62.9%) | 12 (18.2%) | 0.000 |
| . | Recurrence (n = 35) . | Atrial arrhythmia free (n = 66) . | P value . |
|---|---|---|---|
| Age (years) | 63.7 ± 8.4 | 63.0 ± 8.7 | 0.704 |
| Male, n (%) | 28 (80.0%) | 52 (78.8%) | 0.886 |
| Body mass index, kg/m2 | 29.1 ± 4.8 | 29.5 ± 4.3 | 0.699 |
| Hypertension, n (%) | 22 (62.9%) | 35 (53.0%) | 0.343 |
| Diabetes mellitus, n (%) | 2 (5.7%) | 3 (4.5%) | 0.797 |
| Coronary heart disease, n (%) | 6 (17.1%) | 4 (6.1%) | 0.076 |
| Obstructive sleep apnoea, n (%) | 2 (5.7%) | 6 (9.1%) | 0.550 |
| CHA2DS2VASC score | 0.139 | ||
| 0 | 8 (22.9%) | 13 (19.7%) | |
| 1 | 4 (11.4%) | 19 (28.8%) | |
| ≥2 | 23 (65.7%) | 34 (51.5%) | |
| Serum creatinine (μmol/L) | 88.2 ± 16.6 | 88.1 ± 15.8 | 0.974 |
| History of AF | 0.105 | ||
| <1 year | 2 (5.7%) | 14 (21.2%) | |
| 1–2 years | 13 (37.1%) | 24 (36.4%) | |
| >2 years | 20 (57.1%) | 28 (42.4%) | |
| Duration of persistent AF before procedure (months) | 12 (5–24) | 6 (0–12) | 0.004 |
| Long-standing persistent AF, n (%) | 13 (37.1%) | 11 (16.7%) | 0.021 |
| History of previous cavotricuspid isthmus block, n (%) | 1 (2.9%) | 6 (9.1%) | 0.240 |
| Left atrial diameter (cm) | 4.7 ± 0.6 | 4.5 ± 0.6 | 0.175 |
| Left ventricular ejection fraction (%) | 54.8 ± 7.4 | 57.1 ± 7.6 | 0.236 |
| Basal medication, n (%) | |||
| Beta-blocker | 25 (71.4%) | 41 (62.1%) | 0.350 |
| Amiodarone | 8 (22.9%) | 19 (28.8%) | 0.666 |
| Dronedarone | 14 (40.0%) | 31 (47.0%) | 0.502 |
| Flecainide | 2 (3.0%) | 4 (11.4%) | 0.089 |
| Procedure started in sinus rhythm, n (%) | 3 (8.6%) | 14 (21.2%) | 0.106 |
| AF terminated during procedure, n (%) | 2 (5.7%) | 3 (4.5%) | 0.797 |
| AF recurrence in the blanking period, n (%) | 22 (62.9%) | 12 (18.2%) | 0.000 |
AF, atrial fibrillation.
Procedure-related complications
Five major complications were observed in four patients (4%) and no difference was found between the two groups (P = 0.353). One patient in the CBA group suffered phrenic palsy (2%) and recovered after 6 months. Complications presented in the RFA group included bleeding with femoral haematoma in one patient, and chest pain in another with no abnormal finding during coronary angiography. Additionally, one patient suffered a tamponade during the procedure and a mild PV stenosis during the follow-up. No further intervention was needed.
Discussion
This trial was a randomized evaluation of PVI achieved by RFA or CBA in patients with persistent/long-standing persistent AF in Norwegian centres. We investigated the efficacy, safety, and procedural profiles of the two most-used ablation techniques. The clinical features of the patients were comparable to those in other trials.1,11 We found the efficacy of CBA to be similar to that of RFA with regard to the primary endpoint. Moreover, a similar procedure-related complication rate, less AFL recurrence, and shorter procedure and ablation times were observed in the CBA group.
Radiofrequency ablation has emerged as an important, effective treatment for AF in the past 20 years.14,15 For paroxysmal AF, non-inferiority of clinical outcomes of CBA has been verified by the FIRE AND ICE study5 and the CIRCA-DOSE study,6 while the similar durability of PVI has been confirmed by the RAZE-AF study.7 The success rate of CBA for persistent AF ranged from 60% to 70% at 1-year follow-up.8,9 Recently, Chun et al.16 reported a similar outcome of 78% recurrence freedom. In a meta-analysis10 which included 917 patients who underwent CBA for persistent AF from 11 studies, after a mean follow-up of 16.7 ± 3.0 months, 68.9% were free from recurrences (95% CI 63.4–74.7%). According to the results from the multicentre STOP Persistent AF trial, freedom of ATAs at 12 months after PVI was achieved by CBA was 54.8% (95% CI 46.7–62.1%).11 The results of CBA from the present study were in line with that of other investigations. However, the success rate of RFA in this trial was 61.2%, which seemed higher than that from earlier studies.1,17 The freedom rate of documented ATAs after PVI without antiarrhythmic drugs was only 41% in the STAR AF II study.1 This difference was probably due to the employment of a contact-force-sensing RFA catheter in our study, which may significantly improve ablation effect. This conjecture was supported by the recently published EARNEST-PVI trial, which showed a success rate of RFA using a contact-force-sensing catheter in patients with persistent AF of 71.7% at 12 months. In addition, similar atrial arrhythmia recurrence rates between RFA and CBA, with a trend favouring CBA in persistent AF, were reported in a prospective multicentre and multinational observational cluster cohort study based on the FREEZE cohort.13 Our study further strengthens these results, but with a randomized design, by demonstrating a comparable efficacy of CBA to RFA in terms of freedom from ATAs. Another factor that may have contributed to the higher success rate observed in this study was the lower proportion of long-standing persistent AF compared to earlier investigations.2,3,12 Even so, still over half of patients had persistent AF lasting >6 months before the procedure.
Although no difference in AF recurrence was observed, less AFL was recorded in the CBA group during follow-up (Table 2). This was also in line with previous reports.18 These observations may be explained in part by the difficulty of creating continuous circumferential lesion lines when using point to point RFA which may generate a potential substrate for atypical AFL, and in part by increased catheter stability due to freeze-mediated adhesion of the cryoballoon in CBA, resulting in creation of more homogeneous lesions with probably less proarrhythmic effect.19 Remarkably, the proportion of AF recurrence in persistent form was significantly higher in the CBA group. The lesion size, depth, durability, and even lesions covering the posterior wall of the LA created by CBA may differ from RFA and lead to different presenting of AF. These issues demand further investigation.
The procedure and ablation times in this study were significantly shorter in the CBA group. This finding was in line with other published data. Hoffmann et al.13 reported a shorter procedure time and higher radiation exposure in the CBA group. Several features of our study design should be taken into account. First, we performed three-dimensional mapping both before and after PVI according to the study protocol. This led to a relatively longer procedure duration for both groups, particularly for CBA, in which mapping is seldom performed routinely in clinical practice. Second, we did not perform a computed tomography scan before procedures to avoid patient selection based on the anatomy. These features could lead to longer procedure and ablation times for CBA. Finally, we used a fixed freezing regimen of two applications of at least 240 s each time in each PV. This strategy has been challenged in later years with reduced application numbers and durations. Therefore, the procedure duration and ablation time of CBA can be even shorter.
Phrenic nerve palsy was observed in one patient (2%) in the CBA group, while PV stenosis was observed in one patient (2%) in the RFA group. This finding of complication is consistent with previous reports.20 Notably, we found that both the duration of persistent AF before the ablation procedure and early AF recurrence during the blanking period were related to ATA recurrence during follow-up. This is in line with the findings of other studies and suggests that these risk factors could serve as predictors for AF recurrence after ablation. In particular, unlike paroxysmal AF, early AF episodes during the blanking period are highly associated with recurrence during long-term follow-up and should be managed without delay.
Limitations
This study was a national study in Norway with a limited sample size, which was calculated based on the available outcomes of earlier investigations when the study was designed. The success rate of RFA has increased while the catheters and techniques have been improved. Due to the difficulty of anticipating clinical incidence precisely, similar sample sizes have been applied to several clinical trials.21,22 Although the interpretation of the data is limited, these findings still show the real outcomes of daily ablation practice and confirm the results of previous trials. Large-scale randomized clinical trials are demanded to further confirm the conclusions of this study. A measure of combined ablation power, time, and contact force, such as lesion size index, was not available when this study started. We employed force-time-integral over 400 g·s as the target for each application. This may have an impact on procedural data, but probably not much on clinical results since this criterion had been applied in practice for years. We did not compare the radiation exposure because we had observed a large variation of radiation dosage from one lab to another due to different devices and systems. Since the fluoroscopy time was similar between the two groups, the radiation exposure might be higher in the CBA group given that cine angiography of the PVs was applied before every cryoablation, which was not necessary for the RFA group.
Conclusions
Pulmonary vein isolation achieved by CBA was as effective as RFA for treatment of persistent or long-standing persistent AF in terms of ATA-freedom at 12-month follow-up, with less AFL recurrence and shorter procedure and ablation times.
Funding
This study was partly supported by the Helse Vest, Norway (L.-B.S.).
Conflict of interest: J.C. serves as a consultant for Biosense Webster, Johnson & Johnson, and has received research grant from Medtronic and Abbott Medical. Otherwise, there are no conflicts of interest to be disclosed for other co-authors.
Data availability
The data underlying this article are related to all patients enrolled in this study. These cannot be shared publicly due to the privacy of individuals that participated in the study. All data are preserved in the participating centres. The data will be shared at reasonable request to the corresponding author.



