-
PDF
- Split View
-
Views
-
Cite
Cite
Kevin J Um, William F McIntyre, Pablo A Mendoza, Omar Ibrahim, Stephanie T Nguyen, Sabrina H Lin, Emmanuelle Duceppe, Bram Rochwerg, Jeff S Healey, Alex Koziarz, Alexandra P Lengyel, Akash Bhatnagar, Guy Amit, Victor A Chu, Richard P Whitlock, Emilie P Belley-Côté, Pre-treatment with antiarrhythmic drugs for elective electrical cardioversion of atrial fibrillation: a systematic review and network meta-analysis, EP Europace, Volume 24, Issue 10, October 2022, Pages 1548–1559, https://doi.org/10.1093/europace/euac063
Close - Share Icon Share
Abstract
Our objective was to compare the efficacy of pre-treatment with different classes of anti-arrhythmic drugs (AADs) in patients with atrial fibrillation (AF) undergoing electrical cardioversion.
We performed a systematic review and network meta-analysis (NMA) of randomized controlled trials (RCTs) comparing different AADs in patients with AF undergoing electrical cardioversion. We grouped AADs into five network nodes: no treatment or rate control, Class Ia, Class Ic, Class III, and amiodarone. Outcomes were (i) acute restoration and (ii) maintenance of sinus rhythm. We searched MEDLINE and EMBASE from inception until June 2020. We used Python 3.8.3 and R 3.6.2 for data analysis. We evaluated the overall certainty of evidence with the GRADE framework. We included 28 RCTs. Compared with no treatment or rate control, Class III AADs [odds ratio (OR): 2.41; 95% credible interval (CrI): 1.37 to 4.62, high certainty] and amiodarone (OR: 2.58; 95% CrI: 1.54 to 4.37, high certainty) improved restoration of sinus rhythm. Amiodarone improved long-term maintenance of sinus rhythm when compared with no treatment or rate control (OR: 5.37; 95% CrI: 4.00–7.39, high certainty), Class Ic (OR: 1.89; 95% CrI: 1.05–3.45, moderate certainty) and Class III AADs (OR: 2.19; 95% CrI: 1.39–3.26, high certainty).
Before electrical cardioversion of AF, treatment with Class III AADs or amiodarone improves the acute restoration of sinus rhythm. Amiodarone is most likely to improve the maintenance of sinus rhythm after electrical cardioversion, but Class Ic and Class III AADs are also effective.
In addition to pre-treatment with amiodarone, treatment with Class III anti-arrhythmic drugs before electrical cardioversion improves the acute restoration of sinus rhythm with high certainty. Our network meta-analysis expands on published evidence regarding the efficacy of amiodarone before electrical cardioversion.
According to a network model using all published randomized controlled trials data, amiodarone, Class Ic and Class III AADs improve the maintenance of sinus rhythm after electrical cardioversion.
Amiodarone, however, is more likely than Class Ic and Class III AADs to improve the maintenance of sinus rhythm.
Introduction
When clinicians select a rhythm control strategy for patients with atrial fibrillation (AF), they often perform electrical cardioversion.1 Electrical cardioversion restores sinus rhythm in up to 87% of patients although in some populations the success rate is as low as 67%.2–6 Even when sinus rhythm is restored, the AF recurrence rate is high—ranging between 57 and 63% within 4 weeks.2,7
Clinicians may treat patients with anti-arrhythmic drugs (AADs) before electrical cardioversion of AF to improve the acute restoration and long-term maintenance of sinus rhythm.8,9 Based on level ‘B’ evidence, the European Society of Cardiology provides a Class IIa recommendation to consider select AADs for treatment before electrical cardioversion in patients with recurrent AF.10
We previously completed a systematic review and pairwise meta-analysis which found high certainty evidence that amiodarone improves the maintenance of sinus rhythm following electrical cardioversion by 20% in the acute setting and 4.4 times over a follow-up period of up to 13 months.11 Despite this, practice remains variable. If an AAD is used, the optimal agent remains uncertain.9
We performed a systematic review and network meta-analysis (NMA) of randomized controlled trials (RCTs) comparing the effects of treatment with an AAD before (and possibly after) electrical cardioversion for patients with AF. Using a Bayesian framework, we systematically compiled the existing evidence into a single analytic model.12
Methods
We registered the protocol (PROSPERO 2017:CRD42017068877) and pre-specified our search strategy, criteria for study selection, statistical methodology, and approach to evaluating the risk of bias and grading the certainty of evidence.
Search strategy
We performed a search of MEDLINE and EMBASE databases from inception to June 2020 using a strategy designed with input from a research librarian (see Supplementary material online, Appendix S7). We searched clinicaltrials.gov and WHO ICTRP for ongoing or unpublished trials and reviewed the conference proceedings of the last two meetings of the American College of Cardiology, American Heart Association and European Society of Cardiology. We consulted experts to identify other relevant studies.
Eligibility criteria
We included RCTs without language constraints. We included studies of adult patients with AF of any duration undergoing elective electrical cardioversion to restore sinus rhythm. We included RCTs enrolling patients with atrial flutter if they comprised <50% of the population. The intervention of interest was treatment with an AAD via any administration route. We included trials with any comparator, including no treatment, rate control, or placebo. We only included medications approved for use in Canada or the United States. The outcomes of interest were the acute restoration and long-term maintenance of sinus rhythm during follow-up.
Study selection
We uploaded all titles and abstracts from the electronic search into RefWorks (ProQuest, United States) and Covidence (Veritas Health Innovation, Australia). Two reviewers independently performed title and abstract screening followed by full-text eligibility assessment. We resolved conflicts by consensus.
Data extraction and quality assessment
Independently and in duplicate, review authors used pre-defined data collection forms to extract data from included studies. Whenever possible, we applied intention-to-treat analysis using the number of participants randomized to a study arm as the total denominator. We assumed the worst-case scenario—i.e. we assumed participants who were lost to follow-up or withdrew consent for any reason to be in AF. We also applied an intention-to-cardiovert analysis. Patients who converted to sinus rhythm prior to electrical cardioversion were considered to have successfully cardioverted.
We used the Cochrane tool to evaluate the risk of bias for individual studies for each outcome.13 We pre-specified that open label trials would be at low risk of performance bias if they followed a systematic protocol for electrical cardioversion and administration of additional AADs. We pre-specified that blinding would not influence detection bias, as sinus rhythm is an objective outcome. We assessed the overall certainty of evidence for each comparison and each outcome using the GRADE framework.14
Statistical analysis
We grouped the interventions into five distinct network nodes according to mechanism of action (Vaughan Williams classification): (i) no treatment or rate control (i.e. calcium channel blockers, beta-blockers, digoxin), (ii) Class Ia, (iii) Class Ic, (iv) Class III and (v) amiodarone.
We performed pairwise meta-analysis using Python (version 3.8.3; packages ‘matplotlib’, ‘numpy’ and ‘pandas’) and NMA using R (version 3.5.1; packages ‘gemtc’ and ‘rjags’).15
We calculated direct estimates using a frequentist random effects framework. For each direct comparison, we reported the odds ratio (OR) and corresponding 95% confidence interval (CI). We used a Bayesian model with random effects to perform our NMA. We calculated direct and indirect estimates for each treatment comparison and reported the combined ORs and 95% credible intervals (CrIs)—the Bayesian equivalent of CIs.12 We used vague, non-informative prior distributions, and generated the posterior distribution with four chains of 40 000 iterations and a thin of 10.
We calculated the probability of each treatment being ranked best, second-best, third-best, and so on by comparing each treatment with the no treatment or rate control group. We derived these probabilities using the surface under the cumulative ranking curve (SUCRA).12
We assessed between-study heterogeneity in two steps.16 First, we calculated the global I2 statistic for direct comparisons and network consistency, and used node-splitting to assess coherence between direct and indirect estimates. For the node-splitting comparisons, we pre-specified a P-value of <0.05 on the z-test to evaluate incoherence. Second, we assessed the forest plots visually to compare the CIs of direct estimates with the CrIs of network estimates.17
Results
Of an initial 3367 citations, we reviewed the full text of 71 studies and included 28 RCTs with 4348 participants (see Supplementary material online, Appendix S1). We included 24 two-arm and 4 three-arm RCTs. Table 1 presents the characteristics of included studies (also see Supplementary material online, Appendix S2). The mean age as reported in 28 studies was 63 years; mean duration of AF before electrical cardioversion was 304 days (reported in 24 trials); mean LA size was 45 mm (reported in 23 trials). Boos and et al.18 excluded patients with cardioversion in the previous 6 months, and three trials only included patients with recent-onset AF.19–21 Two trials only included patients without a history of unsuccessful electrical cardioversion.22,23
| Study . | Design . | Setting . | Number of patients . | Cardioversion . | Arms . | Follow-up . | Risk of bias (acute | maintenance) . |
|---|---|---|---|---|---|---|---|
| Bianconi et al. 199630 | Single blind | Single centre | 100 |
| Propafenone: pre-cardioversion, three daily doses of 300 mg, 150 mg, and 300 mg for 48 h; post-cardioversion, ongoing propafenone therapy | N/A | Unclear |
| Matching placebo | |||||||
| Boos et al. 200418 | Unblinded | Single centre | 35 |
| Amiodarone: pre-cardioversion, 1 week of 200 mg PO three times daily; post-cardioversion, 3 weeks of 200 mg PO three times daily for 1 week, followed by 200 mg PO twice daily for 1 week, followed by 200 mg PO daily for 1 week | 16 months | Unclear | high |
| No treatment | |||||||
| Capucci 200031 | Unblinded | Single centre | 92 |
| Amiodarone: pre-cardioversion, 1 month of 400 mg PO daily; post-cardioversion, 200 mg PO daily | 2 months | Unclear | high |
| Glucose-insulin-potassium solution: pre-cardioversion, continuous infusion for 24 h prior to cardioversion | |||||||
| No treatment | |||||||
| Channer et al. 200428 | Double blind | Multi-centre | 161 |
| Amiodarone: pre-cardioversion, 400 mg PO twice daily for 2 weeks; post-cardioversion, 200 mg PO once daily for 8 weeks followed by matching placebo for 44 weeks | 52 weeks | High | high |
| Amiodarone: pre-cardioversion, 400 mg PO twice daily for 2 weeks; post-cardioversion, 200 mg PO once daily for 52 weeks | |||||||
| Matching placebo | |||||||
| Climent et al. 200432 | Double blind | Single centre | 54 |
| Flecainide: pre-cardioversion, 2 mg/kg IV over 30 min | 1 month | High | high |
| Matching placebo | |||||||
| De Simone et al. 200333 | Unblinded | Multi-centre | 162 |
| Amiodarone: pre-cardioversion, 600 mg PO daily for 7 days, followed by 400 mg PO daily for 7 days, then 200 mg PO daily for 14 days | 3 months | Unclear | high |
| Flecainide: pre-cardioversion, 200 mg PO daily for 3 days | |||||||
| Galperin et al. 200134 | Double blind | Multi-centre | 95 |
| Amiodarone: pre-cardioversion, 600 mg PO daily for at least 4 weeks, then 200 mg PO daily | 16 months | Unclear | unclear |
| Matching placebo | |||||||
| Hartel et al. 197435 | Double blind | Single centre | 52 |
| Disopyramide: pre-cardioversion, 100 mg PO three times daily starting the day before cardioversion; post-cardioversion, 500 mg PO daily (in 3 doses) for maintenance | 3 months | Unclear | unclear |
| Matching placebo | |||||||
| Hillestad et al. 197236 | Unblinded | Single centre | 124 |
| Quinidine sulphate: pre-cardioversion, 0.2 g PO twice daily until serum quinidine level of between 4 and 6 mg/L | N/A | Unclear |
| No treatment | |||||||
| Jacobs et al. 199837 | Not specified | Single centre | 100 |
| Procainamide: pre-cardioversion, 15 mg/kg IV (up to max of 1250 mg) over 60 min, followed by continuous infusion of 2 mg/mL prior to cardioversion | N/A | Unclear |
| Matching placebo | |||||||
| Jong et al. 199538 | Unblinded | Not specified | 91 |
| Amiodarone: pre-cardioversion, 200 mg PO three times daily for 4 weeks; post-cardioversion, 200 mg daily for 1 month then 200 mg every other day for 1 month | 2 months | Unclear | high |
| Matching placebo | |||||||
| Joseph et al. 200021 | Single blind | Multi-centre | 120 |
| Amiodarone: pre-cardioversion, 5 mg/kg IV over 30 min then 200 mg PO every 8 h for 6 doses | N/A | Low |
| Sotalol: pre-cardioversion, 1.5 mg/kg IV over 30 min then 80 mg PO every 8 h for 6 doses | |||||||
| No treatment | |||||||
| Kanoupakis et al. 200439 | Unblinded | Not specified | 145 |
| Amiodarone: 600 mg PO daily for first 2 weeks then 200 mg daily until end of study | 4 weeks | Low | high |
| Carvedilol: 6.25 mg PO twice daily, titrated up to 25 mg twice daily up to end of study | |||||||
| No treatment | |||||||
| Komatsu et al. 200940 | No specified | Single centre | 70 |
| Cibenzoline: 70 mg IV over 5 min | N/A | Unclear |
| Pilsicainide: 50 mg IV over 5 min | |||||||
| Lombardi et al. 200641 | Double blind | Not specified | 658 |
| Azimilide: pre-cardioversion, 125 mg PO twice daily for 3 days; post-cardioversion, 125 mg PO daily or placebo daily for 26 weeks | N/A | High |
| Sotalol: pre-cardioversion, 160 mg PO twice daily for 3 days; post-cardioversion, 160 mg PO twice daily for 26 weeks | |||||||
| Matching placebo | |||||||
| Manios etal. 200342 | Unblinded | Single centre | 111 |
| Amiodarone: 600 mg PO daily for 2 weeks, then 200 mg daily up to end of study | 6 weeks | Low | high |
| Diltiazem: 270 mg to 360 mg PO daily | |||||||
| No treatment | |||||||
| Mazzocca et al. 200643 | Unblinded | Single centre | 32 |
| Ibutilide: pre-cardioversion, 0.01 mg/kg IV over 10 min | N/A | Unclear |
| No treatment | |||||||
| Okishige et al. 200044 | Not specified | Single centre | 62 |
| Pilsicainide: pre-cardioversion, 150 mg PO daily for 4 weeks; post-cardioversion, 50 mg PO 3 times daily for 2 years | 19 months | Unclear | unclear |
| Matching placebo | |||||||
| Oral et al. 199919 | Unblinded | Single centre | 100 |
| Ibutilide: pre-cardioversion, 1 mg IV over 10 min | 6 months | Unclear | high |
| No treatment | |||||||
| Pritchett et al. 200622 | Double blind | Multi-centre | 446 |
| Azimilide: pre-cardioversion, 125 mg PO twice daily for 3 days; post-cardioversion, 125 mg daily for 6 months | N/A | Unclear |
| Matched placebo | |||||||
| No treatment | |||||||
| Singh et al. 200045 | Double blind | Multi-centre | 325 |
| Dofetilide: pre-cardioversion, 125 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | 12 months | Unclear | unclear |
| Dofetilide: pre-cardioversion, 250 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | |||||||
| Dofetilide: pre-cardioversion, 500 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | |||||||
| Matching placebo | |||||||
| Singh et al. 200527 | Double blind | Multi-centre | 665 |
| Amiodarone: 800 mg PO daily for first 14 days, then 600 mg PO daily for next 14 days, then 300 mg PO daily for first year, then 200 mg PO daily thereafter | 1 year | Low | low |
| Sotalol: 80 mg PO twice daily for first week, then 160 mg PO twice daily thereafter | |||||||
| Matching placebo | |||||||
| Sticherling et al. 200246 | Not specified | Single centre | 20 |
| Verapamil: pre-cardioversion, 0.15 mg/kg IV at rate of 2 mg/min | N/A | Unclear |
| Ibutilide: pre-cardioversion, 1 mg IV over 10 min | |||||||
| Sticherling et al. 200547 | Unblinded | Single centre | 20 |
| Verapamil: pre-cardioversion, 0.15 mg/kg IV at rate of 2 mg/min | N/A | Unclear |
| Amiodarone: pre-cardioversion, 5 mg/kg IV over 5 min | |||||||
| Stroobandt et al. 199748 | Double blind | Multi-centre | 136 |
| Propafenone: pre-cardioversion, 2 mg/kg IV over 30 min followed 2 h later by 150 mg PO every 8 h; post-cardioversion, 150 mg PO 3 times daily for 6 months | 6 months | Unclear | unclear |
| Matching placebo | |||||||
| Thomas et al. 200420 | Unblinded | Single centre | 140 |
| Amiodarone: pre-cardioversion, 10 mg/kg IV amiodarone over 30 min; post-cardioversion, 200 mg PO twice daily until discharge | 24 h | Unclear | high |
| Sotalol: pre-cardioversion, 1.5 mg/kg IV over 10 min; post-cardioversion, 80 mg PO twice daily until discharge | |||||||
| No treatment | |||||||
| Vijayalakshmi et al. 200649 | Unblinded | Single centre | 94 |
| Amiodarone: pre-cardioversion, 200 mg PO three times daily for the first week, twice daily for the second week, and once daily for 4 weeks | 6 months | High | high |
| Sotalol: pre-cardioversion, 160 mg PO twice daily for 6 weeks | |||||||
| No treatment | |||||||
| Villani et al. 200023 | Single blind | Single centre | 120 |
| Amiodarone: pre-cardioversion, 400 mg PO daily for 1 month; post-cardioversion, 200 mg PO daily | 1 month | Unclear | high |
| Diltiazem: pre-cardioversion, 60 mg PO 3 times a day for 1 month (titrated until heart rate <80 beats/min or max dose of 360 mg/day); post-cardioversion, continued at established dose | |||||||
| Digoxin: pre-cardioversion, 0.25 mg PO daily for 1 month; post-cardioversion, 0.25 mg PO daily |
| Study . | Design . | Setting . | Number of patients . | Cardioversion . | Arms . | Follow-up . | Risk of bias (acute | maintenance) . |
|---|---|---|---|---|---|---|---|
| Bianconi et al. 199630 | Single blind | Single centre | 100 |
| Propafenone: pre-cardioversion, three daily doses of 300 mg, 150 mg, and 300 mg for 48 h; post-cardioversion, ongoing propafenone therapy | N/A | Unclear |
| Matching placebo | |||||||
| Boos et al. 200418 | Unblinded | Single centre | 35 |
| Amiodarone: pre-cardioversion, 1 week of 200 mg PO three times daily; post-cardioversion, 3 weeks of 200 mg PO three times daily for 1 week, followed by 200 mg PO twice daily for 1 week, followed by 200 mg PO daily for 1 week | 16 months | Unclear | high |
| No treatment | |||||||
| Capucci 200031 | Unblinded | Single centre | 92 |
| Amiodarone: pre-cardioversion, 1 month of 400 mg PO daily; post-cardioversion, 200 mg PO daily | 2 months | Unclear | high |
| Glucose-insulin-potassium solution: pre-cardioversion, continuous infusion for 24 h prior to cardioversion | |||||||
| No treatment | |||||||
| Channer et al. 200428 | Double blind | Multi-centre | 161 |
| Amiodarone: pre-cardioversion, 400 mg PO twice daily for 2 weeks; post-cardioversion, 200 mg PO once daily for 8 weeks followed by matching placebo for 44 weeks | 52 weeks | High | high |
| Amiodarone: pre-cardioversion, 400 mg PO twice daily for 2 weeks; post-cardioversion, 200 mg PO once daily for 52 weeks | |||||||
| Matching placebo | |||||||
| Climent et al. 200432 | Double blind | Single centre | 54 |
| Flecainide: pre-cardioversion, 2 mg/kg IV over 30 min | 1 month | High | high |
| Matching placebo | |||||||
| De Simone et al. 200333 | Unblinded | Multi-centre | 162 |
| Amiodarone: pre-cardioversion, 600 mg PO daily for 7 days, followed by 400 mg PO daily for 7 days, then 200 mg PO daily for 14 days | 3 months | Unclear | high |
| Flecainide: pre-cardioversion, 200 mg PO daily for 3 days | |||||||
| Galperin et al. 200134 | Double blind | Multi-centre | 95 |
| Amiodarone: pre-cardioversion, 600 mg PO daily for at least 4 weeks, then 200 mg PO daily | 16 months | Unclear | unclear |
| Matching placebo | |||||||
| Hartel et al. 197435 | Double blind | Single centre | 52 |
| Disopyramide: pre-cardioversion, 100 mg PO three times daily starting the day before cardioversion; post-cardioversion, 500 mg PO daily (in 3 doses) for maintenance | 3 months | Unclear | unclear |
| Matching placebo | |||||||
| Hillestad et al. 197236 | Unblinded | Single centre | 124 |
| Quinidine sulphate: pre-cardioversion, 0.2 g PO twice daily until serum quinidine level of between 4 and 6 mg/L | N/A | Unclear |
| No treatment | |||||||
| Jacobs et al. 199837 | Not specified | Single centre | 100 |
| Procainamide: pre-cardioversion, 15 mg/kg IV (up to max of 1250 mg) over 60 min, followed by continuous infusion of 2 mg/mL prior to cardioversion | N/A | Unclear |
| Matching placebo | |||||||
| Jong et al. 199538 | Unblinded | Not specified | 91 |
| Amiodarone: pre-cardioversion, 200 mg PO three times daily for 4 weeks; post-cardioversion, 200 mg daily for 1 month then 200 mg every other day for 1 month | 2 months | Unclear | high |
| Matching placebo | |||||||
| Joseph et al. 200021 | Single blind | Multi-centre | 120 |
| Amiodarone: pre-cardioversion, 5 mg/kg IV over 30 min then 200 mg PO every 8 h for 6 doses | N/A | Low |
| Sotalol: pre-cardioversion, 1.5 mg/kg IV over 30 min then 80 mg PO every 8 h for 6 doses | |||||||
| No treatment | |||||||
| Kanoupakis et al. 200439 | Unblinded | Not specified | 145 |
| Amiodarone: 600 mg PO daily for first 2 weeks then 200 mg daily until end of study | 4 weeks | Low | high |
| Carvedilol: 6.25 mg PO twice daily, titrated up to 25 mg twice daily up to end of study | |||||||
| No treatment | |||||||
| Komatsu et al. 200940 | No specified | Single centre | 70 |
| Cibenzoline: 70 mg IV over 5 min | N/A | Unclear |
| Pilsicainide: 50 mg IV over 5 min | |||||||
| Lombardi et al. 200641 | Double blind | Not specified | 658 |
| Azimilide: pre-cardioversion, 125 mg PO twice daily for 3 days; post-cardioversion, 125 mg PO daily or placebo daily for 26 weeks | N/A | High |
| Sotalol: pre-cardioversion, 160 mg PO twice daily for 3 days; post-cardioversion, 160 mg PO twice daily for 26 weeks | |||||||
| Matching placebo | |||||||
| Manios etal. 200342 | Unblinded | Single centre | 111 |
| Amiodarone: 600 mg PO daily for 2 weeks, then 200 mg daily up to end of study | 6 weeks | Low | high |
| Diltiazem: 270 mg to 360 mg PO daily | |||||||
| No treatment | |||||||
| Mazzocca et al. 200643 | Unblinded | Single centre | 32 |
| Ibutilide: pre-cardioversion, 0.01 mg/kg IV over 10 min | N/A | Unclear |
| No treatment | |||||||
| Okishige et al. 200044 | Not specified | Single centre | 62 |
| Pilsicainide: pre-cardioversion, 150 mg PO daily for 4 weeks; post-cardioversion, 50 mg PO 3 times daily for 2 years | 19 months | Unclear | unclear |
| Matching placebo | |||||||
| Oral et al. 199919 | Unblinded | Single centre | 100 |
| Ibutilide: pre-cardioversion, 1 mg IV over 10 min | 6 months | Unclear | high |
| No treatment | |||||||
| Pritchett et al. 200622 | Double blind | Multi-centre | 446 |
| Azimilide: pre-cardioversion, 125 mg PO twice daily for 3 days; post-cardioversion, 125 mg daily for 6 months | N/A | Unclear |
| Matched placebo | |||||||
| No treatment | |||||||
| Singh et al. 200045 | Double blind | Multi-centre | 325 |
| Dofetilide: pre-cardioversion, 125 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | 12 months | Unclear | unclear |
| Dofetilide: pre-cardioversion, 250 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | |||||||
| Dofetilide: pre-cardioversion, 500 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | |||||||
| Matching placebo | |||||||
| Singh et al. 200527 | Double blind | Multi-centre | 665 |
| Amiodarone: 800 mg PO daily for first 14 days, then 600 mg PO daily for next 14 days, then 300 mg PO daily for first year, then 200 mg PO daily thereafter | 1 year | Low | low |
| Sotalol: 80 mg PO twice daily for first week, then 160 mg PO twice daily thereafter | |||||||
| Matching placebo | |||||||
| Sticherling et al. 200246 | Not specified | Single centre | 20 |
| Verapamil: pre-cardioversion, 0.15 mg/kg IV at rate of 2 mg/min | N/A | Unclear |
| Ibutilide: pre-cardioversion, 1 mg IV over 10 min | |||||||
| Sticherling et al. 200547 | Unblinded | Single centre | 20 |
| Verapamil: pre-cardioversion, 0.15 mg/kg IV at rate of 2 mg/min | N/A | Unclear |
| Amiodarone: pre-cardioversion, 5 mg/kg IV over 5 min | |||||||
| Stroobandt et al. 199748 | Double blind | Multi-centre | 136 |
| Propafenone: pre-cardioversion, 2 mg/kg IV over 30 min followed 2 h later by 150 mg PO every 8 h; post-cardioversion, 150 mg PO 3 times daily for 6 months | 6 months | Unclear | unclear |
| Matching placebo | |||||||
| Thomas et al. 200420 | Unblinded | Single centre | 140 |
| Amiodarone: pre-cardioversion, 10 mg/kg IV amiodarone over 30 min; post-cardioversion, 200 mg PO twice daily until discharge | 24 h | Unclear | high |
| Sotalol: pre-cardioversion, 1.5 mg/kg IV over 10 min; post-cardioversion, 80 mg PO twice daily until discharge | |||||||
| No treatment | |||||||
| Vijayalakshmi et al. 200649 | Unblinded | Single centre | 94 |
| Amiodarone: pre-cardioversion, 200 mg PO three times daily for the first week, twice daily for the second week, and once daily for 4 weeks | 6 months | High | high |
| Sotalol: pre-cardioversion, 160 mg PO twice daily for 6 weeks | |||||||
| No treatment | |||||||
| Villani et al. 200023 | Single blind | Single centre | 120 |
| Amiodarone: pre-cardioversion, 400 mg PO daily for 1 month; post-cardioversion, 200 mg PO daily | 1 month | Unclear | high |
| Diltiazem: pre-cardioversion, 60 mg PO 3 times a day for 1 month (titrated until heart rate <80 beats/min or max dose of 360 mg/day); post-cardioversion, continued at established dose | |||||||
| Digoxin: pre-cardioversion, 0.25 mg PO daily for 1 month; post-cardioversion, 0.25 mg PO daily |
We did not include post-crossover results in our analysis.
| Study . | Design . | Setting . | Number of patients . | Cardioversion . | Arms . | Follow-up . | Risk of bias (acute | maintenance) . |
|---|---|---|---|---|---|---|---|
| Bianconi et al. 199630 | Single blind | Single centre | 100 |
| Propafenone: pre-cardioversion, three daily doses of 300 mg, 150 mg, and 300 mg for 48 h; post-cardioversion, ongoing propafenone therapy | N/A | Unclear |
| Matching placebo | |||||||
| Boos et al. 200418 | Unblinded | Single centre | 35 |
| Amiodarone: pre-cardioversion, 1 week of 200 mg PO three times daily; post-cardioversion, 3 weeks of 200 mg PO three times daily for 1 week, followed by 200 mg PO twice daily for 1 week, followed by 200 mg PO daily for 1 week | 16 months | Unclear | high |
| No treatment | |||||||
| Capucci 200031 | Unblinded | Single centre | 92 |
| Amiodarone: pre-cardioversion, 1 month of 400 mg PO daily; post-cardioversion, 200 mg PO daily | 2 months | Unclear | high |
| Glucose-insulin-potassium solution: pre-cardioversion, continuous infusion for 24 h prior to cardioversion | |||||||
| No treatment | |||||||
| Channer et al. 200428 | Double blind | Multi-centre | 161 |
| Amiodarone: pre-cardioversion, 400 mg PO twice daily for 2 weeks; post-cardioversion, 200 mg PO once daily for 8 weeks followed by matching placebo for 44 weeks | 52 weeks | High | high |
| Amiodarone: pre-cardioversion, 400 mg PO twice daily for 2 weeks; post-cardioversion, 200 mg PO once daily for 52 weeks | |||||||
| Matching placebo | |||||||
| Climent et al. 200432 | Double blind | Single centre | 54 |
| Flecainide: pre-cardioversion, 2 mg/kg IV over 30 min | 1 month | High | high |
| Matching placebo | |||||||
| De Simone et al. 200333 | Unblinded | Multi-centre | 162 |
| Amiodarone: pre-cardioversion, 600 mg PO daily for 7 days, followed by 400 mg PO daily for 7 days, then 200 mg PO daily for 14 days | 3 months | Unclear | high |
| Flecainide: pre-cardioversion, 200 mg PO daily for 3 days | |||||||
| Galperin et al. 200134 | Double blind | Multi-centre | 95 |
| Amiodarone: pre-cardioversion, 600 mg PO daily for at least 4 weeks, then 200 mg PO daily | 16 months | Unclear | unclear |
| Matching placebo | |||||||
| Hartel et al. 197435 | Double blind | Single centre | 52 |
| Disopyramide: pre-cardioversion, 100 mg PO three times daily starting the day before cardioversion; post-cardioversion, 500 mg PO daily (in 3 doses) for maintenance | 3 months | Unclear | unclear |
| Matching placebo | |||||||
| Hillestad et al. 197236 | Unblinded | Single centre | 124 |
| Quinidine sulphate: pre-cardioversion, 0.2 g PO twice daily until serum quinidine level of between 4 and 6 mg/L | N/A | Unclear |
| No treatment | |||||||
| Jacobs et al. 199837 | Not specified | Single centre | 100 |
| Procainamide: pre-cardioversion, 15 mg/kg IV (up to max of 1250 mg) over 60 min, followed by continuous infusion of 2 mg/mL prior to cardioversion | N/A | Unclear |
| Matching placebo | |||||||
| Jong et al. 199538 | Unblinded | Not specified | 91 |
| Amiodarone: pre-cardioversion, 200 mg PO three times daily for 4 weeks; post-cardioversion, 200 mg daily for 1 month then 200 mg every other day for 1 month | 2 months | Unclear | high |
| Matching placebo | |||||||
| Joseph et al. 200021 | Single blind | Multi-centre | 120 |
| Amiodarone: pre-cardioversion, 5 mg/kg IV over 30 min then 200 mg PO every 8 h for 6 doses | N/A | Low |
| Sotalol: pre-cardioversion, 1.5 mg/kg IV over 30 min then 80 mg PO every 8 h for 6 doses | |||||||
| No treatment | |||||||
| Kanoupakis et al. 200439 | Unblinded | Not specified | 145 |
| Amiodarone: 600 mg PO daily for first 2 weeks then 200 mg daily until end of study | 4 weeks | Low | high |
| Carvedilol: 6.25 mg PO twice daily, titrated up to 25 mg twice daily up to end of study | |||||||
| No treatment | |||||||
| Komatsu et al. 200940 | No specified | Single centre | 70 |
| Cibenzoline: 70 mg IV over 5 min | N/A | Unclear |
| Pilsicainide: 50 mg IV over 5 min | |||||||
| Lombardi et al. 200641 | Double blind | Not specified | 658 |
| Azimilide: pre-cardioversion, 125 mg PO twice daily for 3 days; post-cardioversion, 125 mg PO daily or placebo daily for 26 weeks | N/A | High |
| Sotalol: pre-cardioversion, 160 mg PO twice daily for 3 days; post-cardioversion, 160 mg PO twice daily for 26 weeks | |||||||
| Matching placebo | |||||||
| Manios etal. 200342 | Unblinded | Single centre | 111 |
| Amiodarone: 600 mg PO daily for 2 weeks, then 200 mg daily up to end of study | 6 weeks | Low | high |
| Diltiazem: 270 mg to 360 mg PO daily | |||||||
| No treatment | |||||||
| Mazzocca et al. 200643 | Unblinded | Single centre | 32 |
| Ibutilide: pre-cardioversion, 0.01 mg/kg IV over 10 min | N/A | Unclear |
| No treatment | |||||||
| Okishige et al. 200044 | Not specified | Single centre | 62 |
| Pilsicainide: pre-cardioversion, 150 mg PO daily for 4 weeks; post-cardioversion, 50 mg PO 3 times daily for 2 years | 19 months | Unclear | unclear |
| Matching placebo | |||||||
| Oral et al. 199919 | Unblinded | Single centre | 100 |
| Ibutilide: pre-cardioversion, 1 mg IV over 10 min | 6 months | Unclear | high |
| No treatment | |||||||
| Pritchett et al. 200622 | Double blind | Multi-centre | 446 |
| Azimilide: pre-cardioversion, 125 mg PO twice daily for 3 days; post-cardioversion, 125 mg daily for 6 months | N/A | Unclear |
| Matched placebo | |||||||
| No treatment | |||||||
| Singh et al. 200045 | Double blind | Multi-centre | 325 |
| Dofetilide: pre-cardioversion, 125 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | 12 months | Unclear | unclear |
| Dofetilide: pre-cardioversion, 250 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | |||||||
| Dofetilide: pre-cardioversion, 500 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | |||||||
| Matching placebo | |||||||
| Singh et al. 200527 | Double blind | Multi-centre | 665 |
| Amiodarone: 800 mg PO daily for first 14 days, then 600 mg PO daily for next 14 days, then 300 mg PO daily for first year, then 200 mg PO daily thereafter | 1 year | Low | low |
| Sotalol: 80 mg PO twice daily for first week, then 160 mg PO twice daily thereafter | |||||||
| Matching placebo | |||||||
| Sticherling et al. 200246 | Not specified | Single centre | 20 |
| Verapamil: pre-cardioversion, 0.15 mg/kg IV at rate of 2 mg/min | N/A | Unclear |
| Ibutilide: pre-cardioversion, 1 mg IV over 10 min | |||||||
| Sticherling et al. 200547 | Unblinded | Single centre | 20 |
| Verapamil: pre-cardioversion, 0.15 mg/kg IV at rate of 2 mg/min | N/A | Unclear |
| Amiodarone: pre-cardioversion, 5 mg/kg IV over 5 min | |||||||
| Stroobandt et al. 199748 | Double blind | Multi-centre | 136 |
| Propafenone: pre-cardioversion, 2 mg/kg IV over 30 min followed 2 h later by 150 mg PO every 8 h; post-cardioversion, 150 mg PO 3 times daily for 6 months | 6 months | Unclear | unclear |
| Matching placebo | |||||||
| Thomas et al. 200420 | Unblinded | Single centre | 140 |
| Amiodarone: pre-cardioversion, 10 mg/kg IV amiodarone over 30 min; post-cardioversion, 200 mg PO twice daily until discharge | 24 h | Unclear | high |
| Sotalol: pre-cardioversion, 1.5 mg/kg IV over 10 min; post-cardioversion, 80 mg PO twice daily until discharge | |||||||
| No treatment | |||||||
| Vijayalakshmi et al. 200649 | Unblinded | Single centre | 94 |
| Amiodarone: pre-cardioversion, 200 mg PO three times daily for the first week, twice daily for the second week, and once daily for 4 weeks | 6 months | High | high |
| Sotalol: pre-cardioversion, 160 mg PO twice daily for 6 weeks | |||||||
| No treatment | |||||||
| Villani et al. 200023 | Single blind | Single centre | 120 |
| Amiodarone: pre-cardioversion, 400 mg PO daily for 1 month; post-cardioversion, 200 mg PO daily | 1 month | Unclear | high |
| Diltiazem: pre-cardioversion, 60 mg PO 3 times a day for 1 month (titrated until heart rate <80 beats/min or max dose of 360 mg/day); post-cardioversion, continued at established dose | |||||||
| Digoxin: pre-cardioversion, 0.25 mg PO daily for 1 month; post-cardioversion, 0.25 mg PO daily |
| Study . | Design . | Setting . | Number of patients . | Cardioversion . | Arms . | Follow-up . | Risk of bias (acute | maintenance) . |
|---|---|---|---|---|---|---|---|
| Bianconi et al. 199630 | Single blind | Single centre | 100 |
| Propafenone: pre-cardioversion, three daily doses of 300 mg, 150 mg, and 300 mg for 48 h; post-cardioversion, ongoing propafenone therapy | N/A | Unclear |
| Matching placebo | |||||||
| Boos et al. 200418 | Unblinded | Single centre | 35 |
| Amiodarone: pre-cardioversion, 1 week of 200 mg PO three times daily; post-cardioversion, 3 weeks of 200 mg PO three times daily for 1 week, followed by 200 mg PO twice daily for 1 week, followed by 200 mg PO daily for 1 week | 16 months | Unclear | high |
| No treatment | |||||||
| Capucci 200031 | Unblinded | Single centre | 92 |
| Amiodarone: pre-cardioversion, 1 month of 400 mg PO daily; post-cardioversion, 200 mg PO daily | 2 months | Unclear | high |
| Glucose-insulin-potassium solution: pre-cardioversion, continuous infusion for 24 h prior to cardioversion | |||||||
| No treatment | |||||||
| Channer et al. 200428 | Double blind | Multi-centre | 161 |
| Amiodarone: pre-cardioversion, 400 mg PO twice daily for 2 weeks; post-cardioversion, 200 mg PO once daily for 8 weeks followed by matching placebo for 44 weeks | 52 weeks | High | high |
| Amiodarone: pre-cardioversion, 400 mg PO twice daily for 2 weeks; post-cardioversion, 200 mg PO once daily for 52 weeks | |||||||
| Matching placebo | |||||||
| Climent et al. 200432 | Double blind | Single centre | 54 |
| Flecainide: pre-cardioversion, 2 mg/kg IV over 30 min | 1 month | High | high |
| Matching placebo | |||||||
| De Simone et al. 200333 | Unblinded | Multi-centre | 162 |
| Amiodarone: pre-cardioversion, 600 mg PO daily for 7 days, followed by 400 mg PO daily for 7 days, then 200 mg PO daily for 14 days | 3 months | Unclear | high |
| Flecainide: pre-cardioversion, 200 mg PO daily for 3 days | |||||||
| Galperin et al. 200134 | Double blind | Multi-centre | 95 |
| Amiodarone: pre-cardioversion, 600 mg PO daily for at least 4 weeks, then 200 mg PO daily | 16 months | Unclear | unclear |
| Matching placebo | |||||||
| Hartel et al. 197435 | Double blind | Single centre | 52 |
| Disopyramide: pre-cardioversion, 100 mg PO three times daily starting the day before cardioversion; post-cardioversion, 500 mg PO daily (in 3 doses) for maintenance | 3 months | Unclear | unclear |
| Matching placebo | |||||||
| Hillestad et al. 197236 | Unblinded | Single centre | 124 |
| Quinidine sulphate: pre-cardioversion, 0.2 g PO twice daily until serum quinidine level of between 4 and 6 mg/L | N/A | Unclear |
| No treatment | |||||||
| Jacobs et al. 199837 | Not specified | Single centre | 100 |
| Procainamide: pre-cardioversion, 15 mg/kg IV (up to max of 1250 mg) over 60 min, followed by continuous infusion of 2 mg/mL prior to cardioversion | N/A | Unclear |
| Matching placebo | |||||||
| Jong et al. 199538 | Unblinded | Not specified | 91 |
| Amiodarone: pre-cardioversion, 200 mg PO three times daily for 4 weeks; post-cardioversion, 200 mg daily for 1 month then 200 mg every other day for 1 month | 2 months | Unclear | high |
| Matching placebo | |||||||
| Joseph et al. 200021 | Single blind | Multi-centre | 120 |
| Amiodarone: pre-cardioversion, 5 mg/kg IV over 30 min then 200 mg PO every 8 h for 6 doses | N/A | Low |
| Sotalol: pre-cardioversion, 1.5 mg/kg IV over 30 min then 80 mg PO every 8 h for 6 doses | |||||||
| No treatment | |||||||
| Kanoupakis et al. 200439 | Unblinded | Not specified | 145 |
| Amiodarone: 600 mg PO daily for first 2 weeks then 200 mg daily until end of study | 4 weeks | Low | high |
| Carvedilol: 6.25 mg PO twice daily, titrated up to 25 mg twice daily up to end of study | |||||||
| No treatment | |||||||
| Komatsu et al. 200940 | No specified | Single centre | 70 |
| Cibenzoline: 70 mg IV over 5 min | N/A | Unclear |
| Pilsicainide: 50 mg IV over 5 min | |||||||
| Lombardi et al. 200641 | Double blind | Not specified | 658 |
| Azimilide: pre-cardioversion, 125 mg PO twice daily for 3 days; post-cardioversion, 125 mg PO daily or placebo daily for 26 weeks | N/A | High |
| Sotalol: pre-cardioversion, 160 mg PO twice daily for 3 days; post-cardioversion, 160 mg PO twice daily for 26 weeks | |||||||
| Matching placebo | |||||||
| Manios etal. 200342 | Unblinded | Single centre | 111 |
| Amiodarone: 600 mg PO daily for 2 weeks, then 200 mg daily up to end of study | 6 weeks | Low | high |
| Diltiazem: 270 mg to 360 mg PO daily | |||||||
| No treatment | |||||||
| Mazzocca et al. 200643 | Unblinded | Single centre | 32 |
| Ibutilide: pre-cardioversion, 0.01 mg/kg IV over 10 min | N/A | Unclear |
| No treatment | |||||||
| Okishige et al. 200044 | Not specified | Single centre | 62 |
| Pilsicainide: pre-cardioversion, 150 mg PO daily for 4 weeks; post-cardioversion, 50 mg PO 3 times daily for 2 years | 19 months | Unclear | unclear |
| Matching placebo | |||||||
| Oral et al. 199919 | Unblinded | Single centre | 100 |
| Ibutilide: pre-cardioversion, 1 mg IV over 10 min | 6 months | Unclear | high |
| No treatment | |||||||
| Pritchett et al. 200622 | Double blind | Multi-centre | 446 |
| Azimilide: pre-cardioversion, 125 mg PO twice daily for 3 days; post-cardioversion, 125 mg daily for 6 months | N/A | Unclear |
| Matched placebo | |||||||
| No treatment | |||||||
| Singh et al. 200045 | Double blind | Multi-centre | 325 |
| Dofetilide: pre-cardioversion, 125 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | 12 months | Unclear | unclear |
| Dofetilide: pre-cardioversion, 250 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | |||||||
| Dofetilide: pre-cardioversion, 500 μg twice daily for a minimum of 3 days or 5 doses; post-cardioversion, 125 μg twice daily for 12 months | |||||||
| Matching placebo | |||||||
| Singh et al. 200527 | Double blind | Multi-centre | 665 |
| Amiodarone: 800 mg PO daily for first 14 days, then 600 mg PO daily for next 14 days, then 300 mg PO daily for first year, then 200 mg PO daily thereafter | 1 year | Low | low |
| Sotalol: 80 mg PO twice daily for first week, then 160 mg PO twice daily thereafter | |||||||
| Matching placebo | |||||||
| Sticherling et al. 200246 | Not specified | Single centre | 20 |
| Verapamil: pre-cardioversion, 0.15 mg/kg IV at rate of 2 mg/min | N/A | Unclear |
| Ibutilide: pre-cardioversion, 1 mg IV over 10 min | |||||||
| Sticherling et al. 200547 | Unblinded | Single centre | 20 |
| Verapamil: pre-cardioversion, 0.15 mg/kg IV at rate of 2 mg/min | N/A | Unclear |
| Amiodarone: pre-cardioversion, 5 mg/kg IV over 5 min | |||||||
| Stroobandt et al. 199748 | Double blind | Multi-centre | 136 |
| Propafenone: pre-cardioversion, 2 mg/kg IV over 30 min followed 2 h later by 150 mg PO every 8 h; post-cardioversion, 150 mg PO 3 times daily for 6 months | 6 months | Unclear | unclear |
| Matching placebo | |||||||
| Thomas et al. 200420 | Unblinded | Single centre | 140 |
| Amiodarone: pre-cardioversion, 10 mg/kg IV amiodarone over 30 min; post-cardioversion, 200 mg PO twice daily until discharge | 24 h | Unclear | high |
| Sotalol: pre-cardioversion, 1.5 mg/kg IV over 10 min; post-cardioversion, 80 mg PO twice daily until discharge | |||||||
| No treatment | |||||||
| Vijayalakshmi et al. 200649 | Unblinded | Single centre | 94 |
| Amiodarone: pre-cardioversion, 200 mg PO three times daily for the first week, twice daily for the second week, and once daily for 4 weeks | 6 months | High | high |
| Sotalol: pre-cardioversion, 160 mg PO twice daily for 6 weeks | |||||||
| No treatment | |||||||
| Villani et al. 200023 | Single blind | Single centre | 120 |
| Amiodarone: pre-cardioversion, 400 mg PO daily for 1 month; post-cardioversion, 200 mg PO daily | 1 month | Unclear | high |
| Diltiazem: pre-cardioversion, 60 mg PO 3 times a day for 1 month (titrated until heart rate <80 beats/min or max dose of 360 mg/day); post-cardioversion, continued at established dose | |||||||
| Digoxin: pre-cardioversion, 0.25 mg PO daily for 1 month; post-cardioversion, 0.25 mg PO daily |
We did not include post-crossover results in our analysis.
For the acute restoration of sinus rhythm outcome, 20 of 28 studies had an unclear risk of bias with four studies each having a low and high risk of bias (see Table 1 and Supplementary material online, Appendix S2). For the maintenance of sinus rhythm outcome, 12 of 18 studies had a high risk of bias, and one study had a low risk of bias. The most common reason for an unclear risk of bias was an inadequate description of the randomization protocol (9 of 21 acute restoration studies and 4 of 5 long-term maintenance studies). For the maintenance of sinus rhythm outcome, lack of blinding of participants, personnel and/or outcome assessment was the most common cause of high risk of bias (9 of 12 studies).
Pre-treatment regimens
Table 1 details the treatment regimens used prior to electrical cardioversion. Class Ia regimens included disopyramide, procainamide, quinidine, and cibenzoline. Class Ic regimens were flecainide, propafenone, and pilsicainide. Class III regimens included sotalol, ibutilide, azimilide, and dofetilide. Dosing regimens for amiodarone ranged from 5 mg/kg IV over 5–30 min to 200–800 mg PO daily for 1–6 weeks.
Acute restoration of sinus rhythm
The number of trials included in direct comparisons varied from 1 to 13 (Table 3). Figure 1A is the network diagram for acute restoration of sinus rhythm. An indirect estimate was not generated for Class III vs. no treatment or rate control because the studies that comprised the network loop were the same three-arm trials that informed the direct comparison.
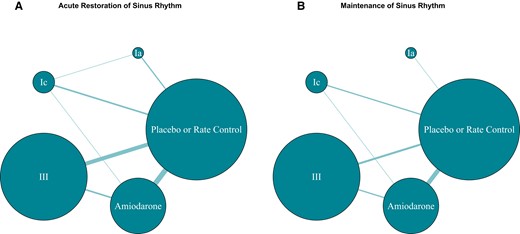
Network diagram of acute restoration and maintenance of sinus rhythm. The size of each node is proportional to the number of participants. The size of each edge reflects the number of studies for that comparison.
| Anti-arrhythmic drug . | Sucra . |
|---|---|
| Acute restoration | |
| Amiodarone | 0.83 |
| Class III | 0.76 |
| Class Ia | 0.50 |
| Class Ic | 0.31 |
| No treatment or rate control | 0.10 |
| Long-term maintenance | |
| Amiodarone | 0.93 |
| Class Ia | 0.63 |
| Class Ic | 0.52 |
| Class III | 0.41 |
| No treatment or rate control | 0.01 |
| Anti-arrhythmic drug . | Sucra . |
|---|---|
| Acute restoration | |
| Amiodarone | 0.83 |
| Class III | 0.76 |
| Class Ia | 0.50 |
| Class Ic | 0.31 |
| No treatment or rate control | 0.10 |
| Long-term maintenance | |
| Amiodarone | 0.93 |
| Class Ia | 0.63 |
| Class Ic | 0.52 |
| Class III | 0.41 |
| No treatment or rate control | 0.01 |
SUCRA, surface under the cumulative ranking curve.
| Anti-arrhythmic drug . | Sucra . |
|---|---|
| Acute restoration | |
| Amiodarone | 0.83 |
| Class III | 0.76 |
| Class Ia | 0.50 |
| Class Ic | 0.31 |
| No treatment or rate control | 0.10 |
| Long-term maintenance | |
| Amiodarone | 0.93 |
| Class Ia | 0.63 |
| Class Ic | 0.52 |
| Class III | 0.41 |
| No treatment or rate control | 0.01 |
| Anti-arrhythmic drug . | Sucra . |
|---|---|
| Acute restoration | |
| Amiodarone | 0.83 |
| Class III | 0.76 |
| Class Ia | 0.50 |
| Class Ic | 0.31 |
| No treatment or rate control | 0.10 |
| Long-term maintenance | |
| Amiodarone | 0.93 |
| Class Ia | 0.63 |
| Class Ic | 0.52 |
| Class III | 0.41 |
| No treatment or rate control | 0.01 |
SUCRA, surface under the cumulative ranking curve.
Network meta-analysis results of five-node analysis, including confidence assessments
| Comparison . | Trials with direct comparisons (n) . | Direct estimate (95% CI) . | Indirect estimate (95% CrI) . | NMA estimate (95% CrI) . | Overall certainty . |
|---|---|---|---|---|---|
| Acute restoration of sinus rhythm | |||||
| Class Ia vs. no treatment or rate control | 3 | 1.04 (0.38, 2.89) | 8.10 (1.22, 55.69) | 1.65 (0.65, 4.24) | High |
| Class Ic vs. no treatment or rate control | 4 | 1.54 (0.60, 4.02) | 0.81 (0.17, 3.88) | 1.30 (0.59, 2.96) | High |
| Class III vs. no treatment or rate control | 10 | 2.41 (1.37, 4.62) | N/A* | 2.41 (1.37, 4.62) | High |
| Amiodarone vs. no treatment or rate control | 13 | 2.82 (1.61, 4.98) | 0.80 (0.07, 8.89) | 2.58 (1.54, 4.37) | High |
| Class Ic vs. Class Ia | 1 | 0.21 (0.04, 1.22) | 1.67 (0.47, 6.46) | 0.79 (0.26, 2.38) | High |
| Amiodarone vs. Class Ic | 1 | 0.74 (0.08, 6.94) | 2.40 (0.88, 6.76) | 1.97 (0.79, 5.02) | High |
| Amiodarone vs. Class III | 4 | 0.75 (0.25, 2.11) | 1.45 (0.43, 4.01) | 1.07 (0.50, 2.12) | High |
| Long-term maintenance of sinus rhythm | |||||
| Class Ia vs. no treatment or rate control | 1 | 3.52 (0.94, 14.08) | N/A* | 3.52 (0.94, 14.08) | High |
| Class Ic vs. no treatment or rate control | 3 | 1.91 (0.96, 4.08) | 4.71 (2.03, 11.03) | 2.83 (1.60, 5.10) | Moderate |
| Class III vs. no treatment or rate control | 5 | 2.50 (1.70, 3.79) | N/A* | 2.50 (1.70, 3.79) | High |
| Amiodarone vs. no treatment or rate control | 11 | 5.86 (4.09, 8.42) | 2.37 (0.68, 8.33) | 5.37 (4.00, 7.39) | High |
| Amiodarone vs. Class Ic | 1 | 1.22 (0.55, 2.81) | 3.08 (1.38, 6.48) | 1.89 (1.05, 3.45) | Moderate |
| Amiodarone vs. Class III | 3 | 2.31 (1.21, 4.18) | 1.97 (0.88, 4.46) | 2.19 (1.39, 3.26) | High |
| Comparison . | Trials with direct comparisons (n) . | Direct estimate (95% CI) . | Indirect estimate (95% CrI) . | NMA estimate (95% CrI) . | Overall certainty . |
|---|---|---|---|---|---|
| Acute restoration of sinus rhythm | |||||
| Class Ia vs. no treatment or rate control | 3 | 1.04 (0.38, 2.89) | 8.10 (1.22, 55.69) | 1.65 (0.65, 4.24) | High |
| Class Ic vs. no treatment or rate control | 4 | 1.54 (0.60, 4.02) | 0.81 (0.17, 3.88) | 1.30 (0.59, 2.96) | High |
| Class III vs. no treatment or rate control | 10 | 2.41 (1.37, 4.62) | N/A* | 2.41 (1.37, 4.62) | High |
| Amiodarone vs. no treatment or rate control | 13 | 2.82 (1.61, 4.98) | 0.80 (0.07, 8.89) | 2.58 (1.54, 4.37) | High |
| Class Ic vs. Class Ia | 1 | 0.21 (0.04, 1.22) | 1.67 (0.47, 6.46) | 0.79 (0.26, 2.38) | High |
| Amiodarone vs. Class Ic | 1 | 0.74 (0.08, 6.94) | 2.40 (0.88, 6.76) | 1.97 (0.79, 5.02) | High |
| Amiodarone vs. Class III | 4 | 0.75 (0.25, 2.11) | 1.45 (0.43, 4.01) | 1.07 (0.50, 2.12) | High |
| Long-term maintenance of sinus rhythm | |||||
| Class Ia vs. no treatment or rate control | 1 | 3.52 (0.94, 14.08) | N/A* | 3.52 (0.94, 14.08) | High |
| Class Ic vs. no treatment or rate control | 3 | 1.91 (0.96, 4.08) | 4.71 (2.03, 11.03) | 2.83 (1.60, 5.10) | Moderate |
| Class III vs. no treatment or rate control | 5 | 2.50 (1.70, 3.79) | N/A* | 2.50 (1.70, 3.79) | High |
| Amiodarone vs. no treatment or rate control | 11 | 5.86 (4.09, 8.42) | 2.37 (0.68, 8.33) | 5.37 (4.00, 7.39) | High |
| Amiodarone vs. Class Ic | 1 | 1.22 (0.55, 2.81) | 3.08 (1.38, 6.48) | 1.89 (1.05, 3.45) | Moderate |
| Amiodarone vs. Class III | 3 | 2.31 (1.21, 4.18) | 1.97 (0.88, 4.46) | 2.19 (1.39, 3.26) | High |
CI, confidence interval; CrI, credible interval; N/A, not applicable.
Network meta-analysis results of five-node analysis, including confidence assessments
| Comparison . | Trials with direct comparisons (n) . | Direct estimate (95% CI) . | Indirect estimate (95% CrI) . | NMA estimate (95% CrI) . | Overall certainty . |
|---|---|---|---|---|---|
| Acute restoration of sinus rhythm | |||||
| Class Ia vs. no treatment or rate control | 3 | 1.04 (0.38, 2.89) | 8.10 (1.22, 55.69) | 1.65 (0.65, 4.24) | High |
| Class Ic vs. no treatment or rate control | 4 | 1.54 (0.60, 4.02) | 0.81 (0.17, 3.88) | 1.30 (0.59, 2.96) | High |
| Class III vs. no treatment or rate control | 10 | 2.41 (1.37, 4.62) | N/A* | 2.41 (1.37, 4.62) | High |
| Amiodarone vs. no treatment or rate control | 13 | 2.82 (1.61, 4.98) | 0.80 (0.07, 8.89) | 2.58 (1.54, 4.37) | High |
| Class Ic vs. Class Ia | 1 | 0.21 (0.04, 1.22) | 1.67 (0.47, 6.46) | 0.79 (0.26, 2.38) | High |
| Amiodarone vs. Class Ic | 1 | 0.74 (0.08, 6.94) | 2.40 (0.88, 6.76) | 1.97 (0.79, 5.02) | High |
| Amiodarone vs. Class III | 4 | 0.75 (0.25, 2.11) | 1.45 (0.43, 4.01) | 1.07 (0.50, 2.12) | High |
| Long-term maintenance of sinus rhythm | |||||
| Class Ia vs. no treatment or rate control | 1 | 3.52 (0.94, 14.08) | N/A* | 3.52 (0.94, 14.08) | High |
| Class Ic vs. no treatment or rate control | 3 | 1.91 (0.96, 4.08) | 4.71 (2.03, 11.03) | 2.83 (1.60, 5.10) | Moderate |
| Class III vs. no treatment or rate control | 5 | 2.50 (1.70, 3.79) | N/A* | 2.50 (1.70, 3.79) | High |
| Amiodarone vs. no treatment or rate control | 11 | 5.86 (4.09, 8.42) | 2.37 (0.68, 8.33) | 5.37 (4.00, 7.39) | High |
| Amiodarone vs. Class Ic | 1 | 1.22 (0.55, 2.81) | 3.08 (1.38, 6.48) | 1.89 (1.05, 3.45) | Moderate |
| Amiodarone vs. Class III | 3 | 2.31 (1.21, 4.18) | 1.97 (0.88, 4.46) | 2.19 (1.39, 3.26) | High |
| Comparison . | Trials with direct comparisons (n) . | Direct estimate (95% CI) . | Indirect estimate (95% CrI) . | NMA estimate (95% CrI) . | Overall certainty . |
|---|---|---|---|---|---|
| Acute restoration of sinus rhythm | |||||
| Class Ia vs. no treatment or rate control | 3 | 1.04 (0.38, 2.89) | 8.10 (1.22, 55.69) | 1.65 (0.65, 4.24) | High |
| Class Ic vs. no treatment or rate control | 4 | 1.54 (0.60, 4.02) | 0.81 (0.17, 3.88) | 1.30 (0.59, 2.96) | High |
| Class III vs. no treatment or rate control | 10 | 2.41 (1.37, 4.62) | N/A* | 2.41 (1.37, 4.62) | High |
| Amiodarone vs. no treatment or rate control | 13 | 2.82 (1.61, 4.98) | 0.80 (0.07, 8.89) | 2.58 (1.54, 4.37) | High |
| Class Ic vs. Class Ia | 1 | 0.21 (0.04, 1.22) | 1.67 (0.47, 6.46) | 0.79 (0.26, 2.38) | High |
| Amiodarone vs. Class Ic | 1 | 0.74 (0.08, 6.94) | 2.40 (0.88, 6.76) | 1.97 (0.79, 5.02) | High |
| Amiodarone vs. Class III | 4 | 0.75 (0.25, 2.11) | 1.45 (0.43, 4.01) | 1.07 (0.50, 2.12) | High |
| Long-term maintenance of sinus rhythm | |||||
| Class Ia vs. no treatment or rate control | 1 | 3.52 (0.94, 14.08) | N/A* | 3.52 (0.94, 14.08) | High |
| Class Ic vs. no treatment or rate control | 3 | 1.91 (0.96, 4.08) | 4.71 (2.03, 11.03) | 2.83 (1.60, 5.10) | Moderate |
| Class III vs. no treatment or rate control | 5 | 2.50 (1.70, 3.79) | N/A* | 2.50 (1.70, 3.79) | High |
| Amiodarone vs. no treatment or rate control | 11 | 5.86 (4.09, 8.42) | 2.37 (0.68, 8.33) | 5.37 (4.00, 7.39) | High |
| Amiodarone vs. Class Ic | 1 | 1.22 (0.55, 2.81) | 3.08 (1.38, 6.48) | 1.89 (1.05, 3.45) | Moderate |
| Amiodarone vs. Class III | 3 | 2.31 (1.21, 4.18) | 1.97 (0.88, 4.46) | 2.19 (1.39, 3.26) | High |
CI, confidence interval; CrI, credible interval; N/A, not applicable.
In absolute terms, the unadjusted average rate of acute conversion in the no treatment or rate control arm was 74.8% (1210/1618). For Classes Ia, Ic, and III AADs, the unadjusted success rates were 81.1% (163/201), 80.7% (276/342), and 83.7% (1145/1368), respectively. The absolute conversion rate for amiodarone was 83.3% (729/875).
Table 2 lists the results of the NMA. Compared with no treatment or rate control, amiodarone and Class III AADs were associated with increased restoration of sinus rhythm: Class III (OR: 2.41; CrI: 1.37–4.62, high certainty); and amiodarone (OR: 2.58; CrI: 1.54–4.37, high certainty). No AAD class was associated with increased odds of cardioversion compared with one another.
According to SUCRA (Table 3), amiodarone (0.81) and Class III AADs (0.73) were most likely to be the best intervention when compared with Class Ia (0.59) and Ic (0.30) AADs and no treatment or rate control (0.07).
Results were consistent between direct and indirect comparisons for all analyses. Although the direction of effect differed between direct and indirect estimates for two comparisons—i.e. Class Ic and amiodarone vs. no treatment or rate control (see Supplementary material online, Appendix S3)—no comparison met the a priori established threshold of P < 0.05 in the node-splitting analysis for inconsistency (see Supplementary material online, Appendix).
Long-term maintenance of sinus rhythm
The number of trials included in direct comparisons ranged from 1 to 11 and the mean duration of follow-up was 7 months (range: 24 h to 18.7 months), (Table 2). Figure 1B is the network diagram for this outcome. An indirect estimate was not generated for two comparisons: Class Ia and Class III vs. no treatment or rate control. Class Ia had a single comparison with no treatment or rate control. An indirect estimate was not generated for Class III vs. no treatment or rate control because the studies that comprised the network loop were the same three-arm trials that informed the direct comparison.
The unadjusted proportion of patients who maintained sinus rhythm in the no treatment or rate control arm was 28.9% (260/900). For Classes Ia, Ic, and III AADs, the unadjusted success rates were 50.0% (13/26), 43.2% (112/259), and 39.5% (250/633), respectively. The absolute maintenance rate for amiodarone was 58.0% (477/822).
Except for Class Ia (OR: 3.52; CrI: 0.94–14.08, high certainty), the other AAD groups were associated with significantly increased odds (see Table 3) of maintaining sinus rhythm when compared with no treatment or rate control: Class Ic (OR: 2.83; CrI: 1.60–5.10, moderate certainty), Class III (OR: 2.50; CrI: 1.70–3.79, high certainty) and amiodarone (OR: 5.37; CrI: 4.00–7.39, high certainty).
Amiodarone was associated with higher odds when compared with Class Ic (OR: 1.89; CrI: 1.05–3.45, moderate certainty) and Class III (OR: 2.19; CrI: 1.39–3.26, high certainty) AADs. SUCRA results were consistent with these findings (see Table 2). Amiodarone ranked first for this outcome (0.93)—followed by Class Ia (0.64), Class Ic (0.52), Class III (0.41), and no treatment or rate control (0.008).
Node-splitting analysis and visual inspection of the forest plot did not demonstrate inconsistency between direct and indirect estimates for all comparisons regarding maintenance of sinus rhythm (see Supplementary material online, Appendix 3).
Assessment of certainty of evidence
We assessed the evidence for all comparisons to be of high certainty for acute restoration of sinus rhythm (see Table 2).24 The certainty for direct and indirect estimates varied between moderate and high.
For maintenance of sinus rhythm, we assessed 4 comparisons to be of high certainty and two to be of moderate certainty (see Table 2). The certainty of evidence for comparisons between Class Ic and no treatment or rate control, and amiodarone was downgraded for serious risk of bias because each comparison was driven by a study with high risk of bias for inconsistent reporting of results and lack of blinding, respectively.
Discussion
In patients with AF undergoing electrical cardioversion, pre-treatment with amiodarone and Class III AADs both enhance the acute restoration of sinus rhythm with comparable efficacy. For the long-term maintenance of sinus rhythm, amiodarone is most likely to have benefit, but other Class III AADs and Class Ic AADs are also effective.
The current guidelines for AF published by the American College of Cardiology provides a Class I recommendation for repeated attempts at cardioversion and includes a brief phrase regarding AAD administration prior to repeat cardioversion—citing a single RCT included in this review and mentioning only ibutilide.19,25 The Canadian Cardiovascular Society makes a similar recommendation based on a single RCT and mentions only amiodarone and ibutilide.26 Based on several individual RCTs that are included in this review, the European Society of Cardiology provides a Class IIa recommendation to specifically consider amiodarone, flecainide, ibutilide, or propafenone to facilitate electrical cardioversion.19,23,27–30
Contemporary data suggest that pre-treatment is only used in a small number of patients undergoing cardioversion. In the X-VeRT trial (an international RCT of two oral anticoagulant strategies in 1504 AF patients undergoing elective cardioversion), fewer than 30% of participants were administered amiodarone, Class I, or III AADs before electrical cardioversion.3
This NMA demonstrated high certainty of evidence for pre-treating patients with amiodarone and Class III AADs before an electrical cardioversion to more than double the odds of the acute restoration of sinus rhythm. It has also shown with high certainty that amiodarone is the optimal AAD regimen for post-cardioversion maintenance of sinus rhythm. These results expand on the recommendations of major American, Canadian, and European guidelines and should lead to broader recommendations for pre-treatment in all patients undergoing elective electrical cardioversion of AF or specific recommendations for Class III AADs or amiodarone for acute restoration and amiodarone for the long-term maintenance of sinus rhythm.
Based on the absolute conversion rate of 75% for acute restoration in the no treatment or rate control arm in our study, pre-treatment with amiodarone may increase the restoration of sinus rhythm to 89%. Based on an absolute rate of 29% in the no treatment or rate control arm in this study, the long-term maintenance of sinus rhythm would have increased to 69% with pre- and post-treatment using amiodarone. Application of the evidence synthesized in this review may lead to improved outcomes for patients with AF by reducing healthcare resource utilization (e.g. repeat electrical cardioversion, unplanned visits), limiting the proportion of symptomatic patients abandoning a rhythm control strategy and decreasing the symptomatic burden during the waiting period for patients pursuing AF ablation. Scientific societies should review the scope and the strength of their recommendations considering these results. Meanwhile, clinicians may consider a broader and more consistent use of AAD pre-treatment before electrical cardioversion of AF.
Strengths
This NMA has several strengths. We performed a comprehensive search with high sensitivity and applied rigorous rule-based methods for data collection and assessment. We assessed the risk of bias for included studies and evaluated the certainty of evidence for outcomes of interest in accordance with GRADE methodology for NMA.
Limitations
To strengthen our network framework, we grouped Classes of AADs together which may have introduced heterogeneity to the network. We only included AADs that were currently available and approved for use in Canada or the United States. Excluded AADs included AZD7009, AZD1305, and bepridil. In addition, we were unable to classify the risk of selection bias for 9 studies due to inadequate reporting in published manuscripts. Finally, given the limited reporting of safety data in our included studies, we did not capture the data for this outcome. The adverse effects of AADs are well known and better addressed by studies with increased scope and the primary intent of answering that clinical question.
Conclusions
Pre-treatment with amiodarone or Class III AADs improves the acute restoration of sinus rhythm in patients with AF undergoing elective electrical cardioversion. Amiodarone is likely superior to other Class III and Ic agents for post-cardioversion maintenance of sinus rhythm.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
We would like to thank Laura Banfield for refining our search strategy. We would also like to acknowledge Serena Sibilio, Cathevine Yang, and Alexandra Sibiga for their translation of Italian, Chinese, and Polish. The authors (W.F.M. and K.J.U.) are members of the Cardiac Arrhythmia Network of Canada (CANet) HQP Association for Trainees (CHAT).
Funding
This work was supported by a Mach-Gaensslen Medical Summer Student Research Grant (2017), McMaster University Department of Medicine Medical Student Research Award (2017), McMaster Medical Student Research Excellence Scholarship (2018), and Heart and Stroke Foundation Ontario Evelyn McGloin Summer Medical Student Scholarship (2018). Emilie Belley-Côté is supported by the E.J. Moran Campbell McMaster Internal Career Award and by a Heart and Stroke Foundation National New Investigator award. R.P.W. holds the Canada Research Chair in Cardiovascular Surgery.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
References
Author notes
Prepared at McMaster University and the Population Health Research Institute.
Conflicts of interest: The authors have no conflict of interest relevant to the submitted work. R.P.W. reports grants and speaker fees from Boeringer-Ingelheim; speaker fees from Atricure, Phasebio and Cryolife; grants from Bayer and BMS-Pfizer, outside the submitted work. E.P.B.-C. reports grants from BMS-Pfizer, Bayer, and Roche Diagnostics, outside the submitted work. J.S.H. reports Research grants and speaking fees from Medtronic, Boston scientific, Abott, BMS/Pfizer, Servier, Bayer, Novartis, Myokardia, ARCA Biopharm, and Cipher Pharm outside the submitted work. W.F.M. reports speaking fees from Servier and Bayer and Boehringer Ingelheim, outside the submitted work. E.D. reports grants from Boeringer-Ingelheim and Roche Diagnostics. The other authors have no conflict of interest to declare.



