-
PDF
- Split View
-
Views
-
Cite
Cite
J N López-Canoa, M Couselo-Seijas, A Baluja, L González-Melchor, A Rozados, V Llorente-Cortés, D de Gonzalo-Calvo, J M Guerra, D Vilades, R Leta, J L Martínez-Sande, F J García-Seara, X A Fernández-López, J R González-Juanatey, S Eiras, M Rodríguez-Mañero, Sex-related differences of fatty acid-binding protein 4 and leptin levels in atrial fibrillation, EP Europace, Volume 23, Issue 5, May 2021, Pages 682–690, https://doi.org/10.1093/europace/euaa284
Close - Share Icon Share
Abstract
Adiposity plays a key role in the pathogenesis of atrial fibrillation (AF). Our aim was to study the sex differences in adipokines levels according to AF burden.
Two independent cohorts of patients were studied: (i) consecutive patients with AF undergoing catheter ablation (n = 217) and (ii) a control group (n = 105). (i) Adipokines, oxidative stress, indirect autonomic markers, and leucocytes mRNA levels were analysed; (ii) correlation between biomarkers was explored with heatmaps and Kendall correlation coefficients; and (iii) logistic regression and random forest model were used to determine predictors of AF recurrence after ablation. Our results showed that: (i) fatty acid-binding protein 4 (FABP4) and leptin levels were higher in women than in men in both cohorts (P < 0.01). In women, FABP4 levels were higher on AF cohort (20 ± 14 control, 29 ± 18 paroxysmal AF and 31 ± 17 ng/mL persistent AF; P < 0.01). In men, leptin levels were lower on AF cohort (22 ± 15 control, 13 ± 16 paroxysmal AF and 13 ± 11 ng/mL persistent AF; P < 0.01). (ii) In female with paroxysmal AF, there was a lower acetylcholinesterase and higher carbonic anhydrase levels with respect to men (P < 0.05). (iii) Adipokines have an important role on discriminate AF recurrence after ablation. In persistent AF, FABP4 was the best predictor of recurrence after ablation (1.067, 95% confidence interval 1–1.14; P = 0.046).
The major finding of the present study is the sex-based differences of FABP4 and leptin levels according to AF burden. These adipokines are associated with oxidative stress, inflammatory and autonomic indirect markers, indicating that they may play a role in AF perpetuation.
Sex-related differences of fatty acid-binding protein 4 (FABP4) and leptin levels according to atrial fibrillation (AF) burden.
The adipose tissue and neutrophil biomarkers differed between male and female in paroxysmal and persistent AF.
The association of FABP4 with oxidative stress marker in persistent but not in paroxysmal AF.
Adipokines had higher importance than oxidative or inflammatory biomarkers for predicting recurrence after catheter ablation.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia worldwide and a major public health problem whose prevalence is increasing in parallel with ageing and obesity.1 These factors predispose to accumulation of epicardial fat tissue (EAT), cardiac structure, and function changes. The effects of this fat tissue may be local or systemic and a strong body of evidence highlights its ability to modulate the cardiovascular system and contribute to the development of arrhythmias.2 Although some effects of adipocytes, such as regulation of inflammation, oxidative stress, or autonomic dysfunction have been postulated, the exact mechanisms of how EAT may drive AF is still not well described. Subsequently, it has been suggested that some adiposity markers might play a role in the pathogenesis of AF and explain this interaction.3 The intensification of research has evidenced its potential in obesity-related cardiovascular disease prevention and treatment. Proteomic studies have identified a fatty acid-binding protein 4 (FABP4), also known as adipocyte protein 2 (aP2), as a predictor of metabolic disorders and a new biomarker for AF risk.4 The main role of FABP4 is to be involved in the intracellular trafficking of fatty acids and lipid signals. But, in macrophages, it can also improve the neutrophils recruitment and oxidative stress. Accordingly, FABP4 has been reported to contribute to structural heart disease and cardiac contractile dysfunction,5 explaining the relationship between FABP4 and AF perpetuation.6 In addition, FABP4 is co-regulated with leptin during adipose inflammatory process.7 Interestingly, the plasma levels of both proteins differ between males and females. This phenomenon likely might explain, among others, the sex differences in AF structural and electrophysiological mechanisms.8 Indeed, an increased risk of stroke, reduced catheter ablation efficacy and major complications have been reported in women compared to men.9 Our study aimed to analyse (i) the role of adipokines on AF and its sex-related differences; (ii) their relationship with inflammatory, oxidative and autonomic markers to elucidate potential mechanisms underlying obesity-AF; and (iii) their role on AF perpetuation.
Methods
Subjects
Two independent cohorts have been used for this study. The case cohort belongs to a cross-sectional study where consecutive patients with paroxysmal or persistent AF were referred for pulmonary vein radiofrequency catheter ablation. The control cohort belongs to a cross-sectional study in which consecutive subjects with suspected coronary artery disease (CAD) were referred for a computed tomography (CT) scan. In the control cohort, subjects with history of AF or at very high risk of silent AF (patients with organic valvular disease, prosthetic valves, more than moderate mitral regurgitation secondary to left ventricular dilatation, pulmonary hypertension, or treated with oral anticoagulant) were excluded. In both groups, the exclusion criteria were age under 18 years, pregnancy, any latent infectious condition, and an active oncology disease. Final analyses were thus based on 322 subjects, of which, 217 belonged to the case cohort (35% women and 65% men) and 105 to the control cohort (51% women and 49% men). All of the patients signed the informed consent. The study protocol follows the ethical guidelines of Declaration of Helsinki and approved by Ethical Committee of Clinical Research of our region according to Helsinki Declaration.
Blood sample collection
Case patients
During the ablation procedure (after a night of fasting), immediately after the transseptal puncture and previous to heparin administration, blood samples were obtained from the left atrium (LA) through the transseptal sheath. At the same time, a peripheral blood sample was obtained from an ante-cubital vein using an 18-G butterfly cannula with a two-syringe technique, discarding the first 5 mL of blood and using the second 5 mL for measures.6 LA and peripheral blood samples were collected in EDTA tubes. Electrical cardioversion was systematically performed at the end of the procedure.
Control patients
Peripheral blood sample collected in EDTA tubes was obtained by venipuncture from an ante-cubital vein after a night of fasting and before the cardiac CT and contrast administration.
From cases and controls, glucose, creatinine, and lipid profile were recorded and considered for the analysis.
Plasma and leucocytes measurements
FABP4, leptin, CAIX levels
After centrifuging at 1800×g for 10 min, the atrial and peripheral plasma samples were stored at −80° C until used. A magnetic Luminex multiplex test kit (R&D Systems, MN, USA) was used. The manufacturer’s instructions were followed when analysing plasma levels of FABP4, leptin, and carbonic anhydrase IX (CAIX). The sensitivity for FABP4, Leptin, and CAIX was 95.7, 10.2, and 2.11 pg/mL, respectively.
Acetylcholinesterase activity
The hydrolysis of acetylthiocholine by plasma acetylcholinesterase after 30 min of incubation was provided by a colorimetric assay to detect mU/mL as it is recommended in the manufacturer’s instructions (Abcam, Cambridge, UK).
Glycerol and H2O2 levels
Plasma glycerol levels were measured by a colorimetric assay based on glycerol kinase and glycerol phosphatase oxidase. The linear range of detection for this kit was 0.01–1 mM (Sigma-Aldrich, St Louis, MO, USA). Hydrogen peroxide (H2O2) levels were determined by a colorimetric assay that utilizes the chromogenic Fe3+ −xylenol orange reaction. The kit has a detection range of 0.2–30 M of H2O2 (Sigma-Aldrich).
IL-6 and DEFA3 mRNA expression levels
Atrial blood leucocytes were isolated after centrifuging. Then, erythrocytes were lysed by 155 mM NH4Cl. Afterwards, RNA was isolated by Allprep RNA/protein kit (Qiagen, Gilden, GE) and complementary DNA was performed by Maxima Reverse Transcriptase activity (Thermo Scientific, Waltham, MA, USA). Real-time polymerase chain reaction with the previous described primers was used for quantifying the mRNA expression levels with respect to β-actin levels as it was previously described.10
Ablation procedure and patient follow-up
Patients underwent point-by-point radiofrequency catheter ablation (without contact force sensing, SmartTouch, Biosense Inc.). The procedural endpoint was ipsilateral pulmonary vein isolation (PVI). Most of the patients were discharged 24–36 h after the ablation procedure. Oral anticoagulation (OA) was maintained for at least 3 months (until the first medical review). Then, OA was continued lifelong in those patients with a CHA2DS2-VASc score ≥2. During the blanking period (3 months), it is the standard of care in our centre to continue or restart previously antiarrhythmic drug therapy (ADT). If the patient is free of recurrence after these 3 months, as evidenced by clinical evaluation and 24-h Holter recording, patients are encouraged to discontinue ADT and only restart them in case of relapse. In case of a second recurrence after ADT or electrical cardioversion if needed, and always outside the blanking period, patients are advised for a Redo procedure. Medical visits were systematically performed at 3, 6, and 12 months after the index procedure. Each visit comprised detailed history, physical examination, and 12-lead electrocardiogram. Moreover, 24-h Holter recording was routinely performed at 3, 6, and 12 months.6
Statistical analyses
Numerical data were tested for normality using the Shapiro–Wilk test, and for homoscedasticity with the Levene’s test, then summarized with mean and standard deviation. Bivariable analysis was performed either with the Wilcoxon rank-sum or Pearson’s χ2 test where appropriate. Kruskal–Wallis test was used for comparison multiple groups. Biomarker data were standardized prior to profile analyses and graphs. Data were matched by propensity scores for the graphical representation of biomarker profiles. Biomarker profiles were graphically explored with boxplots matched for age and body mass index (BMI). Correlation between biomarkers was explored by Kendall correlation coefficients. Logistic regressions and generalized additive models were used to test variables associated to either dichotomic- or continuous-dependent variables, respectively. A random forest model based on the Breiman and Cutler’s method was used to measure the within-study variable importance for classifying patients with or without recurrence 12 months after PVI. Random forests are regression and classification trees (CART) combined with bootstrap feature selection that provides increased classification performance and robustness in trained/validation data pairs with multiple variables. Multiple null hypothesis testing was addressed with false discovery ratio control by the Benjamini–Hochberg procedure.
All analyses were programmed in R 3.5 (R Core Team, Vienna, Austria), using the packages ggplot, dplyr, purrr (Henry, 2019), Hmisc (Harrell, 2019), and P < 0.05 was considered with statistical significance.
Results
Population characteristics
The study population included 322 participants of which 217 belonged to the case cohort (49% paroxysmal AF and 51% persistent AF) and 105 to the control cohort. After classifying patients in the case cohort according to AF pattern, we observed a younger population (57 ± 14 vs. 63 ± 13, P < 0.05) and higher male percentage (80% vs. 49%, P < 0.05) in the persistent AF group respect to the control cohort. Nonetheless, with respect to age and gender, the control cohort and the paroxysmal AF group were very similar. The mean BMI was higher in the case than in the control cohort (30 ± 5 vs. 28 ± 5 kg/m2, P < 0.05). There were no differences regarding the percentage of active smokers, arterial hypertension, or type 2 diabetes mellitus. Neither the mean glucose, total and LDL cholesterol levels, or percentage of statins prescription were different among cohorts. However, lower level of HDL cholesterol was observed in the persistent AF group (P < 0.05). Although the levels of creatinine were higher in the case cohort (P < 0.01), there were no differences in the percentage of chronic kidney disease (estimated glomerular filtration rate less than 60 mL/min/1.73 m2). No between-group differences were observed in left ventricular ejection fraction. Beta-blocker intake was higher in the case cohort. About 60% of patients in the case cohort were receiving ADT at baseline, most frequently Class I in paroxysmal AF and Class III ADT in patients with persistent AF. Baseline characteristics of patients included according AF pattern are presented in Table 1.
Differential characteristics among control, paroxysmal, and persistent AF at baseline
| . | Control (n = 105) . | Paroxysmal AF (n = 107) . | Persistent AF (n = 110) . | P-value . |
|---|---|---|---|---|
| Age (years) | 63 ± 13 | 60 ± 10 | 57 ± 14* | <0.001 |
| BMI (kg/m2) | 28 ± 5 | 30 ± 5* | 30 ± 5* | 0.002 |
| Gender male/female (%) | 51/54 (49%) | 54/53 (50%) | 88/22 (80%)*,≠ | <0.001 |
| AHT (%) | 56 (53%) | 41 (38%) | 53 (48%) | 0.083 |
| Active smokers (%) | 32 (31%) | 30 (28%) | 36 (32%) | 0.750 |
| T2DM (%) | 23 (22%) | 12 (11%) | 14 (13%) | 0.064 |
| Obesity (%) | 31 (30%) | 46 (43%)* | 51 (46%)* | 0.028 |
| CKD (%) | 4 (4%) | 11 (10%) | 11 (10%) | 0.148 |
| Creatinine (mg/dL), mean ± SD | 0.82 ± 0.20 | 0.95 ± 0.27* | 1.06 ± 0.28*,≠ | <0.001 |
| Glucose (mg/dL), mean ± SD | 106 ± 35 | 108 ± 25 | 109 ± 30 | 0.702 |
| TC (mg/dL), mean ± SD | 199 ± 42 | 190 ± 45 | 198 ± 37 | 0.307 |
| LDL-C (mg/dL), mean ± SD | 114 ± 41 (105–122) | 112 ± 36 (104–121) | 125 ± 30(118–131) | 0.076 |
| HDL-C (mg/dL), mean ± SD | 59 ± 26 (53–64) | 52 ± 18 (48–56) | 51 ± 13 (48–54)* | 0.030 |
| FABP4 (ng/mL), mean ± SD | 17.5 ± 12 (15–20) | 23 ± 16 (20–26)* | 19 ± 13 (17–21) | 0.015 |
| Leptin (ng/mL), mean ± SD | 39 ± 42 (31–48) | 24 ± 22 (20–28)* | 21 ± 37 (14–28)* | <0.001 |
| LVEF (%) | 63 ± 7 | 64 ± 6 | 62 ± 8 | 0.220 |
| Statin, yes/no (%) | 48/57 (46%) | 32/44 (42%) | 30/56 (35%) | 0.313 |
| Beta-blocker, n (%) | 23 (22%) | 59 (56%)* | 72 (67%)* | <0.001 |
| ADT class I, n (%) | – | 43 (41%) | 23 (21%)≠ | 0.002 |
| ADT class III, n (%) | – | 23 (22%) | 39 (36%)≠ | 0.020 |
| . | Control (n = 105) . | Paroxysmal AF (n = 107) . | Persistent AF (n = 110) . | P-value . |
|---|---|---|---|---|
| Age (years) | 63 ± 13 | 60 ± 10 | 57 ± 14* | <0.001 |
| BMI (kg/m2) | 28 ± 5 | 30 ± 5* | 30 ± 5* | 0.002 |
| Gender male/female (%) | 51/54 (49%) | 54/53 (50%) | 88/22 (80%)*,≠ | <0.001 |
| AHT (%) | 56 (53%) | 41 (38%) | 53 (48%) | 0.083 |
| Active smokers (%) | 32 (31%) | 30 (28%) | 36 (32%) | 0.750 |
| T2DM (%) | 23 (22%) | 12 (11%) | 14 (13%) | 0.064 |
| Obesity (%) | 31 (30%) | 46 (43%)* | 51 (46%)* | 0.028 |
| CKD (%) | 4 (4%) | 11 (10%) | 11 (10%) | 0.148 |
| Creatinine (mg/dL), mean ± SD | 0.82 ± 0.20 | 0.95 ± 0.27* | 1.06 ± 0.28*,≠ | <0.001 |
| Glucose (mg/dL), mean ± SD | 106 ± 35 | 108 ± 25 | 109 ± 30 | 0.702 |
| TC (mg/dL), mean ± SD | 199 ± 42 | 190 ± 45 | 198 ± 37 | 0.307 |
| LDL-C (mg/dL), mean ± SD | 114 ± 41 (105–122) | 112 ± 36 (104–121) | 125 ± 30(118–131) | 0.076 |
| HDL-C (mg/dL), mean ± SD | 59 ± 26 (53–64) | 52 ± 18 (48–56) | 51 ± 13 (48–54)* | 0.030 |
| FABP4 (ng/mL), mean ± SD | 17.5 ± 12 (15–20) | 23 ± 16 (20–26)* | 19 ± 13 (17–21) | 0.015 |
| Leptin (ng/mL), mean ± SD | 39 ± 42 (31–48) | 24 ± 22 (20–28)* | 21 ± 37 (14–28)* | <0.001 |
| LVEF (%) | 63 ± 7 | 64 ± 6 | 62 ± 8 | 0.220 |
| Statin, yes/no (%) | 48/57 (46%) | 32/44 (42%) | 30/56 (35%) | 0.313 |
| Beta-blocker, n (%) | 23 (22%) | 59 (56%)* | 72 (67%)* | <0.001 |
| ADT class I, n (%) | – | 43 (41%) | 23 (21%)≠ | 0.002 |
| ADT class III, n (%) | – | 23 (22%) | 39 (36%)≠ | 0.020 |
ADT, antiarrhythmic drug therapy; AF, atrial fibrillation; AHT, arterial hypertension; BMI, body mass index; CKD, chronic kidney disease (eGFR < 60 mL/min/1.73 m2); FABP4, fatty acid-binding protein 4; LVEF, left ventricular ejection fraction; SD, standard deviation; T2DM, type 2 diabetes mellitus; TC, total cholesterol.
Post hoc differences between paroxysmal or persistent AF vs. control* or paroxysmal vs. persistent AF≠.
Differential characteristics among control, paroxysmal, and persistent AF at baseline
| . | Control (n = 105) . | Paroxysmal AF (n = 107) . | Persistent AF (n = 110) . | P-value . |
|---|---|---|---|---|
| Age (years) | 63 ± 13 | 60 ± 10 | 57 ± 14* | <0.001 |
| BMI (kg/m2) | 28 ± 5 | 30 ± 5* | 30 ± 5* | 0.002 |
| Gender male/female (%) | 51/54 (49%) | 54/53 (50%) | 88/22 (80%)*,≠ | <0.001 |
| AHT (%) | 56 (53%) | 41 (38%) | 53 (48%) | 0.083 |
| Active smokers (%) | 32 (31%) | 30 (28%) | 36 (32%) | 0.750 |
| T2DM (%) | 23 (22%) | 12 (11%) | 14 (13%) | 0.064 |
| Obesity (%) | 31 (30%) | 46 (43%)* | 51 (46%)* | 0.028 |
| CKD (%) | 4 (4%) | 11 (10%) | 11 (10%) | 0.148 |
| Creatinine (mg/dL), mean ± SD | 0.82 ± 0.20 | 0.95 ± 0.27* | 1.06 ± 0.28*,≠ | <0.001 |
| Glucose (mg/dL), mean ± SD | 106 ± 35 | 108 ± 25 | 109 ± 30 | 0.702 |
| TC (mg/dL), mean ± SD | 199 ± 42 | 190 ± 45 | 198 ± 37 | 0.307 |
| LDL-C (mg/dL), mean ± SD | 114 ± 41 (105–122) | 112 ± 36 (104–121) | 125 ± 30(118–131) | 0.076 |
| HDL-C (mg/dL), mean ± SD | 59 ± 26 (53–64) | 52 ± 18 (48–56) | 51 ± 13 (48–54)* | 0.030 |
| FABP4 (ng/mL), mean ± SD | 17.5 ± 12 (15–20) | 23 ± 16 (20–26)* | 19 ± 13 (17–21) | 0.015 |
| Leptin (ng/mL), mean ± SD | 39 ± 42 (31–48) | 24 ± 22 (20–28)* | 21 ± 37 (14–28)* | <0.001 |
| LVEF (%) | 63 ± 7 | 64 ± 6 | 62 ± 8 | 0.220 |
| Statin, yes/no (%) | 48/57 (46%) | 32/44 (42%) | 30/56 (35%) | 0.313 |
| Beta-blocker, n (%) | 23 (22%) | 59 (56%)* | 72 (67%)* | <0.001 |
| ADT class I, n (%) | – | 43 (41%) | 23 (21%)≠ | 0.002 |
| ADT class III, n (%) | – | 23 (22%) | 39 (36%)≠ | 0.020 |
| . | Control (n = 105) . | Paroxysmal AF (n = 107) . | Persistent AF (n = 110) . | P-value . |
|---|---|---|---|---|
| Age (years) | 63 ± 13 | 60 ± 10 | 57 ± 14* | <0.001 |
| BMI (kg/m2) | 28 ± 5 | 30 ± 5* | 30 ± 5* | 0.002 |
| Gender male/female (%) | 51/54 (49%) | 54/53 (50%) | 88/22 (80%)*,≠ | <0.001 |
| AHT (%) | 56 (53%) | 41 (38%) | 53 (48%) | 0.083 |
| Active smokers (%) | 32 (31%) | 30 (28%) | 36 (32%) | 0.750 |
| T2DM (%) | 23 (22%) | 12 (11%) | 14 (13%) | 0.064 |
| Obesity (%) | 31 (30%) | 46 (43%)* | 51 (46%)* | 0.028 |
| CKD (%) | 4 (4%) | 11 (10%) | 11 (10%) | 0.148 |
| Creatinine (mg/dL), mean ± SD | 0.82 ± 0.20 | 0.95 ± 0.27* | 1.06 ± 0.28*,≠ | <0.001 |
| Glucose (mg/dL), mean ± SD | 106 ± 35 | 108 ± 25 | 109 ± 30 | 0.702 |
| TC (mg/dL), mean ± SD | 199 ± 42 | 190 ± 45 | 198 ± 37 | 0.307 |
| LDL-C (mg/dL), mean ± SD | 114 ± 41 (105–122) | 112 ± 36 (104–121) | 125 ± 30(118–131) | 0.076 |
| HDL-C (mg/dL), mean ± SD | 59 ± 26 (53–64) | 52 ± 18 (48–56) | 51 ± 13 (48–54)* | 0.030 |
| FABP4 (ng/mL), mean ± SD | 17.5 ± 12 (15–20) | 23 ± 16 (20–26)* | 19 ± 13 (17–21) | 0.015 |
| Leptin (ng/mL), mean ± SD | 39 ± 42 (31–48) | 24 ± 22 (20–28)* | 21 ± 37 (14–28)* | <0.001 |
| LVEF (%) | 63 ± 7 | 64 ± 6 | 62 ± 8 | 0.220 |
| Statin, yes/no (%) | 48/57 (46%) | 32/44 (42%) | 30/56 (35%) | 0.313 |
| Beta-blocker, n (%) | 23 (22%) | 59 (56%)* | 72 (67%)* | <0.001 |
| ADT class I, n (%) | – | 43 (41%) | 23 (21%)≠ | 0.002 |
| ADT class III, n (%) | – | 23 (22%) | 39 (36%)≠ | 0.020 |
ADT, antiarrhythmic drug therapy; AF, atrial fibrillation; AHT, arterial hypertension; BMI, body mass index; CKD, chronic kidney disease (eGFR < 60 mL/min/1.73 m2); FABP4, fatty acid-binding protein 4; LVEF, left ventricular ejection fraction; SD, standard deviation; T2DM, type 2 diabetes mellitus; TC, total cholesterol.
Post hoc differences between paroxysmal or persistent AF vs. control* or paroxysmal vs. persistent AF≠.
In 69% of the control cohort, coronary atherosclerosis was identified on cardiac CT [50% non-obstructive (≥20 but <50% stenosis) and 19% obstructive].
All but one female from case cohort had reached menopause at the time of the ablation. This information was missing in the control cohort.
Peripheral plasma FABP4 and leptin levels in control cohort, paroxysmal AF, and persistent AF groups
Higher peripheral plasma FABP4 levels were detected in patients with paroxysmal AF (23 ± 16 ng/mL) as compared to the control cohort (17.5 ± 12 ng/mL) (P < 0.05). Leptin levels were lower in AF cohort (P < 0.001) (Table 1).
While age was the main predictor for FABP4 levels in the control cohort, gender and BMI were in the AF cohort (Supplementary material online, Table S1a). Regarding leptin levels, gender and BMI were the main predictors in the control and the AF cohort (Supplementary material online, Table S1b).
Peripheral plasma FABP4 and leptin levels in women and men
Gender, BMI, or ageing were the main factors associated with FABP4 or leptin levels in AF. Compared to men, women presented higher FABP4 and leptin levels in all cohorts (Figure 1). In women, despite similar age and BMI among control, paroxysmal, and persistent AF groups, the FABP4 levels were 20 ± 14 ng/mL, 29 ± 18 ng/mL, and 31 ± 17 ng/mL, respectively. So, the higher FABP4 levels were dependent on AF burden (P = 0.007) (Supplementary material online, Table S2). In men, there were not differences among groups regarding FABP4 levels. A decline in leptin levels was detected on AF cohort (Supplementary material online, Table S3).
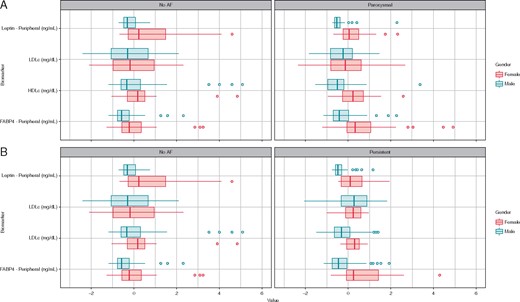
Box plots represents median and interquartile range of adipokines and lipoproteins regarding gender and AF burden, data matched for age and BMI.
In the multivariable analysis, higher FABP4 levels were independently associated with ageing and higher BMI in male and female. However, only in female patients, it was dependent on AF (Table 2). Leptin levels were dependent on BMI, FABP4 levels, and history of AF in male but not in female (Table 3).
| Women . | . | Men . | ||||
|---|---|---|---|---|---|---|
| . | Coeff. . | SE . | P-value . | Coeff. . | SE . | P-value . |
| (Intercept) | −1.69 | 0.092 | −2.62 | 0.009 | ||
| Age | 0.181 | 2.23 | 0.027 | 0.217 | 3.30 | 0.001 |
| BMI | 0.215 | 2.41 | 0.017 | 0.246 | 3.12 | 0.002 |
| AF presence/ control | 0.232 | 2.81 | 0.006 | 0.129 | 1.80 | 0.074 |
| Leptin | 0.147 | 1.65 | 0.101 | 0.261 | 3.28 | 0.001 |
| Women . | . | Men . | ||||
|---|---|---|---|---|---|---|
| . | Coeff. . | SE . | P-value . | Coeff. . | SE . | P-value . |
| (Intercept) | −1.69 | 0.092 | −2.62 | 0.009 | ||
| Age | 0.181 | 2.23 | 0.027 | 0.217 | 3.30 | 0.001 |
| BMI | 0.215 | 2.41 | 0.017 | 0.246 | 3.12 | 0.002 |
| AF presence/ control | 0.232 | 2.81 | 0.006 | 0.129 | 1.80 | 0.074 |
| Leptin | 0.147 | 1.65 | 0.101 | 0.261 | 3.28 | 0.001 |
Dependent variable: FABP4 levels on women or men.
AF, atrial fibrillation; BMI, body mass index; Coeff., coefficient; FABP4, fatty acid-binding protein 4; SE, standard error.
| Women . | . | Men . | ||||
|---|---|---|---|---|---|---|
| . | Coeff. . | SE . | P-value . | Coeff. . | SE . | P-value . |
| (Intercept) | −1.69 | 0.092 | −2.62 | 0.009 | ||
| Age | 0.181 | 2.23 | 0.027 | 0.217 | 3.30 | 0.001 |
| BMI | 0.215 | 2.41 | 0.017 | 0.246 | 3.12 | 0.002 |
| AF presence/ control | 0.232 | 2.81 | 0.006 | 0.129 | 1.80 | 0.074 |
| Leptin | 0.147 | 1.65 | 0.101 | 0.261 | 3.28 | 0.001 |
| Women . | . | Men . | ||||
|---|---|---|---|---|---|---|
| . | Coeff. . | SE . | P-value . | Coeff. . | SE . | P-value . |
| (Intercept) | −1.69 | 0.092 | −2.62 | 0.009 | ||
| Age | 0.181 | 2.23 | 0.027 | 0.217 | 3.30 | 0.001 |
| BMI | 0.215 | 2.41 | 0.017 | 0.246 | 3.12 | 0.002 |
| AF presence/ control | 0.232 | 2.81 | 0.006 | 0.129 | 1.80 | 0.074 |
| Leptin | 0.147 | 1.65 | 0.101 | 0.261 | 3.28 | 0.001 |
Dependent variable: FABP4 levels on women or men.
AF, atrial fibrillation; BMI, body mass index; Coeff., coefficient; FABP4, fatty acid-binding protein 4; SE, standard error.
| Women . | . | Men . | ||||
|---|---|---|---|---|---|---|
| . | Coeff. . | SE . | P-value . | Coeff. . | SE . | P-value . |
| (Intercept) | −1.33 | 0.185 | −4.74 | 0.000 | ||
| Age | 0.019 | 0.196 | 0.845 | 0.096 | 1.39 | 0.165 |
| BMI | 0.413 | 4.40 | 0.000 | 0.595 | 8.73 | 0.000 |
| AHT | 0.062 | 0.634 | 0.528 | −0.078 | −1.10 | 0.273 |
| AF presence/ control | −0.141 | −1.50 | 0.136 | −0.413 | −6.23 | 0.000 |
| Total cholesterol | −0.037 | −0.403 | 0.688 | 0.034 | 0.541 | 0.589 |
| Women . | . | Men . | ||||
|---|---|---|---|---|---|---|
| . | Coeff. . | SE . | P-value . | Coeff. . | SE . | P-value . |
| (Intercept) | −1.33 | 0.185 | −4.74 | 0.000 | ||
| Age | 0.019 | 0.196 | 0.845 | 0.096 | 1.39 | 0.165 |
| BMI | 0.413 | 4.40 | 0.000 | 0.595 | 8.73 | 0.000 |
| AHT | 0.062 | 0.634 | 0.528 | −0.078 | −1.10 | 0.273 |
| AF presence/ control | −0.141 | −1.50 | 0.136 | −0.413 | −6.23 | 0.000 |
| Total cholesterol | −0.037 | −0.403 | 0.688 | 0.034 | 0.541 | 0.589 |
Dependent variable: leptin levels on women or men.
AF, atrial fibrillation; AHT, arterial hypertension; BMI, body mass index; Coeff., coefficient; SE, standard error.
| Women . | . | Men . | ||||
|---|---|---|---|---|---|---|
| . | Coeff. . | SE . | P-value . | Coeff. . | SE . | P-value . |
| (Intercept) | −1.33 | 0.185 | −4.74 | 0.000 | ||
| Age | 0.019 | 0.196 | 0.845 | 0.096 | 1.39 | 0.165 |
| BMI | 0.413 | 4.40 | 0.000 | 0.595 | 8.73 | 0.000 |
| AHT | 0.062 | 0.634 | 0.528 | −0.078 | −1.10 | 0.273 |
| AF presence/ control | −0.141 | −1.50 | 0.136 | −0.413 | −6.23 | 0.000 |
| Total cholesterol | −0.037 | −0.403 | 0.688 | 0.034 | 0.541 | 0.589 |
| Women . | . | Men . | ||||
|---|---|---|---|---|---|---|
| . | Coeff. . | SE . | P-value . | Coeff. . | SE . | P-value . |
| (Intercept) | −1.33 | 0.185 | −4.74 | 0.000 | ||
| Age | 0.019 | 0.196 | 0.845 | 0.096 | 1.39 | 0.165 |
| BMI | 0.413 | 4.40 | 0.000 | 0.595 | 8.73 | 0.000 |
| AHT | 0.062 | 0.634 | 0.528 | −0.078 | −1.10 | 0.273 |
| AF presence/ control | −0.141 | −1.50 | 0.136 | −0.413 | −6.23 | 0.000 |
| Total cholesterol | −0.037 | −0.403 | 0.688 | 0.034 | 0.541 | 0.589 |
Dependent variable: leptin levels on women or men.
AF, atrial fibrillation; AHT, arterial hypertension; BMI, body mass index; Coeff., coefficient; SE, standard error.
Relationship between FABP4 and leptin with inflammatory, oxidative stress, lipid transport and metabolism and indirect autonomic markers on atrial blood samples
As previously stated, we observed a clear positive association between FABP4 and leptin levels in patients with paroxysmal or persistent AF. Similarly, on leucocytes, defensin-3 (DEFA-3) levels, expressed mainly by neutrophils, were associated with interleukin 6 (IL-6). Also, there was a significative association among H2O2 levels, which induces oxidative stress, and IL-6. However, FABP4 was inversely associated with glycerol, lipolytic metabolite, in persistent AF (Figure 2). The gender and AF type matched by age and BMI showed that the adipose tissue and neutrophil biomarkers differed between male and female in persistent and paroxysmal AF. In women with paroxysmal AF, there was a lower acetylcholinesterase, indirect parasympathetic activity, and higher CAIX (an intrinsic markers of hypoxia-acidosis) (Figure 3).
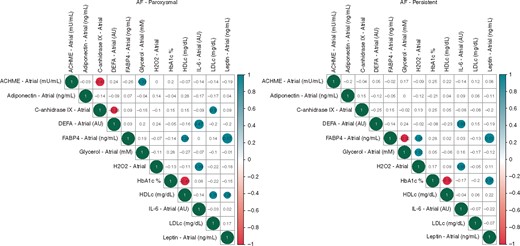
Heatmaps of Kendall’s correlation among levels of biomarkers on atrial plasma.
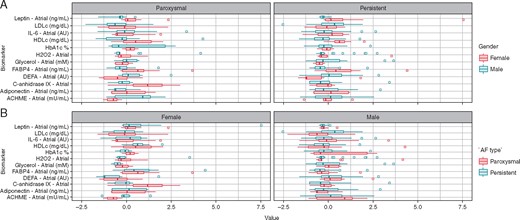
Box plots represents median and interquartile range of biomarkers levels on atrial plasma regarding gender and AF burden, data matched for age and BMI.
Variable importance to classify patients with recurrence after (PVI)
In a random forest model fitted to assess covariable importance, adipocyte markers were the highest ranked variables in predicting AF recurrence after PVI (Figure 4).
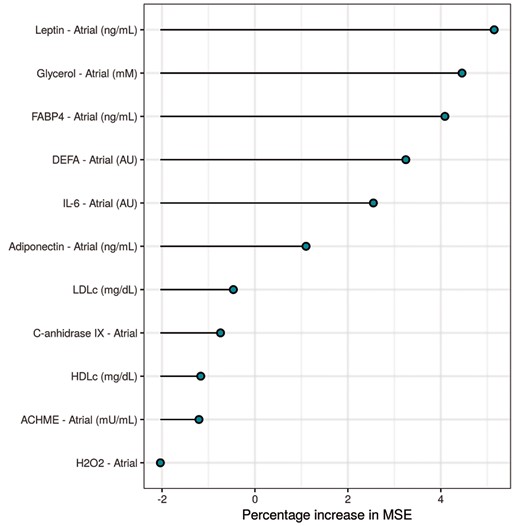
Random forest plot represents the main important variables for discriminating AF recurrence after catheter ablation.
Logistic regression analysis was also performed and showed that FABP4 was the stronger predictor for persistent AF recurrence (adjusted by BMI, age, gender, and leptin levels) (P < 0.05), but not in patients with paroxysmal AF. Its interaction with leptin improved the predictive value of FABP4 (Table 4).
| . | Coeff. . | 95% CI . | P-value . |
|---|---|---|---|
| (Intercept) | 1.0603195 | 0–480.6 | 0.9849 |
| FABP4 | 1.0673804 | 1–1.14 | 0.0466 |
| Leptin | 0.9584996 | 0.9–1 | 0.1204 |
| Gender | 0.2758803 | 0.05–1.72 | 0.1527 |
| BMI | 1.0092720 | 0.84–1.2 | 0.9180 |
| Age | 0.9767280 | 0.92–1.03 | 0.4149 |
| . | Coeff. . | 95% CI . | P-value . |
|---|---|---|---|
| (Intercept) | 1.0603195 | 0–480.6 | 0.9849 |
| FABP4 | 1.0673804 | 1–1.14 | 0.0466 |
| Leptin | 0.9584996 | 0.9–1 | 0.1204 |
| Gender | 0.2758803 | 0.05–1.72 | 0.1527 |
| BMI | 1.0092720 | 0.84–1.2 | 0.9180 |
| Age | 0.9767280 | 0.92–1.03 | 0.4149 |
BMI, body mass index; CI, confidence interval; Coeff., coefficient; FABP4, fatty acid-binding protein 4; PVI, pulmonary vein isolation.
| . | Coeff. . | 95% CI . | P-value . |
|---|---|---|---|
| (Intercept) | 1.0603195 | 0–480.6 | 0.9849 |
| FABP4 | 1.0673804 | 1–1.14 | 0.0466 |
| Leptin | 0.9584996 | 0.9–1 | 0.1204 |
| Gender | 0.2758803 | 0.05–1.72 | 0.1527 |
| BMI | 1.0092720 | 0.84–1.2 | 0.9180 |
| Age | 0.9767280 | 0.92–1.03 | 0.4149 |
| . | Coeff. . | 95% CI . | P-value . |
|---|---|---|---|
| (Intercept) | 1.0603195 | 0–480.6 | 0.9849 |
| FABP4 | 1.0673804 | 1–1.14 | 0.0466 |
| Leptin | 0.9584996 | 0.9–1 | 0.1204 |
| Gender | 0.2758803 | 0.05–1.72 | 0.1527 |
| BMI | 1.0092720 | 0.84–1.2 | 0.9180 |
| Age | 0.9767280 | 0.92–1.03 | 0.4149 |
BMI, body mass index; CI, confidence interval; Coeff., coefficient; FABP4, fatty acid-binding protein 4; PVI, pulmonary vein isolation.
Discussion
The major findings of the present study are the sex-related differences of two fat markers, FABP4 and leptin, on AF patients. This sex-dependent behaviour might be related to differences in the pro-inflammatory potential of leucocytes in the context of AF. Importantly, FABP4 was the best predictor for persistent AF recurrence after catheter ablation in our sample population. These findings portray an association between adiposity and AF, even in terms of AF severity with specific differences amongst gender. These findings would give rise to further insights regarding the role of the adipose tissue in AF development, to design directed therapies according to sex and finally it may help select individuals most likely to benefit from invasive strategies.
Adipocyte biomarker and its relationship with inflammatory and oxidative markers in patients with and without AF according to gender
We found a positive relationship between plasma FABP4 and leptin levels with BMI and female sex. It is assumed that the concentration of these adipokines increases with obesity due to the greater amount of body fat. This argument is also used to explain the highest levels in women, but sex-specific associations between sexual hormones and adipose tissue secretion have also been described.11 Accordingly, with the same amount of visceral fat, there are differences in the secretome from adipose tissue between women and men, which could be one of the reasons for the important sex differences detected in AF patients. In the present study, plasma FABP4 and leptin levels were different among cohorts despite fairly similar cardiovascular risk factors and irrespective of age, BMI, and sex. FABP4 levels were higher and leptin levels were lower in the AF cohort compared to the control cohort. After splitting the population according to gender, we found significant differences on plasma FABP4 levels in women according to AF burden. The lack of differences in female mean age among groups (64 ± 13 in the control group, 64 ± 8 in paroxysmal AF patients, and 64 ± 8 year old in persistent AF patients; P = 0.9) could indicate that this finding is not due to hormonal differences (menopausal), which as it has been described has influence on FABP4 levels.11
A similar behaviour was observed in coronary atherosclerosis, where women presented higher levels of FABP4 than men.12 A higher predominance of the parasympathetic nervous system in women and the antilipolytic effect of beta-blockers (ADT Class II) treatment might explain, at least in part, a higher fat accumulation and fatty acid transport into the adipose tissue storage in women as compared to men. Whether this potential different response is due to specific fat metabolism is unknown but under our point of view would deserve further investigations.
On the other hand, in men, leptin levels were lower in patients with persistent AF as compared to those with paroxysmal AF and the control group, but without significant changes on FABP4 levels. Though, it has to be emphasized that the high proportion of coronary heart disease (69%) in the control cohort may justify pathological FABP4 levels in this subgroup. These findings could have masked the difference of FABP4 levels among groups since this protein increases with age and is related also to atherosclerosis. Nevertheless, it does not seem to be the explanation for the differences detected in leptin levels because is decreased with ageing and increased with obesity. These results suggest a new mechanism associated with the gender-dependent increased FABP4 and decreased leptin levels in AF patients. One of the potential explanations for the differences seen in FABP4 and leptin levels according to AF type could be based on the pro-inflammatory substances release by the adipose tissue. For instance, epicardial fat becomes dysfunctional in obesity, resulting in an increased production of proinflammatory factors and cytokines targeting the vascular wall, inducing endothelial dysfunction and inflammation. The results of the present study show that FABP4 levels were positively correlated with H2O2 (oxidative stress radical) and inversely with glycerol (lipolysis metabolite) in persistent AF. The increment of fat accumulation suggests a lower lipolytic activity, which can be associated with autonomic disbalance, higher oxidative stress, and pro-inflammatory activity. This mechanism might favour the arrhythmogenic substrate on the atrium and perpetuate AF. It is known the important role of obesity therapeutic effectiveness of sodium vs. potassium channels blocker antiarrhythmics drugs. One of the mechanisms suggested is the oxidative stress. In line with this hypothesis, our data showed an increment of hydrogen peroxide in the LA (as compared to peripheral blood) (see Supplementary material online, Table S4). Importantly, this difference was higher in persistent than paroxysmal AF.13 It might indicate that the oxidative stress could be a potential mechanism involved in AF perpetuation, from paroxysmal to persistent forms. Likewise, the consistent associations of DEFA-3, IL-6, and H2O2, which promote endothelial dysfunction and prompt alterations in vascular structure, reinforced this potential relationship between pro-inflammatory and oxidative stress environment and AF progression.
While FABP4 and leptin levels differed among male and females in paroxysmal or persistent AF, there was a lower acetylcholinesterase and higher carbonic anhydrase levels in female patients with paroxysmal AF respect to men. These findings might explain in part the differential mechanism underlying paroxysmal AF between male and female.
Value of these biomarkers as predictors of recurrence after AF catheter ablation
Myocardial lipidosis, inflammation and proliferation of fibroblasts induced by epicardial fat-secreted adipokines contribute to the progressive fibrotic remodelling of the atrium.14 This disorganization and loss of homogeneity of the atrial myocardium is considered the substrate for the development and maintenance of electrophysiological disturbances.15 Atrial fibrillation is a clinically manifestation of this pathological changes. As a matter of fact, the EHRAS classification (EHRAS Class I–IV) is a first attempt to characterize these atrial pathologies/stages into discrete cohorts. Specifically, based on the adipocyte infiltration into the myocardium and atrial fibrosis, cardiomyopathy due to obesity was classified as EHRAS Class IVf and EHRAS III as collagen depositions are also present.16 The biomarkers herein analyse could help characterizing the atrial substrate according to sex and this characterization is very important since the different predictive capacity according to AF type seems to vary over time and be different according to the underlying substrate: the presence of pulmonary vein triggers frequently seen in paroxysmal AF vs. a heavier weight placed on a modified and complex substrate, with extrapulmonary vein triggers, seen in persistent AF. According with this rationality and in agreement with our prior study,6 the random forest determined that the adipocyte markers were the most important variable for discriminating long-term AF recurrence after catheter ablation, being FABP4 levels were the best predictor of recurrence in persistent but not in paroxysmal AF.
Clinical implications and future directions
Based on the fact that adipokines can provide information about both the amount and activity of fat, these results could open the door to an intensification of research to exploit a more precise relationship between adipose tissue and AF. In fact, the sex-related differences in adipokines may provide an explanation for the important gender differences in the epidemiology, pathophysiology, and prognosis of AF. Several studies found that FABP4 levels increase drastically after menopause,17 which would justify the later presentation of AF in this population. Other studies showed that high levels of FABP4 are associated with a worse prognosis after a stroke,18 which would explain why women might have an increased risk of stroke/TIA and all-cause mortality compared with men. Herein, we describe the potential role of FABP4 as a predictor of success after catheter ablation in patients with persistent AF. From our point of view, this is not a negligible point since at the present time there are few and poorly studies markers of atrial disease beyond atrial size. This may help select individuals most likely to benefit from invasive strategies.
On the other hand, changes in lifestyle and increasing aerobic physical activity can decrease FABP4 levels,19 and therefore it could reduce the adverse events and improve the efficacy of catheter ablation. Whether it could be used for monitoring changes in fat activity is still uncertain and will deserve further investigations.
Limitations of the study
We acknowledge that our study has several limitations. The lack of association with some other variable could be caused by a lack of statistical power due to the presence of missing data. Two independent cohorts were used in this study. Importantly, although none of the patients in the control cohort reported previous episodes of AF, silent episodes cannot be strictly ruled out. We tried to minimize this limitation by excluding patients at very high risk for AF (patients with organic valvular disease, prosthetic valves, more than moderate mitral regurgitation secondary to left ventricular dilatation, pulmonary hypertension, or treated with oral anticoagulant), a very similar profile of patients referred for AF ablation. Hence, although this is a limitation that has to be taken into account, from our point of view it should not have altered the conclusions of the present study.
The sex ratio was different between cohorts. Due to the inclusion of consecutives patients (not selected) the proportion of females was higher in the control cohort than in the case cohort (51% vs. 35%). Moreover, the vast majority of women referred for AF ablation experience menopause at the time of the procedure and this information was missing in the control cohort. Subsequently, the conclusions of the present study could not be applicable to non-menopausal females.
This study found that higher FABP4 levels were independently associated with ageing and higher BMI in male and female. However, we did not get the body fat percentage by Dual-Energy X-ray Absorptiometry. Thus, although in our previous study FABP4 levels were associated with the amount of left atrial adipose tissue (LAAT), we cannot rule out the possibility that FABP4 levels from our patients are associated with both LAAT and total body fat volumes. Because there is a higher extracardiac adiposity, the FABP4 levels were also higher in peripheral than in atrial blood. However, the correlation between atrial FABP4 levels and hydrogen peroxide, levels that were higher in atrial than in peripheral blood (Supplementary material online, Table S4), might explain the therapeutic inefficiency against adiposity-oxidative stress and the AF perpetuation.
Long treatments with atorvastatin can reduce the FABP4 expression levels induced by oxidized-LDL.20 It is conceivable hence that baseline level of adipocytes marker can be affected by baseline medical treatment. However, due to the lack of baseline differences (Table 1), this fact should not have distorted the conclusions of the study.
In a high proportion of the subjects enrolled in the control cohort (69%), a CAD was detected by CT scan. As it has been described, perivascular adipose tissue is involved in atherosclerosis pathogeny via several possible mechanisms, among other due to the pro-atherogenic effect of FABP4 and leptin. Consequently, it is likely that if the study had it been done in patients without CAD, the differences seen in adipocytes markers could have been even higher.
In the present study, sex-specific differences in the associations between various adipokines and AF burden have been pursuit. However, mechanistic studies aiming at revealing the mechanistic role of these adipokines for AF perpetuation have not yet been performed and are clearly warranted to better appreciate the relevance of these findings for sex differences in AF.
Conclusion
The major finding of the present study is the sex-related differences of FABP4 and leptin according to AF burden and its relationship with oxidative stress, inflammatory and autonomic indirect markers.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
We would like to thank the patients for their participation and Miguel Villamayor Blanco for his technical assistance.
Funding
This study was supported by projects (PI16/01282 and PI18/01584) integrated in the Plan Estatal de I+D+I 2016–2019 and cofounded by ISCIII-Subdirección General de Evaluación y Fomento de la Investigación del Fondo Europeo de Desarrollo Regional (FEDER). J.N.L.-C. and M.R.-M. were a recipient of a Sociedade Galega de Cardioloxía (SOGACAR) research grant. D.d.G.-C. was a recipient of a Juan de la Cierva-Incorporación grant from the Ministry of Science Innovation and Universities (IJCI-2016-29393). CIBER Cardiovascular (CB16/11/00403 to V.Ll.-C. and D.d.G.-C.) is a project from Carlos III Health Institute.
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Author notes
S Eiras and M Rodríguez-Mañero authors contributed equally to this study.



