-
PDF
- Split View
-
Views
-
Cite
Cite
David Duncker, Martin Svetlosak, Federico Guerra, Klaudia Vivien Nagy, Philippe Vanduynhoven, Evgeny N Mikhaylov, Jedrzej Kosiuk, Reprocessing of electrophysiology material in EHRA countries: an EHRA Young EP survey, EP Europace, Volume 23, Issue 3, March 2021, Pages 479–485, https://doi.org/10.1093/europace/euaa250
Close - Share Icon Share
Abstract
Data on reprocessing of electrophysiology (EP) materials are sparse. Reprocessing of catheters and other materials in daily routine varies through countries and may depend on specific material characteristics, supplier, or federal law. The aim of this study was to collect data on reprocessing usage through EHRA countries. An online survey consisting of 27 questions was distributed to EHRA Young EP members and members of national EP working groups. Two hundred and two participants from 34 EHRA countries completed the survey. One hundred and seven respondents (53.0%) reported having used and using reprocessed EP material, 30 (14.9%) respondents have used reprocessed EP material in the past but not at the time of the survey, 65 (32.2%) had never used reprocessed EP material. The most reprocessed EP materials include cables (70%), diagnostic EP catheters with deflectable (64%) or fixed curve (63%), non-irrigated ablation catheters (51%), and other conventional diagnostic catheters (41%). The most durable material was diagnostic EP catheters with a fixed curve (61%), the most sensitive material was ablation catheters with contact force sensors (21%). Important benefits were seen in reducing costs for the providing hospital (65%) and the healthcare provider (42%) and making EP procedures available for a greater number of patients (42%). Main concerns were on quality aspects (58%), contamination (52%), and loss of precision (47%). Reprocessing of EP materials is heterogeneously managed among EHRA countries. The present survey shows that European electrophysiologists consider the use of reprocessed EP material as generally safe and cost-effective.
Data on reprocessing of electrophysiology (EP) material in EHRA countries are sparse. This represents the first data collection on use of reprocessed material in everyday clinical practice in EHRA countries.
Over two-thirds (67.8%) of responders are using or have used reprocessed EP material, with substantial heterogeneity in the types and management of reprocessed materials.
Important benefits were seen in reducing costs for the providing hospital (65%) and the healthcare provider (42%) and making EP procedures available for a greater number of patients (42%).
Main concerns were on quality aspects (58%), contamination (52%), and loss of precision (47%).
Introduction
For the most part, materials used in invasive cardiac electrophysiology (EP) are designated for single-use only. Electrophysiology catheters are introduced to the heart for either diagnostic or therapeutic purposes during EP studies in patients with conduction disturbancies, supraventricular, or ventricular arrhythmias.1 Although EP catheters are officially single-use devices, in several countries it is not restricted to reuse them and there is constant use of reprocessed EP materials worldwide.2,3 Generally, EP catheters represent high-cost medical devices, which are used in huge numbers. However, their designs are relatively simple—especially, regarding diagnostic catheters. Therefore, they can be considered for reprocessing and have a long history of repeated usage in hospitals in the last 30 years.4 From an economic point of view, using reprocessed EP material helps to improve cost-effectiveness—an effect used in lower as well as higher income countries throughout Europe. However, repeated use of EP catheters may cause concerns in terms of material durability, infections, or procedural risks.
We have minimal data on the personal judgement of electrophysiologists and the specific regulations of reprocessing EP materials among European countries. Some earlier publications highlighted, though, that reprocessing is a safe and feasible method to reduce procedural costs and increase the number of patients that could be potentially treated in the EP lab.5
The aim of the present study therefore was to collect information about reprocessing, use of reprocessed EP materials, and the experience of electrophysiologists with these materials throughout European Heart Rhythm Association (EHRA) countries.
Methods
An online questionnaire (www.surveymonkey.com) was prepared using the EHRA Young EP infrastructure and distributed to EHRA Young EP members and members of national EP working groups on a voluntary basis. The questionnaire consisted of 27 questions in four blocks (see Supplementary material online).
The first block consisted of five questions regarding personal information including age, gender, working environment, working position, and years of expertise in the field of invasive EP.
The topic of the second block was reprocessing, asking the EP physician if she/he was aware of the local regulations of reprocessing EP materials and then to rate the importance of the topic. Then the questionnaire asked about current or former use of reprocessed EP materials.
If the respondent never used reprocessed EP material before or stopped using reprocessed EP material, the physician was asked if she/he would consider using reprocessed material in the future, why she/he does not use it currently, and which EP materials she/he would like to reuse eventually.
If the respondent answered that she/he uses reprocessed EP materials currently or has used it before, further questions were concerning the practice of reprocessing: duration of using reprocessed EP material, type of reprocessed EP material, location of reprocessing process (on-site vs. third party), tracking or limitation of reprocessing cycles. Furthermore, there were some questions regarding the quality, durability, and sensitivity of these reprocessed EP materials. The final three questions were concerning the economic impact, the potential risks, and the benefits of using a reprocessed EP material. Potential risks of the use of reprocessed materials were assessed by a question suggesting a possible association of reprocessing and a complication developed. The types of adverse events and reprocessed items were surveyed.
Statistical analysis
The Kolmogorov–Smirnov test was used to assess normality of quantitative variables. Continuous variables are presented as mean and standard deviation for normally distributed variables or median and 1st to 3rd quartile for not-normally distributed variables. Categorical variables are presented as absolute and relative numbers. SPSS 25.0 for Windows (SPSS Inc., Chicago, IL, USA), and R (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
Results
Two hundred and two participants from 34 EHRA countries completed the survey. Centre distribution by nation in alphabetical order was Algeria 2, Armenia 1, Austria 5, Belarus 2, Belgium 4, Bosnia and Herzegovina 1, Bulgaria 1, Croatia 1, Denmark 1, Egypt 4, France 7, Republic of Georgia 2, Germany 11, Greece 4, Hungary 3, Iceland 1, Israel 1, Italy 18, Kazakhstan 4, The Netherlands 2, Norway 1, Poland 8, Portugal 3, Romania 2, Russian Federation 11, Slovak Republic 1, Slovenia 2, Spain 4, Switzerland 5, Tunisia 1, Turkey 6, Ukraine 1, UK 4, Uzbekistan 1. Seventy-seven responders (38.1%) preferred not to mention their country.
The majority of responders worked in a university hospital (n = 116, 57.4%), with less respondents working in specialized public cardiology centres (n = 44, 21.8%), district or community hospitals (n = 14, 6.9%), or other settings (n = 7, 3.5%).
Respondents working position were defined as follows: 89 EP specialists (44.1%), 50 EP team leaders (24.8%), 42 EP fellows (20.8%), 13 clinical cardiologists (6.4%), 5 cardiology fellows (2.5%), 2 residents (1.0%), and 1 student (0.5%). Respondents had a mean experience of 7.0 ± 5.3 years in EP field.
Nearly three-quarters of all respondents (n = 148, 73.3%) were aware of their local regulations and legislations concerning EP material reprocessing at the time of the survey.
The perceived benefits and risks of reprocessing EP material are shown in Figure 1.
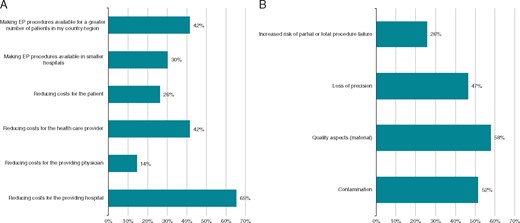
Benefits (A) and risks (B) relevant for reprocessing of EP materials. EP, electrophysiology.
One hundred and seven respondents (53.0%) reported that they have used and are still using reprocessed EP material, 30 (14.9%) that they have used reprocessed EP material in the past but not at the time of the survey and 65 (32.2%) that they never used reprocessed EP material.
Respondents with personal experience with reprocessed electrophysiology material
Questions regarding reprocessing practice were submitted only to respondents who replied to currently have or have had any kind of experience with reprocessed materials (n = 137, 67.8%). The median experience time in using reprocessed EP material was five years (1st–3rd quartile 2–9 years).
The most reprocessed EP materials include cables (70%), diagnostic EP catheters with a deflectable curve (64%), diagnostic EP catheters with a fixed curve (63%), non-irrigated ablation catheters (51%), and other conventional diagnostic catheters (41%) (Figure 2).
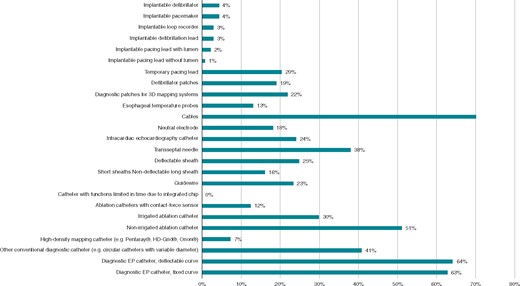
Respondents actually using reprocessed EP material. EP, electrophysiology.
A median of 50% of all used EP catheters undergoes the reprocessing process at least once (1st–3rd quartile 20–70%). The maximum limit of times that a catheter can be reprocessed has been reported as six for diagnostic catheter (1st–3rd quartile 3–10 times) and five for ablation catheter (1st–3rd quartile 3–10 times). Thirty-two respondents (27.8%) reported the use of reprocessed mapping catheters within 24 h.
Table 1 shows the top five reprocessed items considered to be the most durable or the most sensitive.
Top five most durable and most sensitive EP materials, according to survey respondents.
| . | Most durable . | . | Most sensitive . | . |
|---|---|---|---|---|
| 1st | Diagnostic EP catheters, fixed curve | 61% | Ablation catheters, contact-force sensors | 21% |
| 2nd | Cables | 42% | Irrigated ablation catheters | 19% |
| 3rd | Diagnostic EP catheters, deflectable curve | 40% | Diagnostic EP catheters, deflectable curve | 19% |
| 4th | Non-irrigated ablation catheters | 33% | High-density mapping catheter | 18% |
| 5th | Transseptal needles | 23% | Other conventional diagnostic catheters | 12% |
| . | Most durable . | . | Most sensitive . | . |
|---|---|---|---|---|
| 1st | Diagnostic EP catheters, fixed curve | 61% | Ablation catheters, contact-force sensors | 21% |
| 2nd | Cables | 42% | Irrigated ablation catheters | 19% |
| 3rd | Diagnostic EP catheters, deflectable curve | 40% | Diagnostic EP catheters, deflectable curve | 19% |
| 4th | Non-irrigated ablation catheters | 33% | High-density mapping catheter | 18% |
| 5th | Transseptal needles | 23% | Other conventional diagnostic catheters | 12% |
EP, electrophysiology.
Top five most durable and most sensitive EP materials, according to survey respondents.
| . | Most durable . | . | Most sensitive . | . |
|---|---|---|---|---|
| 1st | Diagnostic EP catheters, fixed curve | 61% | Ablation catheters, contact-force sensors | 21% |
| 2nd | Cables | 42% | Irrigated ablation catheters | 19% |
| 3rd | Diagnostic EP catheters, deflectable curve | 40% | Diagnostic EP catheters, deflectable curve | 19% |
| 4th | Non-irrigated ablation catheters | 33% | High-density mapping catheter | 18% |
| 5th | Transseptal needles | 23% | Other conventional diagnostic catheters | 12% |
| . | Most durable . | . | Most sensitive . | . |
|---|---|---|---|---|
| 1st | Diagnostic EP catheters, fixed curve | 61% | Ablation catheters, contact-force sensors | 21% |
| 2nd | Cables | 42% | Irrigated ablation catheters | 19% |
| 3rd | Diagnostic EP catheters, deflectable curve | 40% | Diagnostic EP catheters, deflectable curve | 19% |
| 4th | Non-irrigated ablation catheters | 33% | High-density mapping catheter | 18% |
| 5th | Transseptal needles | 23% | Other conventional diagnostic catheters | 12% |
EP, electrophysiology.
Regarding where the reprocessing process is performed, 93 respondents (67.9%) reported the presence of a facility within their institution, 26(19.0%) use a third-party reprocessing facility, and 6 (4.4%) lean on private companies which rebrand the material. Twelve respondents (8.7%) did not know or preferred not to say.
A tracing system allowing identification and use of reprocessed EP material have been reported as in place for all EP materials by 37 respondents (27.0%) and for some EP materials only by 20 respondents (14.6%). Fifty-eight respondents (42.3%) do not use any kind of tracing system for reprocessed EP material and 22 (16.1%) do not know or prefer not to say.
Respondents reported that reprocessed EP material had to be changed during the procedure due to insufficient functionality 15% of the times (1st–3rd quartile 8–30%). Major complications potentially due to reprocessing (as assessed by the responder) have been signalled by 16 respondents (11.6%). These include 15 cases of iatrogenic embolism, one snare extraction of the catheter and one artery dissection.
The hospital/clinical institution has been reported as the first direct beneficiary of the reduced costs due to reprocessing by the majority of respondents (n = 59, 43.0%). The national healthcare system (n = 36, 26.2%), the patients (n = 14, 10.2%), the insurance companies (n = 14, 10.2%), and the treating physicians (n = 7, 5.1%) were reported as other potential direct beneficiaries.
Respondents with no personal experience with reprocessed electrophysiology material
Sixty-five respondents (32.2%) reported having no experience with reprocessed EP material. Of those, 40 (61.5%) stated that they are interested in or are considering using reprocessed EP material in the future.
Reasons for not using reprocessed EP material include national law against reprocessing (n = 24, 36.9%), local restrictions (n = 22, 33.8%), personal concerns (n = 17, 26.1%), lack of reprocessing facilities (n = 5, 7.7%), or other motivations (n = 4, 6.2%).
Among respondents who do not currently use reprocessed EP material, diagnostic EP catheters with a fixed curve (65%), cables (57%), diagnostic EP catheters with a deflectable curve (49%), intracardiac echocardiography catheters (46%), and non-irrigated ablation catheters (37%) were the most commonly chosen items that would be gladly used if reprocessed (Figure 3).
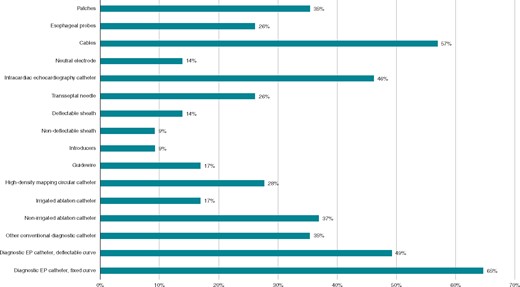
Material respondents would like to reuse who are actually not using reprocessed EP material. EP, electrophysiology.
Figure 4shows how strongly all the respondents would consider reusing EP material without considering legal issues related to their country. Values range from one (this material should never be reprocessed) to five (this material could be routinely reprocessed).
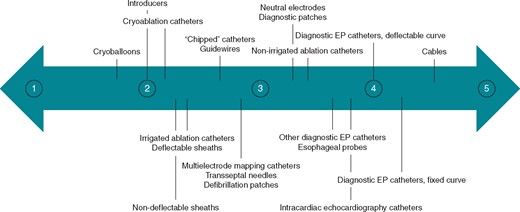
Median rating for each material how strongly respondents would consider reusing it or not, without considering any legal issues (scale 1 ‘This material should never be reused’ to 5 ‘This material could routinely be used more than one time’).
Discussion
More than 200 electrophysiologists from at least 34 different EHRA countries participated in the present survey using EHRA Young EP infrastructures. To the best of our knowledge, this represents the first data collection on use of reprocessed material in everyday clinical practice in EHRA countries.
Over two-thirds (67.8%) of responders are using or have used reprocessed EP material, with substantial heterogeneity in the types and management of reprocessed materials. Most of the responders (88.3%) have not experienced any complication related to reprocessed materials and find using it beneficial, particularly in reducing costs (65%). A majority (61.5%) of those who have never used it would consider using reprocessed EP material in the future.
Reprocessing of EP materials has been discussed for years.6,7 The present survey recorded a broad spectrum of used reprocessed EP material. It ranged from items, that would be from the technical and practical point of view most suitable for reprocessing, like cables and diagnostic catheters with fixed or deflectable curves (used by 63–70% of respondents). It continued with non-irrigated ablation catheters, requiring a more vigorous maintenance of the original accuracy and physical properties during reprocessing (used by 51% of respondents). On the other end of the spectrum, it included materials with a lumen, which might be more challenging to be safely reprocessed, like transseptal needles, irrigated ablation catheters, or even different types of sheaths. Of note, reprocessing of these items with lumen was not that rare (25–38% of respondents, depending on type of material). In some institutions, even implantable materials (i.e. implantable pacemakers, defibrillators, and corresponding leads) were reprocessed, still, their use was marginal (1–4% of respondents). However, a recent study even reported the safety of the reusage of implantable defibrillators as an option for low-income countries.8
One reason for the observed heterogeneity could be particular differences in legislations and related hospital policies allowing a less strict approach to this issue in some countries. European Union (EU) countries constituted at least 53% of the involved countries. There is not a uniform legislation regarding reprocessing in EU. It is forbidden in some of the EU countries, in others it is legal.2 Countries, such as Spain, Italy, and France, have clearly banned this. Germany has a regulatory framework with guidelines allowing reprocessing is certain standards are met. It is also accepted in Belgium, Portugal, and Sweden. Little is known about the regulations of many other European countries. According to a scientific report published by European Commissions’ Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR), the whole reprocessing cycle starting with the collection of single-use devices after their use until the final sterilization and delivery step, including its functional performance, needs to be evaluated and validated to identify and reduce potential hazards associated with reprocessing of a specific single-use device.9 It also states that a traceability system has to be in place. The validation and monitoring of reprocessing process might hence become quite challenging, reducing the financial benefit of using reprocessed materials. Moreover, according to more recent EU legislation (expected for May 2020 but postponed by one year as a result of the COVID-19 pandemic), the reprocessor of a single-use device should be considered to be the manufacturer of the reprocessed device. It should assume the obligations incumbent on manufacturers, putting possible legal implications on the reprocessor in case a complication occurs.10 In a NASPE Task Force Report on reprocessing of EP catheters, a similar regulation set by the US Food and Drug Administration (FDA) was deemed to cause a shift from reprocessing performed in hospitals towards professional reprocessing companies charging in general about 50% of the original catheter costs for reprocessing.11 In the present survey, most of the reprocessing was performed at the responders’ institutions and third-party reprocessed EP material providers made up only about one-fourth of reported reprocessing facilities. However, less than half of the responders reported using any kind of traceability system of reprocessed EP materials. Taking in consideration that more than one-third of all responders preferred not to mention their country, it is not possible to determine to what extent this underuse of EU recommended reprocessed material tracing could be explained by the fact that the corresponding legislation has not yet been implemented by the respondent’s institution or simply does not apply in his country. Legislative limitation and local restrictions represented the main reasons for not using reprocessed EP materials and might have also been the cause why nearly a third of responders stopped using them.
A major complication potentially due to reprocessing (mostly embolization of material) was experienced by 11.3% of respondents having any experience with reprocessed EP materials, no death was reported. It has been shown earlier that the complication rate is <1% for diagnostic or EP catheters12,13 or deflectable ablation catheters.4,14 However, as the present study did not collect data on the total number of EP procedures with reprocessed materials or total number of used reprocessed catheters per respondent, it is not possible to calculate an exact complication rate. Regarding the fact that the reported median experience time with reprocessed EP materials in the present survey was 5 years, one could assume that most of the respondents used reprocessed EP materials much more than once. Therefore, the actual complication rate in the present cohort would be probably much lower. The other factors that are associated with the development of adverse events might coincide with the use of reprocessed materials. For instance, the following factors leading to an adverse event, are highly related to the reprocessing process: poor quality of intracardiac signals, misleading in the diagnosis and defining target ablation sites, air embolism from re-used long intracardiac sheaths with insufficient tightness of a haemostatic valve, or broken distal part of a diagnostic or mapping catheter. On the other hand, thromboembolism related to insufficient anticoagulation or endocardial damage, and temperature-triggered fibrinogen denaturation during radiofrequency ablation are well-known procedure-related complications with no direct relation to the reprocessing. Even though the question on complications while using re-processed materials was formulated implying a clear connection between reprocessing and the development of complications, we assume that in some cases it might be difficult to differentiate. Published real-word data on complications rates with reprocessed EP materials are scarce. One former study from the USA reported a series of allergic reactions to a soap residue used during the sterilization process.15 The authors of a NASPE Task Force Report on reprocessing of EP catheters, having considered the adverse events reported to FDA, state that there seems to be no disproportion in complications when using single-use devices or reprocessed EP materials. They consider using reprocessed material generally being safe, if the reprocessing is done by the current standards of practice, including validation and control of the process.11 The reprocessing procedure should follow current standards and should be monitored, e.g. concerning the maximum number of reprocessing cycles.16
Reducing the costs was the most important perceived benefit of using reprocessed EP material (65% of respondents). Tessarolo et al.16 calculated that reprocessing of EP material is associated with a potential cost reduction of 41.2% for diagnostic and 32.9% for ablation procedures. Based on our survey results, the maximum limit of times that a catheter can be reprocesses was reported as five for ablation catheters and six for diagnostic catheters and might be even higher for cables. These multiple reprocessing cycles further reduce costs relevantly. Re-usage of single-use devices more than once could lead to a greater availability of EP procedures (42% of respondents), particularly in countries with weaker health economics. Using reprocessed EP materials could also contribute to wider use of techniques that increase the safety of EP procedures or reduce X-ray exposure. For instance, reprocessing might lead to a broader use of intracardiac echocardiography (ICE).17,18 In the present survey, reprocessed ICE catheters were used by 24% of respondents. Last but not least, using reprocessed EP materials could reduce the burden of medical waste and help saving world raw stock resources, for example on precious metals.2,16 There are some published data supporting a positive environmental impact of reusable instruments in surgery but exact data balancing the environmental aspects of reprocessing vs. disposing single-use materials in cardiology are lacking.19,20 However, given the actually growing public emphasis on preservation of nature and sustainability, this potential benefit of reprocessing is of great importance and interest.
Limitations
The present survey-based study has limitations attributed to target respondents and questionnaire design. The survey was mainly spread through the scientific network of EHRA Young EP and participation was entirely voluntary, therefore, being prone to selection bias. Especially, since reprocessing is subject to local legal regulations, some underreporting and social desirability bias leading to over- or underreporting cannot be excluded. The reported frequency of reprocessing particular materials might have been biased by the type of procedures performed by the centre, leading to underreporting the reuse of some more complex items (e.g. transseptal needles or ICE probes by centres not using them).
Conclusions
Reprocessing of EP material is heterogeneously managed among the EHRA countries, as vast differences are present in terms of national and local regulations, hospital policies, and spectrum of reprocessed items. Nonetheless, the current data show that European electrophysiologists consider the use of reprocessed EP materials as generally safe and cost-effective. Looking at the potential financial and environmental benefits of reprocessing and developments and changes in the spectrum of used material as well as in the legislative requirements during the last decades, more actual and robust real-world data on safety and cost-effectiveness is needed.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
The authors are deeply thankful to Vanessa Meyen for her support in conducting this survey.
Conflict of interest: D.D. has received lecture honorary and/or travel grants from Abbott, Astra Zeneca, Biotronik, Boehringer Ingelheim, Boston Scientific, Medtronic, Pfizer, and Zoll. M.S. has received consultant fees (device troubleshooting and remote monitoring) from Abbott and travel grants from Boston Scientific and Biotronik. F.G. has received speaker fees, travel grants, and research grants from Actelion, Bayer, Boehringer Ingelheim, Boston Scientific, Daiichi-Sankyo, Medtronic, Novartis, and Sanofi. P.V. has received speaker honoraria from Abbott and Daiichi-Sankyo. E.M. has received speaker honoraria from Biosense Webster, Medtronic, Boston Scientific, Boehringer Ingelheim, Pfizer; has performed consultations for Biosense Webster and Boehringer Ingelheim. The other authors have no conflict of interest to declare.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Scientific Committee on Emerging and Newly Identified Health Risks. The safety of reprocessed medical devices marketed for single-use.
REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL—of 5 April 2017—on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC.



