-
PDF
- Split View
-
Views
-
Cite
Cite
Daehoon Kim, Pil-Sung Yang, Eunsun Jang, Hee Tae Yu, Tae-Hoon Kim, Jae-Sun Uhm, Jong-Youn Kim, Jung-Hoon Sung, Hui-Nam Pak, Moon-Hyoung Lee, Gregory Y H Lip, Boyoung Joung, The optimal drug adherence to maximize the efficacy and safety of non-vitamin K antagonist oral anticoagulant in real-world atrial fibrillation patients, EP Europace, Volume 22, Issue 4, April 2020, Pages 547–557, https://doi.org/10.1093/europace/euz273
Close - Share Icon Share
Abstract
To investigate the association between adherence to non-vitamin K antagonist oral anticoagulant (NOAC) and clinical outcomes and to determine the optimal cut-off level of NOAC adherence among patients with atrial fibrillation (AF).
Using the Korean National Health Insurance Service database, we identified 96 197 patients with non-valvular AF who initiated NOAC or warfarin in 2013–16. We compared clinical outcomes between adherent [proportion of days covered (PDC) ≥80%] vs. non-adherent (PDC <80%) NOAC users, and further with warfarin users. We assessed the outcomes according to different levels of adherence. The proportion of adherent NOAC users was 64.0%. Compared with non-adherent NOAC users, adherent NOAC users were at lower risks of ischaemic stroke/systemic embolism (SE) [adjusted hazard ratio (aHR) 0.73, 95% confidence interval (CI) 0.69–0.79], and myocardial infarction (aHR 0.82, 95% CI 0.72–0.93), whereas there was no significant risk alteration for major bleeding (aHR 1.01, 95% CI 0.91–1.11). Compared with warfarin, non-adherent NOAC use failed to have better efficacy against ischaemic stroke/SE (aHR 0.99, 95% CI 0.93–1.05) and rather had increased risk of myocardial infarction (aHR 1.13, 95% CI 1.03–1.25). In NOAC users, the risks of adverse outcomes decreased according to gradual increase of adherence rates with the lowest risks in ≥90%, except for major bleeding in which there were no significant associations.
In an adherence level-dependent fashion, adherent use of NOAC showed better clinical outcomes without increasing bleeding risk. Maintaining ≥90% of adherence optimizes effectiveness of NOAC therapy without compromising its safety.
In real-world clinical practice, adherence to NOAC is still suboptimal in AF patients.
Adherent NOAC use was associated with favourable clinical outcomes without any alteration of bleeding risk.
If a patient with AF was estimated or predicted to be non-adherent to NOAC, NOAC might be no longer a comparable option to warfarin in efficacy.
Although most trials consider adherence rates greater than 80% to be acceptable, we should aim even ‘higher-than-90%’ for the best efficacy of NOAC therapy without compromising its safety.
Introduction
Stroke prevention is the principal management priority in patients with atrial fibrillation (AF) given its association with a five-fold increase in stroke risk, and given that one in five cases of stroke can be attributed to this arrhythmia.1,2 The non-vitamin K antagonist oral anticoagulants (NOACs) have all been shown to be at least as effective and safe as warfarin in large randomized controlled trials.3–6 However, poor adherence to medication in daily life could lead to less effectiveness compared with clinical trial efficacy.7 Compared with warfarin, NOACs may improve adherence with less burden of treatment.8,9 However, there are limited real-world data describing the adherence levels of individual NOAC agents. Further, the impact of adherence to NOACs on efficacy and safety outcomes has not been well studied.
There is no consensual standard for what constitutes adequate adherence for specific medication. Some trials consider adherence rates of ≥80% to be acceptable, whereas others consider rates of ≥95% to be mandatory for adequate adherence, particularly among patients with serious conditions such as infection with human immunodeficiency virus.10 We are not certain about whether we should aim to the NOAC adherence rate of just ≥80% or even higher.
Therefore, we sought to characterize (i) the rates of adherence to three NOACs (dabigatran, rivaroxaban, and apixaban) in real-world practice; (ii) associations between adherence to NOAC and risks of adverse outcomes; (iii) comparison of clinical outcomes between non-adherent and adherent NOAC use and warfarin use; and (iv) effects of the degree of NOAC adherence on these outcomes to determine the optimal cut-off level of NOAC adherence.
Methods
This study is a retrospective analysis based on the national health claims database (NHIS-2016-4-026) established by the national health insurance service (NHIS) of Korea. The NHIS is the single insurer managed by the Korean government and the NHIS database represents the entire Korean population.11,12 The following information is provided: patient sociodemographic information, use of inpatient and outpatient services, pharmacy dispensing claims, and mortality data. This study was approved by the Institutional Review Board of Yonsei University Health System (4-2016-0179), and informed consent was waived.
Study population
From the entire Korean population in the Korean NHIS database, we initially identified 197 436 patients with AF who were aged 20 years or older and initiated treatment with dabigatran, rivaroxaban, apixaban, edoxaban, or warfarin from 1 January 2013 to 31 December 2016. Patients with AF were identified using the International Classification of Disease 10th Revision codes I48, only when it was a discharge diagnosis or confirmed more than twice in the outpatient department to ensure diagnostic accuracy. The AF diagnosis was previously validated in the NHIS database with a positive predictive value of 94.1%.12–14 Detailed exclusion criteria are presented in Supplementary material online, Methods.
We finally enrolled 96 197 adults with non-valvular AF and the CHA2DS2-VASc score ≥1 point, who initiated treatment with dabigatran (150 mg or 110 mg bid), rivaroxaban (20 mg or 15 mg qd), apixaban (5 mg or 2.5 mg bid), or warfarin from 1 January 2013 to 31 December 2016 (Figure 1). Patients taking edoxaban were excluded from this study due to their shorter follow-up times (the median follow-up of 5.6 months), which could potentially lead to bias regarding level of adherence. We defined the date of the first oral anticoagulant (OAC) prescription as the index date.
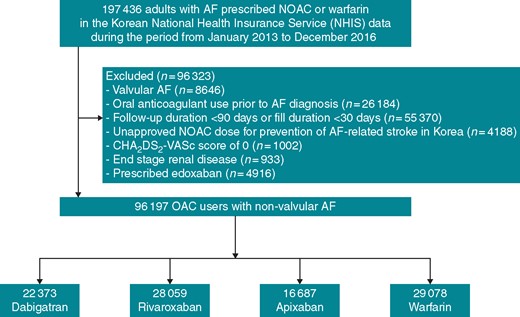
A flowchart of study population enrolment and analyses. AF, atrial fibrillation; NOAC, non-vitamin K antagonist oral anticoagulant; OAC, oral anticoagulant.
Exposure and outcomes
As the primary exposure variable, patient adherence to NOAC was defined as proportion of days covered (PDC) equal to or greater than 80%.10 Non-adherence was defined as PDC <80%. Proportion of days covered has been a validated adherence measure showing that it correlates with other direct measures of adherence and NOAC outcomes.3,8,10,13–16 Proportion of days covered was defined as the total number of outpatient days covered by the index medication divided by the observation time interval, based on fill date and the days of supply on the pharmacy claims.8,13–16 Consistent with prior literature, if outpatient supply was interrupted secondary to hospitalization for any cause, duration of inpatient stay was excluded from the denominator but resumed after discharge.8
Baseline comorbidities were defined using the medical claims and prescription medication prior to the index date. To ensure diagnostic accuracy, the patients were considered to have comorbidities when the condition was a discharge diagnosis or was confirmed at least twice in an outpatient setting, which was similar to previous studies with NHIS (Supplementary material online, Table S1).11,12 The CHA2DS2-VASc score, HAS-BLED score, and SAMe-TT2R2 score were calculated using baseline comorbidities and medication use (Supplementary material online, Table S2). One element of HAS-BLED, the labile international normalized ratios, is not available before the initiation of warfarin and is not applicable to NOACs. Therefore, the range of HAS-BLED score in this study was 0–8 instead of 0–9 in the original score. Among the elements of SAMe-TT2R2 score, tobacco use (for 2 points) was also not available. The range of SAMe-TT2R2 score was 0–6 in this study instead of 0–8 in the original score. Household income was evaluated based on the total national health insurance premiums paid by the insured individual in the index year, which is proportional to the individual’s income. Prescription medication use was ascertained by identifying NHIS database claims within 90 days before the index date.
Patients were followed from their index date until the time of switching to other OACs, at the end of follow-up (e.g., death or emigration), or at the end of the study period (31 December 2016), whichever occurred first. We assessed clinical outcomes including ischaemic stroke, systemic embolism (SE), major bleeding, intracranial haemorrhage, gastrointestinal (GI) bleeding, and myocardial infarction. Major cardiovascular event was defined as a composite of ischaemic stroke, intracranial haemorrhage, and myocardial infarction. When calculating incidence rates and hazard ratios for each outcome, patient’s adherence, the primary exposure variable, was estimated time-dependently until the occurrence of each outcome of interest.8 Each endpoint was analysed independently of the other without being censored. The definitions of clinical outcomes are presented in Supplementary material online, Table S3.
Statistical analysis
To determine the predictive variables of NOAC adherence, multivariable logistic regression analyses using the baseline characteristics listed in Table 1 were performed in NOAC users. The threshold for entry was 0.05 and removal was 0.10.
| . | All NOACs pooled . | |||
|---|---|---|---|---|
| Variables . | Total . | Adherent . | Non-adherent . | P-value . |
| (n = 67 119) . | (n = 42 974) . | (n = 24 145) . | ||
| Age (years) | 72.1 ± 9.4 | 72.2 ± 9.0 | 72.0 ± 10.2 | 0.001 |
| Male | 37 025 (55.2) | 23 480 (54.6) | 13 545 (56.1) | <0.001 |
| Prior warfarin use | 35 856 (53.4) | 25 157 (58.5) | 10 699 (44.3) | <0.001 |
| Reduced NOAC dosinga | 35 397 (52.7) | 22 370 (52.1) | 13 027 (54.0) | <0.001 |
| Indicated dose reduction (%)b | 51.5 | 49.2 | 57.2 | <0.001 |
| Class of NOAC | <0.001 | |||
| Dabigatran | 16 687 (24.9) | 10 828 (25.2) | 5859 (24.3) | |
| Rivaroxaban | 22 373 (33.3) | 14 619 (34.0) | 7754 (32.1) | |
| Apixaban | 28 059 (41.8) | 17 527 (40.8) | 10 532 (43.6) | |
| Household income (tertiles) | 0.029 | |||
| Low | 18 759 (27.9) | 11 902 (27.7) | 6857 (28.4) | |
| Intermediate | 14 140 (21.1) | 8998 (20.9) | 5142 (21.3) | |
| High | 34 220 (51.0) | 22 074 (51.4) | 12 146 (50.3) | |
| Heart failure | 42 365 (63.1) | 27 312 (63.6) | 15 053 (62.3) | 0.002 |
| Hypertension | 60 432 (90.0) | 39 244 (91.3) | 21 188 (87.8) | <0.001 |
| Diabetes mellitus | 22 619 (33.7) | 14 651 (34.1) | 7968 (33.0) | 0.004 |
| Dyslipidaemia | 61 898 (92.2) | 40 019 (93.1) | 21 879 (90.6) | <0.001 |
| Prior ischaemic stroke | 30 802 (45.9) | 20 595 (47.9) | 10 207 (42.3) | <0.001 |
| Prior transient ischaemic attack | 9785 (14.6) | 6489 (15.1) | 3296 (13.7) | <0.001 |
| Prior intracranial haemorrhage | 2762 (4.1) | 1685 (3.9) | 1077 (4.5) | 0.001 |
| Prior myocardial infarction | 8690 (12.9) | 5533 (12.9) | 3157 (13.1) | 0.466 |
| Peripheral artery disease | 14 084 (21.0) | 9074 (21.1) | 5010 (20.7) | 0.269 |
| Chronic kidney disease | 5974 (8.9) | 3854 (9.0) | 2120 (8.8) | 0.420 |
| Proteinuria | 3507 (5.2) | 2298 (5.3) | 1209 (5.0) | 0.060 |
| CHA2DS2-VASc score | 4.9 ± 1.8 | 5.0 ± 1.8 | 4.8 ± 1.9 | <0.001 |
| HAS-BLED score | 3.0 ± 1.1 | 3.0 ± 1.1 | 3.1 ± 1.1 | <0.001 |
| SAMe-TT2R2 score | 3.7 ± 0.7 | 3.7 ± 0.7 | 3.7 ± 0.7 | 0.789 |
| Cardiovascular medications | ||||
| Aspirin | 10 655 (15.9) | 5133 (11.9) | 5522 (22.9) | <0.001 |
| P2Y12 inhibitor | 6588 (9.8) | 3269 (7.6) | 3319 (13.7) | <0.001 |
| Statin | 39 715 (59.2) | 26 501 (61.7) | 13 214 (54.7) | <0.001 |
| β-blocker | 38 432 (57.3) | 24 995 (58.2) | 13 437 (55.7) | <0.001 |
| RAS blocker | 35 682 (53.2) | 23 296 (54.2) | 12 386 (51.3) | <0.001 |
| DHP CCB | 14 693 (21.9) | 9453 (22.0) | 5240 (21.7) | 0.381 |
| Non-DHP CCB | 8405 (12.5) | 5385 (12.5) | 3020 (12.5) | 0.941 |
| Loop/thiazide diuretics | 33 839 (50.4) | 22 116 (51.5) | 11 723 (48.6) | <0.001 |
| K+ sparing diuretics | 13 233 (19.7) | 8511 (19.8) | 4722 (19.6) | 0.444 |
| Digoxin | 13 604 (20.3) | 8848 (20.6) | 4756 (19.7) | 0.006 |
| Anti-arrhythmic drug | 15 014 (22.4) | 9419 (21.9) | 5595 (23.2) | <0.001 |
| Amiodarone | 6008 (9.0) | 3667 (8.5) | 2341 (9.7) | <0.001 |
| Other medications | ||||
| Rifampin | 535 (0.8) | 331 (0.8) | 204 (0.8) | 0.318 |
| Phenytoin | 133 (0.2) | 76 (0.2) | 57 (0.2) | 0.117 |
| Corticosteroid | 5470 (8.1) | 3496 (8.1) | 1974 (8.2) | 0.866 |
| Azole antifungal agent | 791 (1.2) | 545 (1.3) | 246 (1.0) | 0.005 |
| Proton pump inhibitor | 21 854 (32.6) | 14 073 (32.7) | 7781 (32.2) | 0.169 |
| . | All NOACs pooled . | |||
|---|---|---|---|---|
| Variables . | Total . | Adherent . | Non-adherent . | P-value . |
| (n = 67 119) . | (n = 42 974) . | (n = 24 145) . | ||
| Age (years) | 72.1 ± 9.4 | 72.2 ± 9.0 | 72.0 ± 10.2 | 0.001 |
| Male | 37 025 (55.2) | 23 480 (54.6) | 13 545 (56.1) | <0.001 |
| Prior warfarin use | 35 856 (53.4) | 25 157 (58.5) | 10 699 (44.3) | <0.001 |
| Reduced NOAC dosinga | 35 397 (52.7) | 22 370 (52.1) | 13 027 (54.0) | <0.001 |
| Indicated dose reduction (%)b | 51.5 | 49.2 | 57.2 | <0.001 |
| Class of NOAC | <0.001 | |||
| Dabigatran | 16 687 (24.9) | 10 828 (25.2) | 5859 (24.3) | |
| Rivaroxaban | 22 373 (33.3) | 14 619 (34.0) | 7754 (32.1) | |
| Apixaban | 28 059 (41.8) | 17 527 (40.8) | 10 532 (43.6) | |
| Household income (tertiles) | 0.029 | |||
| Low | 18 759 (27.9) | 11 902 (27.7) | 6857 (28.4) | |
| Intermediate | 14 140 (21.1) | 8998 (20.9) | 5142 (21.3) | |
| High | 34 220 (51.0) | 22 074 (51.4) | 12 146 (50.3) | |
| Heart failure | 42 365 (63.1) | 27 312 (63.6) | 15 053 (62.3) | 0.002 |
| Hypertension | 60 432 (90.0) | 39 244 (91.3) | 21 188 (87.8) | <0.001 |
| Diabetes mellitus | 22 619 (33.7) | 14 651 (34.1) | 7968 (33.0) | 0.004 |
| Dyslipidaemia | 61 898 (92.2) | 40 019 (93.1) | 21 879 (90.6) | <0.001 |
| Prior ischaemic stroke | 30 802 (45.9) | 20 595 (47.9) | 10 207 (42.3) | <0.001 |
| Prior transient ischaemic attack | 9785 (14.6) | 6489 (15.1) | 3296 (13.7) | <0.001 |
| Prior intracranial haemorrhage | 2762 (4.1) | 1685 (3.9) | 1077 (4.5) | 0.001 |
| Prior myocardial infarction | 8690 (12.9) | 5533 (12.9) | 3157 (13.1) | 0.466 |
| Peripheral artery disease | 14 084 (21.0) | 9074 (21.1) | 5010 (20.7) | 0.269 |
| Chronic kidney disease | 5974 (8.9) | 3854 (9.0) | 2120 (8.8) | 0.420 |
| Proteinuria | 3507 (5.2) | 2298 (5.3) | 1209 (5.0) | 0.060 |
| CHA2DS2-VASc score | 4.9 ± 1.8 | 5.0 ± 1.8 | 4.8 ± 1.9 | <0.001 |
| HAS-BLED score | 3.0 ± 1.1 | 3.0 ± 1.1 | 3.1 ± 1.1 | <0.001 |
| SAMe-TT2R2 score | 3.7 ± 0.7 | 3.7 ± 0.7 | 3.7 ± 0.7 | 0.789 |
| Cardiovascular medications | ||||
| Aspirin | 10 655 (15.9) | 5133 (11.9) | 5522 (22.9) | <0.001 |
| P2Y12 inhibitor | 6588 (9.8) | 3269 (7.6) | 3319 (13.7) | <0.001 |
| Statin | 39 715 (59.2) | 26 501 (61.7) | 13 214 (54.7) | <0.001 |
| β-blocker | 38 432 (57.3) | 24 995 (58.2) | 13 437 (55.7) | <0.001 |
| RAS blocker | 35 682 (53.2) | 23 296 (54.2) | 12 386 (51.3) | <0.001 |
| DHP CCB | 14 693 (21.9) | 9453 (22.0) | 5240 (21.7) | 0.381 |
| Non-DHP CCB | 8405 (12.5) | 5385 (12.5) | 3020 (12.5) | 0.941 |
| Loop/thiazide diuretics | 33 839 (50.4) | 22 116 (51.5) | 11 723 (48.6) | <0.001 |
| K+ sparing diuretics | 13 233 (19.7) | 8511 (19.8) | 4722 (19.6) | 0.444 |
| Digoxin | 13 604 (20.3) | 8848 (20.6) | 4756 (19.7) | 0.006 |
| Anti-arrhythmic drug | 15 014 (22.4) | 9419 (21.9) | 5595 (23.2) | <0.001 |
| Amiodarone | 6008 (9.0) | 3667 (8.5) | 2341 (9.7) | <0.001 |
| Other medications | ||||
| Rifampin | 535 (0.8) | 331 (0.8) | 204 (0.8) | 0.318 |
| Phenytoin | 133 (0.2) | 76 (0.2) | 57 (0.2) | 0.117 |
| Corticosteroid | 5470 (8.1) | 3496 (8.1) | 1974 (8.2) | 0.866 |
| Azole antifungal agent | 791 (1.2) | 545 (1.3) | 246 (1.0) | 0.005 |
| Proton pump inhibitor | 21 854 (32.6) | 14 073 (32.7) | 7781 (32.2) | 0.169 |
Values are mean ± standard deviation or n (%). Adherence was estimated over a patient’s entire follow-up.
CCB, calcium channel blockers; DHP, dihydropyridine; NOAC, non-vitamin K antagonist oral anticoagulant; RAS, renin–angiotensin system.
Dabigatran 110 mg bid, rivaroxaban 15 mg qd, or apixaban 2.5 mg bid.
Proportion of those meeting indications for dose reduction among patients with reduced dosing. These proportions were calculated in limited patients (19 043 of 35 397 patients with reduced dosing) with available data about body weight, serum creatinine level, and creatinine clearance. The criteria for dose reduction are presented in Supplementary material online, Table S5.
| . | All NOACs pooled . | |||
|---|---|---|---|---|
| Variables . | Total . | Adherent . | Non-adherent . | P-value . |
| (n = 67 119) . | (n = 42 974) . | (n = 24 145) . | ||
| Age (years) | 72.1 ± 9.4 | 72.2 ± 9.0 | 72.0 ± 10.2 | 0.001 |
| Male | 37 025 (55.2) | 23 480 (54.6) | 13 545 (56.1) | <0.001 |
| Prior warfarin use | 35 856 (53.4) | 25 157 (58.5) | 10 699 (44.3) | <0.001 |
| Reduced NOAC dosinga | 35 397 (52.7) | 22 370 (52.1) | 13 027 (54.0) | <0.001 |
| Indicated dose reduction (%)b | 51.5 | 49.2 | 57.2 | <0.001 |
| Class of NOAC | <0.001 | |||
| Dabigatran | 16 687 (24.9) | 10 828 (25.2) | 5859 (24.3) | |
| Rivaroxaban | 22 373 (33.3) | 14 619 (34.0) | 7754 (32.1) | |
| Apixaban | 28 059 (41.8) | 17 527 (40.8) | 10 532 (43.6) | |
| Household income (tertiles) | 0.029 | |||
| Low | 18 759 (27.9) | 11 902 (27.7) | 6857 (28.4) | |
| Intermediate | 14 140 (21.1) | 8998 (20.9) | 5142 (21.3) | |
| High | 34 220 (51.0) | 22 074 (51.4) | 12 146 (50.3) | |
| Heart failure | 42 365 (63.1) | 27 312 (63.6) | 15 053 (62.3) | 0.002 |
| Hypertension | 60 432 (90.0) | 39 244 (91.3) | 21 188 (87.8) | <0.001 |
| Diabetes mellitus | 22 619 (33.7) | 14 651 (34.1) | 7968 (33.0) | 0.004 |
| Dyslipidaemia | 61 898 (92.2) | 40 019 (93.1) | 21 879 (90.6) | <0.001 |
| Prior ischaemic stroke | 30 802 (45.9) | 20 595 (47.9) | 10 207 (42.3) | <0.001 |
| Prior transient ischaemic attack | 9785 (14.6) | 6489 (15.1) | 3296 (13.7) | <0.001 |
| Prior intracranial haemorrhage | 2762 (4.1) | 1685 (3.9) | 1077 (4.5) | 0.001 |
| Prior myocardial infarction | 8690 (12.9) | 5533 (12.9) | 3157 (13.1) | 0.466 |
| Peripheral artery disease | 14 084 (21.0) | 9074 (21.1) | 5010 (20.7) | 0.269 |
| Chronic kidney disease | 5974 (8.9) | 3854 (9.0) | 2120 (8.8) | 0.420 |
| Proteinuria | 3507 (5.2) | 2298 (5.3) | 1209 (5.0) | 0.060 |
| CHA2DS2-VASc score | 4.9 ± 1.8 | 5.0 ± 1.8 | 4.8 ± 1.9 | <0.001 |
| HAS-BLED score | 3.0 ± 1.1 | 3.0 ± 1.1 | 3.1 ± 1.1 | <0.001 |
| SAMe-TT2R2 score | 3.7 ± 0.7 | 3.7 ± 0.7 | 3.7 ± 0.7 | 0.789 |
| Cardiovascular medications | ||||
| Aspirin | 10 655 (15.9) | 5133 (11.9) | 5522 (22.9) | <0.001 |
| P2Y12 inhibitor | 6588 (9.8) | 3269 (7.6) | 3319 (13.7) | <0.001 |
| Statin | 39 715 (59.2) | 26 501 (61.7) | 13 214 (54.7) | <0.001 |
| β-blocker | 38 432 (57.3) | 24 995 (58.2) | 13 437 (55.7) | <0.001 |
| RAS blocker | 35 682 (53.2) | 23 296 (54.2) | 12 386 (51.3) | <0.001 |
| DHP CCB | 14 693 (21.9) | 9453 (22.0) | 5240 (21.7) | 0.381 |
| Non-DHP CCB | 8405 (12.5) | 5385 (12.5) | 3020 (12.5) | 0.941 |
| Loop/thiazide diuretics | 33 839 (50.4) | 22 116 (51.5) | 11 723 (48.6) | <0.001 |
| K+ sparing diuretics | 13 233 (19.7) | 8511 (19.8) | 4722 (19.6) | 0.444 |
| Digoxin | 13 604 (20.3) | 8848 (20.6) | 4756 (19.7) | 0.006 |
| Anti-arrhythmic drug | 15 014 (22.4) | 9419 (21.9) | 5595 (23.2) | <0.001 |
| Amiodarone | 6008 (9.0) | 3667 (8.5) | 2341 (9.7) | <0.001 |
| Other medications | ||||
| Rifampin | 535 (0.8) | 331 (0.8) | 204 (0.8) | 0.318 |
| Phenytoin | 133 (0.2) | 76 (0.2) | 57 (0.2) | 0.117 |
| Corticosteroid | 5470 (8.1) | 3496 (8.1) | 1974 (8.2) | 0.866 |
| Azole antifungal agent | 791 (1.2) | 545 (1.3) | 246 (1.0) | 0.005 |
| Proton pump inhibitor | 21 854 (32.6) | 14 073 (32.7) | 7781 (32.2) | 0.169 |
| . | All NOACs pooled . | |||
|---|---|---|---|---|
| Variables . | Total . | Adherent . | Non-adherent . | P-value . |
| (n = 67 119) . | (n = 42 974) . | (n = 24 145) . | ||
| Age (years) | 72.1 ± 9.4 | 72.2 ± 9.0 | 72.0 ± 10.2 | 0.001 |
| Male | 37 025 (55.2) | 23 480 (54.6) | 13 545 (56.1) | <0.001 |
| Prior warfarin use | 35 856 (53.4) | 25 157 (58.5) | 10 699 (44.3) | <0.001 |
| Reduced NOAC dosinga | 35 397 (52.7) | 22 370 (52.1) | 13 027 (54.0) | <0.001 |
| Indicated dose reduction (%)b | 51.5 | 49.2 | 57.2 | <0.001 |
| Class of NOAC | <0.001 | |||
| Dabigatran | 16 687 (24.9) | 10 828 (25.2) | 5859 (24.3) | |
| Rivaroxaban | 22 373 (33.3) | 14 619 (34.0) | 7754 (32.1) | |
| Apixaban | 28 059 (41.8) | 17 527 (40.8) | 10 532 (43.6) | |
| Household income (tertiles) | 0.029 | |||
| Low | 18 759 (27.9) | 11 902 (27.7) | 6857 (28.4) | |
| Intermediate | 14 140 (21.1) | 8998 (20.9) | 5142 (21.3) | |
| High | 34 220 (51.0) | 22 074 (51.4) | 12 146 (50.3) | |
| Heart failure | 42 365 (63.1) | 27 312 (63.6) | 15 053 (62.3) | 0.002 |
| Hypertension | 60 432 (90.0) | 39 244 (91.3) | 21 188 (87.8) | <0.001 |
| Diabetes mellitus | 22 619 (33.7) | 14 651 (34.1) | 7968 (33.0) | 0.004 |
| Dyslipidaemia | 61 898 (92.2) | 40 019 (93.1) | 21 879 (90.6) | <0.001 |
| Prior ischaemic stroke | 30 802 (45.9) | 20 595 (47.9) | 10 207 (42.3) | <0.001 |
| Prior transient ischaemic attack | 9785 (14.6) | 6489 (15.1) | 3296 (13.7) | <0.001 |
| Prior intracranial haemorrhage | 2762 (4.1) | 1685 (3.9) | 1077 (4.5) | 0.001 |
| Prior myocardial infarction | 8690 (12.9) | 5533 (12.9) | 3157 (13.1) | 0.466 |
| Peripheral artery disease | 14 084 (21.0) | 9074 (21.1) | 5010 (20.7) | 0.269 |
| Chronic kidney disease | 5974 (8.9) | 3854 (9.0) | 2120 (8.8) | 0.420 |
| Proteinuria | 3507 (5.2) | 2298 (5.3) | 1209 (5.0) | 0.060 |
| CHA2DS2-VASc score | 4.9 ± 1.8 | 5.0 ± 1.8 | 4.8 ± 1.9 | <0.001 |
| HAS-BLED score | 3.0 ± 1.1 | 3.0 ± 1.1 | 3.1 ± 1.1 | <0.001 |
| SAMe-TT2R2 score | 3.7 ± 0.7 | 3.7 ± 0.7 | 3.7 ± 0.7 | 0.789 |
| Cardiovascular medications | ||||
| Aspirin | 10 655 (15.9) | 5133 (11.9) | 5522 (22.9) | <0.001 |
| P2Y12 inhibitor | 6588 (9.8) | 3269 (7.6) | 3319 (13.7) | <0.001 |
| Statin | 39 715 (59.2) | 26 501 (61.7) | 13 214 (54.7) | <0.001 |
| β-blocker | 38 432 (57.3) | 24 995 (58.2) | 13 437 (55.7) | <0.001 |
| RAS blocker | 35 682 (53.2) | 23 296 (54.2) | 12 386 (51.3) | <0.001 |
| DHP CCB | 14 693 (21.9) | 9453 (22.0) | 5240 (21.7) | 0.381 |
| Non-DHP CCB | 8405 (12.5) | 5385 (12.5) | 3020 (12.5) | 0.941 |
| Loop/thiazide diuretics | 33 839 (50.4) | 22 116 (51.5) | 11 723 (48.6) | <0.001 |
| K+ sparing diuretics | 13 233 (19.7) | 8511 (19.8) | 4722 (19.6) | 0.444 |
| Digoxin | 13 604 (20.3) | 8848 (20.6) | 4756 (19.7) | 0.006 |
| Anti-arrhythmic drug | 15 014 (22.4) | 9419 (21.9) | 5595 (23.2) | <0.001 |
| Amiodarone | 6008 (9.0) | 3667 (8.5) | 2341 (9.7) | <0.001 |
| Other medications | ||||
| Rifampin | 535 (0.8) | 331 (0.8) | 204 (0.8) | 0.318 |
| Phenytoin | 133 (0.2) | 76 (0.2) | 57 (0.2) | 0.117 |
| Corticosteroid | 5470 (8.1) | 3496 (8.1) | 1974 (8.2) | 0.866 |
| Azole antifungal agent | 791 (1.2) | 545 (1.3) | 246 (1.0) | 0.005 |
| Proton pump inhibitor | 21 854 (32.6) | 14 073 (32.7) | 7781 (32.2) | 0.169 |
Values are mean ± standard deviation or n (%). Adherence was estimated over a patient’s entire follow-up.
CCB, calcium channel blockers; DHP, dihydropyridine; NOAC, non-vitamin K antagonist oral anticoagulant; RAS, renin–angiotensin system.
Dabigatran 110 mg bid, rivaroxaban 15 mg qd, or apixaban 2.5 mg bid.
Proportion of those meeting indications for dose reduction among patients with reduced dosing. These proportions were calculated in limited patients (19 043 of 35 397 patients with reduced dosing) with available data about body weight, serum creatinine level, and creatinine clearance. The criteria for dose reduction are presented in Supplementary material online, Table S5.
Incidence rates of outcomes were calculated by dividing the number of events by person-time at risk, with the 95% confidence interval (CI) estimated by exact Poisson distribution. We used multivariable Cox proportional hazards models to estimate the effect of adherence (PDC ≥ 80%) to NOAC (for all NOACs pooled and each NOAC) on clinical outcomes compared with non-adherence use (PDC < 80%). We also used different Cox models to estimate the risks of adverse outcomes according to gradual PDC increase in NOAC users [PDC: 40 to <70% (reference), 70 to <80%, 80 to <90%, and 90–100%] and to assess the risk of adverse outcomes according to three groups including all warfarin users, adherent NOAC users, and non-adherent NOAC users. The Cox regression model analyses were adjusted for the baseline characteristics listed in Table 1 as time-fixed variables and also for heart failure, hypertension, diabetes mellitus, ischaemic stroke, transient ischaemic attack (TIA), myocardial infarction, intracranial haemorrhage, peripheral artery disease, dyslipidaemia, chronic kidney disease, and proteinuria as time-dependent variables.
For sensitivity analyses, we estimated the effect of adherence to NOAC on clinical outcomes compared with non-adherent users with a PDC of 40–79% in order to exclude the effect of extremely low PDC (<40%). Also, we censored all patients at the earliest date of occurrence of ischaemic stroke/SE, major bleeding, and myocardial infarction when assessing every clinical outcome because each unique event can affect patient adherence by either prompting treatment interruption (e.g., bleeding) or promoting treatment compliance (e.g., ischaemic stroke, SE, and myocardial infarction).15 Statistical significance was defined as P < 0.05. Statistical analyses were conducted by using SAS version 9.4 (SAS Institute, Inc.) and R version 3.5.2 (The R Foundation for Statistical Computing; www.R-project.org).
Results
Adherence to non-vitamin K antagonist oral anticoagulant
The majority of the patients (69.8%, n = 67 119) was treated with NOACs (23.2% of dabigatran, 29.2% of rivaroxaban, and 17.3% of apixaban), while 30.2% was treated with warfarin. The median follow-up duration of NOAC users was 12.7 (7.6–16.8) months. The proportion of adherent NOAC users with PDC ≥80% (measured over a patient’s entire follow-up) was 64.0%. Among NOACs, dabigatran accounted for the highest percentage of adherent users (65.3%), followed by apixaban (64.9%), and rivaroxaban (62.5%), while apixaban showed the highest mean PDC level (79.8 ± 22.0%) (Figure 2A). In all NOACs pooled and each of the three individual NOACs, the proportion of patients increased gradually with increase of PDC (Figure 2B). Proportions of adherent users decreased as patients were followed up beyond 1 year in all NOAC pooled and individual NOAC groups (all P values by McNemar test <0.001) (Figure 2C).
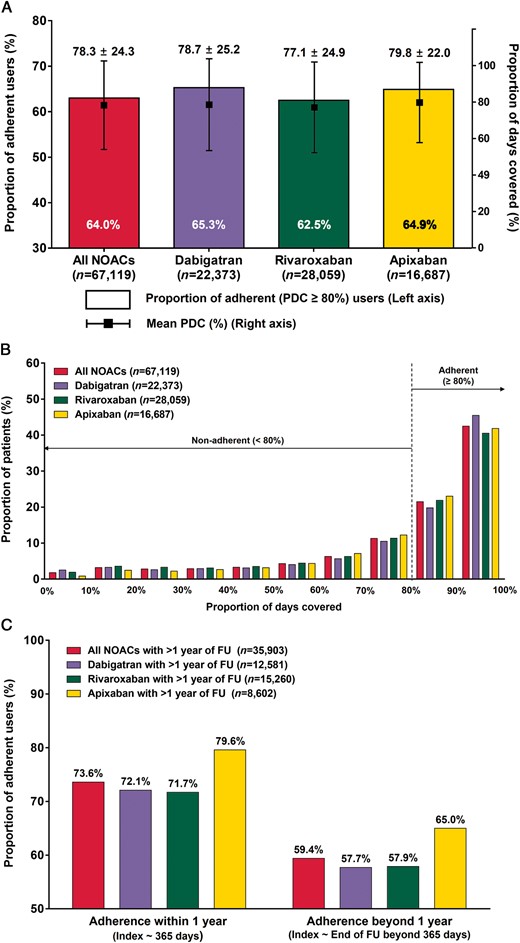
Adherence to NOAC. (A) Proportions of adherent users (PDC ≥80%) and mean PDC levels. (B) Proportions of patients according to PDC. (C) Proportions of adherent users (PDC ≥80%) within or beyond 1 year of follow-up. Adherence was estimated over a patient’s entire follow-up. NOAC, non-vitamin K antagonist oral anticoagulant; PDC, proportion of days covered.
Table 1 shows baseline characteristics of patients according to adherence to NOAC. Compared with non-adherent NOAC users, adherent NOAC users were more likely to be older and female. They had more frequent prior warfarin use, histories of heart failure, hypertension, diabetes mellitus, dyslipidaemia, and ischaemic stroke/TIA; and a higher CHA2DS2-VASc score. In contrast, they had lower frequencies of reduced NOAC dosing and history of intracranial haemorrhage; and a lower HAS-BLED score. Characteristics of patients using each NOAC and warfarin are presented in Supplementary material online, Table S4.
Predictors of adherence to non-vitamin K antagonist oral anticoagulant
From the multivariate logistic analysis, twice-daily dosing, hypertension, dyslipidaemia, prior ischaemic stroke, a higher CHA2DS2-VASc score, and concomitant medication use including statin, β-blocker, renin-angiotensin system blocker, calcium channel blocker, diuretics, digoxin, and proton-pump inhibitor were found to be associated with adherence. Reduced NOAC dosing, de novo OAC starter, lower income, prior intracranial haemorrhage, a higher SAMe-TT2R2 score, polypharmacy (more concomitant medication), and concomitant antiplatelet use were associated with non-adherence (Table 2).
| Variables . | Adjusted OR (95% CI) . | P-value . |
|---|---|---|
| Positive predictors | ||
| Twice-daily dosing (Once-daily dosing as reference) | 1.11 (1.08–1.15) | <0.001 |
| Hypertension | 1.40 (1.32–1.49) | <0.001 |
| Dyslipidaemia | 1.14 (1.07–1.21) | 0.001 |
| Prior ischaemic stroke | 1.22 (1.16–1.29) | <0.001 |
| CHA2DS2-VASc score (per 1 increase) | 1.02 (1.00–1.04) | 0.016 |
| Concomitant medication | ||
| Statin use | 1.77 (1.63–1.91) | <0.001 |
| β-blocker use | 1.46 (1.35–1.58) | <0.001 |
| RAS blocker use | 1.40 (1.29–1.51) | <0.001 |
| Calcium channel blocker use | 1.36 (1.25–1.48) | <0.001 |
| Loop/thiazide diuretics use | 1.47 (1.36–1.59) | <0.001 |
| Digoxin use | 1.36 (1.26–1.48) | <0.001 |
| Proton-pump inhibitor use | 1.05 (1.02–1.09) | 0.003 |
| Negative predictors | ||
| Reduced NOAC dose | 0.93 (0.90–0.96) | <0.001 |
| De novo OAC starter (prior warfarin use as reference) | 0.60 (0.58–0.62) | <0.001 |
| Lower household income (per 1 tertile decrease) | 0.97 (0.96–0.99) | 0.008 |
| Prior intracranial haemorrhage | 0.77 (0.71–0.84) | <0.001 |
| High SAMe-TT2R2 score (per 1 increase) | 0.84 (0.80–0.88) | <0.001 |
| Polypharmacy (per 1 increase of concomitant medication) | 0.78 (0.73–0.83) | <0.001 |
| Concomitant medication | ||
| Antiplatelet use | 0.55 (0.50–0.60) | <0.001 |
| Variables . | Adjusted OR (95% CI) . | P-value . |
|---|---|---|
| Positive predictors | ||
| Twice-daily dosing (Once-daily dosing as reference) | 1.11 (1.08–1.15) | <0.001 |
| Hypertension | 1.40 (1.32–1.49) | <0.001 |
| Dyslipidaemia | 1.14 (1.07–1.21) | 0.001 |
| Prior ischaemic stroke | 1.22 (1.16–1.29) | <0.001 |
| CHA2DS2-VASc score (per 1 increase) | 1.02 (1.00–1.04) | 0.016 |
| Concomitant medication | ||
| Statin use | 1.77 (1.63–1.91) | <0.001 |
| β-blocker use | 1.46 (1.35–1.58) | <0.001 |
| RAS blocker use | 1.40 (1.29–1.51) | <0.001 |
| Calcium channel blocker use | 1.36 (1.25–1.48) | <0.001 |
| Loop/thiazide diuretics use | 1.47 (1.36–1.59) | <0.001 |
| Digoxin use | 1.36 (1.26–1.48) | <0.001 |
| Proton-pump inhibitor use | 1.05 (1.02–1.09) | 0.003 |
| Negative predictors | ||
| Reduced NOAC dose | 0.93 (0.90–0.96) | <0.001 |
| De novo OAC starter (prior warfarin use as reference) | 0.60 (0.58–0.62) | <0.001 |
| Lower household income (per 1 tertile decrease) | 0.97 (0.96–0.99) | 0.008 |
| Prior intracranial haemorrhage | 0.77 (0.71–0.84) | <0.001 |
| High SAMe-TT2R2 score (per 1 increase) | 0.84 (0.80–0.88) | <0.001 |
| Polypharmacy (per 1 increase of concomitant medication) | 0.78 (0.73–0.83) | <0.001 |
| Concomitant medication | ||
| Antiplatelet use | 0.55 (0.50–0.60) | <0.001 |
CI, confidence interval; NOAC, non-vitamin K antagonist oral anticoagulant; OAC, oral anticoagulant; OR, odds ratio; RAS, renin–angiotensin system.
| Variables . | Adjusted OR (95% CI) . | P-value . |
|---|---|---|
| Positive predictors | ||
| Twice-daily dosing (Once-daily dosing as reference) | 1.11 (1.08–1.15) | <0.001 |
| Hypertension | 1.40 (1.32–1.49) | <0.001 |
| Dyslipidaemia | 1.14 (1.07–1.21) | 0.001 |
| Prior ischaemic stroke | 1.22 (1.16–1.29) | <0.001 |
| CHA2DS2-VASc score (per 1 increase) | 1.02 (1.00–1.04) | 0.016 |
| Concomitant medication | ||
| Statin use | 1.77 (1.63–1.91) | <0.001 |
| β-blocker use | 1.46 (1.35–1.58) | <0.001 |
| RAS blocker use | 1.40 (1.29–1.51) | <0.001 |
| Calcium channel blocker use | 1.36 (1.25–1.48) | <0.001 |
| Loop/thiazide diuretics use | 1.47 (1.36–1.59) | <0.001 |
| Digoxin use | 1.36 (1.26–1.48) | <0.001 |
| Proton-pump inhibitor use | 1.05 (1.02–1.09) | 0.003 |
| Negative predictors | ||
| Reduced NOAC dose | 0.93 (0.90–0.96) | <0.001 |
| De novo OAC starter (prior warfarin use as reference) | 0.60 (0.58–0.62) | <0.001 |
| Lower household income (per 1 tertile decrease) | 0.97 (0.96–0.99) | 0.008 |
| Prior intracranial haemorrhage | 0.77 (0.71–0.84) | <0.001 |
| High SAMe-TT2R2 score (per 1 increase) | 0.84 (0.80–0.88) | <0.001 |
| Polypharmacy (per 1 increase of concomitant medication) | 0.78 (0.73–0.83) | <0.001 |
| Concomitant medication | ||
| Antiplatelet use | 0.55 (0.50–0.60) | <0.001 |
| Variables . | Adjusted OR (95% CI) . | P-value . |
|---|---|---|
| Positive predictors | ||
| Twice-daily dosing (Once-daily dosing as reference) | 1.11 (1.08–1.15) | <0.001 |
| Hypertension | 1.40 (1.32–1.49) | <0.001 |
| Dyslipidaemia | 1.14 (1.07–1.21) | 0.001 |
| Prior ischaemic stroke | 1.22 (1.16–1.29) | <0.001 |
| CHA2DS2-VASc score (per 1 increase) | 1.02 (1.00–1.04) | 0.016 |
| Concomitant medication | ||
| Statin use | 1.77 (1.63–1.91) | <0.001 |
| β-blocker use | 1.46 (1.35–1.58) | <0.001 |
| RAS blocker use | 1.40 (1.29–1.51) | <0.001 |
| Calcium channel blocker use | 1.36 (1.25–1.48) | <0.001 |
| Loop/thiazide diuretics use | 1.47 (1.36–1.59) | <0.001 |
| Digoxin use | 1.36 (1.26–1.48) | <0.001 |
| Proton-pump inhibitor use | 1.05 (1.02–1.09) | 0.003 |
| Negative predictors | ||
| Reduced NOAC dose | 0.93 (0.90–0.96) | <0.001 |
| De novo OAC starter (prior warfarin use as reference) | 0.60 (0.58–0.62) | <0.001 |
| Lower household income (per 1 tertile decrease) | 0.97 (0.96–0.99) | 0.008 |
| Prior intracranial haemorrhage | 0.77 (0.71–0.84) | <0.001 |
| High SAMe-TT2R2 score (per 1 increase) | 0.84 (0.80–0.88) | <0.001 |
| Polypharmacy (per 1 increase of concomitant medication) | 0.78 (0.73–0.83) | <0.001 |
| Concomitant medication | ||
| Antiplatelet use | 0.55 (0.50–0.60) | <0.001 |
CI, confidence interval; NOAC, non-vitamin K antagonist oral anticoagulant; OAC, oral anticoagulant; OR, odds ratio; RAS, renin–angiotensin system.
Adherence to non-vitamin K antagonist oral anticoagulant and adverse outcomes
Figure 3 shows the risks of adverse outcomes of adherence users compared with non-adherent users (PDC <80%) for all NOACs pooled and each NOAC. Compared with non-adherence, adherence to NOAC was associated with lower risks of major cardiovascular event [adjusted hazard ratio (aHR) 0.79, 95% CI 0.74–0.84] and ischaemic stroke/SE (aHR 0.73, 95% CI 0.69–0.79). These favourable effects of adherence were consistently observed in the separate analyses of each individual NOAC. Adherence to NOAC was also associated with decreased risks of myocardial infarction (aHR 0.82, 95% CI 0.72–0.93). In contrast, there was no significant association observed with the risk of major bleeding (aHR 1.01, 95% CI 0.91–1.11).
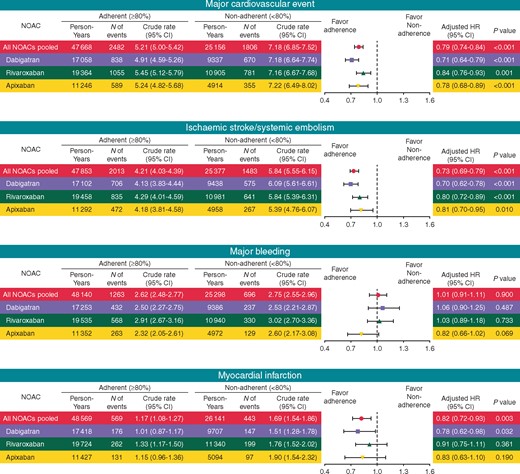
Risk of adverse outcomes of adherent NOAC use (PDC ≥80%) compared with non-adherent use (PDC <80%). CI, confidence interval; HR, hazard ratio; NOAC, non-vitamin K antagonist oral anticoagulant; PDC, proportion of days covered.
Risks of individual components of outcomes including ischaemic stroke, SE, intracranial haemorrhage, and GI bleeding were presented in Supplementary material online, Figure S1. Similarly, adherence to NOAC was associated with lower risks of ischaemic stroke and SE, while there were no significant effects on risks of intracranial and GI bleeding.
The subgroup analysis was performed to investigate whether the effects of adherence to NOAC were consistent across different subgroups (Figure 4). Significant risk reductions of major cardiovascular event were observed in all subgroups. The risk reductions of major cardiovascular event and stroke/SE were more prominent for those with a longer follow-up duration (≥1 year). No significant association with the risk of major bleeding was observed in all subgroups. For the risk of myocardial infarction, no statistically significant interactions were present although the risk reductions associated with adherence to NOAC were generally less significant in subgroups than in overall.
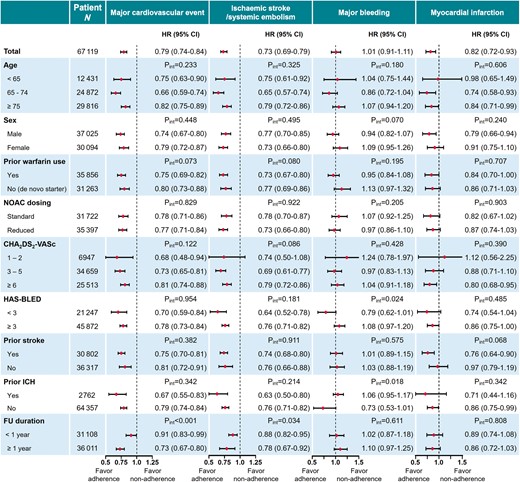
Subgroup analysis: risk of adverse outcomes of adherent NOAC use (PDC ≥80%) compared with non-adherent use (PDC <80%) in each subgroup. CI, confidence interval; HR, hazard ratio; ICH, intracranial haemorrhage; NOAC, non-vitamin K antagonist oral anticoagulant; PDC, proportion of days covered.
The results of the sensitivity analysis which excluded patients with extremely low PDC (<40%) (Supplementary material online, Figure S2) and the analysis in which patients were censored at the earliest occurrence of any adverse outcome (Supplementary material online, Figure S3) were consistent with the main findings.
Outcomes of adherent or non-adherent non-vitamin K antagonist oral anticoagulant use compared with warfarin use
Risks of adverse outcomes by adherent and non-adherent NOAC use compared with warfarin use are presented in Figure 5 (individual components of outcomes in Supplementary material online, Figure S4). Compared with warfarin use, adherent NOAC use was associated with decreased risks of major cardiovascular event (aHR 0.71, 95% CI 0.67–0.76), ischaemic stroke/SE (aHR 0.63, 95% CI 0.60–0.65), and major bleeding (aHR 0.77, 95% CI 0.71–0.83). Consistently, non-adherent NOAC use was associated with a lower risk of major bleeding. However, non-adherent NOAC use was not more effective against ischaemic stroke/SE (aHR 0.99, 95% 0.93–1.05), and rather had an increased risk of myocardial infarction (aHR 1.13, 95% CI 1.03–1.25) compared with warfarin.
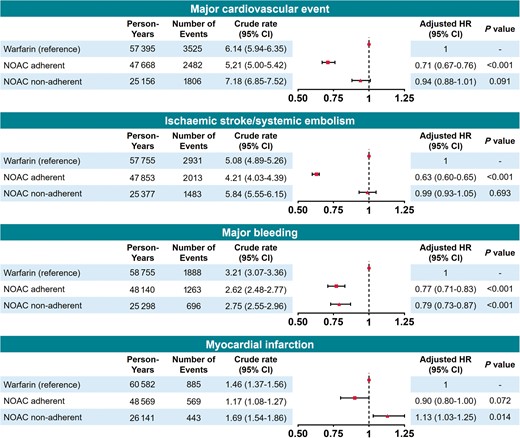
Risk of adverse outcomes of adherent (PDC ≥80%) or non-adherent (PDC <80%) NOAC use compared with warfarin use. CI, confidence interval; HR, hazard ratio; NOAC, non-vitamin K antagonist oral anticoagulant; PDC, proportion of days covered.
Outcomes according to different levels of non-vitamin K antagonist oral anticoagulant adherence
Figure 6 shows the risks of adverse outcomes according to gradual PDC increase in NOAC users. The risks of all adverse outcomes decreased in a PDC-dependent fashion (P for trend <0.001) with the lowest risks in those with PDC over 90%, except for major bleeding in which there was no significant trend observed (P for trend = 0.619).
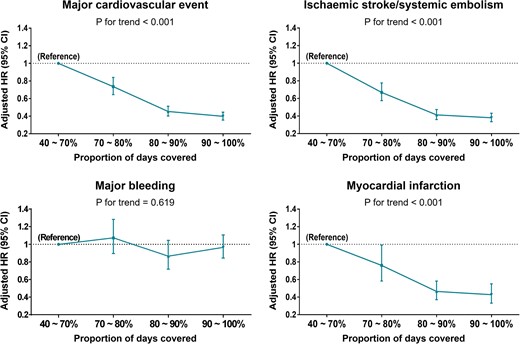
Risk of adverse outcomes according to gradual increase of NOAC adherence. CI, confidence interval; HR, hazard ratio.
Discussion
Our study includes four principal findings, as follows: (i) adherence to three NOACs was suboptimal in real-world practice; (ii) adherent use of NOAC was associated with lower risks of major cardiovascular event, ischaemic stroke/SE, and myocardial infarction without increasing bleeding risk; (iii) compared with warfarin use, adherent use of NOAC was associated with lower risks of major cardiovascular event, ischaemic stroke/SE, and major bleeding whereas non-adherent use of NOAC failed to have better efficacy against ischaemic stroke/SE and rather had increased risk of myocardial infarction; and (iv) the risks of adverse outcomes were decreased in a NOAC adherence level-dependent manner, with the lowest risks in patients with PDC ≥90%, but no significant association was observed between better adherence and bleeding risk.
In this study, only 64.0% of patients were adherent to NOAC with PDC ≥80%. This low adherence rate was consistent with previous studies reporting adherence to NOAC.8,9,15–17 Most studies have described adherence to dabigatran, reporting adherence rates of 72.1% (of 5376 patients) to 88.9% (of 103 patients).8,9,17 Two recent studies from the United States investigating adherence to three NOACs including dabigatran, rivaroxaban, and apixaban showed lower adherence rates of 47.5% (of 26 471 patients) and 72.4% (of 2882 patients) than the studies with dabigatran only.15,16 Our study furthers these findings with the largest NOAC cohort ever. Our finding of suboptimal adherence highlights critical differences between real-world practices and randomized trial settings (reporting over 95% adherence) and the importance of multi-modal interventions for improving adherence.3,14
This study provides evidence of possible predictors of adherence to determine which factors can be targeted to maximize adherence. Prior stroke, chronic diseases including hypertension and dyslipidaemia, and use of statin and blood pressure-lowering medications were positively associated with adherence, reflecting that patients who had sustained cardiovascular events or high cardiovascular risks were more likely to be treatment adherent, hoping to prevent further adverse outcomes. Conversely, this implies that patients with less comorbidity who take only NOAC as daily medication might be in danger of non-adherence. Once-daily dosing is expected to have better adherence due to its simplicity; however, we found that twice-daily dosing was associated with better adherence, consistently with previous real-world investigations about NOAC adherence.15,16 Although further research is required to verify this finding, we suggest avoiding prescription of rivaroxaban only for better adherence.
Among the negative predictors of adherence, lower income could produce financial burden for regular clinic visits and prescriptions. Patients with prior intracranial haemorrhage or concomitant antiplatelet use might fear adverse bleeding events. Reduced NOAC dosing was a negative predictor. However, 49.5% of those taking reduced NOAC dose were underdosed (without any indications for dose reduction) in this study (Table 1). Non-adherent use will have a more critical negative impact on clinical outcomes of these patients with underdosing. Poorer adherence of de novo OAC starter (without prior warfarin use) was consistently observed in previous studies.8,16 The SAMe-TT2R2 score, originally developed to predict poor anticoagulation control in warfarin users, might help to identify non-adherent users to NOAC.18 Polypharmacy is becoming an increasing problem due to ageing population with multiple comorbidities and has been linked with an increased risk of adverse drug events and medication non-adherence.19 For patients with such high-risk conditions for non-adherence, specific pharmacist-based activities including pharmacist-led longer duration of monitoring and more intensive care, which were shown to be associated with greater adherence to dabigatran, may be needed.14
Our finding of decreased risks of ischaemic stroke associated with adherence is consistent with previous studies investigating the effects of discontinuation or adherent use of NOAC on clinical outcomes.8,15,16 However, in our study, adherent use of NOAC was also associated with a lower risk of myocardial infarction, although statistical significance was observed only for dabigatran among individual NOACs. For bleeding, there was no significant risk alteration by NOAC adherence for any outcome including major bleeding and intracranial or GI bleeding. These findings suggest that better adherence to NOAC should be pursued for positive net clinical benefit.
Compared with warfarin use, adherent NOAC use was associated with lower risks of all adverse events except myocardial infarction (no significant difference for myocardial infarction). In contrast, non-adherent NOAC use showed no significant risk reductions for ischaemic stroke/SE and rather had 13% increased risk of myocardial infarction compared with warfarin use. These findings suggest that, if a patient with AF was estimated or predicted to be non-adherent to NOAC, NOAC might be no longer a comparable option to warfarin in efficacy.
Although data on adherence are often reported as dichotomous variables (adherence vs. non-adherence), adherence can vary along a continuum from 0 to more than 100%, since patients sometimes take more than the prescribed amount of medication.10,20 Although most trials consider rates greater than 80% to be acceptable, there is no clinical evidence for what constitutes adequate adherence to NOAC.10 This study clearly demonstrated that all efficacy outcomes improved according to gradual increase of NOAC adherence, with the lowest risks in patients having PDC ≥90%. There was no significant increase of bleeding risk even in those with PDC ≥90%. Therefore, emphasis on a ‘higher than 90%, not 80%’ adherence to NOAC might be appropriate when educating physicians and patients.
Limitations
The present study has several limitations. First, this retrospective and non-randomized study cannot prove or disprove causal relationships. Second, we measured adherence based on dispensed prescriptions and days of supply from each dispensing, assuming dispensed medications were actually taken. This approach could overestimate drug adherence. While there are a number of direct and indirect methods to measure adherence, none is considered the gold standard because laboratory tests for concentrations of NOAC (direct methods) are currently not available and other indirect methods are not readily feasible (electronic monitors recording bottle opening) or are susceptible to misrepresentation and overestimation (using a questionnaire).10 Proportion of days covered has been validated as an accurate way of measuring drug adherence, representing real-world practice without being susceptible to social desirability and recall bias.3,4,13,15,20 Third, we were unable to determine the exact reasons for non-adherence and to distinguish temporary interruptions from permanent discontinuations, which are common limitations of such studies using claims data.8,15–17 Fourth, we did not have access to information on time in therapeutic range among warfarin users. Our comparisons between NOAC and warfarin users should be interpreted carefully. Fifth, certain clinical and health behaviour parameters, such as left ventricular ejection fraction, body mass index, physical activity, and smoking/alcohol status, are not available in this database. Sixth, such studies using administrative databases could be susceptible to errors arising from coding inaccuracies. To minimize this problem, we applied definitions that we had already validated in previous studies which used the Korean NHIS cohort.11,12 Finally, in the present study, we enrolled only patients of East Asian ancestry, and therefore, whether the results can be extrapolated to other populations remains uncertain. Despite these limitations, this study presents the largest population dataset available in the literature to investigate the relationships between adherence and cardiovascular outcomes in AF patients using the entire population of one country.
Conclusions
In real-world clinical practice, adherence to NOAC is suboptimal. Adherent use of NOAC was associated with decreased risks of major cardiovascular event, ischaemic stroke/SE, and myocardial infarction, without any alteration of bleeding risk. Moreover, the risks of these adverse outcomes were decreased in an adherence level-dependent fashion, suggesting that maintaining ≥90% of adherence optimizes the effectiveness of NOAC therapy without compromising its safety.
Conflict of interest: G.Y.H.L. has served as a consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi-Sankyo, and as a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo; no fees were directly received personally. B.J. has served as a speaker for Bayer, BMS/Pfizer, Medtronic, and Daiichi-Sankyo, and has received research funding from Medtronic and Abbott; no fees were directly received personally. And all other authors have no conflict of interest to declare.
Funding
This work was supported by a research grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology [grant number NRF-2017R1A2B3003303] and grants from the Korean Healthcare Technology R&D project funded by the Ministry of Health & Welfare [grant numbers HI16C0058, HI15C1200].
References
Author notes
Daehoon Kim and Pil-Sung Yang contributed equally to this work.
Gregory Y.H. Lip and Boyoung Joung are joint senior authors.



