-
PDF
- Split View
-
Views
-
Cite
Cite
Gesa von Olshausen, Tara Bourke, Jonas Schwieler, Nikola Drca, Hamid Bastani, Jari Tapanainen, Ott Saluveer, Lina Benson, Alexander Goedel, Göran Kennebäck, Per Insulander, Mats Jensen-Urstad, Frieder Braunschweig, Long-term outcome of patients with invasive electrophysiology procedure-related cardiac tamponade, EP Europace, Volume 22, Issue 10, October 2020, Pages 1547–1557, https://doi.org/10.1093/europace/euaa155
Close - Share Icon Share
Abstract
Iatrogenic cardiac tamponades are a rare but dreaded complication of invasive electrophysiology procedures (EPs). Their long-term impact on clinical outcomes is unknown. This study analysed the risk of death or serious cardiovascular events in patients suffering from EP-related cardiac tamponade requiring pericardiocentesis during long-term follow-up.
Out of 19 997 invasive EPs at the Karolinska University Hospital between January 1998 and September 2018, all patients with EP-related periprocedural cardiac tamponade were identified (n = 60) and matched (1:3 ratio) to a control group (n = 180). After a follow-up of 5 years, the composite primary endpoint — death from any cause, acute myocardial infarction, transitory ischaemic attack (TIA)/stroke, and hospitalization for heart failure — occurred in significantly more patients in the tamponade than in the control group [12 patients (20.0%) vs. 19 patients (10.6%); hazard ratio (HR) 2.53 (95% confidence interval, CI 1.15–5.58); P = 0.021]. This was mainly driven by a higher incidence of TIA/stroke in the tamponade than in the control group [HR 3.75 (95% CI 1.01–13.97); P = 0.049]. Death from any cause, acute myocardial infarction, and hospitalization for heart failure did not show a significant difference between the groups. Hospitalization for pericarditis occurred in significantly more patients in the tamponade than in the control group [HR 36.0 (95% CI 4.68–276.86); P = 0.001].
Patients with EP-related cardiac tamponade are at higher risk for cerebrovascular events during the first 2 weeks and hospitalization for pericarditis during the first months after index procedure. Despite the increased risk for early complications tamponade patients have a good long-term prognosis without increased risk for mortality or other serious cardiovascular events.
This is the first study reporting the clinical long-term outcome of patients with invasive electrophysiology procedure-related cardiac tamponades.
Patients with cardiac tamponade are at significantly higher risk for cerebrovascular events during the first 2 weeks as well as hospitalization for post-traumatic pericarditis during the first months after index procedure. Therefore, a close monitoring of these patients during the first months after index procedure is advisable.
Despite the increased risk for early complications cardiac tamponade patients have a good long-term prognosis without increased risk for mortality or other serious cardiovascular events.
Introduction
Catheter ablation has been established as an effective treatment of cardiac arrhythmias1–3 and the number of electrophysiology procedures (EPs) is continuously rising.4 Procedure-related complication rates are generally low5 but due to high patient volumes even rare complications occur at sizable numbers and constitute an important challenge in clinical practice. While there is comprehensive knowledge about the acute management of these complications, their potential long-term impact on clinical outcomes has not been appropriately addressed so far.
Cardiac tamponade with an overall rate of around 1% is the most frequent potentially fatal complication associated with EPs. It is particularly common after catheter ablation of atrial fibrillation as these procedures warrant transseptal puncture, extensive application of atrial ablation lesions and are performed with ongoing anticoagulation.6,7 However, cardiac tamponade may also occur during other types of invasive EPs.
Acute cardiac tamponade may cause cardiogenic shock or cardiac arrest with a risk of myocardial injury and peripheral organ damage potentially increasing the risk for future cardiovascular events as well as death. Moreover, it is suggested that bleeding triggers an inflammatory response in the pericardium which in turn may promote chronic pericardial disease.8 Early detection and treatment of cardiac tamponade via pericardiocentesis in unstable patients is necessary.9 However, pericardiocentesis itself may lead to further complications.
It is known that cardiac tamponades related to percutaneous coronary interventions are associated with a substantially increased risk for myocardial infarction and mortality.10 In contrast, information on the clinical outcome after tamponades caused by diagnostic electrophysiology and ablation procedures is sparse and limited by sample size, short follow-up or lack of relevant clinical outcome measures other than arrhythmia recurrence.11–13 Therefore, the purpose of this study was to investigate whether iatrogenic cardiac tamponades related to invasive EPs and requiring pericardiocentesis are associated with an increased risk of death or serious cardiovascular events during long-term follow-up.
Methods
Design, study population, and control group
Relevant patient characteristics and procedural details of all invasive EPs for all types of cardiac arrhythmia at the Karolinska University Hospital between January 1998 and September 2018 were prospectively collected at the time of the EP and recorded in a computerized database. Electrophysiology procedures were performed according to conventional and local standards as described previously.14,15 All patients who suffered from an invasive EP-related cardiac tamponade proven by echocardiographic imaging and requiring pericardiocentesis were included. Cardiac tamponades had to be detected during hospital stay. Patients developing a cardiac tamponade after discharge from hospital were not included. A control group not experiencing any kind of cardiac effusion was generated from the same catheter ablation cohort and matched with cardiac tamponade patients in a ratio of 1:3 based on age, gender, treated arrhythmia type at index procedure, and timepoint of index procedure (range of 5 years for the first and last criteria). The study complied with the Declaration of Helsinki. All data collection was approved by the regional ethics committee and patient data were collected in accordance with the institutional ethics guidelines.
Data collection and post-ablation follow-up
Patient-related demographics, medical history, comorbidities, procedural details, clinical course, and follow-up information (clinical endpoints, survival data, and causes of death) were derived from the electronic medical record (TakeCare, CompuGroup Medical Sweden, Uppsala, Sweden) which covers most hospitals and medical practices in Stockholm county. All patients were followed-up from the time of their index procedure until the date of death or the end of the study (30 April 2019). In four patients in the tamponade group without complete follow-up in the electronic medical record, follow-up data were completed by telephone interview and copies with relevant chart information were required from the responsible caregivers. In one patient of the tamponade group, follow-up data were only available until June 2014, i.e. 10.4 years after the index procedure.
Anticoagulant strategy
With regard to thromboembolic prophylaxis, the stroke risk in patients with atrial fibrillation was estimated using the CHADS2 scale until the European Society of Cardiology (ESC) guideline update in 2012 recommended CHA2DS2-VASc score as the preferred instrument for risk prediction. While warfarin or acetylsalicylic acid (ASA) were the recommended drugs before 2012, novel oral anticoagulants (NOACs) were preferably used from that time and ASA became obsolete.
Concerning strategies for periprocedural anticoagulation, in the first part of the study until 2006, patients requiring oral anticoagulation were treated with warfarin [therapeutic international normalized ratio (INR) 2–3] for at least 1 month before as well as for at least 2 months after the procedure. However, warfarin was discontinued 3 days before ablation and subcutaneous low-molecular-weight heparin (LMWH) was started 1 day prior to the planned operation. Low-molecular-weight heparin was not administered in the morning of the procedure. After the procedure, oral anticoagulation was reinstituted and LMWH given until a therapeutic INR was reached. From 2006, patients continued warfarin at INR 2–3 throughout the periprocedural phase. In case of subtherapeutic INR levels during the periprocedural period, LMWH was administered. The procedure was postponed if the INR exceeded 3.5 in the morning of the EP. With the ESC guideline update in 2012, NOAC became the preferred choice for thromboembolic prophylaxis. However, patients were switched to warfarin at least 1 month before the ablation procedure. With the approval of idarucizumab in 2015, NOAC patients were put on dabigatran before and after the procedure.
Regarding procedural anticoagulation during atrial fibrillation ablation, which was the most common procedure in our study, a continuous infusion plus single bolus injections of heparin were maintained to achieve an activated clotting time (ACT) of >300 s and ACT measurements were routinely done every 30 min throughout the procedure.
Agents for antagonization of anticoagulation were administered according to local standards and/or manufacturer’s instructions.
Study outcomes
The primary endpoint was defined as composite of death from any cause, acute myocardial infarction, transitory ischaemic attack (TIA), or stroke and heart failure that led to an unplanned overnight hospitalization. Transitory ischaemic attack or stroke was diagnosed during ambulatory or inpatient visit according to neurological guidelines and documented by a neurologist. Cerebrovascular events occurring within the first 2 weeks post-index procedure were defined as ‘early post-procedural TIA/strokes’.
Secondary endpoints were the single components of the primary endpoint as well as cardiovascular death as previously defined,16 planned overnight hospitalization for repeat catheter ablation, hospitalization for arrhythmia [unplanned overnight hospitalization (hospitalization for repeat catheter ablation excluded), emergency room, and ambulant cardioversion] and unplanned overnight hospitalization for pericarditis. Hospitalization for pericarditis was defined as either new admission to hospital or significant prolongation (>2 days) of index procedure-related hospital stay due to pericarditis.
Statistical analysis
The matching was performed in R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria) using the Matching package.17 All continuous variables are presented as mean ± standard deviation or median with interquartile range (IQR), were appropriate and were compared by using Student’s t-test or Mann–Whitney test, respectively, according to their distributions. Categorical variables are expressed as frequencies/percentages and were compared by χ2 tests. Clinical outcomes were examined as a time-to-first-event analysis within 5 years which was deemed a meaningful period to reflect long-term follow-up and during which time more than half of patients were still at risk. For endpoints which do not include mortality patients were censored at death. Hazard ratios (HRs) with 95% confidence intervals and P-values from Cox regression analyses, stratifying for matched pairs are provided. In case of zero events in one group, P-values from log-rank analysis are provided. In the 30 days of follow-up analysis, due to convergence problems, HRs with 95% confidence intervals and P-values from Cox regression analyses were not stratified for matched pairs. As all available tamponade patients were included, no sample size calculation was performed. All statistical tests and confidence intervals were two-sided, with a significance level of 0.05. Statistical analyses were performed using SPSS software, version 25 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics of patients with and without periprocedural cardiac tamponade
Between January 1998 and September 2018, a total of 19 997 invasive EPs were performed in 14 712 patients. In this cohort, a total of 60 patients with 61 periprocedural cardiac tamponades requiring pericardiocentesis were identified and included in the study. In one patient suffering from two tamponades during two procedures, the first procedure was considered as baseline resulting in a total of 60 tamponades for analysis. The overall procedural risk of tamponade was 0.31%. Among patients undergoing catheter ablation for atrial fibrillation the procedural risk of tamponade was 0.72% (43 tamponades among 5955 atrial fibrillation catheter ablations). The control group comprised 180 patients without any complication during the index procedure except for one arterial puncture not requiring further medical attention. In addition to the matched variables, the remaining clinical baseline characteristics of both groups were similar without any statistically significant difference (Table 1).
Baseline characteristics of patients with and without periprocedural cardiac tamponade
| Baseline characteristics . | Tamponade group (n = 60) . | Control group (n = 180) . | P-value . |
|---|---|---|---|
| Age (years)a | 61.5 ± 10.4 | 61.7 ± 10.2 | 0.882 |
| Male, n (%)a | 28 (46.7) | 84 (46.7) | 1.000 |
| BMI (kg/m2) | 26.6 ± 4.5 | 26.6 ± 4.3 | 0.973 |
| Previous AADs, n (%) | 46 (76.7) | 122 (67.8) | 0.255 |
| Structural heart disease, n (%) | 0.567 | ||
| Ischaemic cardiomyopathy | 2 (3.3) | 13 (7.2) | |
| Dilated cardiomyopathy | 3 (5.0) | 6 (3.3) | |
| Other cardiomyopathy | 2 (3.3) | 3 (1.7) | |
| CHA2DS2-VASc Score, n (%) | 0.902 | ||
| 0 | 8 (13.3) | 32 (17.8) | |
| 1 | 21 (35.0) | 60 (33.3) | |
| 2 | 13 (21.7) | 44 (24.4) | |
| ≥3 | 18 (30.0) | 44 (24.4) | |
| Congestive heart failure, n (%) | 4 (6.7) | 14 (7.8) | 0.777 |
| Arterial hypertension, n (%) | 30 (50) | 77 (42.8) | 0.369 |
| Diabetes mellitus, n (%) | 5 (8.3) | 16 (8.9) | 0.895 |
| Hyperlipidaemia, n (%) | 18 (30) | 52 (28.9) | 0.871 |
| Smoker, n (%) | 5 (8.3) | 10 (5.6) | 0.537 |
| Chronic obstructive pulmonary disease/asthma, n (%) | 8 (13.3) | 15 (8.3) | 0.310 |
| Peripheral vascular disease, n (%) | 1 (1.7) | 5 (2.8) | 0.633 |
| Previous TIA/stroke, n (%) | 7 (11.7) | 10 (5.6) | 0.143 |
| Previous pulmonary embolism, n (%) | 2 (3.3) | 3 (1.7) | 0.601 |
| Pacemaker/ICD carrier, n (%) | 3 (5.0) | 13 (7.2) | 0.423 |
| Left ventricular ejection fraction (%) | 55.9 ± 7.8 | 55.9 ± 8.1 | 0.963 |
| Oral anticoagulation/antiplatelet therapy, n (%) | 0.974 | ||
| None | 12 (20.0) | 37 (20.6) | |
| Warfarin | 39 (65.0) | 118 (65.6) | |
| NOAC | 3 (5.0) | 9 (5.0) | |
| ASA | 5 (8.3) | 11 (6.1) | |
| ASA + ADP inhibitor | 1 (1.7) | 4 (2.2) | |
| ASA + Warfarin | 0 (0.0) | 1 (0.6) |
| Baseline characteristics . | Tamponade group (n = 60) . | Control group (n = 180) . | P-value . |
|---|---|---|---|
| Age (years)a | 61.5 ± 10.4 | 61.7 ± 10.2 | 0.882 |
| Male, n (%)a | 28 (46.7) | 84 (46.7) | 1.000 |
| BMI (kg/m2) | 26.6 ± 4.5 | 26.6 ± 4.3 | 0.973 |
| Previous AADs, n (%) | 46 (76.7) | 122 (67.8) | 0.255 |
| Structural heart disease, n (%) | 0.567 | ||
| Ischaemic cardiomyopathy | 2 (3.3) | 13 (7.2) | |
| Dilated cardiomyopathy | 3 (5.0) | 6 (3.3) | |
| Other cardiomyopathy | 2 (3.3) | 3 (1.7) | |
| CHA2DS2-VASc Score, n (%) | 0.902 | ||
| 0 | 8 (13.3) | 32 (17.8) | |
| 1 | 21 (35.0) | 60 (33.3) | |
| 2 | 13 (21.7) | 44 (24.4) | |
| ≥3 | 18 (30.0) | 44 (24.4) | |
| Congestive heart failure, n (%) | 4 (6.7) | 14 (7.8) | 0.777 |
| Arterial hypertension, n (%) | 30 (50) | 77 (42.8) | 0.369 |
| Diabetes mellitus, n (%) | 5 (8.3) | 16 (8.9) | 0.895 |
| Hyperlipidaemia, n (%) | 18 (30) | 52 (28.9) | 0.871 |
| Smoker, n (%) | 5 (8.3) | 10 (5.6) | 0.537 |
| Chronic obstructive pulmonary disease/asthma, n (%) | 8 (13.3) | 15 (8.3) | 0.310 |
| Peripheral vascular disease, n (%) | 1 (1.7) | 5 (2.8) | 0.633 |
| Previous TIA/stroke, n (%) | 7 (11.7) | 10 (5.6) | 0.143 |
| Previous pulmonary embolism, n (%) | 2 (3.3) | 3 (1.7) | 0.601 |
| Pacemaker/ICD carrier, n (%) | 3 (5.0) | 13 (7.2) | 0.423 |
| Left ventricular ejection fraction (%) | 55.9 ± 7.8 | 55.9 ± 8.1 | 0.963 |
| Oral anticoagulation/antiplatelet therapy, n (%) | 0.974 | ||
| None | 12 (20.0) | 37 (20.6) | |
| Warfarin | 39 (65.0) | 118 (65.6) | |
| NOAC | 3 (5.0) | 9 (5.0) | |
| ASA | 5 (8.3) | 11 (6.1) | |
| ASA + ADP inhibitor | 1 (1.7) | 4 (2.2) | |
| ASA + Warfarin | 0 (0.0) | 1 (0.6) |
AADs, antiarrhythmic drugs; ADP, adenosine diphosphate receptor; ASA, acetylsalicylic acid; BMI, body mass index; ICD, implantable cardioverter-defibrillator; NOAC, novel oral anticoagulant; TIA, transitory ischaemic attack.
Matched variable.
Baseline characteristics of patients with and without periprocedural cardiac tamponade
| Baseline characteristics . | Tamponade group (n = 60) . | Control group (n = 180) . | P-value . |
|---|---|---|---|
| Age (years)a | 61.5 ± 10.4 | 61.7 ± 10.2 | 0.882 |
| Male, n (%)a | 28 (46.7) | 84 (46.7) | 1.000 |
| BMI (kg/m2) | 26.6 ± 4.5 | 26.6 ± 4.3 | 0.973 |
| Previous AADs, n (%) | 46 (76.7) | 122 (67.8) | 0.255 |
| Structural heart disease, n (%) | 0.567 | ||
| Ischaemic cardiomyopathy | 2 (3.3) | 13 (7.2) | |
| Dilated cardiomyopathy | 3 (5.0) | 6 (3.3) | |
| Other cardiomyopathy | 2 (3.3) | 3 (1.7) | |
| CHA2DS2-VASc Score, n (%) | 0.902 | ||
| 0 | 8 (13.3) | 32 (17.8) | |
| 1 | 21 (35.0) | 60 (33.3) | |
| 2 | 13 (21.7) | 44 (24.4) | |
| ≥3 | 18 (30.0) | 44 (24.4) | |
| Congestive heart failure, n (%) | 4 (6.7) | 14 (7.8) | 0.777 |
| Arterial hypertension, n (%) | 30 (50) | 77 (42.8) | 0.369 |
| Diabetes mellitus, n (%) | 5 (8.3) | 16 (8.9) | 0.895 |
| Hyperlipidaemia, n (%) | 18 (30) | 52 (28.9) | 0.871 |
| Smoker, n (%) | 5 (8.3) | 10 (5.6) | 0.537 |
| Chronic obstructive pulmonary disease/asthma, n (%) | 8 (13.3) | 15 (8.3) | 0.310 |
| Peripheral vascular disease, n (%) | 1 (1.7) | 5 (2.8) | 0.633 |
| Previous TIA/stroke, n (%) | 7 (11.7) | 10 (5.6) | 0.143 |
| Previous pulmonary embolism, n (%) | 2 (3.3) | 3 (1.7) | 0.601 |
| Pacemaker/ICD carrier, n (%) | 3 (5.0) | 13 (7.2) | 0.423 |
| Left ventricular ejection fraction (%) | 55.9 ± 7.8 | 55.9 ± 8.1 | 0.963 |
| Oral anticoagulation/antiplatelet therapy, n (%) | 0.974 | ||
| None | 12 (20.0) | 37 (20.6) | |
| Warfarin | 39 (65.0) | 118 (65.6) | |
| NOAC | 3 (5.0) | 9 (5.0) | |
| ASA | 5 (8.3) | 11 (6.1) | |
| ASA + ADP inhibitor | 1 (1.7) | 4 (2.2) | |
| ASA + Warfarin | 0 (0.0) | 1 (0.6) |
| Baseline characteristics . | Tamponade group (n = 60) . | Control group (n = 180) . | P-value . |
|---|---|---|---|
| Age (years)a | 61.5 ± 10.4 | 61.7 ± 10.2 | 0.882 |
| Male, n (%)a | 28 (46.7) | 84 (46.7) | 1.000 |
| BMI (kg/m2) | 26.6 ± 4.5 | 26.6 ± 4.3 | 0.973 |
| Previous AADs, n (%) | 46 (76.7) | 122 (67.8) | 0.255 |
| Structural heart disease, n (%) | 0.567 | ||
| Ischaemic cardiomyopathy | 2 (3.3) | 13 (7.2) | |
| Dilated cardiomyopathy | 3 (5.0) | 6 (3.3) | |
| Other cardiomyopathy | 2 (3.3) | 3 (1.7) | |
| CHA2DS2-VASc Score, n (%) | 0.902 | ||
| 0 | 8 (13.3) | 32 (17.8) | |
| 1 | 21 (35.0) | 60 (33.3) | |
| 2 | 13 (21.7) | 44 (24.4) | |
| ≥3 | 18 (30.0) | 44 (24.4) | |
| Congestive heart failure, n (%) | 4 (6.7) | 14 (7.8) | 0.777 |
| Arterial hypertension, n (%) | 30 (50) | 77 (42.8) | 0.369 |
| Diabetes mellitus, n (%) | 5 (8.3) | 16 (8.9) | 0.895 |
| Hyperlipidaemia, n (%) | 18 (30) | 52 (28.9) | 0.871 |
| Smoker, n (%) | 5 (8.3) | 10 (5.6) | 0.537 |
| Chronic obstructive pulmonary disease/asthma, n (%) | 8 (13.3) | 15 (8.3) | 0.310 |
| Peripheral vascular disease, n (%) | 1 (1.7) | 5 (2.8) | 0.633 |
| Previous TIA/stroke, n (%) | 7 (11.7) | 10 (5.6) | 0.143 |
| Previous pulmonary embolism, n (%) | 2 (3.3) | 3 (1.7) | 0.601 |
| Pacemaker/ICD carrier, n (%) | 3 (5.0) | 13 (7.2) | 0.423 |
| Left ventricular ejection fraction (%) | 55.9 ± 7.8 | 55.9 ± 8.1 | 0.963 |
| Oral anticoagulation/antiplatelet therapy, n (%) | 0.974 | ||
| None | 12 (20.0) | 37 (20.6) | |
| Warfarin | 39 (65.0) | 118 (65.6) | |
| NOAC | 3 (5.0) | 9 (5.0) | |
| ASA | 5 (8.3) | 11 (6.1) | |
| ASA + ADP inhibitor | 1 (1.7) | 4 (2.2) | |
| ASA + Warfarin | 0 (0.0) | 1 (0.6) |
AADs, antiarrhythmic drugs; ADP, adenosine diphosphate receptor; ASA, acetylsalicylic acid; BMI, body mass index; ICD, implantable cardioverter-defibrillator; NOAC, novel oral anticoagulant; TIA, transitory ischaemic attack.
Matched variable.
The mean duration of follow-up was 7.5 ± 4.4 years (median 6.2 years; IQR 4.1–10.2 years) in the tamponade and 7.3 ± 4.2 years (median 6.2 years; IQR 4.1–10.4 years) in the control group (P = 0.762 for mean and P = 0.796 for median follow-up).
Index procedure-related characteristics in patients with and without periprocedural cardiac tamponade
All timepoints (year) of index EPs in tamponade and control patients are provided in Supplementary material online, Table S1. In 38 patients (63.3%) of the tamponade group, the ablation procedure was stopped early due to pericardial complications—in 26 patients during ablation (43.3%) and in 12 patients (20.0%) even before ablation was started. All patients in the control group completed the ablation procedure uneventfully (P < 0.001). In the tamponade group, three patients were planned for epicardial electrophysiological procedure [due to ventricular extrasystole (VES)/ventricular tachycardia (VT)]. Of those, one patient received only epicardial puncture but no ablation (due to tamponade in association with transseptal puncture), one patient received epicardial puncture and ablation, but ablation was interrupted, and one patient received epicardial puncture and complete epicardial ablation. In the control group, no epicardial puncture/ablation was performed (P = 0.015). Steam pop which can lead to cardiac perforation only occurred in the tamponade group (3 patients vs. 0 patients, P = 0.015). Duration of hospital stay after index ablation procedure was significantly longer in the tamponade than in the control group (6.1 ± 3.8 vs. 1.8 ± 2.9 days, P < 0.001). The remainder of index procedure-related characteristics was not different between the groups as presented in Table 2.
Index procedure-related characteristics in patients with and without periprocedural cardiac tamponade
| Baseline characteristics . | Tamponade group (n = 60) . | Control group (n = 180) . | P-value . |
|---|---|---|---|
| Type of arrhythmia to be treated, n (%)a | 1.000 | ||
| Atrial fibrillation | 42 (70) | 126 (70) | |
| VES/VT | 11 (18.3) | 33 (18.3) | |
| AVNRT | 3 (5.0) | 9 (5.0) | |
| AVRT | 2 (3.3) | 6 (3.3) | |
| Atrial tachycardia | 2 (3.3) | 6 (3.3) | |
| Repeat ablation, n (%) | 22 (36.7) | 69 (38.3) | 0.879 |
| Initiated ablation but interrupted due to complications, n (%) | 26 (43.3) | 0 (0) | <0.001 |
| No ablation (early interruption) due to complications, n (%) | 12 (20.0) | 0 (0) | <0.001 |
| Epicardial puncture/electrophysiological procedureb | 3 (5.0) | 0 (0) | 0.015 |
| Type of ablation technique, n (%)c | 0.319 | ||
| PVI only | 28 (58.3) | 88 (48.9) | |
| PVI + CFAE and/or lines | 4 (8.3) | 38 (21.1) | |
| VES/VT ablation (left and right sided) | 5 (10.4) | 27 (15.0) | |
| Ablation of slow-pathway (AV node) | 3 (6.3) | 9 (5.0) | |
| Ablation of left sided accessory pathway | 2 (4.2) | 6 (3.3) | |
| EAT ablation (left and right sided) | 2 (4.2) | 6 (3.3) | |
| Diagnostic electrophysiological examination | 4 (8.3) | 6 (3.3) | |
| Energy delivery, n (%)c | 0.219 | ||
| RF energy | 40 (85.1) | 167 (92.8) | |
| Cryo-energy | 3 (6.4) | 7 (3.9) | |
| Only diagnostic procedure | 4 (8.5) | 6 (3.3) | |
| Steam pop, n (%) | 3 (5.0) | 0 (0.0) | 0.015 |
| Procedure time (min) | 191.7 ± 79.7 | 187.9 ± 71.1 | 0.729 |
| Fluoroscopy time (min) | 29.3 ± 22.8 | 27.3 ± 20.8 | 0.545 |
| Duration of hospital stay (days)d,e | 4.5 (4.0–8.0) | 1.0 (1.0–2.0) | <0.001 |
| Baseline characteristics . | Tamponade group (n = 60) . | Control group (n = 180) . | P-value . |
|---|---|---|---|
| Type of arrhythmia to be treated, n (%)a | 1.000 | ||
| Atrial fibrillation | 42 (70) | 126 (70) | |
| VES/VT | 11 (18.3) | 33 (18.3) | |
| AVNRT | 3 (5.0) | 9 (5.0) | |
| AVRT | 2 (3.3) | 6 (3.3) | |
| Atrial tachycardia | 2 (3.3) | 6 (3.3) | |
| Repeat ablation, n (%) | 22 (36.7) | 69 (38.3) | 0.879 |
| Initiated ablation but interrupted due to complications, n (%) | 26 (43.3) | 0 (0) | <0.001 |
| No ablation (early interruption) due to complications, n (%) | 12 (20.0) | 0 (0) | <0.001 |
| Epicardial puncture/electrophysiological procedureb | 3 (5.0) | 0 (0) | 0.015 |
| Type of ablation technique, n (%)c | 0.319 | ||
| PVI only | 28 (58.3) | 88 (48.9) | |
| PVI + CFAE and/or lines | 4 (8.3) | 38 (21.1) | |
| VES/VT ablation (left and right sided) | 5 (10.4) | 27 (15.0) | |
| Ablation of slow-pathway (AV node) | 3 (6.3) | 9 (5.0) | |
| Ablation of left sided accessory pathway | 2 (4.2) | 6 (3.3) | |
| EAT ablation (left and right sided) | 2 (4.2) | 6 (3.3) | |
| Diagnostic electrophysiological examination | 4 (8.3) | 6 (3.3) | |
| Energy delivery, n (%)c | 0.219 | ||
| RF energy | 40 (85.1) | 167 (92.8) | |
| Cryo-energy | 3 (6.4) | 7 (3.9) | |
| Only diagnostic procedure | 4 (8.5) | 6 (3.3) | |
| Steam pop, n (%) | 3 (5.0) | 0 (0.0) | 0.015 |
| Procedure time (min) | 191.7 ± 79.7 | 187.9 ± 71.1 | 0.729 |
| Fluoroscopy time (min) | 29.3 ± 22.8 | 27.3 ± 20.8 | 0.545 |
| Duration of hospital stay (days)d,e | 4.5 (4.0–8.0) | 1.0 (1.0–2.0) | <0.001 |
AV node, atrioventricular node; AVNRT, atrioventricular node re-entry tachycardia; AVRT, atrioventricular re-entrant tachycardia; CFAE, complex fractionated atrial electrogram; EAT, ectopic atrial tachycardia; PVI, pulmonary vein isolation; RF, radiofrequency; VES, ventricular extrasystole; VT, ventricular tachycardia.
Matched variable.
For details see text in the results.
Initiated or completed catheter ablation procedures only.
Reflects the amount of days spent in the hospital, including admissions to the day care unit.
Non-normally distributed continuous variables are expressed as median and interquartile range (25th and 75th percentile).
Index procedure-related characteristics in patients with and without periprocedural cardiac tamponade
| Baseline characteristics . | Tamponade group (n = 60) . | Control group (n = 180) . | P-value . |
|---|---|---|---|
| Type of arrhythmia to be treated, n (%)a | 1.000 | ||
| Atrial fibrillation | 42 (70) | 126 (70) | |
| VES/VT | 11 (18.3) | 33 (18.3) | |
| AVNRT | 3 (5.0) | 9 (5.0) | |
| AVRT | 2 (3.3) | 6 (3.3) | |
| Atrial tachycardia | 2 (3.3) | 6 (3.3) | |
| Repeat ablation, n (%) | 22 (36.7) | 69 (38.3) | 0.879 |
| Initiated ablation but interrupted due to complications, n (%) | 26 (43.3) | 0 (0) | <0.001 |
| No ablation (early interruption) due to complications, n (%) | 12 (20.0) | 0 (0) | <0.001 |
| Epicardial puncture/electrophysiological procedureb | 3 (5.0) | 0 (0) | 0.015 |
| Type of ablation technique, n (%)c | 0.319 | ||
| PVI only | 28 (58.3) | 88 (48.9) | |
| PVI + CFAE and/or lines | 4 (8.3) | 38 (21.1) | |
| VES/VT ablation (left and right sided) | 5 (10.4) | 27 (15.0) | |
| Ablation of slow-pathway (AV node) | 3 (6.3) | 9 (5.0) | |
| Ablation of left sided accessory pathway | 2 (4.2) | 6 (3.3) | |
| EAT ablation (left and right sided) | 2 (4.2) | 6 (3.3) | |
| Diagnostic electrophysiological examination | 4 (8.3) | 6 (3.3) | |
| Energy delivery, n (%)c | 0.219 | ||
| RF energy | 40 (85.1) | 167 (92.8) | |
| Cryo-energy | 3 (6.4) | 7 (3.9) | |
| Only diagnostic procedure | 4 (8.5) | 6 (3.3) | |
| Steam pop, n (%) | 3 (5.0) | 0 (0.0) | 0.015 |
| Procedure time (min) | 191.7 ± 79.7 | 187.9 ± 71.1 | 0.729 |
| Fluoroscopy time (min) | 29.3 ± 22.8 | 27.3 ± 20.8 | 0.545 |
| Duration of hospital stay (days)d,e | 4.5 (4.0–8.0) | 1.0 (1.0–2.0) | <0.001 |
| Baseline characteristics . | Tamponade group (n = 60) . | Control group (n = 180) . | P-value . |
|---|---|---|---|
| Type of arrhythmia to be treated, n (%)a | 1.000 | ||
| Atrial fibrillation | 42 (70) | 126 (70) | |
| VES/VT | 11 (18.3) | 33 (18.3) | |
| AVNRT | 3 (5.0) | 9 (5.0) | |
| AVRT | 2 (3.3) | 6 (3.3) | |
| Atrial tachycardia | 2 (3.3) | 6 (3.3) | |
| Repeat ablation, n (%) | 22 (36.7) | 69 (38.3) | 0.879 |
| Initiated ablation but interrupted due to complications, n (%) | 26 (43.3) | 0 (0) | <0.001 |
| No ablation (early interruption) due to complications, n (%) | 12 (20.0) | 0 (0) | <0.001 |
| Epicardial puncture/electrophysiological procedureb | 3 (5.0) | 0 (0) | 0.015 |
| Type of ablation technique, n (%)c | 0.319 | ||
| PVI only | 28 (58.3) | 88 (48.9) | |
| PVI + CFAE and/or lines | 4 (8.3) | 38 (21.1) | |
| VES/VT ablation (left and right sided) | 5 (10.4) | 27 (15.0) | |
| Ablation of slow-pathway (AV node) | 3 (6.3) | 9 (5.0) | |
| Ablation of left sided accessory pathway | 2 (4.2) | 6 (3.3) | |
| EAT ablation (left and right sided) | 2 (4.2) | 6 (3.3) | |
| Diagnostic electrophysiological examination | 4 (8.3) | 6 (3.3) | |
| Energy delivery, n (%)c | 0.219 | ||
| RF energy | 40 (85.1) | 167 (92.8) | |
| Cryo-energy | 3 (6.4) | 7 (3.9) | |
| Only diagnostic procedure | 4 (8.5) | 6 (3.3) | |
| Steam pop, n (%) | 3 (5.0) | 0 (0.0) | 0.015 |
| Procedure time (min) | 191.7 ± 79.7 | 187.9 ± 71.1 | 0.729 |
| Fluoroscopy time (min) | 29.3 ± 22.8 | 27.3 ± 20.8 | 0.545 |
| Duration of hospital stay (days)d,e | 4.5 (4.0–8.0) | 1.0 (1.0–2.0) | <0.001 |
AV node, atrioventricular node; AVNRT, atrioventricular node re-entry tachycardia; AVRT, atrioventricular re-entrant tachycardia; CFAE, complex fractionated atrial electrogram; EAT, ectopic atrial tachycardia; PVI, pulmonary vein isolation; RF, radiofrequency; VES, ventricular extrasystole; VT, ventricular tachycardia.
Matched variable.
For details see text in the results.
Initiated or completed catheter ablation procedures only.
Reflects the amount of days spent in the hospital, including admissions to the day care unit.
Non-normally distributed continuous variables are expressed as median and interquartile range (25th and 75th percentile).
Periprocedural anticoagulation
In the tamponade group, 42 patients received Warfarin or NOAC (Warfarin = 39 and NOAC = 3). In 34 out of these 42 patients, the anticoagulation was interrupted post-cardiac tamponade ranging from 0.5 to 6 days (mean duration 1.4 ± 1.5 days). Details about the management of periprocedural anticoagulation in the tamponade group are provided in Supplementary material online, Table S2. In the control group, 128 patients received Warfarin or NOAC (Warfarin = 119 and NOAC = 9). A total of 105 patients in the warfarin group had therapeutic INR levels throughout the periprocedural phase. The other 14 patients in the warfarin group received additional LMWH treatment (either Enoxaparin 200 IE/kg/day or Dalteparin 200 IE/kg/day) due to subtherapeutic INR levels during the periprocedural period. In all nine control patients receiving NOAC, dabigatran 150 mg twice daily was administered throughout the periprocedural phase. In summary, all control patients had no interruption of their therapeutic anticoagulation throughout the periprocedural phase.
Tamponade-related characteristics during index hospitalization
Tamponade-related characteristics during hospital stay are presented in Table 3. According to the inclusion criteria, all cardiac tamponades were detected and treated either during (48 patients) or within 4 h after the index procedure (1 patient after 1 h, 8 patients after 2 h, 1 patient after 2.5 h, 1 patient after 3 h, and 1 patient after 4 h). The pericardiocentesis itself was carried out successfully and without further complications in all patients except for one punctio sicca. After pericardiocentesis, the pericardial drainage was removed in two patients before leaving the EP laboratory and in 57 patients on the ward (mean duration of pericardial drainage 33.5 ± 31.7 h). Five patients were temporarily intubated, three patients required cardiopulmonary resuscitation, and three patients required cardiac surgery (details of patients undergoing cardiac surgery are provided in Supplementary material online, Table S3). Nine tamponade patients suffered from pericarditis during index procedure-related hospital stay as compared to no patient in the control group (P < 0.001). Medical treatment of pericarditis was performed with non-steroidal anti-inflammatory drugs (NSAIDs) (n = 8), steroids (n = 2), and colchicine (n = 2) either alone or in combination. In 43 tamponade patients (71.7%) anticoagulation was antagonized with protamine (n = 39), prothrombin complex concentrates (n = 31), fresh frozen plasma (n = 4), fibrinogen (n = 1), phytomenadion (n = 1), and idarucizumab (n = 2) either alone or in combination. In the control group, one patient received antagonization of anticoagulation with prothrombin complex concentrate due to a high INR.
Tamponade-related characteristics in patients with periprocedural cardiac tamponade
| Baseline characteristics . | Tamponade group (n = 60) . |
|---|---|
| Location of cardiac perforation, n (%)a | |
| Proven during cardiac surgery | |
| Left atrial appendage | 1 (1.6) |
| Right atrium | 2 (3.3) |
| Right ventricle | 1 (1.6) |
| Suspected perforation site | |
| Left atrium | 32 (52.5) |
| Right atrium | 1 (1.6) |
| Left ventricle | 4 (6.6) |
| Right ventricle | 2 (3.3) |
| Unknown perforation site | |
| During transseptal puncture | 8 (13.1) |
| Right atrium or ventricle | 8 (13.1) |
| Left or right atrium | 1 (1.6) |
| Left atrium or ventricle | 1 (1.6) |
| Size of tamponade (echocardiographic), n (%) | |
| <10 mm | 16 (26.7) |
| 10–20 mm | 39 (65.0) |
| >20 mm | 5 (8.3) |
| Mean amount of removed tamponade fluid (mL) | 541 ± 410 |
| Mean duration of pericardial drainage (h) | 33.5 ± 31.7 |
| Blood reinfusion of pericardiocentesis, n (%) | 30 (50.0) |
| Antagonization of blood-thinners, n (%) | 43 (71.7) |
| Temporary intubation, n (%) | 5 (8.3) |
| Cardiopulmonary resuscitation, n (%) | 3 (5.0) |
| Cardiac surgery, n (%) | 3 (5.0) |
| Death during hospital stay | 0 (0) |
| Pericarditis during hospital stay, n (%) | 9 (15.0) |
| Baseline characteristics . | Tamponade group (n = 60) . |
|---|---|
| Location of cardiac perforation, n (%)a | |
| Proven during cardiac surgery | |
| Left atrial appendage | 1 (1.6) |
| Right atrium | 2 (3.3) |
| Right ventricle | 1 (1.6) |
| Suspected perforation site | |
| Left atrium | 32 (52.5) |
| Right atrium | 1 (1.6) |
| Left ventricle | 4 (6.6) |
| Right ventricle | 2 (3.3) |
| Unknown perforation site | |
| During transseptal puncture | 8 (13.1) |
| Right atrium or ventricle | 8 (13.1) |
| Left or right atrium | 1 (1.6) |
| Left atrium or ventricle | 1 (1.6) |
| Size of tamponade (echocardiographic), n (%) | |
| <10 mm | 16 (26.7) |
| 10–20 mm | 39 (65.0) |
| >20 mm | 5 (8.3) |
| Mean amount of removed tamponade fluid (mL) | 541 ± 410 |
| Mean duration of pericardial drainage (h) | 33.5 ± 31.7 |
| Blood reinfusion of pericardiocentesis, n (%) | 30 (50.0) |
| Antagonization of blood-thinners, n (%) | 43 (71.7) |
| Temporary intubation, n (%) | 5 (8.3) |
| Cardiopulmonary resuscitation, n (%) | 3 (5.0) |
| Cardiac surgery, n (%) | 3 (5.0) |
| Death during hospital stay | 0 (0) |
| Pericarditis during hospital stay, n (%) | 9 (15.0) |
The total number of perforation sites exceeds the total patient number 60 since in one case two perforation sites were detected.
Tamponade-related characteristics in patients with periprocedural cardiac tamponade
| Baseline characteristics . | Tamponade group (n = 60) . |
|---|---|
| Location of cardiac perforation, n (%)a | |
| Proven during cardiac surgery | |
| Left atrial appendage | 1 (1.6) |
| Right atrium | 2 (3.3) |
| Right ventricle | 1 (1.6) |
| Suspected perforation site | |
| Left atrium | 32 (52.5) |
| Right atrium | 1 (1.6) |
| Left ventricle | 4 (6.6) |
| Right ventricle | 2 (3.3) |
| Unknown perforation site | |
| During transseptal puncture | 8 (13.1) |
| Right atrium or ventricle | 8 (13.1) |
| Left or right atrium | 1 (1.6) |
| Left atrium or ventricle | 1 (1.6) |
| Size of tamponade (echocardiographic), n (%) | |
| <10 mm | 16 (26.7) |
| 10–20 mm | 39 (65.0) |
| >20 mm | 5 (8.3) |
| Mean amount of removed tamponade fluid (mL) | 541 ± 410 |
| Mean duration of pericardial drainage (h) | 33.5 ± 31.7 |
| Blood reinfusion of pericardiocentesis, n (%) | 30 (50.0) |
| Antagonization of blood-thinners, n (%) | 43 (71.7) |
| Temporary intubation, n (%) | 5 (8.3) |
| Cardiopulmonary resuscitation, n (%) | 3 (5.0) |
| Cardiac surgery, n (%) | 3 (5.0) |
| Death during hospital stay | 0 (0) |
| Pericarditis during hospital stay, n (%) | 9 (15.0) |
| Baseline characteristics . | Tamponade group (n = 60) . |
|---|---|
| Location of cardiac perforation, n (%)a | |
| Proven during cardiac surgery | |
| Left atrial appendage | 1 (1.6) |
| Right atrium | 2 (3.3) |
| Right ventricle | 1 (1.6) |
| Suspected perforation site | |
| Left atrium | 32 (52.5) |
| Right atrium | 1 (1.6) |
| Left ventricle | 4 (6.6) |
| Right ventricle | 2 (3.3) |
| Unknown perforation site | |
| During transseptal puncture | 8 (13.1) |
| Right atrium or ventricle | 8 (13.1) |
| Left or right atrium | 1 (1.6) |
| Left atrium or ventricle | 1 (1.6) |
| Size of tamponade (echocardiographic), n (%) | |
| <10 mm | 16 (26.7) |
| 10–20 mm | 39 (65.0) |
| >20 mm | 5 (8.3) |
| Mean amount of removed tamponade fluid (mL) | 541 ± 410 |
| Mean duration of pericardial drainage (h) | 33.5 ± 31.7 |
| Blood reinfusion of pericardiocentesis, n (%) | 30 (50.0) |
| Antagonization of blood-thinners, n (%) | 43 (71.7) |
| Temporary intubation, n (%) | 5 (8.3) |
| Cardiopulmonary resuscitation, n (%) | 3 (5.0) |
| Cardiac surgery, n (%) | 3 (5.0) |
| Death during hospital stay | 0 (0) |
| Pericarditis during hospital stay, n (%) | 9 (15.0) |
The total number of perforation sites exceeds the total patient number 60 since in one case two perforation sites were detected.
Primary endpoint
After a follow-up of 5 years, the composite primary endpoint — death from any cause, acute myocardial infarction, TIA/stroke, and hospitalization for heart failure — occurred in significantly more patients in the tamponade than in the control group [12 patients (20.0%) vs. 19 patients (10.6%); HR 2.53 (95% confidence interval, CI 1.15–5.58); P = 0.021] (Table 4). The Kaplan–Meier curve of the primary endpoint is provided in Figure 1.
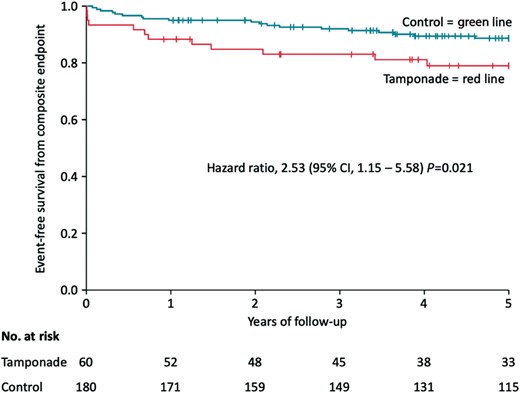
Kaplan–Meier curve showing event-free survival from the primary endpoint (death from any cause, acute myocardial infarction, TIA/stroke, hospitalization for heart failure) in the tamponade compared to the control group after a follow-up of 5 years. Day 0 is the timepoint of index procedure. CI, confidence interval; TIA, transitory ischaemic attack.
| Endpoint . | Tamponade group (n = 60), n (%) . | Control group (n = 180), n (%) . | Hazard ratio (95% CI) . | P-value Cox regression . |
|---|---|---|---|---|
| Primarya | 12 (20.0) | 19 (10.6) | 2.53 (1.15–5.58) | 0.021 |
| Secondary | ||||
| Death from any cause | 4 (6.7) | 9 (5.0) | 1.17 (0.36–3.82) | 0.795 |
| Acute myocardial infarction | 1 (1.7) | 4 (2.2) | 0.63 (0.70–5.68) | 0.681 |
| TIA/stroke | 5 (8.3) | 4 (2.2) | 3.75 (1.01–13.97) | 0.049 |
| Hospitalization for heart failure | 5 (8.3) | 9 (5.0) | 1.40 (0.46–4.23) | 0.551 |
| Cardiovascular death | 2 (3.3) | 3 (1.7) | 1.69 (0.28–10.23) | 0.565 |
| Hospitalization for repeat catheter ablation | 28 (46.7) | 50 (27.8) | 2.21 (1.32–3.70) | 0.002 |
| Hospitalization for arrhythmia | 26 (43.3) | 65 (36.1) | 1.26 (0.78–2.04) | 0.348 |
| Hospitalization for pericarditis | 12 (20.0) | 1 (0.6) | 36.00 (4.68–276.86) | 0.001 |
| Endpoint . | Tamponade group (n = 60), n (%) . | Control group (n = 180), n (%) . | Hazard ratio (95% CI) . | P-value Cox regression . |
|---|---|---|---|---|
| Primarya | 12 (20.0) | 19 (10.6) | 2.53 (1.15–5.58) | 0.021 |
| Secondary | ||||
| Death from any cause | 4 (6.7) | 9 (5.0) | 1.17 (0.36–3.82) | 0.795 |
| Acute myocardial infarction | 1 (1.7) | 4 (2.2) | 0.63 (0.70–5.68) | 0.681 |
| TIA/stroke | 5 (8.3) | 4 (2.2) | 3.75 (1.01–13.97) | 0.049 |
| Hospitalization for heart failure | 5 (8.3) | 9 (5.0) | 1.40 (0.46–4.23) | 0.551 |
| Cardiovascular death | 2 (3.3) | 3 (1.7) | 1.69 (0.28–10.23) | 0.565 |
| Hospitalization for repeat catheter ablation | 28 (46.7) | 50 (27.8) | 2.21 (1.32–3.70) | 0.002 |
| Hospitalization for arrhythmia | 26 (43.3) | 65 (36.1) | 1.26 (0.78–2.04) | 0.348 |
| Hospitalization for pericarditis | 12 (20.0) | 1 (0.6) | 36.00 (4.68–276.86) | 0.001 |
Composite of death from any cause, acute myocardial infarction, TIA/stroke and hospitalization for heart failure.
CI, confidence interval; TIA, transitory ischaemic attack.
| Endpoint . | Tamponade group (n = 60), n (%) . | Control group (n = 180), n (%) . | Hazard ratio (95% CI) . | P-value Cox regression . |
|---|---|---|---|---|
| Primarya | 12 (20.0) | 19 (10.6) | 2.53 (1.15–5.58) | 0.021 |
| Secondary | ||||
| Death from any cause | 4 (6.7) | 9 (5.0) | 1.17 (0.36–3.82) | 0.795 |
| Acute myocardial infarction | 1 (1.7) | 4 (2.2) | 0.63 (0.70–5.68) | 0.681 |
| TIA/stroke | 5 (8.3) | 4 (2.2) | 3.75 (1.01–13.97) | 0.049 |
| Hospitalization for heart failure | 5 (8.3) | 9 (5.0) | 1.40 (0.46–4.23) | 0.551 |
| Cardiovascular death | 2 (3.3) | 3 (1.7) | 1.69 (0.28–10.23) | 0.565 |
| Hospitalization for repeat catheter ablation | 28 (46.7) | 50 (27.8) | 2.21 (1.32–3.70) | 0.002 |
| Hospitalization for arrhythmia | 26 (43.3) | 65 (36.1) | 1.26 (0.78–2.04) | 0.348 |
| Hospitalization for pericarditis | 12 (20.0) | 1 (0.6) | 36.00 (4.68–276.86) | 0.001 |
| Endpoint . | Tamponade group (n = 60), n (%) . | Control group (n = 180), n (%) . | Hazard ratio (95% CI) . | P-value Cox regression . |
|---|---|---|---|---|
| Primarya | 12 (20.0) | 19 (10.6) | 2.53 (1.15–5.58) | 0.021 |
| Secondary | ||||
| Death from any cause | 4 (6.7) | 9 (5.0) | 1.17 (0.36–3.82) | 0.795 |
| Acute myocardial infarction | 1 (1.7) | 4 (2.2) | 0.63 (0.70–5.68) | 0.681 |
| TIA/stroke | 5 (8.3) | 4 (2.2) | 3.75 (1.01–13.97) | 0.049 |
| Hospitalization for heart failure | 5 (8.3) | 9 (5.0) | 1.40 (0.46–4.23) | 0.551 |
| Cardiovascular death | 2 (3.3) | 3 (1.7) | 1.69 (0.28–10.23) | 0.565 |
| Hospitalization for repeat catheter ablation | 28 (46.7) | 50 (27.8) | 2.21 (1.32–3.70) | 0.002 |
| Hospitalization for arrhythmia | 26 (43.3) | 65 (36.1) | 1.26 (0.78–2.04) | 0.348 |
| Hospitalization for pericarditis | 12 (20.0) | 1 (0.6) | 36.00 (4.68–276.86) | 0.001 |
Composite of death from any cause, acute myocardial infarction, TIA/stroke and hospitalization for heart failure.
CI, confidence interval; TIA, transitory ischaemic attack.
Secondary endpoints
The secondary endpoints death from any cause [6.7% vs. 5.0%; HR 1.17 (95% CI 0.36–3.82); P = 0.795], acute myocardial infarction [1.7% vs. 2.2%; HR 0.63 (95% CI 0.70–5.68); P = 0.681], and hospitalization for heart failure [8.3% vs. 5.0%; HR 1.40 (95% CI 0.46–4.23); P = 0.551] did not show a significant difference between the tamponade and control patients after a follow-up of 5 years (Table 4). The Kaplan–Meier curves of these endpoints are presented in Figure 2A, B, and D. Notably, neither during hospital stay nor during the first year of follow-up any patient in the tamponade group died. The first death occurred after 456 days in the tamponade and 124 days in the control group.
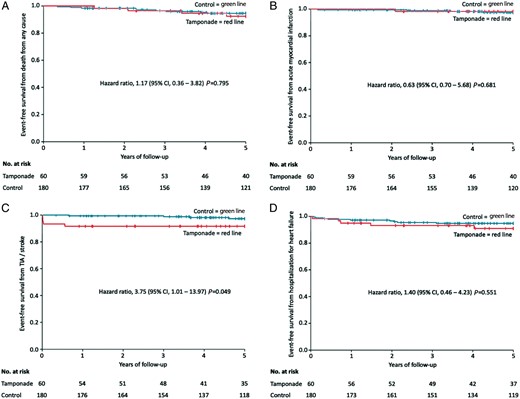
Kaplan–Meier curve showing event-free survival from (A) death from any cause, (B) acute myocardial infarction, (C) TIA/stroke, and (D) hospitalization for heart failure in the tamponade compared to the control group after a follow-up of 5 years. Day 0 is the timepoint of index procedure. CI, confidence interval; TIA, transitory ischaemic attack.
TIA or stroke occurred in significantly more patients in the tamponade than in the control group [8.3% vs. 2.2%; HR 3.75 (95% CI 1.01–13.97); P = 0.049] (Table 4). The Kaplan–Meier curve of this endpoint is provided in Figure 2C. All five events of TIA or stroke (one TIA and four strokes) in tamponade patients occurred during the first year of follow-up (after 2, 4, 5, 10, and 204 days). The patient with a stroke at 204 days after the index procedure was insufficiently anticoagulated with warfarin (INR was 1.3) despite atrial fibrillation. Among the remaining four patients (one TIA and three strokes after 2, 4, 5, and 10 days), two patients developed the cerebrovascular event after cardiac surgery [one patient undergoing diagnostic EP for VT (perforation of right atrium) and one patient with known atrial fibrillation undergoing pulmonary vein isolation + mitral line at index procedure (perforation of left atrial appendage)]. The patient with atrial fibrillation received subtherapeutic anticoagulation after cardiac surgery and before the cerebrovascular event. The other two patients underwent invasive EPs for VT (only diagnostic) and atrioventricular node re-entry tachycardia (AVNRT) but also had known atrial fibrillation. However, both only received antiplatelet therapy with ASA having a CHADS2 of 0 and 1, respectively in keeping with treatment recommendations at the time of the events (years 2002–2003). All detailed tamponade patient characteristics related to TIA/stroke during the first 10 days after index procedure are presented in Table 5. In the control group, a total of four TIA/stroke events occurred (two TIAs and two strokes). The first TIA/stroke occurred after 240 days and all other cerebrovascular events after 3 years and more.
Characteristics of patients with early post-procedural cerebrovascular events in the tamponade group
| Patient number . | Gender . | Age . | Time to cerebrovascular event after index EP (days) . | TIA or stroke (symptoms) . | To be treated arrhythmia at index EP . | Catheter technique at index EP . | Known atrial fibrillation . | Anticoagulation/antiplatelet therapy before index EP . | Agent of antagonization of anticoagulation . | Anticoagulation/antiplatelet therapy after index EP and before stroke . | Blood reinfusion of pericardiocentesis (amount) . | Cardiac surgery . | CHA2DS2-VASc score . | Congestive heart failure . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Woman | 72 | 5 | TIA (sight disorder) | VT | Epicardial puncture, failed transseptal puncture, no ablation | No | ASA 75 mg/day, one-time Ticagrelor 180 mg/day, Fondaparinux 2.5 mg/day | Protamine, fibrinogen, fresh frozen plasma | ASA 75 mg/day, Fondaparinux 2.5 mg/day | Yes (800 mL) | Yes | 3 | Yes (EF 25%) |
| 2 | Man | 65 | 4 | Stroke (sight disorder, apraxia) | VT | Only diagnostic EP | Yes | ASA 160 mg/day | None | ASA 160 mg/day | No | No | 2 | No |
| 3 | Woman | 64 | 2 | Stroke (aphasia) | Atrial fibrillation | Transseptal puncture, PVI + mitral line, ablation interrupted | Yes | Warfarin | Protamine | Dalteparin 100 IE/kg/day | Yes (750 mL) | Yes | 3 | No |
| 4 | Woman | 74 | 10 | Stroke (aphasia, right-sided impairment) | AVNRT | Modification of AV-node, ablation interrupted | Yes | ASA 160 mg/day | None | ASA 160 mg/day | No | No | 2 | No |
| Patient number . | Gender . | Age . | Time to cerebrovascular event after index EP (days) . | TIA or stroke (symptoms) . | To be treated arrhythmia at index EP . | Catheter technique at index EP . | Known atrial fibrillation . | Anticoagulation/antiplatelet therapy before index EP . | Agent of antagonization of anticoagulation . | Anticoagulation/antiplatelet therapy after index EP and before stroke . | Blood reinfusion of pericardiocentesis (amount) . | Cardiac surgery . | CHA2DS2-VASc score . | Congestive heart failure . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Woman | 72 | 5 | TIA (sight disorder) | VT | Epicardial puncture, failed transseptal puncture, no ablation | No | ASA 75 mg/day, one-time Ticagrelor 180 mg/day, Fondaparinux 2.5 mg/day | Protamine, fibrinogen, fresh frozen plasma | ASA 75 mg/day, Fondaparinux 2.5 mg/day | Yes (800 mL) | Yes | 3 | Yes (EF 25%) |
| 2 | Man | 65 | 4 | Stroke (sight disorder, apraxia) | VT | Only diagnostic EP | Yes | ASA 160 mg/day | None | ASA 160 mg/day | No | No | 2 | No |
| 3 | Woman | 64 | 2 | Stroke (aphasia) | Atrial fibrillation | Transseptal puncture, PVI + mitral line, ablation interrupted | Yes | Warfarin | Protamine | Dalteparin 100 IE/kg/day | Yes (750 mL) | Yes | 3 | No |
| 4 | Woman | 74 | 10 | Stroke (aphasia, right-sided impairment) | AVNRT | Modification of AV-node, ablation interrupted | Yes | ASA 160 mg/day | None | ASA 160 mg/day | No | No | 2 | No |
ASA, acetylsalicylic acid; AV node, atrioventricular node; AVNRT, atrioventricular node re-entry tachycardia; EF, ejection fraction; EP, electrophysiology procedure; PVI, pulmonary vein isolation; TIA, transitory ischaemic attack; VT, ventricular tachycardia.
Characteristics of patients with early post-procedural cerebrovascular events in the tamponade group
| Patient number . | Gender . | Age . | Time to cerebrovascular event after index EP (days) . | TIA or stroke (symptoms) . | To be treated arrhythmia at index EP . | Catheter technique at index EP . | Known atrial fibrillation . | Anticoagulation/antiplatelet therapy before index EP . | Agent of antagonization of anticoagulation . | Anticoagulation/antiplatelet therapy after index EP and before stroke . | Blood reinfusion of pericardiocentesis (amount) . | Cardiac surgery . | CHA2DS2-VASc score . | Congestive heart failure . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Woman | 72 | 5 | TIA (sight disorder) | VT | Epicardial puncture, failed transseptal puncture, no ablation | No | ASA 75 mg/day, one-time Ticagrelor 180 mg/day, Fondaparinux 2.5 mg/day | Protamine, fibrinogen, fresh frozen plasma | ASA 75 mg/day, Fondaparinux 2.5 mg/day | Yes (800 mL) | Yes | 3 | Yes (EF 25%) |
| 2 | Man | 65 | 4 | Stroke (sight disorder, apraxia) | VT | Only diagnostic EP | Yes | ASA 160 mg/day | None | ASA 160 mg/day | No | No | 2 | No |
| 3 | Woman | 64 | 2 | Stroke (aphasia) | Atrial fibrillation | Transseptal puncture, PVI + mitral line, ablation interrupted | Yes | Warfarin | Protamine | Dalteparin 100 IE/kg/day | Yes (750 mL) | Yes | 3 | No |
| 4 | Woman | 74 | 10 | Stroke (aphasia, right-sided impairment) | AVNRT | Modification of AV-node, ablation interrupted | Yes | ASA 160 mg/day | None | ASA 160 mg/day | No | No | 2 | No |
| Patient number . | Gender . | Age . | Time to cerebrovascular event after index EP (days) . | TIA or stroke (symptoms) . | To be treated arrhythmia at index EP . | Catheter technique at index EP . | Known atrial fibrillation . | Anticoagulation/antiplatelet therapy before index EP . | Agent of antagonization of anticoagulation . | Anticoagulation/antiplatelet therapy after index EP and before stroke . | Blood reinfusion of pericardiocentesis (amount) . | Cardiac surgery . | CHA2DS2-VASc score . | Congestive heart failure . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Woman | 72 | 5 | TIA (sight disorder) | VT | Epicardial puncture, failed transseptal puncture, no ablation | No | ASA 75 mg/day, one-time Ticagrelor 180 mg/day, Fondaparinux 2.5 mg/day | Protamine, fibrinogen, fresh frozen plasma | ASA 75 mg/day, Fondaparinux 2.5 mg/day | Yes (800 mL) | Yes | 3 | Yes (EF 25%) |
| 2 | Man | 65 | 4 | Stroke (sight disorder, apraxia) | VT | Only diagnostic EP | Yes | ASA 160 mg/day | None | ASA 160 mg/day | No | No | 2 | No |
| 3 | Woman | 64 | 2 | Stroke (aphasia) | Atrial fibrillation | Transseptal puncture, PVI + mitral line, ablation interrupted | Yes | Warfarin | Protamine | Dalteparin 100 IE/kg/day | Yes (750 mL) | Yes | 3 | No |
| 4 | Woman | 74 | 10 | Stroke (aphasia, right-sided impairment) | AVNRT | Modification of AV-node, ablation interrupted | Yes | ASA 160 mg/day | None | ASA 160 mg/day | No | No | 2 | No |
ASA, acetylsalicylic acid; AV node, atrioventricular node; AVNRT, atrioventricular node re-entry tachycardia; EF, ejection fraction; EP, electrophysiology procedure; PVI, pulmonary vein isolation; TIA, transitory ischaemic attack; VT, ventricular tachycardia.
Hospitalization for repeat catheter ablation occurred in significantly more patients in the tamponade as compared to the control group [46.7% vs. 27.8%; HR 2.21 (95% CI 1.32–3.70); P = 0.002] (Table 4). No cerebrovascular events occurred during any repeat ablation procedure. Antiarrhythmic drugs (AADs) were significantly more frequently administered to patients in the tamponade (63.3%) than in the control group (36.1%) (P < 0.001).
Hospitalization for pericarditis occurred in significantly more patients in the tamponade than in the control group [20.0% vs. 0.6%; HR 36.0 (95% CI 4.68–276.86); P = 0.001] (Table 4). The Kaplan–Meier curve of this endpoint is provided in Figure 3. All patients with hospitalization for pericarditis fulfilled the diagnostic criteria for post-cardiac injury syndrome, more specifically post-traumatic pericarditis.8,9 Incidence of clinical symptoms/signs of patients with hospitalization for pericarditis are presented in Supplementary material online, Table S4. In one tamponade patient, the pericarditis occurred after a repeat catheter ablation (237 days after index EP). All other hospitalizations for pericarditis occurred during the first four months of follow-up in both groups (median 15 days, range 1–105 days).

Kaplan–Meier curve showing event-free survival from hospitalization for pericarditis in the tamponade compared to the control group after a follow-up of 5 years. Day 0 is the timepoint of index procedure. CI, confidence interval.
The duration of pericardial drainage after the pericardiocentesis did not differ between tamponade patients with hospitalization for pericarditis (n = 12) vs. tamponade patients with no hospitalization for pericarditis (n = 48) [mean 32.2 ± 23.4 h, median 36.5 h (IQR 6.8–48.0 h) vs. mean 33.8 ± 33.7, median 23.5 h (IQR 19.3–30.0 h), P = 0.874 (mean) and P = 0.566 (median)].
Consistency analysis
In order to investigate whether the observed events are index procedure related, we performed a consistency analysis considering only the first 30 days and first year of follow-up. The results of the primary endpoint and its subcomponents from this analysis were in concordance with the results of the primary analysis with a higher relative risk of the primary endpoint in the tamponade compared to the control group [4 patients (6.7%) vs. 1 patient (0.6%); HR 12.42 (95% CI 1.39–111.13); P = 0.024 after 30 days and 7 patients (11.7%) vs. 9 patients (5.0%); HR 2.92 (95% CI 1.02–8.32); P = 0.046 after 1 year of follow-up]. All results for the primary and secondary endpoints after 30 days and 1 year of follow-up are provided in Supplementary material online, Tables S5 and S6.
In order to investigate whether the observed events are also found in the different subgroups of arrhythmia, we performed a consistency analysis only considering patients treated for atrial fibrillation as well as VES/VT. From these analyses, the results of the primary endpoint and its subcomponents after a 5-year follow-up were similar to the results of the primary analysis with a higher relative risk of the primary endpoint in the tamponade compared to the control group [6 patients (14.3%) vs. 10 patient (7.9%); HR 1.63 (95% CI 0.59–4.53); P = 0.348] in patients treated for atrial fibrillation and 4 patients (36.4%) vs. 5 patients (15.2%); HR 11.15 (95% CI 1.24–100.17); P = 0.031 in patients treated for VES/VT). All results for the primary and secondary endpoints in patients treated for atrial fibrillation and VES/VT after a 5-year follow-up are provided in Supplementary material online, Tables S7 and S8.
Discussion
Along with the increasing number of catheter ablations for the treatment of cardiac arrhythmias a small but sizable portion of patients is suffering from periprocedural iatrogenic cardiac tamponade. The short- and long-term impact of this severe complication on cardiovascular outcomes is not known. In this study, we observed that patients with invasive EP-related cardiac tamponades as compared to a well-matched control group after 5-year follow-up (i) experienced significantly more cerebrovascular events which mainly occurred during the first 2 weeks after index procedure, (ii) had a significant higher rate of hospitalization for post-traumatic pericarditis during the first months after index procedure, and (iii) underwent significantly more repeat catheter ablation procedures. However, mortality, acute myocardial infarction, and hospitalization for heart failure were not increased.
To the best of our knowledge, this is the first study reporting the clinical long-term outcome of patients with EP-related cardiac tamponades. The rate of cardiac tamponade requiring pericardiocentesis with 0.31% per ablation procedure was in the lower range compared to previous data.11 In our study, the most common procedure associated with tamponade was ablation for atrial fibrillation (70%) followed by ablation for VES or VT (18.3%) which is in concordance with previous studies.1,18 The most frequently suspected location of cardiac perforation was the left atrium related to ablation procedures of atrial fibrillation, accessory pathways, and ectopic atrial tachycardias. In many cases, transseptal puncture, required to establish catheter access to the left atrium, was the suspected cause. In addition, epicardial EPs seem to carry an increased risk for cardiac tamponades.
While cardiac tamponade in the context of ablation carries an acute mortality risk of <0.1%,6 adverse events may develop later on, for example secondary to cardiogenic shock, due to complications of evacuation, the reversal of anticoagulation or induced by inflammatory or immunological responses to pericardial bleeding.
In our study, the composite primary endpoint of death from any cause, acute coronary syndromes, cerebrovascular complications, and hospitalization for heart failure occurred in significantly more patients in the tamponade than in the control group. This was mainly driven by the increased incidence of TIA or stroke during the first 10 days. One stroke occurring after 204 days in a patient with insufficient oral anticoagulation during atrial fibrillation was not considered as procedure/complication related.
Overall, the risk of periprocedural thromboembolic complications during catheter ablation is approximately 0.5% even in the absence of tamponade.19 In the four patients with TIA or stroke within 10 days post-tamponade, several factors might have increased the risk for early post-procedural cerebrovascular events such as reversal of anticoagulation, bleeding itself, blood reinfusion, cardiac surgery, and insufficient anticoagulation in patients with atrial fibrillation. Therefore, our findings highlight that thorough adjustment of anticoagulation and awareness of an increased cerebrovascular complication risk are mandatory in the initial phase after cardiac tamponade requiring interventional evacuation. Interestingly, only one out of the four patients underwent left-sided ablation assuming that several other risk factors rather than left-sided ablation alone increased the risk for early post-procedural cerebrovascular events in the tamponade group.
Notably, of three patients in whom cardiac surgery was needed to stop pericardial bleeding, two suffered from post-operative TIA or stroke. Of those, none suffered from ventricular but atrial perforation and therefore ventricular perforation did not seem to be a risk factor for TIA/stroke in these patients. While it remains unclear whether this is related to the severity of the complication during ablation or the surgical procedure itself, it is reasonable to believe that acute heart surgery in the setting of haemodynamic compromise and reversed anticoagulation therapy carries an increased risk for cerebrovascular and other adverse events. Hence, in patients with known atrial fibrillation requiring cardiac surgery, resumption of anticoagulation as early as possible and/or concomitant left atrial appendage closure should be considered. Two patients with TIA/stroke ablated for VT and AVNRT during ‘the early years’ had concomitant atrial fibrillation but were only protected by ASA. While this was in line with clinical routine at that time, periprocedural anticoagulation management according to modern recommendations may have prevented these events.
The rate of hospitalization for pericarditis was significantly higher in the tamponade compared to the control group. Trauma and bleeding trigger inflammatory reactions in the pericardium and this phenomenon has been previously described.8 In this regard, pericardial irritation may be even more promoted by a longer time of keeping the pericardial drainage in situ. In our study, however, we did not observe a correlation of longer duration of pericardial drainage in situ and hospitalization for pericarditis. This might be due to the fact that in most of the patients the pericardial drainage was removed later on the ward and only in two patients the pericardial drainage was removed before leaving the EP laboratory. Hence, the number of pericardial drainages with short in situ duration was too small to draw a significant conclusion from it. Regarding the high incidence of hospitalization for post-traumatic pericarditis in the tamponade group, a routine application of NSAIDs, colchicine or intrapericardial administration of steroids after pericardiocentesis should be considered in order to prevent or at least attenuate the intrapericardial inflammatory reactions. In both groups, hospitalization for pericarditis occurred during the first year after index procedure (after a maximum of 105 days). Occasionally, intrapericardial inflammatory processes may lead to fibrinous scarring and the development of pericardial constriction with symptoms of heart failure. However, we did not observe a higher rate of hospitalization for heart failure after long-term follow-up of patients in the tamponade group.
Patients in the tamponade group received significantly more repeat catheter ablations and AADs than the control group. This might be explained by the fact that over half of the planned catheter ablations in the tamponade group were either interrupted or not even initiated and the underlying arrhythmia was not sufficiently treated by ablation. Hence, further catheter ablations were necessary. As ‘bridging’ therapy, AADs were often administered until the next catheter ablation. Interestingly, rates of hospitalization for arrhythmia (planned hospitalization for repeat ablation excluded) were similar in both groups.
Death from any cause, acute myocardial infarction, or hospitalization for heart failure did not show any statistical difference between both groups. Pericardial tamponades are a life-threatening condition, especially when the bleeding is very large, the pericardiocentesis fails or is performed too late.7 Although some tamponade patients in our study required a temporary intubation, cardiopulmonary resuscitation or cardiac surgery, none of the patients died during hospital stay. Moreover, we did not observe a death among the tamponade group in the first year of follow-up. These results differ from cardiac tamponades related to percutaneous coronary intervention where a substantial increased risk for myocardial infarction and mortality has been described.10 This difference can be explained by the fact that a perforation of coronary arteries bears the risk of side-branch loss with periprocedural myocardial infarction and ongoing ischaemia due to untreated coronary stenosis20 all phenomena that usually do not occur during cardiac perforation related to catheter ablation.
Limitations
Only in-hospital cardiac tamponades were included in the study. Hence, these results may not be applicable to patients with out-of-hospital tamponades which rarely occur. As only a subset of variables was available in the initial ablation database, we performed an exact matching over a propensity score matching. Despite this limitation, our matching resulted in a well-matched cohort. As all data have been collected at a single electrophysiology centre results can differ from other centres. However, this study comprises the largest patient cohort and longest follow-up regarding EP-related cardiac tamponades, so far.
Conclusion
Patients with EP-related cardiac tamponade requiring pericardiocentesis are at significantly higher risk for cerebrovascular events during the first 2 weeks and hospitalization for post-traumatic pericarditis during the first months after index procedure. Hence, an extra-careful follow-up of these patients especially during the first months after cardiac tamponade is advisable. Despite the increased risk for early complications tamponade patients have a good long-term prognosis without increased risk for mortality or other serious cardiovascular events.
Supplementary material
Supplementary material is available at Europace online.
Funding
This project was supported by the Deutsche Forschungsgemeinschaft[to G.v.O. (OL 605/1-1)and A.G. (GO 3220/1-1)].
Conflict of interest: M.J.-U. is a consultant for Medtronic and Johnson & Johnson and has received research grants from Medtronic (modest). F.B is a consultant for Medtronic and Biotronik and has received speaker fees from Biotronik, Boston Scientific, Abbott, Pfizer and Orion (modest). For the remaining authors, no conflicts of interest are declared.
Data availability
The data underlying this article cannot be shared publicly due to privacy of individuals that were investigated in the study. The data will be shared on reasonable request to the corresponding author provided that this in accordance with the institutional ethical guidelines as well as regulation and legislation.
References
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;
Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;



