-
PDF
- Split View
-
Views
-
Cite
Cite
Guillem Muntané-Carol, David del Val, Lucía Junquera, Laurent Faroux, Robert Delarochellière, Jean-Michel Paradis, Siamak Mohammadi, Dimitri Kalavrouziotis, Eric Dumont, François Philippon, Josep Rodés-Cabau, Timing and evolution of advanced conduction disturbances in patients with right bundle branch block undergoing transcatheter aortic valve replacement, EP Europace, Volume 22, Issue 10, October 2020, Pages 1537–1546, https://doi.org/10.1093/europace/euaa149
Close - Share Icon Share
Abstract
This study sought to determine the timing and evolution over time of advanced conduction disturbances (CDs) in patients with baseline right bundle branch block (RBBB) undergoing transcatheter aortic valve replacement (TAVR).
One hundred and ten consecutive patients with pre-existing RBBB were included (out of 1341, 8.2%). All arrhythmias during the hospitalization period were recorded. Follow-up was performed at 30 days, 1 year, and yearly thereafter. Conduction recovery and ventricular pacing percentage (VPP) was evaluated at 30 days in those patients with permanent pacemaker implantation (PPMI). Sixty-one (55.5%) patients suffered advanced CDs [97% complete or high-degree atrioventricular block (CHB/HAVB)], and the vast majority (98%) occurred within the first 3 days post-procedure (intraprocedural: 85%). Fifty-two (47.3%) patients had PPMI (vs. 11.0% in non-RBBB patients, P < 0.001). Ventricular pacing percentage at 1 month was higher in patients with persistent-intraprocedural CHB/HAVB compared to those with transient-intraprocedural or post-procedural CHB/HAVB [99 (interquartile range, IQR 97–100)% vs. 72 (IQR 30–99)%, P = 0.02]. Complete recovery (VPP < 1%) was observed in only one patient (2%) with CHB/HAVB. After hospital discharge, no symptomatic bradyarrhythmias or sudden death occurred within 30 days. Patients with pre-existing RBBB exhibited a higher risk of PPMI at 4-year follow-up (26% vs. 8% in non-RBBB patients, P < 0.001).
In patients with pre-existing RBBB, the vast majority of advanced CDs occurred within the 3 days following TAVR, and most did not recover at 1-month, particularly those with intra-procedural persistent CHB/HAVB. These results should help to determine the hospitalization length and timing of PPMI in RBBB patients undergoing TAVR.
Among patients with pre-existing right bundle branch block undergoing transcatheter aortic valve replacement (TAVR), complete or high-degree atrioventricular block (CHB/HAVB) occurred in 55% of patients. The vast majority (98%) of CHB/HAVB episodes appeared within the first 3 days post-procedure (intraprocedural: 85%).
Older age and pre-existing first-degree atrioventricular block were found to be independent predictors of permanent pacemaker implantation (PPMI) in this group of patients.
Among patients with PPMI, ventricular pacing percentage at 1 month was higher in patients with persistent-intraprocedural CHB/HAVB compared to those with transient-intraprocedural or post-procedural CHB/HAVB [99 (interquartile range, IQR 97–100) % vs. 72 (IQR 30–99)%, P = 0.02].
These results suggest that a strategy of relatively short hospitalization (3 days after TAVR) period in such patients would be safe. Also, early PPMI (within 24 h) following the occurrence of the bradyarrhythmic event would be a reasonable strategy.
Introduction
The advent of transcatheter aortic valve replacement (TAVR) has brought a new era in the treatment of degenerative aortic stenosis. During the last decade, TAVR has become a well-established treatment for patients at high and intermediate surgical risk, and it will likely expand towards the treatment of most patients with aortic stenosis in the near future. However, new-onset rhythm and conduction disturbances (CDs) remain the main drawback of the procedure,1 and its management is still under debate.2 Among patient-related predictors of permanent pacemaker implantation (PPMI) post-TAVR, pre-existing right bundle branch block (RBBB) has been the most consistent factor,3 resulting in PPMI rates of about 40%.4–6 In addition, prior studies have shown an increased risk of all-cause and cardiovascular mortality among TAVR recipients with pre-existing RBBB.4,7
The current trend towards a simplification of TAVR procedures including an early discharge strategy may be controversial in the subset of pre-existing RBBB patients due to the increased risk of CDs in the early phase post-TAVR. Also, two studies using continuous electrocardiographic monitoring showed that pre-existing RBBB was associated with a higher rate of delayed CDs.8,9 However, no detailed data exist regarding the timing of CDs occurrence (during initial hospitalization) in these patients. Thus, we aimed to determine the timing of CDs occurrence in patients with baseline RBBB undergoing TAVR and the degree of conduction recovery [assessing the ventricular pacing percentage (VPP)] at the first follow-up after hospital discharge.
Methods
This was a single-centre study that analysed 1359 consecutive TAVR patients from 2007 to 2019. Patients with intra-procedural death (18 patients, including 2 TAVR recipients with prior RBBB) were excluded, leading to a final study population of 1341 patients. Among them, 110 (8.2%) patients had an RBBB pre-TAVR. The electrocardiogram (ECG) was recorded at baseline, post-procedure and daily until hospital discharge. Intraventricular conduction abnormalities were classified according to the American Heart Association, American College of Cardiology Foundation and Heart Rhythm Society recommendations for standardization and interpretation of the ECG.10 At the beginning of the procedure, a temporary transvenous pacing lead was placed in the right ventricle in all patients with baseline RBBB. The diagnosis of intraprocedural complete or high-degree atrioventricular block (CHB/HAVB) was made by continuous electrocardiographic monitoring during the intervention. Right bundle branch block was defined as: (i) QRS duration greater than 120 ms, (ii) S wave of greater duration than R wave or greater than 40 ms in leads I and V6, (iii) R peak greater than50 ms in lead V1, and (iv) Rsr’, rsR’, or rSR’ in leads V1 or V2 (wide and notched R wave pattern in lead V1 and/or V2). Intraprocedural persistent complete or high-degree atrioventricular block (IP-CHB/HAVB) was defined as any procedural CHB/HAVB that persisted until the end of the TAVR procedure (minimum of 30 min from the occurrence of the CHB/HAVB episode). Intraprocedural transient complete or high-degree atrioventricular block (IT-CHB/HAVB) was defined as a HAVB that recovered before the end of the TAVR procedure. Post-procedural complete or high-degree atrioventricular block (PP-CHB/HAVB) was defined as a CHB/HAVB that occurred after the end of the TAVR procedure. PPMI was performed if CHB/HAVB occurred and was not expected to resolve or in the presence of sinus node dysfunction and documented symptomatic bradycardia, in agreement with current recommendations.11 Procedural aspects, valve type, access, and post-procedural management were at the discretion of the local heart team assessment. Baseline, procedural and follow-up data were prospectively collected. Follow-up (at 1 and 12 months, and yearly thereafter) was completed in all patients but 54 (4% of patients lost to follow-up; 0% among patients with pre-existing RBBB). Pacemaker interrogation including the VPP was obtained at 1-month follow-up. Complete CHB/HAVB recovery was defined as a percentage of ventricular stimulation <1% and clinically relevant pacemaker dependency was defined as VPP >40%.12 Clinical outcomes were defined according to Valve Academic Research Consortium-2 (VARC-2) criteria.13
Statistical analysis
Qualitative variables were reported as percentages and continuous data as mean (standard deviation) or median [interquartile range (IQR)], depending on their distribution. Continuous variables were compared using t-test (two-tailed) or Mann–Whitney rank-sum tests as appropriate. Qualitative variables were compared with χ2 or Fisher exact tests. Survival curves were summarized using Kaplan–Meier estimates, and log-rank tests were used to compare groups. A multivariate regression analysis was performed to identify independent predictors of PPMI. Variables with clinical interest and with P < 0.05 on univariate analysis were entered in a multivariate analysis. The multivariate analysis was performed using backward stepwise regression. A two-sided alpha level of 0.05 was used for all statistical testing. All statistical analyses were performed using STATA version 14.0 (StataCorp, College Station, TX, USA).
Results
Baseline and procedural characteristics of the global study population and according to the presence of RBBB are outlined in Table 1. The mean age of the patients was 79 ± 8 years, 46.1% were women, and the mean STS-PROM (Society of Thoracic Surgeons Predicted Risk of Mortality) score was 4.9% (IQR 3.4–7.6). The transfemoral approach was used in 58.8% of cases. Regarding patients with non-transfemoral approach, the transapical, transaortic, trans-subclavian, and transcarotid accesses were used in 22.2%, 6.3%, 0.2%, and 12.5% of cases, respectively. Balloon- and self-expanding transcatheter valves were used in 77% and 23% of cases, respectively.
Baseline, procedural characteristics, and in-hospital outcomes, overall and according to pre-existing RBBB
| . | Overall (n = 1341) . | RBBB (n = 110) . | Non-RBBB (n = 1231) . | P-value . |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 79 ± 8 | 79 ± 8 | 79 ± 8 | 0.693 |
| Female sex | 618 (46.1) | 36 (32.7) | 582 (47.3) | 0.003 |
| HTN | 1161 (86.6) | 101 (91.8) | 1060 (86.1) | 0.092 |
| DM | 477 (35.6) | 48 (43.6) | 429 (34.9) | 0.065 |
| History of smoking | 351 (26.2) | 34 (30.9) | 317 (25.8) | 0.238 |
| COPD | 363 (27.1) | 38 (34.6) | 325 (26.4) | 0.066 |
| Cerebrovascular disease | 188 (14.0) | 15 (13.6) | 173 (14.1) | 0.901 |
| Peripheral artery disease | 377 (28.1) | 41 (37.3) | 336 (27.3) | 0.026 |
| Coronary artery disease | 851 (63.5) | 69 (62.7) | 782 (63.6) | 0.859 |
| Atrial fibrillation or flutter | 422 (31.6) | 32 (29.1) | 390 (31.8) | 0.605 |
| Previous heart surgery | 501 (37.4) | 48 (43.6) | 453 (36.8) | 0.156 |
| Chronic renal disease (eGFR < 60 mL/min) | 684 (51.1) | 56 (50.9) | 628 (51.1) | 0.949 |
| STS-PROM | 4.9 (3.4–7.6) | 5.3 (3.7–8) | 4.9 (3.3–7.5) | 0.194 |
| Treatment at baseline | ||||
| Beta blocker | 669 (49.9) | 45 (40.9) | 624 (50.7) | 0.067 |
| Calcium channel blockers | 94 (7.0) | 10 (9.1) | 84 (6.8) | 0.372 |
| Amiodarone | 67 (5.0) | 6 (5.5) | 61 (5.0) | 0.807 |
| Digoxin | 29 (2.2) | 2 (1.8) | 27 (2.2) | 0.999 |
| Computed tomography | ||||
| Annulus area (mm2) | 436 (373–503) | 469 (408–515) | 435 (370–503) | 0.027 |
| Perimeter | 74 (69–80) | 77 (70–81) | 74 (69–80) | 0.269 |
| Agatston score | 1971 (1300–2985) | 2132 (1637–2950) | 1948 (1260–2988) | 0.021 |
| Echocardiography at baseline | ||||
| LVEF (%) | 54 ± 13 | 53 ± 12 | 54 ± 13 | 0.821 |
| Mean aortic gradient (mmHg) | 40 (31–52) | 40 (32–50) | 40 (30–52) | 0.985 |
| MR ≥3 | 301 (22.5) | 14 (12.7) | 287 (23.3) | 0.011 |
| sPAP | 39 (31–50) | 38 (29–50) | 39 (32–50) | 0.201 |
| Procedural characteristics | ||||
| Valve-in-valve | 149 (11.1) | 10 (9.1) | 139 (11.3) | 0.477 |
| Predilatation | 656 (48.9) | 54 (49.1) | 602 (48.9) | 0.970 |
| Primary access, n (%) | ||||
| Transfemoral | 788 (58.8) | 58 (52.7) | 730 (59.3) | 0.180 |
| Non-transfemoral | 553 (41.2) | 52 (47.3) | 501 (40.7) | |
| Valve type, n (%) | ||||
| Balloon-expandable | 1037 (77.4) | 90 (81.8) | 947 (77.0) | 0.246 |
| Self-expandable | 303(22.6) | 18 (18.2) | 283 (23.0) | |
| Prosthesis size, n (%) | ||||
| ≤23 mm | 442(33.0) | 23 (20.9) | 419 (34.1) | 0.005 |
| >23 mm | 898(67.0) | 84 (79.1) | 811(65.9) | |
| Post-dilatation | 257 (19.3) | 17 (15.5) | 240 (19.5) | 0.290 |
| PPMI post-TAVR | 187 (14.0) | 52 (47.3) | 135 (11.0) | <0.001 |
| Time to PPMI, median | 3 (1–5) | 2 (0–4) | 3 (1–6) | 0.001 |
| New-onset atrial fibrillation | 167 (12.5) | 13 (11.8) | 154 (12.5) | 0.826 |
| Length of stay (days) | 6 (4–10) | 6 (4–9) | 6 (4–10) | 0.718 |
| Echocardiography post-procedure | ||||
| Mean valve gradient (mmHg) | 10 (8–14) | 10 (8–13) | 10 (8–14) | 0.854 |
| Aortic valve area (cm2) | 1.5 (1.2–1.8) | 1.4 (1.2–1.8) | 1.5 (1.2–1.8) | 0.903 |
| AR ≥3 | 45 (3.4) | 4 (3.6) | 41 (3.3) | 0.832 |
| . | Overall (n = 1341) . | RBBB (n = 110) . | Non-RBBB (n = 1231) . | P-value . |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 79 ± 8 | 79 ± 8 | 79 ± 8 | 0.693 |
| Female sex | 618 (46.1) | 36 (32.7) | 582 (47.3) | 0.003 |
| HTN | 1161 (86.6) | 101 (91.8) | 1060 (86.1) | 0.092 |
| DM | 477 (35.6) | 48 (43.6) | 429 (34.9) | 0.065 |
| History of smoking | 351 (26.2) | 34 (30.9) | 317 (25.8) | 0.238 |
| COPD | 363 (27.1) | 38 (34.6) | 325 (26.4) | 0.066 |
| Cerebrovascular disease | 188 (14.0) | 15 (13.6) | 173 (14.1) | 0.901 |
| Peripheral artery disease | 377 (28.1) | 41 (37.3) | 336 (27.3) | 0.026 |
| Coronary artery disease | 851 (63.5) | 69 (62.7) | 782 (63.6) | 0.859 |
| Atrial fibrillation or flutter | 422 (31.6) | 32 (29.1) | 390 (31.8) | 0.605 |
| Previous heart surgery | 501 (37.4) | 48 (43.6) | 453 (36.8) | 0.156 |
| Chronic renal disease (eGFR < 60 mL/min) | 684 (51.1) | 56 (50.9) | 628 (51.1) | 0.949 |
| STS-PROM | 4.9 (3.4–7.6) | 5.3 (3.7–8) | 4.9 (3.3–7.5) | 0.194 |
| Treatment at baseline | ||||
| Beta blocker | 669 (49.9) | 45 (40.9) | 624 (50.7) | 0.067 |
| Calcium channel blockers | 94 (7.0) | 10 (9.1) | 84 (6.8) | 0.372 |
| Amiodarone | 67 (5.0) | 6 (5.5) | 61 (5.0) | 0.807 |
| Digoxin | 29 (2.2) | 2 (1.8) | 27 (2.2) | 0.999 |
| Computed tomography | ||||
| Annulus area (mm2) | 436 (373–503) | 469 (408–515) | 435 (370–503) | 0.027 |
| Perimeter | 74 (69–80) | 77 (70–81) | 74 (69–80) | 0.269 |
| Agatston score | 1971 (1300–2985) | 2132 (1637–2950) | 1948 (1260–2988) | 0.021 |
| Echocardiography at baseline | ||||
| LVEF (%) | 54 ± 13 | 53 ± 12 | 54 ± 13 | 0.821 |
| Mean aortic gradient (mmHg) | 40 (31–52) | 40 (32–50) | 40 (30–52) | 0.985 |
| MR ≥3 | 301 (22.5) | 14 (12.7) | 287 (23.3) | 0.011 |
| sPAP | 39 (31–50) | 38 (29–50) | 39 (32–50) | 0.201 |
| Procedural characteristics | ||||
| Valve-in-valve | 149 (11.1) | 10 (9.1) | 139 (11.3) | 0.477 |
| Predilatation | 656 (48.9) | 54 (49.1) | 602 (48.9) | 0.970 |
| Primary access, n (%) | ||||
| Transfemoral | 788 (58.8) | 58 (52.7) | 730 (59.3) | 0.180 |
| Non-transfemoral | 553 (41.2) | 52 (47.3) | 501 (40.7) | |
| Valve type, n (%) | ||||
| Balloon-expandable | 1037 (77.4) | 90 (81.8) | 947 (77.0) | 0.246 |
| Self-expandable | 303(22.6) | 18 (18.2) | 283 (23.0) | |
| Prosthesis size, n (%) | ||||
| ≤23 mm | 442(33.0) | 23 (20.9) | 419 (34.1) | 0.005 |
| >23 mm | 898(67.0) | 84 (79.1) | 811(65.9) | |
| Post-dilatation | 257 (19.3) | 17 (15.5) | 240 (19.5) | 0.290 |
| PPMI post-TAVR | 187 (14.0) | 52 (47.3) | 135 (11.0) | <0.001 |
| Time to PPMI, median | 3 (1–5) | 2 (0–4) | 3 (1–6) | 0.001 |
| New-onset atrial fibrillation | 167 (12.5) | 13 (11.8) | 154 (12.5) | 0.826 |
| Length of stay (days) | 6 (4–10) | 6 (4–9) | 6 (4–10) | 0.718 |
| Echocardiography post-procedure | ||||
| Mean valve gradient (mmHg) | 10 (8–14) | 10 (8–13) | 10 (8–14) | 0.854 |
| Aortic valve area (cm2) | 1.5 (1.2–1.8) | 1.4 (1.2–1.8) | 1.5 (1.2–1.8) | 0.903 |
| AR ≥3 | 45 (3.4) | 4 (3.6) | 41 (3.3) | 0.832 |
Values are presented as mean ± standard deviation, median and interquartile range, or n (%).
AR, aortic regurgitation; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; PPMI, permanent pacemaker implantation; RBBB, right bundle branch block; sPAP, systolic pulmonary artery pressure; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TAVR, transcatheter aortic valve replacement.
Baseline, procedural characteristics, and in-hospital outcomes, overall and according to pre-existing RBBB
| . | Overall (n = 1341) . | RBBB (n = 110) . | Non-RBBB (n = 1231) . | P-value . |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 79 ± 8 | 79 ± 8 | 79 ± 8 | 0.693 |
| Female sex | 618 (46.1) | 36 (32.7) | 582 (47.3) | 0.003 |
| HTN | 1161 (86.6) | 101 (91.8) | 1060 (86.1) | 0.092 |
| DM | 477 (35.6) | 48 (43.6) | 429 (34.9) | 0.065 |
| History of smoking | 351 (26.2) | 34 (30.9) | 317 (25.8) | 0.238 |
| COPD | 363 (27.1) | 38 (34.6) | 325 (26.4) | 0.066 |
| Cerebrovascular disease | 188 (14.0) | 15 (13.6) | 173 (14.1) | 0.901 |
| Peripheral artery disease | 377 (28.1) | 41 (37.3) | 336 (27.3) | 0.026 |
| Coronary artery disease | 851 (63.5) | 69 (62.7) | 782 (63.6) | 0.859 |
| Atrial fibrillation or flutter | 422 (31.6) | 32 (29.1) | 390 (31.8) | 0.605 |
| Previous heart surgery | 501 (37.4) | 48 (43.6) | 453 (36.8) | 0.156 |
| Chronic renal disease (eGFR < 60 mL/min) | 684 (51.1) | 56 (50.9) | 628 (51.1) | 0.949 |
| STS-PROM | 4.9 (3.4–7.6) | 5.3 (3.7–8) | 4.9 (3.3–7.5) | 0.194 |
| Treatment at baseline | ||||
| Beta blocker | 669 (49.9) | 45 (40.9) | 624 (50.7) | 0.067 |
| Calcium channel blockers | 94 (7.0) | 10 (9.1) | 84 (6.8) | 0.372 |
| Amiodarone | 67 (5.0) | 6 (5.5) | 61 (5.0) | 0.807 |
| Digoxin | 29 (2.2) | 2 (1.8) | 27 (2.2) | 0.999 |
| Computed tomography | ||||
| Annulus area (mm2) | 436 (373–503) | 469 (408–515) | 435 (370–503) | 0.027 |
| Perimeter | 74 (69–80) | 77 (70–81) | 74 (69–80) | 0.269 |
| Agatston score | 1971 (1300–2985) | 2132 (1637–2950) | 1948 (1260–2988) | 0.021 |
| Echocardiography at baseline | ||||
| LVEF (%) | 54 ± 13 | 53 ± 12 | 54 ± 13 | 0.821 |
| Mean aortic gradient (mmHg) | 40 (31–52) | 40 (32–50) | 40 (30–52) | 0.985 |
| MR ≥3 | 301 (22.5) | 14 (12.7) | 287 (23.3) | 0.011 |
| sPAP | 39 (31–50) | 38 (29–50) | 39 (32–50) | 0.201 |
| Procedural characteristics | ||||
| Valve-in-valve | 149 (11.1) | 10 (9.1) | 139 (11.3) | 0.477 |
| Predilatation | 656 (48.9) | 54 (49.1) | 602 (48.9) | 0.970 |
| Primary access, n (%) | ||||
| Transfemoral | 788 (58.8) | 58 (52.7) | 730 (59.3) | 0.180 |
| Non-transfemoral | 553 (41.2) | 52 (47.3) | 501 (40.7) | |
| Valve type, n (%) | ||||
| Balloon-expandable | 1037 (77.4) | 90 (81.8) | 947 (77.0) | 0.246 |
| Self-expandable | 303(22.6) | 18 (18.2) | 283 (23.0) | |
| Prosthesis size, n (%) | ||||
| ≤23 mm | 442(33.0) | 23 (20.9) | 419 (34.1) | 0.005 |
| >23 mm | 898(67.0) | 84 (79.1) | 811(65.9) | |
| Post-dilatation | 257 (19.3) | 17 (15.5) | 240 (19.5) | 0.290 |
| PPMI post-TAVR | 187 (14.0) | 52 (47.3) | 135 (11.0) | <0.001 |
| Time to PPMI, median | 3 (1–5) | 2 (0–4) | 3 (1–6) | 0.001 |
| New-onset atrial fibrillation | 167 (12.5) | 13 (11.8) | 154 (12.5) | 0.826 |
| Length of stay (days) | 6 (4–10) | 6 (4–9) | 6 (4–10) | 0.718 |
| Echocardiography post-procedure | ||||
| Mean valve gradient (mmHg) | 10 (8–14) | 10 (8–13) | 10 (8–14) | 0.854 |
| Aortic valve area (cm2) | 1.5 (1.2–1.8) | 1.4 (1.2–1.8) | 1.5 (1.2–1.8) | 0.903 |
| AR ≥3 | 45 (3.4) | 4 (3.6) | 41 (3.3) | 0.832 |
| . | Overall (n = 1341) . | RBBB (n = 110) . | Non-RBBB (n = 1231) . | P-value . |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 79 ± 8 | 79 ± 8 | 79 ± 8 | 0.693 |
| Female sex | 618 (46.1) | 36 (32.7) | 582 (47.3) | 0.003 |
| HTN | 1161 (86.6) | 101 (91.8) | 1060 (86.1) | 0.092 |
| DM | 477 (35.6) | 48 (43.6) | 429 (34.9) | 0.065 |
| History of smoking | 351 (26.2) | 34 (30.9) | 317 (25.8) | 0.238 |
| COPD | 363 (27.1) | 38 (34.6) | 325 (26.4) | 0.066 |
| Cerebrovascular disease | 188 (14.0) | 15 (13.6) | 173 (14.1) | 0.901 |
| Peripheral artery disease | 377 (28.1) | 41 (37.3) | 336 (27.3) | 0.026 |
| Coronary artery disease | 851 (63.5) | 69 (62.7) | 782 (63.6) | 0.859 |
| Atrial fibrillation or flutter | 422 (31.6) | 32 (29.1) | 390 (31.8) | 0.605 |
| Previous heart surgery | 501 (37.4) | 48 (43.6) | 453 (36.8) | 0.156 |
| Chronic renal disease (eGFR < 60 mL/min) | 684 (51.1) | 56 (50.9) | 628 (51.1) | 0.949 |
| STS-PROM | 4.9 (3.4–7.6) | 5.3 (3.7–8) | 4.9 (3.3–7.5) | 0.194 |
| Treatment at baseline | ||||
| Beta blocker | 669 (49.9) | 45 (40.9) | 624 (50.7) | 0.067 |
| Calcium channel blockers | 94 (7.0) | 10 (9.1) | 84 (6.8) | 0.372 |
| Amiodarone | 67 (5.0) | 6 (5.5) | 61 (5.0) | 0.807 |
| Digoxin | 29 (2.2) | 2 (1.8) | 27 (2.2) | 0.999 |
| Computed tomography | ||||
| Annulus area (mm2) | 436 (373–503) | 469 (408–515) | 435 (370–503) | 0.027 |
| Perimeter | 74 (69–80) | 77 (70–81) | 74 (69–80) | 0.269 |
| Agatston score | 1971 (1300–2985) | 2132 (1637–2950) | 1948 (1260–2988) | 0.021 |
| Echocardiography at baseline | ||||
| LVEF (%) | 54 ± 13 | 53 ± 12 | 54 ± 13 | 0.821 |
| Mean aortic gradient (mmHg) | 40 (31–52) | 40 (32–50) | 40 (30–52) | 0.985 |
| MR ≥3 | 301 (22.5) | 14 (12.7) | 287 (23.3) | 0.011 |
| sPAP | 39 (31–50) | 38 (29–50) | 39 (32–50) | 0.201 |
| Procedural characteristics | ||||
| Valve-in-valve | 149 (11.1) | 10 (9.1) | 139 (11.3) | 0.477 |
| Predilatation | 656 (48.9) | 54 (49.1) | 602 (48.9) | 0.970 |
| Primary access, n (%) | ||||
| Transfemoral | 788 (58.8) | 58 (52.7) | 730 (59.3) | 0.180 |
| Non-transfemoral | 553 (41.2) | 52 (47.3) | 501 (40.7) | |
| Valve type, n (%) | ||||
| Balloon-expandable | 1037 (77.4) | 90 (81.8) | 947 (77.0) | 0.246 |
| Self-expandable | 303(22.6) | 18 (18.2) | 283 (23.0) | |
| Prosthesis size, n (%) | ||||
| ≤23 mm | 442(33.0) | 23 (20.9) | 419 (34.1) | 0.005 |
| >23 mm | 898(67.0) | 84 (79.1) | 811(65.9) | |
| Post-dilatation | 257 (19.3) | 17 (15.5) | 240 (19.5) | 0.290 |
| PPMI post-TAVR | 187 (14.0) | 52 (47.3) | 135 (11.0) | <0.001 |
| Time to PPMI, median | 3 (1–5) | 2 (0–4) | 3 (1–6) | 0.001 |
| New-onset atrial fibrillation | 167 (12.5) | 13 (11.8) | 154 (12.5) | 0.826 |
| Length of stay (days) | 6 (4–10) | 6 (4–9) | 6 (4–10) | 0.718 |
| Echocardiography post-procedure | ||||
| Mean valve gradient (mmHg) | 10 (8–14) | 10 (8–13) | 10 (8–14) | 0.854 |
| Aortic valve area (cm2) | 1.5 (1.2–1.8) | 1.4 (1.2–1.8) | 1.5 (1.2–1.8) | 0.903 |
| AR ≥3 | 45 (3.4) | 4 (3.6) | 41 (3.3) | 0.832 |
Values are presented as mean ± standard deviation, median and interquartile range, or n (%).
AR, aortic regurgitation; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; PPMI, permanent pacemaker implantation; RBBB, right bundle branch block; sPAP, systolic pulmonary artery pressure; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TAVR, transcatheter aortic valve replacement.
A total of 110 patients (8.2%) had pre-existent RBBB and no permanent pacemaker before the TAVR procedure. Patients in the RBBB group were more frequently male, had a larger annulus area, had less frequently significant mitral regurgitation, had higher Agatston scores and received less frequently small prostheses (P < 0.03 for all). PPMI post-TAVR occurred in 52 (47.3%) and 135 (11.0%) patients in the RBBB and non-RBBB groups, respectively (P < 0.001). Median time to PPMI was shorter in RBBB compared to non-RBBB patients [2 (0–4) days vs. 3 (1–6) days; P = 0.001]. The type of PPMI was single-chamber (VVI) in 4/51 (8%) and double-chamber (DDD) in 47/51 (92%) of RBBB patients. No patient received a biventricular pacemaker. Four intra-hospital deaths occurred in RBBB patients, none of them related to arrhythmic events. The median length of stay in RBBB patients was 6 (IQR 4–9) days, with a minimal length of stay of 3 days in patients without PPMI. The PPMI rate in RBBB and non-RBBB groups according to transcatheter aortic valve generation is shown in Supplementary material online, Figure S1. The PPMI rate increased with the use of newer generation valves in both groups, reaching statistical significance in non-RBBB patients (8% vs. 14%, P = 0.001).
Among patients with pre-existing RBBB, 61 (55%) suffered significant CDs, which consisted of CHB/HAVB in the vast majority (97%) of cases. One event was related to sinus node disease (severe bradycardia at Day 3 post-TAVR) and one patient suffered extreme PR interval lengthening (>300 ms) immediately post-procedure (Supplementary material online, Figure S2).
Regarding CHB/HAVB, almost all events [58/59 (98%)] occurred within the 3 days post-TAVR and led to PPMI in 50/59 (85%) of cases (Figure 1). The timing of CHB/HAVB events was as follows: 51/59 (86%) were intraprocedural (IP-CHB/HAVB: 63%, IT-CHB/HAVB: 37%) and 8/59 (14%) post-procedural (PP-CHB/HAVB). Among PP-CHB/HAVB episodes, 1 (2%), 2 (3%), 1 (2%), 3 (5%), and 1 (2%) episodes occurred within the same day and at days 1, 2, 3, and 7 post-procedure, respectively.
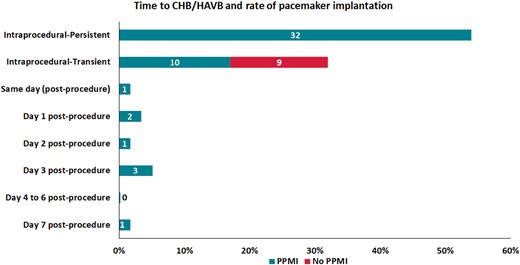
Time to bradyarrhythmic event and rate of PPMI. CHB/HAVB, complete or high-degree atrioventricular block; PPMI, permanent pacemaker implantation.
All patients with IP-CHB/HAVB had PPMI that was mostly implanted the same day of the procedure [median 0 (IQR 0–1) days after TAVR]. In patients with IT-CHB/HAVB, 10 (53%) required PPMI, implanted at 4 (IQR 2–5) days post-TAVR, and 9 (47%) had no PPMI (Figure 1). All IT-CHB/HAVB patients without PPMI suffered short episodes of intraprocedural HAVB and did not experience additional bradyarrhythmic episodes during the hospitalization period. The clinical evolution at 30 days in this group was excellent, with no new arrhythmic events or sudden death. Table 2 depicts the clinical characteristics and follow-up of patients with IT-CHB/HAVB and no PPMI. Table 1 in the Supplementary material online describes the individual characteristics of patients with IT-CHB/HAVB and PPMI during initial hospitalization.
Clinical characteristics and evolution in patients with IT-HAVB and no PPMI.
| Age . | Gender . | Valve . | Comments . | Clinical events at 30 days . | Last FU . |
|---|---|---|---|---|---|
| 71 | Female | TA Sapien 23 mm | Intrahospital death | NA | NA |
| 83 | Male | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 82 months post-procedure. No bradyarrhythmic events. |
| 79 | Male | TA Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 94 months post-procedure. No bradyarrhythmic events. |
| 83 | Male | TA Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Non-cardiovascular death 49 months post-procedure. No bradyarrhythmic events. |
| 82 | Male | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. Hospitalization due to pneumonia. | Death due to heart failure 38 months post-procedure. No bradyarrhythmic events. |
| 88 | Female | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 50 months post-procedure. No bradyarrhythmic events. |
| 89 | Female | TAo Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. Hospitalization due to NSTEMI. | Sudden death 21 months post-procedure. No previous bradyarrhythmic events. |
| 79 | Male | TAo Sapien XT 29 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 45 months post-procedure. No bradyarrhythmic events. |
| 74 | Male | TF Sapien 3 26mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 3 months post-procedure. Syncope and PPMI (3 months post-procedure). |
| Age . | Gender . | Valve . | Comments . | Clinical events at 30 days . | Last FU . |
|---|---|---|---|---|---|
| 71 | Female | TA Sapien 23 mm | Intrahospital death | NA | NA |
| 83 | Male | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 82 months post-procedure. No bradyarrhythmic events. |
| 79 | Male | TA Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 94 months post-procedure. No bradyarrhythmic events. |
| 83 | Male | TA Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Non-cardiovascular death 49 months post-procedure. No bradyarrhythmic events. |
| 82 | Male | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. Hospitalization due to pneumonia. | Death due to heart failure 38 months post-procedure. No bradyarrhythmic events. |
| 88 | Female | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 50 months post-procedure. No bradyarrhythmic events. |
| 89 | Female | TAo Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. Hospitalization due to NSTEMI. | Sudden death 21 months post-procedure. No previous bradyarrhythmic events. |
| 79 | Male | TAo Sapien XT 29 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 45 months post-procedure. No bradyarrhythmic events. |
| 74 | Male | TF Sapien 3 26mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 3 months post-procedure. Syncope and PPMI (3 months post-procedure). |
CDs, conduction disturbances; ECG, electrocardiogram; FU, follow-up; IT-HAVB, intraprocedural transient or high-degree atrioventricular block; NA, non available; NSTEMI, non-ST-elevation myocardial infarction; PPMI, permanent pacemaker implantation; TA, trans-apical; TAo, trans-aortic; TAVR, transcatheter aortic valve replacement; TF, trans-femoral.
Clinical characteristics and evolution in patients with IT-HAVB and no PPMI.
| Age . | Gender . | Valve . | Comments . | Clinical events at 30 days . | Last FU . |
|---|---|---|---|---|---|
| 71 | Female | TA Sapien 23 mm | Intrahospital death | NA | NA |
| 83 | Male | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 82 months post-procedure. No bradyarrhythmic events. |
| 79 | Male | TA Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 94 months post-procedure. No bradyarrhythmic events. |
| 83 | Male | TA Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Non-cardiovascular death 49 months post-procedure. No bradyarrhythmic events. |
| 82 | Male | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. Hospitalization due to pneumonia. | Death due to heart failure 38 months post-procedure. No bradyarrhythmic events. |
| 88 | Female | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 50 months post-procedure. No bradyarrhythmic events. |
| 89 | Female | TAo Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. Hospitalization due to NSTEMI. | Sudden death 21 months post-procedure. No previous bradyarrhythmic events. |
| 79 | Male | TAo Sapien XT 29 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 45 months post-procedure. No bradyarrhythmic events. |
| 74 | Male | TF Sapien 3 26mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 3 months post-procedure. Syncope and PPMI (3 months post-procedure). |
| Age . | Gender . | Valve . | Comments . | Clinical events at 30 days . | Last FU . |
|---|---|---|---|---|---|
| 71 | Female | TA Sapien 23 mm | Intrahospital death | NA | NA |
| 83 | Male | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 82 months post-procedure. No bradyarrhythmic events. |
| 79 | Male | TA Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 94 months post-procedure. No bradyarrhythmic events. |
| 83 | Male | TA Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Non-cardiovascular death 49 months post-procedure. No bradyarrhythmic events. |
| 82 | Male | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. Hospitalization due to pneumonia. | Death due to heart failure 38 months post-procedure. No bradyarrhythmic events. |
| 88 | Female | TF Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 50 months post-procedure. No bradyarrhythmic events. |
| 89 | Female | TAo Sapien XT 26 mm | No new ECG changes or CDs post-TAVR | Patient alive. Hospitalization due to NSTEMI. | Sudden death 21 months post-procedure. No previous bradyarrhythmic events. |
| 79 | Male | TAo Sapien XT 29 mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 45 months post-procedure. No bradyarrhythmic events. |
| 74 | Male | TF Sapien 3 26mm | No new ECG changes or CDs post-TAVR | Patient alive. No clinical events. | Patient alive. Last FU: 3 months post-procedure. Syncope and PPMI (3 months post-procedure). |
CDs, conduction disturbances; ECG, electrocardiogram; FU, follow-up; IT-HAVB, intraprocedural transient or high-degree atrioventricular block; NA, non available; NSTEMI, non-ST-elevation myocardial infarction; PPMI, permanent pacemaker implantation; TA, trans-apical; TAo, trans-aortic; TAVR, transcatheter aortic valve replacement; TF, trans-femoral.
The characteristics of the RBBB population according to PPMI during the hospitalization period are shown in Table 3. Patients who had PPMI were older (P = 0.024), had more frequently pre-existing first-degree atrioventricular block (1-AVB) (P = 0.01), and less frequently prior aortic bioprosthesis (valve-in-valve procedures, P = 0.013). No differences regarding 30-day outcomes were observed between groups, including rehospitalization or sudden death (Table 3). In the multivariate analysis depicted in Table 4, the factors associated with PPMI were older age [hazard ratio (HR) 1.07 for each increase of 1 year, 95% confidence interval (CI) 1.00–1.13, p=0.039] and pre-existing 1-AVB (HR 3.89, 95% CI 1.39–10.93, p=0.01).
Baseline, procedural characteristics, and early outcomes according to PPMI in patients with pre-existing RBBB
| . | PPMI post-TAVR (n = 52) . | Non-PPMI post-TAVR (n = 58) . | P-value . |
|---|---|---|---|
| Age | 81 ± 8 | 77 ± 9 | 0.024 |
| Female | 17 (32.7) | 19 (32.8) | 0.994 |
| HTN | 49 (94.2) | 52 (89.7) | 0.382 |
| DM | 25 (48.1) | 23 (39.6) | 0.374 |
| COPD | 15 (28.9) | 23 (39.7) | 0.234 |
| Coronary artery disease | 36 (69.2) | 33 (56.9) | 0.182 |
| Atrial fibrillation | 15 (28.9) | 17 (29.3) | 0.911 |
| STS-PROM | 6.2 (3.9–8.9) | 5.0 (3.7–7.4) | 0.250 |
| Beta blocker treatment | 20 (38.5) | 25 (43.1) | 0.625 |
| Calcium channel blockers | 5 (9.6) | 5 (8.6) | 0.999 |
| Amiodarone treatment | 2 (3.9) | 4 (6.9) | 0.683 |
| Digoxin treatment | 1 (2.0) | 1 (1.7) | 0.999 |
| ECG at baseline | |||
| Sinus rhythm | 46 (88.5) | 49 (84.5) | 0.544 |
| PR (ms) | 204 ± 50 | 182 ± 37 | 0.023 |
| 1-AVB | 21 (43.8) | 10 (20.4) | 0.014 |
| QRS (ms) | 141 ± 14 | 143 ± 15 | 0.394 |
| Bifascicular blocka | 16 (30.8) | 19 (32.8) | 0.823 |
| 1-AVB + bifascicular block | |||
| Number of patients | 6 (13.0) | 6 (12.2) | 0.907 |
| PR (ms) | 249 ± 36 | 219 ± 30 | 0.145 |
| QRS (ms) | 153 ± 16 | 153 ± 10 | 0.983 |
| Echocardiography at baseline | |||
| LVEF (%) | 55 ± 10 | 52 ± 13 | 0.164 |
| Mean gradient | 42 (33–50) | 39 (30–47) | 0.345 |
| MR ≥3 | 6 (11.5) | 8 (13.8) | 0.723 |
| PAPs | 39 (29–49) | 38 (30–50) | 0.858 |
| Procedural characteristics | |||
| Valve-in-valve | 1 (1.9) | 9 (15.5) | 0.013 |
| Predilatation | 25 (48.1) | 29 (50.0) | 0.840 |
| Primary access, n (%) | |||
| Transfemoral | 32 (61.5) | 26 (44.8) | 0.07 |
| Non-transfemoral | 20 (38.5) | 32 (55.2) | |
| Valve type, n (%) | |||
| Balloon-expandable | 40 (80.0) | 50 (86.2) | 0.388 |
| Self-expandable | 10 (20.0) | 8 (13.8) | |
| Prosthesis size, n (%) | |||
| ≤23 mm | 10 (19.6) | 13 (23.2) | 0.650 |
| >23 mm | 41 (80.4) | 43 (76.8) | |
| Post-dilatation | 8 (15.4) | 9 (15.6) | 0.985 |
| Length of stay (days) | 6 (4–10) | 6 (4–8) | 0.608 |
| Intrahospital death | 2 (3.9) | 2 (3.5) | 0.999 |
| Echocardiography post-procedure | |||
| Mean valve gradient (mmHg) | 10 (8–12) | 10 (8–14) | 0.358 |
| Aortic valve area (cm2) | 1.6 (1.3–1.8) | 1.4 (1.2–1.8) | 0.075 |
| 30-day outcomes (after discharge) | |||
| Rehospitalization | 9 (18.0) | 7 (12.5) | 0.430 |
| Rehospitalization due to bradyarrhythmic events | NA | 0 (0) | NA |
| Sudden death | 0 (0) | 0 (0) | 0.999 |
| Cardiovascular death | 0 (0) | 0 (0) | 0.999 |
| Mortality | 0 (0) | 1 (1.8) | 0.999 |
| . | PPMI post-TAVR (n = 52) . | Non-PPMI post-TAVR (n = 58) . | P-value . |
|---|---|---|---|
| Age | 81 ± 8 | 77 ± 9 | 0.024 |
| Female | 17 (32.7) | 19 (32.8) | 0.994 |
| HTN | 49 (94.2) | 52 (89.7) | 0.382 |
| DM | 25 (48.1) | 23 (39.6) | 0.374 |
| COPD | 15 (28.9) | 23 (39.7) | 0.234 |
| Coronary artery disease | 36 (69.2) | 33 (56.9) | 0.182 |
| Atrial fibrillation | 15 (28.9) | 17 (29.3) | 0.911 |
| STS-PROM | 6.2 (3.9–8.9) | 5.0 (3.7–7.4) | 0.250 |
| Beta blocker treatment | 20 (38.5) | 25 (43.1) | 0.625 |
| Calcium channel blockers | 5 (9.6) | 5 (8.6) | 0.999 |
| Amiodarone treatment | 2 (3.9) | 4 (6.9) | 0.683 |
| Digoxin treatment | 1 (2.0) | 1 (1.7) | 0.999 |
| ECG at baseline | |||
| Sinus rhythm | 46 (88.5) | 49 (84.5) | 0.544 |
| PR (ms) | 204 ± 50 | 182 ± 37 | 0.023 |
| 1-AVB | 21 (43.8) | 10 (20.4) | 0.014 |
| QRS (ms) | 141 ± 14 | 143 ± 15 | 0.394 |
| Bifascicular blocka | 16 (30.8) | 19 (32.8) | 0.823 |
| 1-AVB + bifascicular block | |||
| Number of patients | 6 (13.0) | 6 (12.2) | 0.907 |
| PR (ms) | 249 ± 36 | 219 ± 30 | 0.145 |
| QRS (ms) | 153 ± 16 | 153 ± 10 | 0.983 |
| Echocardiography at baseline | |||
| LVEF (%) | 55 ± 10 | 52 ± 13 | 0.164 |
| Mean gradient | 42 (33–50) | 39 (30–47) | 0.345 |
| MR ≥3 | 6 (11.5) | 8 (13.8) | 0.723 |
| PAPs | 39 (29–49) | 38 (30–50) | 0.858 |
| Procedural characteristics | |||
| Valve-in-valve | 1 (1.9) | 9 (15.5) | 0.013 |
| Predilatation | 25 (48.1) | 29 (50.0) | 0.840 |
| Primary access, n (%) | |||
| Transfemoral | 32 (61.5) | 26 (44.8) | 0.07 |
| Non-transfemoral | 20 (38.5) | 32 (55.2) | |
| Valve type, n (%) | |||
| Balloon-expandable | 40 (80.0) | 50 (86.2) | 0.388 |
| Self-expandable | 10 (20.0) | 8 (13.8) | |
| Prosthesis size, n (%) | |||
| ≤23 mm | 10 (19.6) | 13 (23.2) | 0.650 |
| >23 mm | 41 (80.4) | 43 (76.8) | |
| Post-dilatation | 8 (15.4) | 9 (15.6) | 0.985 |
| Length of stay (days) | 6 (4–10) | 6 (4–8) | 0.608 |
| Intrahospital death | 2 (3.9) | 2 (3.5) | 0.999 |
| Echocardiography post-procedure | |||
| Mean valve gradient (mmHg) | 10 (8–12) | 10 (8–14) | 0.358 |
| Aortic valve area (cm2) | 1.6 (1.3–1.8) | 1.4 (1.2–1.8) | 0.075 |
| 30-day outcomes (after discharge) | |||
| Rehospitalization | 9 (18.0) | 7 (12.5) | 0.430 |
| Rehospitalization due to bradyarrhythmic events | NA | 0 (0) | NA |
| Sudden death | 0 (0) | 0 (0) | 0.999 |
| Cardiovascular death | 0 (0) | 0 (0) | 0.999 |
| Mortality | 0 (0) | 1 (1.8) | 0.999 |
1-AVB, first-degree atrioventricular block; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NA, non available; PAP, pulmonary artery pressure; PPMI, permanent pacemaker implantation; RBBB, right bundle branch block; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TAVR, transcatheter aortic valve replacement.
RBBB + anterior (n = 34) or posterior (n = 1) fascicular block.
Baseline, procedural characteristics, and early outcomes according to PPMI in patients with pre-existing RBBB
| . | PPMI post-TAVR (n = 52) . | Non-PPMI post-TAVR (n = 58) . | P-value . |
|---|---|---|---|
| Age | 81 ± 8 | 77 ± 9 | 0.024 |
| Female | 17 (32.7) | 19 (32.8) | 0.994 |
| HTN | 49 (94.2) | 52 (89.7) | 0.382 |
| DM | 25 (48.1) | 23 (39.6) | 0.374 |
| COPD | 15 (28.9) | 23 (39.7) | 0.234 |
| Coronary artery disease | 36 (69.2) | 33 (56.9) | 0.182 |
| Atrial fibrillation | 15 (28.9) | 17 (29.3) | 0.911 |
| STS-PROM | 6.2 (3.9–8.9) | 5.0 (3.7–7.4) | 0.250 |
| Beta blocker treatment | 20 (38.5) | 25 (43.1) | 0.625 |
| Calcium channel blockers | 5 (9.6) | 5 (8.6) | 0.999 |
| Amiodarone treatment | 2 (3.9) | 4 (6.9) | 0.683 |
| Digoxin treatment | 1 (2.0) | 1 (1.7) | 0.999 |
| ECG at baseline | |||
| Sinus rhythm | 46 (88.5) | 49 (84.5) | 0.544 |
| PR (ms) | 204 ± 50 | 182 ± 37 | 0.023 |
| 1-AVB | 21 (43.8) | 10 (20.4) | 0.014 |
| QRS (ms) | 141 ± 14 | 143 ± 15 | 0.394 |
| Bifascicular blocka | 16 (30.8) | 19 (32.8) | 0.823 |
| 1-AVB + bifascicular block | |||
| Number of patients | 6 (13.0) | 6 (12.2) | 0.907 |
| PR (ms) | 249 ± 36 | 219 ± 30 | 0.145 |
| QRS (ms) | 153 ± 16 | 153 ± 10 | 0.983 |
| Echocardiography at baseline | |||
| LVEF (%) | 55 ± 10 | 52 ± 13 | 0.164 |
| Mean gradient | 42 (33–50) | 39 (30–47) | 0.345 |
| MR ≥3 | 6 (11.5) | 8 (13.8) | 0.723 |
| PAPs | 39 (29–49) | 38 (30–50) | 0.858 |
| Procedural characteristics | |||
| Valve-in-valve | 1 (1.9) | 9 (15.5) | 0.013 |
| Predilatation | 25 (48.1) | 29 (50.0) | 0.840 |
| Primary access, n (%) | |||
| Transfemoral | 32 (61.5) | 26 (44.8) | 0.07 |
| Non-transfemoral | 20 (38.5) | 32 (55.2) | |
| Valve type, n (%) | |||
| Balloon-expandable | 40 (80.0) | 50 (86.2) | 0.388 |
| Self-expandable | 10 (20.0) | 8 (13.8) | |
| Prosthesis size, n (%) | |||
| ≤23 mm | 10 (19.6) | 13 (23.2) | 0.650 |
| >23 mm | 41 (80.4) | 43 (76.8) | |
| Post-dilatation | 8 (15.4) | 9 (15.6) | 0.985 |
| Length of stay (days) | 6 (4–10) | 6 (4–8) | 0.608 |
| Intrahospital death | 2 (3.9) | 2 (3.5) | 0.999 |
| Echocardiography post-procedure | |||
| Mean valve gradient (mmHg) | 10 (8–12) | 10 (8–14) | 0.358 |
| Aortic valve area (cm2) | 1.6 (1.3–1.8) | 1.4 (1.2–1.8) | 0.075 |
| 30-day outcomes (after discharge) | |||
| Rehospitalization | 9 (18.0) | 7 (12.5) | 0.430 |
| Rehospitalization due to bradyarrhythmic events | NA | 0 (0) | NA |
| Sudden death | 0 (0) | 0 (0) | 0.999 |
| Cardiovascular death | 0 (0) | 0 (0) | 0.999 |
| Mortality | 0 (0) | 1 (1.8) | 0.999 |
| . | PPMI post-TAVR (n = 52) . | Non-PPMI post-TAVR (n = 58) . | P-value . |
|---|---|---|---|
| Age | 81 ± 8 | 77 ± 9 | 0.024 |
| Female | 17 (32.7) | 19 (32.8) | 0.994 |
| HTN | 49 (94.2) | 52 (89.7) | 0.382 |
| DM | 25 (48.1) | 23 (39.6) | 0.374 |
| COPD | 15 (28.9) | 23 (39.7) | 0.234 |
| Coronary artery disease | 36 (69.2) | 33 (56.9) | 0.182 |
| Atrial fibrillation | 15 (28.9) | 17 (29.3) | 0.911 |
| STS-PROM | 6.2 (3.9–8.9) | 5.0 (3.7–7.4) | 0.250 |
| Beta blocker treatment | 20 (38.5) | 25 (43.1) | 0.625 |
| Calcium channel blockers | 5 (9.6) | 5 (8.6) | 0.999 |
| Amiodarone treatment | 2 (3.9) | 4 (6.9) | 0.683 |
| Digoxin treatment | 1 (2.0) | 1 (1.7) | 0.999 |
| ECG at baseline | |||
| Sinus rhythm | 46 (88.5) | 49 (84.5) | 0.544 |
| PR (ms) | 204 ± 50 | 182 ± 37 | 0.023 |
| 1-AVB | 21 (43.8) | 10 (20.4) | 0.014 |
| QRS (ms) | 141 ± 14 | 143 ± 15 | 0.394 |
| Bifascicular blocka | 16 (30.8) | 19 (32.8) | 0.823 |
| 1-AVB + bifascicular block | |||
| Number of patients | 6 (13.0) | 6 (12.2) | 0.907 |
| PR (ms) | 249 ± 36 | 219 ± 30 | 0.145 |
| QRS (ms) | 153 ± 16 | 153 ± 10 | 0.983 |
| Echocardiography at baseline | |||
| LVEF (%) | 55 ± 10 | 52 ± 13 | 0.164 |
| Mean gradient | 42 (33–50) | 39 (30–47) | 0.345 |
| MR ≥3 | 6 (11.5) | 8 (13.8) | 0.723 |
| PAPs | 39 (29–49) | 38 (30–50) | 0.858 |
| Procedural characteristics | |||
| Valve-in-valve | 1 (1.9) | 9 (15.5) | 0.013 |
| Predilatation | 25 (48.1) | 29 (50.0) | 0.840 |
| Primary access, n (%) | |||
| Transfemoral | 32 (61.5) | 26 (44.8) | 0.07 |
| Non-transfemoral | 20 (38.5) | 32 (55.2) | |
| Valve type, n (%) | |||
| Balloon-expandable | 40 (80.0) | 50 (86.2) | 0.388 |
| Self-expandable | 10 (20.0) | 8 (13.8) | |
| Prosthesis size, n (%) | |||
| ≤23 mm | 10 (19.6) | 13 (23.2) | 0.650 |
| >23 mm | 41 (80.4) | 43 (76.8) | |
| Post-dilatation | 8 (15.4) | 9 (15.6) | 0.985 |
| Length of stay (days) | 6 (4–10) | 6 (4–8) | 0.608 |
| Intrahospital death | 2 (3.9) | 2 (3.5) | 0.999 |
| Echocardiography post-procedure | |||
| Mean valve gradient (mmHg) | 10 (8–12) | 10 (8–14) | 0.358 |
| Aortic valve area (cm2) | 1.6 (1.3–1.8) | 1.4 (1.2–1.8) | 0.075 |
| 30-day outcomes (after discharge) | |||
| Rehospitalization | 9 (18.0) | 7 (12.5) | 0.430 |
| Rehospitalization due to bradyarrhythmic events | NA | 0 (0) | NA |
| Sudden death | 0 (0) | 0 (0) | 0.999 |
| Cardiovascular death | 0 (0) | 0 (0) | 0.999 |
| Mortality | 0 (0) | 1 (1.8) | 0.999 |
1-AVB, first-degree atrioventricular block; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NA, non available; PAP, pulmonary artery pressure; PPMI, permanent pacemaker implantation; RBBB, right bundle branch block; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TAVR, transcatheter aortic valve replacement.
RBBB + anterior (n = 34) or posterior (n = 1) fascicular block.
| Variables . | Univariate model . | Multivariate model . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Age (years)a | 1.07 (1.02–1.14) | 0.009 | 1.07 (1.00–1.13) | 0.039 |
| 1-AVB at baseline | 3.33 (1.31–8.44) | 0.011 | 3.89 (1.39–10.93) | 0.010 |
| Valve-in-valve | 0.1 (0.01–0.81) | 0.031 | 0.14 (0.01–1.29) | 0.083 |
| Variables . | Univariate model . | Multivariate model . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Age (years)a | 1.07 (1.02–1.14) | 0.009 | 1.07 (1.00–1.13) | 0.039 |
| 1-AVB at baseline | 3.33 (1.31–8.44) | 0.011 | 3.89 (1.39–10.93) | 0.010 |
| Valve-in-valve | 0.1 (0.01–0.81) | 0.031 | 0.14 (0.01–1.29) | 0.083 |
1-AVB, first-degree atrioventricular block; CI, confidence interval; OR, odds ratio; PPMI, permanent pacemaker implantation; RBBB, right bundle branch block.
For each increase in 1 year.
| Variables . | Univariate model . | Multivariate model . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Age (years)a | 1.07 (1.02–1.14) | 0.009 | 1.07 (1.00–1.13) | 0.039 |
| 1-AVB at baseline | 3.33 (1.31–8.44) | 0.011 | 3.89 (1.39–10.93) | 0.010 |
| Valve-in-valve | 0.1 (0.01–0.81) | 0.031 | 0.14 (0.01–1.29) | 0.083 |
| Variables . | Univariate model . | Multivariate model . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Age (years)a | 1.07 (1.02–1.14) | 0.009 | 1.07 (1.00–1.13) | 0.039 |
| 1-AVB at baseline | 3.33 (1.31–8.44) | 0.011 | 3.89 (1.39–10.93) | 0.010 |
| Valve-in-valve | 0.1 (0.01–0.81) | 0.031 | 0.14 (0.01–1.29) | 0.083 |
1-AVB, first-degree atrioventricular block; CI, confidence interval; OR, odds ratio; PPMI, permanent pacemaker implantation; RBBB, right bundle branch block.
For each increase in 1 year.
An analysis of the VPP was performed at first follow-up post-discharge in patients with PPMI and CHB/HAVB. Patients with PPMI were divided according to the timing of occurrence of the event (IP-CHB/HAVB vs IT-CHB/HAVB/PP-CHB/HAVB). Ventricular pacing percentage was available in 41/50 (82%) of PPMI patients and was evaluated at 36 (IQR 33–48) days post-hospital discharge. The median VPP was 98 (IQR 60–99) %. Ventricular pacing percentage in patients with PPMI due to IP-CHB/HAVB was higher compared to IT-CHB/HAVB or PP-CHB/HAVB [99% (IQR 97–100%) vs. 72% (IQR 30–99%], P = 0.02)]. As depicted in Figure 2, only one patient (the initial bradyarrhythmic event was an IP-CHB/HAVB) had complete conduction recovery at 1-month follow-up. A total of 34/41 (83%) patients [22/24 (92%)] in the case of IP-HAVB) had clinically-relevant pacemaker dependency (VPP > 40%).
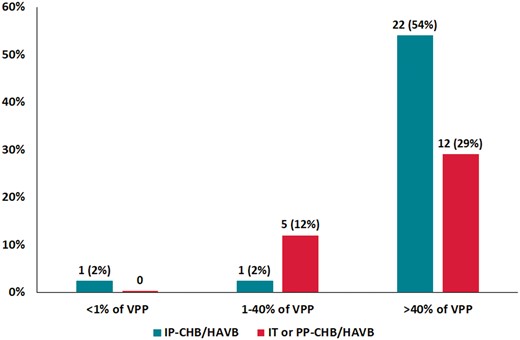
Rate of patients in each category of ventricular pacing percentage (<1%, 1–40%, and >40%) at first follow-up and according to the timing of CHB/HAVB (n = 41). The Y-axis represents the percentage of patients in each category. The X-axis classifies the patients according to VPP and the timing of CHB/HAVB (IP-CHB/HAVB and IT or PP CHB/HAVB). CHB/HAVB, complete or high-degree atrioventricular block; IP-CHB/HAVB, intraprocedural persistent complete or high-degree atrioventricular block; IT or PP CHB/HAVB, intraprocedural transient or post-procedural complete or high-degree atrioventricular block; VPP, ventricular pacing percentage.
After discharge, 9/48 (19%) patients with baseline RBBB had PPMI after a median follow-up of 17 months (IQR 12–22 months), with an annual rate of 7% (95% CI 4–13%). No patient had PPMI within the 30 days following hospital discharge. Table 5 shows the main individual characteristics of the nine patients with PPMI during the follow-up period.
| Age (years) (sex) . | Valve type . | Timing of PPMI or ICD (days) . | Reasons for PPMI or ICD . |
|---|---|---|---|
| 74 (male) | Sapien 3 | 88 | Syncope. ECG with RBBB (QRS 140 ms). |
| 84 (male) | Sapien | 342 | Asymptomatic HAVB. |
| 81 (male) | Sapien | 359 | Pre-syncope (3 episodes). ECG with 1-AVB + bifascicular block. |
| 82 (female) | Sapien XT | 505 | Pre-syncope. CHB. |
| 68 (male) | Sapien 3 | 505 | Syncope. ECG with bifascicular block. |
| 80 (female) | Sapien XT | 658 | Syncope. CHB. |
| 83 (male) | Sapien XT | 775 | Syncope. ECG with bifascicular block. |
| 66 (male) | Symetis | 950 | Asymptomatic. Sinus pauses during hospitalization for heart failure. ECG with bifascicular block. |
| 85 (female) | Sapien | 1197 | Syncope. ECG with bifascicular block. |
| Age (years) (sex) . | Valve type . | Timing of PPMI or ICD (days) . | Reasons for PPMI or ICD . |
|---|---|---|---|
| 74 (male) | Sapien 3 | 88 | Syncope. ECG with RBBB (QRS 140 ms). |
| 84 (male) | Sapien | 342 | Asymptomatic HAVB. |
| 81 (male) | Sapien | 359 | Pre-syncope (3 episodes). ECG with 1-AVB + bifascicular block. |
| 82 (female) | Sapien XT | 505 | Pre-syncope. CHB. |
| 68 (male) | Sapien 3 | 505 | Syncope. ECG with bifascicular block. |
| 80 (female) | Sapien XT | 658 | Syncope. CHB. |
| 83 (male) | Sapien XT | 775 | Syncope. ECG with bifascicular block. |
| 66 (male) | Symetis | 950 | Asymptomatic. Sinus pauses during hospitalization for heart failure. ECG with bifascicular block. |
| 85 (female) | Sapien | 1197 | Syncope. ECG with bifascicular block. |
1-AVB, first-degree atrioventricular block; CHB, complete heart block; ECG, electrocardiogram; HAVB, high-degree atrioventricular block; ICD, implantable cardioverter-defibrillator; PPMI, permanent pacemaker implantation.
| Age (years) (sex) . | Valve type . | Timing of PPMI or ICD (days) . | Reasons for PPMI or ICD . |
|---|---|---|---|
| 74 (male) | Sapien 3 | 88 | Syncope. ECG with RBBB (QRS 140 ms). |
| 84 (male) | Sapien | 342 | Asymptomatic HAVB. |
| 81 (male) | Sapien | 359 | Pre-syncope (3 episodes). ECG with 1-AVB + bifascicular block. |
| 82 (female) | Sapien XT | 505 | Pre-syncope. CHB. |
| 68 (male) | Sapien 3 | 505 | Syncope. ECG with bifascicular block. |
| 80 (female) | Sapien XT | 658 | Syncope. CHB. |
| 83 (male) | Sapien XT | 775 | Syncope. ECG with bifascicular block. |
| 66 (male) | Symetis | 950 | Asymptomatic. Sinus pauses during hospitalization for heart failure. ECG with bifascicular block. |
| 85 (female) | Sapien | 1197 | Syncope. ECG with bifascicular block. |
| Age (years) (sex) . | Valve type . | Timing of PPMI or ICD (days) . | Reasons for PPMI or ICD . |
|---|---|---|---|
| 74 (male) | Sapien 3 | 88 | Syncope. ECG with RBBB (QRS 140 ms). |
| 84 (male) | Sapien | 342 | Asymptomatic HAVB. |
| 81 (male) | Sapien | 359 | Pre-syncope (3 episodes). ECG with 1-AVB + bifascicular block. |
| 82 (female) | Sapien XT | 505 | Pre-syncope. CHB. |
| 68 (male) | Sapien 3 | 505 | Syncope. ECG with bifascicular block. |
| 80 (female) | Sapien XT | 658 | Syncope. CHB. |
| 83 (male) | Sapien XT | 775 | Syncope. ECG with bifascicular block. |
| 66 (male) | Symetis | 950 | Asymptomatic. Sinus pauses during hospitalization for heart failure. ECG with bifascicular block. |
| 85 (female) | Sapien | 1197 | Syncope. ECG with bifascicular block. |
1-AVB, first-degree atrioventricular block; CHB, complete heart block; ECG, electrocardiogram; HAVB, high-degree atrioventricular block; ICD, implantable cardioverter-defibrillator; PPMI, permanent pacemaker implantation.
The Kaplan–Meier curves up to 4 years post-procedure regarding PPMI post-discharge are depicted in Figure 3. Patients with pre-existing RBBB and no PPMI during the hospitalization period exhibited a higher incidence of PPMI at follow-up compared to non-RBBB patients (cumulative rate 26% vs. 8%, log-rank P < 0.001).
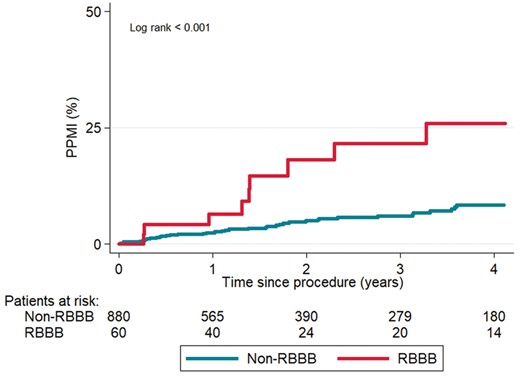
Kaplan-Meier curves of PPMI during follow-up, according to pre-existing RBBB. PPMI, permanent pacemaker implantation; RBBB, right bundle branch block.
Discussion
The main results of the study can be summarized as follows: (i) the vast majority of bradyarrhythmic events in RBBB patients undergoing TAVR consisted of CHB/HAVB and occurred within the first 3 days post-procedure (most of them -85%- intraprocedural); (ii) among patients requiring PPMI (47%), a very low rate of conduction recovery and high VPP were observed in the first follow-up post-discharge, particularly in patients with IP-CHB/HAVB; (iii) In the early phase after hospital discharge (30-day follow-up), no episodes of symptomatic bradyarrhythmia or sudden death were observed among RBBB patients with no in-hospital PPMI. However, up to one out of four of these patients required PPMI at 4-year follow-up.
TAVR therapy has become nowadays a straightforward procedure. The successive iterations in transcatheter heart valve systems, the growing experience of the operators and the increasing rate of moderate- to low-risk patients undergoing TAVR have translated into a reduction of the majority of periprocedural complications. Thus, the adoption of a ‘minimalist’ approach with a short length of stay (24–48 h) has been progressively implemented. However, CDs post-TAVR, particularly in high-risk subsets of patients like those with prior RBBB could hamper this strategy. In this regard, a recent scientific expert panel2 recommended to maintain a temporary pacing wire 24 h along with telemetry and daily ECG for a minimum of 2 days post-procedure in RBBB patients. After this initial period, if no ECG changes (e.g. PR prolongation or QRS widening) or significant bradyarrhythmias occurred within the 2–3 days following the procedure the patient could be discharged. Also, it was suggested that continuous ECG monitoring systems (up to 4 weeks) could be considered after the hospitalization period. This strategy was recommended without published data regarding the timing of bradyarrhythmic events in these patients and should be interpreted as an experts’ opinion. The results from the present study showing that almost all CHB/HAVB episodes (98%) occurred within the 3 days following the procedure add new data to the field and suggest that a strategy including a minimum hospitalization period of 3 days post-procedure may be safe in this setting. Of note, our study showed no episodes of symptomatic bradyarrhythmic events or sudden death occurring in the first 30 days following hospital discharge. In this direction, two recent studies analysed the usefulness and safety of 30-day ECG monitoring in all TAVR patients (with and without RBBB) following hospital discharge.8,9 Up to 8% of patients suffered delayed CHB/HAVB within the first 30 days post-TAVR. The episodes were asymptomatic in most cases and, in accordance with our results, no mortality at 30 days was reported. Thus, the occurrence of early silent bradyarrhythmic events could not be completely excluded in our cohort. In addition, baseline RBBB was identified as an independent factor of delayed HAVB/CHB.8 Finally, no data regarding the length of stay was reported in these studies. Larger multicentre studies are needed to elucidate the utility of ECG monitoring in the challenging conundrum of late CDs post-TAVR, especially in patients at higher risk as those with baseline RBBB.
Close to one-half of RBBB patients had PPMI during the post-TAVR hospitalization period. This high PPMI rate is consistent across the vast majority of TAVR studies1 and one may wonder if a preventive indication of PPMI could be implemented in this context. Older age and pre-existing 1-AVB increased the risk of PPMI during the hospitalization period and the presence of such factors, likely indicating a more impaired conduction system, should further enhance the awareness about the potential occurrence of severe bradyarrhythmias in RBBB patients. Also, future studies should determine the potential usefulness of prolonged continuous ECG monitoring in this high-risk group. Interestingly, promising recent data has been reported on valve type and valve positioning optimization to reduce CDs post-TAVR, including patients with pre-existing RBBB.6,14 Thus, while awaiting for definitive data to determine the optimal management of RBBB patients undergoing TAVR, avoiding preventive PPMI, a hospital stay no shorter than 3 days, and continuous ECG monitoring at hospital discharge seems to be a safe option in such patients.
Conduction recovery in the follow-up period may occur in patients with PPMI post-TAVR. Nevertheless, baseline RBBB has been identified as a factor predicting the persistence of AV block at follow-up.15,16 Our findings confirm that conduction recovery is unlikely in RBBB patients, especially in the case of IP-CHB/HAVB.12 While current guidelines11 still recommend a 7-day waiting period before PPMI, the present data suggest that early PPMI (within 24 h post-TAVR) is reasonable in patients with IP-CHB/HAVB and pre-existing RBBB. Also, considering that most patients exhibited a very high VPP at follow-up, resynchronization therapy might be considered in patients with significant ventricular dysfunction.
The present study showed high rates of PPMI during follow-up among patients with baseline RBBB (and no periprocedural PPMI) compared to the rest of TAVR patients. The annual PPMI rate of 7% was 14-fold higher than the ∼0.5% rate observed in elderly people (≥75 years) from a population-based observational study,17 and was also significantly higher compared to the 1–2% rate from a non-TAVR population with bundle branch block.18 Moreover, data from a recent publication19 showed an annual rate of PPMI of 1.1% in patients without ECG-CDs post-TAVR, also lower compared to RBBB patients in the present cohort. It is known that degenerative aortic stenosis per se can have a deleterious effect on the conduction system20 and a more rapid progression of CDs in this high-risk population may be expected. However, one may wonder if a delayed late interaction between the transcatheter heart valve and the conduction system may contribute to PPMI at follow-up. More studies with longer follow-up are needed to better understand late bradyarrhythmic events post-TAVR. Meanwhile, a closer follow-up would be advisable in this subset of patients. Unfortunately, the relatively small cohort of patients hindered the identification of clinical factors associated with bradyarrhythmic events leading to PPMI at follow-up.
Study limitations
Although data were recorded prospectively, this analysis was of retrospective nature. No event adjudication committee was available, nor echocardiographic and ECG core laboratories. Due to the retrospective nature of the study, it was not possible to standardize the pacemaker programming algorithm. In addition, 18% of patients did not have available VPP at 1-month follow-up. In the absence of definite recommendations guiding the management of CDs after TAVR, the final decision regarding PPMI was made by the cardiologist responsible for the patient (along with the EEP team), and this may have contributed to some degree of variability regarding the timing/indications of PPMI during the hospitalization period. In the absence of a standardized definition for persistent vs. transient intraprocedural CHB/HAVB, we proposed an arbitrary definition taken into consideration the persistence of the CHB/HAVB at the end of the procedure (with a minimum duration of 30 min), but this will need further validation in future studies. Also, the exact timing of the CHB/HAVB event was not recorded, and this precluded the possibility of providing the exact duration of the event during the procedure. Finally, ECG-monitoring post-discharge was not used in any RBBB patient during the study period.
Conclusion
In conclusion, baseline RBBB was found in close to 1 out of 10 TAVR recipients and close to half of them required PPMI during the initial hospitalization. The fact that the vast majority of severe bradyarrhythmic events occurred within the initial 3 days following the procedure along with the lack of severe symptomatic arrhythmias or sudden death within the month following the procedure (after hospital discharge) would support a strategy of a relatively short hospitalization (3 days after TAVR) period in such patients. Also, the extremely low conduction recovery rate and high VPP in most patients at 1-month follow-up suggest that early PPMI (within 24 h) following the occurrence of the bradyarrhythmic event would be a reasonable strategy. Future studies are needed to determine the potential usefulness of prolonged continuous ECG monitoring following hospital discharge in this high-risk group of patients.
Supplementary material
Supplementary material is available at Europace online.
Funding
G.M.-C., L.J., and D.d.V. are supported by a research grant from the Fundación Alfonso Martín Escudero (Madrid, Spain). J.R.-C. holds the Research Chair ‘Fondation Famille Jacques Larivière’ for the Development of Structural Heart Disease Interventions.
Conflict of interest: J.R.-C. has received institutional research grants from Medtronic and Edwards Lifesciences. The rest of authors have not reported any potential conflict of interest with respect to this article.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013;



