-
PDF
- Split View
-
Views
-
Cite
Cite
Raphaël P Martins, Nathalie Behar, Vincent Galand, Adeline Basquin, Jean-Claude Daubert, Philippe Mabo, Dominique Pavin, Christophe Leclercq, Radiofrequency ablation of right ventricular tachycardia in patients with no femoral access: safety and efficacy of a superior approach, EP Europace, Volume 21, Issue 5, May 2019, Pages 803–809, https://doi.org/10.1093/europace/euy298
Close - Share Icon Share
Abstract
Ventricular tachycardia (VT) ablation has been proven to be effective and safe to avoid arrhythmia recurrences in patients with repaired congenital heart disease (CHD). However, some of these patients may present right ventricular (RV) access issues [agenesia or thrombosis of inferior vena cava (IVC)], making impossible to access the right ventricle through an inferior approach. In such patients, only a superior approach would theoretically be feasible.
All VT ablations performed through a jugular or subclavian approach in CHD patients between 2012 and 2017 were included. Among 247 patients scheduled for VT ablation, two patients underwent three VT ablation procedures via a superior approach for due to the inability to access the right ventricle through a conventional IVC access (IVC interruption with azygos continuation in one patient and IVC thrombosis in the other). Ablation was performed using a three-dimensional system through a superior approach, using a subclavian access in both cases. A redo ablation had to be performed in the first patient using a jugular approach. Large curve catheters were used to facilitate RV outflow tract access. Supposed critical isthmuses could be localized and ablated. Patients remained free from arrhythmias during follow-up.
In patients with repaired CHD and ‘no femoral access’, ablation of RV tachycardia can be performed using a subclavian or a jugular approach. Mapping may be challenging, requiring large curve catheters. Conventional isthmuses can be mapped and ablated successfully, and such patients should not be denied radiofrequency ablation.
Some patients with congenital heart disease may present right ventricular access issues [agenesia or thrombosis of inferior vena cava (IVC)], making impossible to access the right ventricle through a femoral approach.
Critical isthmuses can be localized and ablated using large curve catheters through the subclavian or the jugular vein.
Patients with ‘no femoral access’ should not be denied radiofrequency ablation.
Introduction
Ventricular tachycardia (VT) ablation has been proven to be effective and safe to avoid arrhythmia recurrences in patients with repaired congenital heart disease (CHD). However, some of these patients may present inferior vena cava (IVC) access issues, making impossible to enter the right ventricle through an inferior approach. In such patients, only a superior approach would theoretically be feasible.
Methods
All VT ablations between 2012 and 2017 at the CHU Rennes were reviewed. All VT procedures performed using a superior approach in CHD patients were included. Procedure time, radiofrequency time, electroanatomical substrate and activation maps, endpoints, complications and follow-up were analysed. Ablation was performed using a conventional 3.5 mm ablation catheter (SmartTouch, Biosense Webster) with a mean power of 30–40 W and an irrigation at 17 mL/min during 60–90 s. Follow-up data were collected using data from patients’ general practitioner and cardiologist, hospital files, and device interrogations. The patients were routinely seen at our institution at 3 and 6 months. The patients described in this manuscript gave their inform consent for publication and the study was approved by the Institutional Committee on Human Research of our institution.
Results
A total of 247 patients underwent a VT ablation procedure at CHU Rennes from 2012 to 2017. A subset of two patients underwent three VT ablation procedures via a superior approach for due to the inability to access the right ventricle through a conventional IVC access. We describe here the technique used and the procedural characteristics in both cases.
Case 1
A 37-year-old woman with a complex CHD was referred for a well-tolerated VT. The CHD associated double outlet right ventricle (DORV), abnormal pulmonary vein connection (right pulmonary veins draining into the right atrium), pulmonary valve stenosis, intra-abdominal situs inversus, left-subclavian vein, and IVC interruption with azygos continuation (heterotaxy syndrome). At 10 years of age, a Blalock–Taussig shunt was performed. In February 2015, aged 37 years, she had a full repair of the DORV with enlargement of the pulmonary tract with a transannular patch, implantation of a bioprosthetic pulmonary valve, ventricular septal defect (VSD) closure with construction of a ventricular tunnel connecting the left ventricle to the aorta, correction of partial anomalous pulmonary veins connection, and implantation of an epicardial dual chamber pacemaker for a post-operative large right bundle branch block and sinus node dysfunction.
In October 2015, she had sudden onset palpitations, but only went to the emergency department 20 h later. The electrocardiogram (ECG) at admission showed a broad QRS complex tachycardia with positive concordance and inferior axis at 200 b.p.m. suggestive of VT (Supplementary material online, Figure S1A). After amiodarone failure, the patient was cardioverted and sinus rhythm obtained (Supplementary material online, Figure S1B). Beta-blockers were initiated, but badly tolerated by the patient, and a VT ablation was scheduled. Since the patient had IVC agenesis with azygos continuation, a superior approach was decided using a right subclavian approach.
Procedural data are described in Table 1. Under general anaesthesia, a single subclavian access was obtained, and two catheters were alternatively used to map the right ventricle, a F-curve Pentaray and a 3.5 mm tip SmartTouch bidirectional large curve F-J (Biosense-Webster Inc., Diamond Bar, CA, USA). First, a right ventricular (RV) high density voltage map was performed (Figure1A and B). A large scar surrounded by a low-voltage area was observed in the anterior aspect of the RV outflow tract (RVOT) and the interventricular septum, at the location of the transannular enlargement patch of the RVOT and of the VSD closure patch, respectively. Pace-mapping was performed in multiple points all-around the RVOT and the VSD patch but a perfect matching to the clinical VT was not obtained.

Right ventricular voltage map performed during the first procedure (A: antero-posterior view and B: postero-anterior view) demonstrating the presence of a large scar surrounded by a low-voltage area in the anterior aspect of the RVOT and the interventricular septum, at the location of the transannular enlargement patch of the RVOT (arrow) and of the VSD closure patch (star), respectively. The activation map of the non-clinical VT induced (right postero-lateral view) is shown in C. The entire cycle could not be mapped in the RV (local activation time from −203 to + 138 ms, i.e. 341 ms for a VT cycle length of 428 ms). (D) Fluoroscopic antero-posterior view showing the ablation catheter entering the RV through a superior approach (arrows). The bioprosthetic pulmonic valve (star) and the epicardial pacemaker leads (arrowheads) can be seen in the image. RV, right ventricle; RVOT, right ventricular outflow tract; VSD, ventricular septal defect; VT, ventricular tachycardia.
| Procedures . | Age . | CHD . | RV access route . | LV access . | Multielectrode catheter . | Points for the RV voltage map . | Points for the activation map during VT . | Contact force during ablation (g) . | Successful procedure . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Double outlet right ventricle | Right subclavian vein | No | Pentaray | 3391 | 1182 RV | 11.4 ± 2.2 | No |
| 2 | Right jugular vein | Yes | Pentaray | 1000 | 851 RV, 651 LV, and 133 aortic cusps | 14.7 ± 3.9 | Yes | ||
| 3 | 34 | Transposition of the great arteries | Right subclavian vein | No | Duodecapolar | 695 | 0 | 14.8 ± 5.4 | Yes |
| Procedures . | Age . | CHD . | RV access route . | LV access . | Multielectrode catheter . | Points for the RV voltage map . | Points for the activation map during VT . | Contact force during ablation (g) . | Successful procedure . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Double outlet right ventricle | Right subclavian vein | No | Pentaray | 3391 | 1182 RV | 11.4 ± 2.2 | No |
| 2 | Right jugular vein | Yes | Pentaray | 1000 | 851 RV, 651 LV, and 133 aortic cusps | 14.7 ± 3.9 | Yes | ||
| 3 | 34 | Transposition of the great arteries | Right subclavian vein | No | Duodecapolar | 695 | 0 | 14.8 ± 5.4 | Yes |
CHD, congenital heart disease; LV, left ventricle; RV, right ventricle; VT, ventricular tachycardia.
| Procedures . | Age . | CHD . | RV access route . | LV access . | Multielectrode catheter . | Points for the RV voltage map . | Points for the activation map during VT . | Contact force during ablation (g) . | Successful procedure . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Double outlet right ventricle | Right subclavian vein | No | Pentaray | 3391 | 1182 RV | 11.4 ± 2.2 | No |
| 2 | Right jugular vein | Yes | Pentaray | 1000 | 851 RV, 651 LV, and 133 aortic cusps | 14.7 ± 3.9 | Yes | ||
| 3 | 34 | Transposition of the great arteries | Right subclavian vein | No | Duodecapolar | 695 | 0 | 14.8 ± 5.4 | Yes |
| Procedures . | Age . | CHD . | RV access route . | LV access . | Multielectrode catheter . | Points for the RV voltage map . | Points for the activation map during VT . | Contact force during ablation (g) . | Successful procedure . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Double outlet right ventricle | Right subclavian vein | No | Pentaray | 3391 | 1182 RV | 11.4 ± 2.2 | No |
| 2 | Right jugular vein | Yes | Pentaray | 1000 | 851 RV, 651 LV, and 133 aortic cusps | 14.7 ± 3.9 | Yes | ||
| 3 | 34 | Transposition of the great arteries | Right subclavian vein | No | Duodecapolar | 695 | 0 | 14.8 ± 5.4 | Yes |
CHD, congenital heart disease; LV, left ventricle; RV, right ventricle; VT, ventricular tachycardia.
A ventricular stimulation protocol (RVOT and RV apex, three extrastimuli) failed to reproduce the clinical VT, but a second non-clinical VT was induced (Supplementary material online, Figure S1C), with a negative concordance and superior axis, at 140 b.p.m. An activation map was performed but the entire cycle could not be mapped in the right ventricle (see Figure 1C, local activation time from −203 to + 138 ms, i.e. 341 ms for a VT cycle length of 428 ms). The ‘early-meets-late’ region was located superiorly to the VSD patch, with a supposed VT isthmus between this patch and the pulmonary annulus. At this site, concealed entrainment was proven with a post-pacing interval of +20 ms. Ablation was conducted during VT at the supposed isthmus but VT did not stop despite multiple radiofrequency (RF) energy delivery. The position of the catheter during ablation is shown in Figure 1D. This non-clinical VT was terminated by antitachycardia pacing but could be systematically reinduced. A possible left-sided septal origin of the VT was hypothesized but the left ventricle was not mapped due to the duration of the procedure (240 min, 36.4 min of fluoroscopy) and the supposed non-clinically relevance of the VT. No complications occurred.
The ablation was considered unsuccessful and a conventional dual-chamber implantable cardioverter-defibrillator (ICD) was implanted, with the RV lead inserted through the right subclavian vein. The patient was discharged from the hospital without antiarrhythmic drugs (neither beta-blocker nor amiodarone).
In December 2016, the patient experienced multiple VT recurrences treated by antitachycardia pacing. A redo procedure was scheduled. Since the ICD lead was implanted through the subclavian vein, a jugular approach was this time decided. Under general anaesthesia, two approaches were used: a venous right jugular access and an arterial right femoral access, and two catheters were alternatively used to map the right ventricle, a F-curve Pentaray and a 3.5 mm tip SmartTouch bidirectional large curve F-J (Biosense-Webster Inc., Diamond Bar, CA, USA). Right and left ventricular (LV) voltage maps were performed (Figure 2). Right ventricular scar was unchanged compared with the first procedure. A fluoroscopy movie of the ablation catheter positioned in the RVOT is shown in Supplementary material online, Movie S1. A large scar area was observed in the LV outflow tract, although a thin region of normal voltage was seen along its’ anterior aspect. A ventricular stimulation protocol failed to reproduce the initial clinical VT, but led to the induction of the VT recorded during the first procedure. Left and RV activation maps were performed. Mid-diastolic potentials were found in the anterior aspect of the LV outflow tract and in the junction between right- and left-coronary cusps (Figure 3A–C). Concealed entrainment was proven in this region (Figure3D and E). A propagation map of the VT is shown in Supplementary material online,Movie S2. Radiofrequency was delivered in this area leading to VT slowing and interruption.
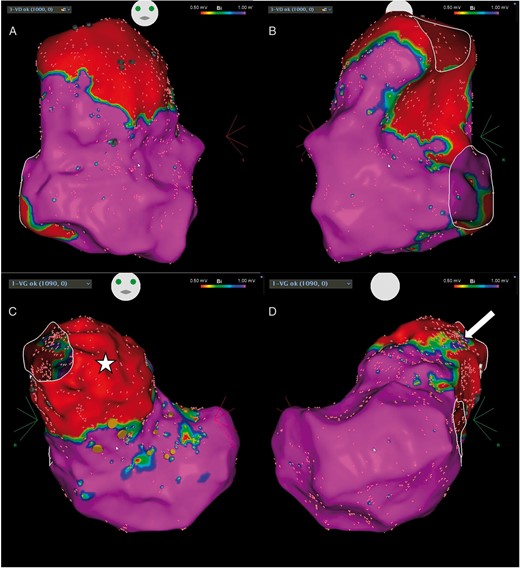
Right and left ventricular voltage maps performed during the redo procedure (A and B: RV in antero-posterior and postero-anterior views, respectively; C and D: LV in antero-posterior and postero-anterior views, respectively). The scar areas are similar to those found during the initial procedure in the RV. A large zone of scar was found in the LV outflow tract (C, star) except in its’ anterior segment (D, arrow). Orange dots denote the position of the His-bundle recording. LV, left ventricle; RV, right ventricle.
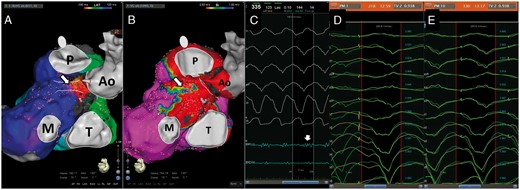
(A) Activation map of the VT induced (left postero-lateral view, Ao, aorta; M, mitral annulus; P, pulmonary annulus; T, tricuspid annulus). The position of the ablation lesions are indicated by the arrow. (B) The zone harbouring the diastolic potential, where ablation was performed was located in a small region of normal voltage along the anterior aspect of the LV outflow tract (arrow). (C) A mid-diastolic potential found in this given spot (blue line, arrow). The QRS pattern during entrainment in the middle and at the exit site of VT isthmus are shown in D and E. A 93.8% correlation was observed between the clinical VT and the pacing, with a long S-QRS in the middle of the isthmus and a short one at the exit site. LV, left ventricle; VT, ventricular tachycardia.
A ventricular stimulation protocol (two sites, three extrastimuli) performed at the end of the procedure failed to induce ventricular arrhythmias, even after isoprenaline infusion. There were no procedural complications and the patient remained free of arrhythmias for an 18-months follow-up.
Case 2
A 34-year-old man with a history of repaired CHD was admitted for VT. He had a transposition of the great arteries, with VSD and pulmonary stenosis surgically repaired during childhood (direct reimplantation of pulmonary artery on the right ventricle, VSD closure and construction of a ventricular tunnel connecting the left ventricle to the aorta). When he was 11, he underwent a second surgery to enlarge his RVOT, due to calcific PA patch retraction.
The patient was admitted for persistent palpitations due to well-tolerated VT. The ECG during VT showed a broad QRS complex tachycardia with left bundle branch block pattern and inferior axis (Supplementary material online, Figure S2A). Amiodarone was unsuccessful to stop the arrhythmia and an external cardioversion was performed to restore sinus rhythm (Supplementary material online, Figure S2B). Transthoracic echocardiography revealed a RV dilatation secondary to a severe pulmonary regurgitation. Cardiac magnetic resonance imaging showed left and right ejection fractions of 58% and 40%, respectively, but also found the presence of an IVC thrombosis. A right heart catheterization confirmed the presence of a massive pulmonary regurgitation and a bioprosthetic pulmonary valve replacement was performed, associated to the implantation of an epicardial single-chamber ICD and a cryoablation of supposed re-entrant VT isthmuses was performed. However, details regarding the ablation could not be obtained.
One month later, he was referred to our institution for an arrhythmic storm and multiple shocks delivered by the ICD without any lightheadedness nor syncope. Multiple sustained VT episodes (cycle length 360 ms) were confirmed by ICD interrogation, most of them successfully terminated by ATP. Despite 80 mg of nadolol, multiple recurrent episodes occurred. A VT ablation was scheduled, through a superior approach due to the pre-existing IVC thrombosis.
Under general anaesthesia, two subclavian accesses were obtained, and two catheters were introduced to map the right ventricle, a steerable duodecapolar catheter (Webster, Johnson & Johnson Medical) and a 3.5 mm tip SmartTouch bidirectional large curve F-J (Biosense-Webster Inc., Diamond Bar, CA, USA). A RV high density voltage map was performed (Supplementary material online, Figure S3A and B). As previously described in the first case, a large scar surrounded by a low-voltage area was observed in the anterior RVOT and the interventricular septum, at the location of the transventricular incision and of the VSD closure patch, respectively. Unfortunately, VT was non-inducible even after isoprenaline infusion. Pace-mapping was performed in multiple points all-around the RVOT and the VSD patch. A close match with the clinical VT was found in the isthmus between pulmonary valve and VSD scar, where the location of the critical isthmus was hypothesized. Potentials recorded by catheter at this spot were delayed and fractionated, occurring after the QRS complex (Supplementary material online, Figure S3B). An ablation line was performed through this supposed critical isthmus. The positioning of the RF catheter during ablation is shown in Supplementary material online, Figure S3C. A ventricular stimulation protocol (RVOT and RV apex, three extrastimuli) performed at the end of the procedure failed to induce any VT. An attempt was performed to prove the conduction block by through the ablation line using pacing manoeuvres with the duodecapolar and the ablation catheters, but positioning both catheters in optimal spots was challenging and the block could not be definitely proven. However, complete late potentials abolition was proven when the right ventricle was remapped after ablation. There were no procedural complications and the patient remained free of arrhythmias for a 18-months follow-up.
Discussion
Main results
The main results of this study are: (i) ablation of RV tachycardia in patients with repaired CHD and ‘no femoral access’ is feasible using a superior subclavian or jugular approach; (ii) multielectrode catheters can be used through this superior access to better define the scar and border zone areas; (iii) mapping may be challenging, requiring large curve catheters to target areas of interest; and (iv) putative isthmuses described in the literature can be mapped and successfully ablated.
Ventricular tachycardia ablation in patients with congenital heart disease
The population of adults with repaired CHD is growing.1 Consequently, an increasing number of these patients suffer from ventricular arrhythmias, contributing to a significant morbidity and responsible for arrhythmic sudden cardiac deaths.2 The critical RV isthmuses contributing to VT after repair of CHD have been described in detail by electroanatomic mapping studies,3,4 particularly for those patients with repaired tetralogy of Fallot.5 Four distinct types of anatomical isthmuses were described by the Zeppenfeld group, i.e. (i) isthmus 1 between the tricuspid annulus and a RV incision/RVOT patch; (ii) isthmus 2 between a RV incision and pulmonary valve; (iii) isthmus 3 between the pulmonary valve and VSD patch; and (iv) isthmus 4 between the VSD patch and the tricuspid annulus.3–5 All these anatomical isthmuses can be treated by RF catheter ablation, although hypertrophied myocardium or prosthetic material can hamper ablation success, sometimes requiring alternative approaches like a left-sided ablation for those VTs depending on septal anatomical isthmuses.6
Superior approaches for ablation of arrhythmias
The usual approach for RV mapping and ablation is the femoral access route. However, this approach may be difficult or not feasible in some patients, such as those with anatomical anomalies (bifurcating or tortuous veins), IVC interruption or agenesis, or IVC obstruction by prosthetic material or thrombus. Congenital anomalies of the IVC are not uncommon. The prevalence of azygos and hemiazygos continuation of the IVC is 0.6% of the general population. In such anomalies, IVC ends above the renal veins and venous blood flows directly into azygos or hemyazygos veins.7,8 These anomalies may be isolated or associated with other congenital anomalies, including congenital heart defects. In those cases, right cavities can be accessed using a transhepatic approach, as previously described in some case reports for atrial arrhythmias.9–11 The other possibility is to use a superior approach, either using a subclavian or a jugular access. Such approaches have been described for ablation of atrial flutter,12 atrioventricular nodal re-entrant tachycardia,13 or even atrial fibrillation.14 Rare cases of ablation of RV premature ventricular contractions15,16 and LV tachycardia17,18 have also been described. However, there are no reports about the feasibility and safety of performing VT mapping and ablation via a superior approach in patients with repaired CHD, who frequently have right atrial and ventricular dilatation that may make catheter manipulation, specifically multielectrode catheters, more complex.
Challenges of ventricular tachycardia ablation in congenital heart disease patients with no inferior vena cava access
In this article, we report the procedural characteristics of VT ablation in patients with surgically repaired CHD and no RV access through the IVC.
The first patient had IVC agenesis, associated with a complex repaired CHD. The isthmus suspected after mapping the VT induced was the 3rd described by the Zeppenfeld group,4,5 i.e. between the pulmonary valve and VSD patch. However, the entire cycle could not be mapped. Indeed, during the second procedure, a left-sided involvement was discovered, and the entire cycle could be mapped. Mapping at the region of the right coronary cusp and in the anterior aspect of the LV outflow tract revealed diastolic activity and concealed entrainment was proven. The pattern and activation of the VT induced in our patient resembles the Case 2 described by Kapel et al.,6 a counterclockwise activation of the isthmus 3, for whom ablation was performed within the right coronary cusp, whereas the clinical VT would be compatible with a clockwise activation of this same isthmus. Both VTs probably shared a common isthmus with opposite activations. The second patient had IVC thrombosis, impeding a regular RV access. Although the VT was not inducible, a good matching was found using pace-mapping in the region between the pulmonary valve and the VSD patch, the 3rd isthmus described by the Zeppenfeld group,4,5 and the ablation at the likely VT circuit was performed successfully.
In both patients, accessing the RVOT was feasible through a superior approach, although challenging. Catheter manipulation required an extra effort and time at the beginning of the procedure to understand and anticipate catheter movements and to learn innovative manoeuvres to access the required positions for ablation.19 Indeed, the access required large curve catheters that had to be torqued enough to point towards the pulmonary artery and pushed to the RVOT. As described, multielectrode catheter were used. The largest available Pentaray has a F-curve (76 mm torque diameter), making RVOT access difficult since, in our experience, the catheter was systematically going to the RV apex when pushed, instead of going to the RVOT. A generous curve was created using the distal 10 cm of the catheter to allow correct RVOT access and mapping. The 3.5 mm tip SmartTouch bidirectional large curve F-J (Biosense-Webster Inc., Diamond Bar, CA, USA) was used in all three procedures since the J curve is the largest currently available with the CARTO system, with a 102 mm torque diameter. Contact force catheters were used and ablation with >10 g could be performed in all three cases. We recommend the use of such catheters for superior access in order to warrant a proper contact in the zones requiring ablation delivery.
One drawback of the technique is that radiation exposure for the operator is higher compared with the femoral approach, due in part to increased fluoroscopy times (a consequence of the time required to position the catheters in a given spot) but also to the proximity of the operator to X-ray tube. Using robotic systems, such as the Stereotaxis, in such patients may help to overcome this drawback.20
Lastly, patient discomfort may be an issue. Indeed, catheter manipulation is the neck and the shoulder region may cause anxiety to the patient, a potential source of chest movement causing map shifts and procedure failure. To avoid such events, we recommend performing the procedure under general anaesthesia, as described here.
Limitations
This a single-centre experience of right VT ablation in a very limited number of patients with no femoral access. A larger population will be needed to validate the safety and feasibility of this approach. Lastly, we did not use long steerable sheaths, but such sheaths may facilitate catheter manipulation and could be used when a superior approach is required.
Conclusion
Ablation of RV tachycardia in patients with repaired CHD may be hampered by hypertrophied myocardium and prosthetic material, but also by RV access issues. In patients with ‘no-IVC access’, a superior approach is feasible using subclavian or jugular approaches as demonstrated in this manuscript. Mapping may be challenging, requiring large curve catheters. Conventional isthmuses can be mapped and ablated successfully, and such patients should not be denied RF ablation.
Conflict of interest: none declared.



